Abstract
One-third of men with obesity or type 2 diabetes have subnormal free testosterone concentrations. The lower free testosterone concentrations are observed in obese men at all ages, including adolescents at completion of puberty. The gonadotropin concentrations in these males are inappropriately normal; thus, these patients have hypogonadotropic hypogonadism (HH). The causative mechanism of diabesity-induced HH is yet to be defined but is likely multifactorial. Decreased insulin and leptin signaling in the central nervous system are probably significant contributors. Contrary to popular belief, estrogen concentrations are lower in men with HH. Men with diabesity and HH have more fat mass and are more insulin resistant than eugonadal men. In addition, they have a high prevalence of anemia and higher mortality rates than eugonadal men. Testosterone replacement therapy results in a loss of fat mass, gain in lean mass, and increase in insulin sensitivity in men with diabesity and HH. This is accompanied by an increase in insulin-signaling genes in adipose tissue and a reduction in inflammatory mediators that interfere with insulin signaling. There is also an improvement in sexual symptoms, anemia, LDL cholesterol, and lipoprotein (a). However, testosterone therapy does not consistently affect HbA1c in men with diabetes. The effect of testosterone replacement on cardiovascular events or mortality in men with diabesity is not known and remains to be studied in prospective trials.
Introduction
Approximately one-third of men with obesity, type 2 diabetes, or metabolic syndrome have subnormal free testosterone concentrations (1–3). Testosterone concentrations are inversely related to BMI and insulin resistance (3). One-third of obese young males (14–35 years of age) also have subnormal free testosterone concentrations. The gonadotropin concentrations in these males are inappropriately normal; thus, these patients have hypogonadotropic hypogonadism (HH). This review will detail the metabolic consequences of HH and the effects of testosterone replacement in men with obesity, type 2 diabetes, and metabolic syndrome. Because the pathophysiological mechanisms of all of these conditions are closely related, we have decided to refer to this group of patients as having “diabesity” in the rest of the review. Please note that this term is not limited to only those with the combination of diabetes and obesity.
Measurement of Testosterone Concentrations in the Obese
Prior to a detailed discussion about subnormal testosterone concentrations in obesity, it is important to clarify that obesity is associated with a decrease in both total and free testosterone. Because testosterone is largely bound to sex hormone–binding globulin (SHBG) (40–80%) and albumin (20–50%), total testosterone does not reflect the bioavailability of testosterone at the cellular level. Insulin-resistant states such as obesity and type 2 diabetes are known to be associated with low SHBG concentrations, and, in fact, there are data demonstrating that low SHBG may be a marker for the development of diabetes (4). Thus, there is a physiological lowering of total testosterone concentrations in obese men. Hence, free or bioavailable (non-SHBG–bound) testosterone measurement is essential in obese men to assess the gonadal status. The current gold standard method of measuring free testosterone involves separation of free testosterone by equilibrium dialysis and measurement by mass spectrometry (5). Although equilibrium dialysis is a tedious, expensive, and time-consuming technique, it is readily available at many commercial laboratories in the U.S. Free testosterone measured by radioimmunoassay (an assay that is still commonly used) is unreliable (5). Bioavailable testosterone can be accurately measured by ammonium sulfate precipitation. Free and bioavailable testosterone can also be calculated from SHBG, albumin, and total testosterone concentrations. Although the calculation equation continues to be refined, this calculated free/bioavailable testosterone has been shown to correlate very well with directly measured free or bioavailable testosterone and is well suited for epidemiological and clinical studies (5). Recent data indicate that linear models used to estimate free and bioavailable testosterone are based on binding affinity assumptions that are not entirely accurate and result in a systematic bias. Multistep ensemble allosteric models of testosterone binding to SHBG provide estimates of free testosterone levels that closely match free testosterone measured by equilibrium dialysis (5).
Accuracy of calculated free/bioavailable testosterone is predicated upon the precise measurement of total testosterone. Liquid or gas chromatography/tandem mass spectrometry is the gold standard method to measure total testosterone (5). The Endocrine Society has recently concluded a project to harmonize testosterone mass spectrometry assays across the U.S. This project calculated that the harmonized reference range (2.5–97.5 percentiles) for total testosterone was 264–916 ng/dL for nonobese men aged 19–39 years (5). Further work is now needed to harmonize assays that accurately measure free or bioavailable testosterone across all laboratories and to provide validated equations for calculation of free/bioavailable testosterone. Free and bioavailable testosterone concentrations correlate strongly with each other, and measurement of only one of them is required for clinical purposes. It is not yet clear whether free or bioavailable testosterone is a better marker of delivery of testosterone molecule to the tissue. Nor is a good test available to measure tissue androgen action. In the meantime, it is prudent to measure free testosterone by equilibrium dialysis/mass spectrometry and follow the reference ranges provided by the laboratory (5). Although these assays are more widely available in the U.S. than those for measurement of bioavailable testosterone, they usually require shipping of the sample by the local laboratory to a central laboratory. When ordering the free testosterone test, the clinician must specify that free testosterone is to be measured by the above-mentioned technique so that the sample is adequately processed and not measured by radioimmunoassay.
Association of Type 2 Diabetes With Low Free Testosterone
It has been known for over two decades that males with type 2 diabetes frequently have low total testosterone concentrations (6). These studies did not report free testosterone or gonadotropin concentrations, nor did they place low testosterone concentrations in a clinically relevant context. Subnormal free testosterone concentrations in association with inappropriately low luteinizing hormone (LH) and follicle-stimulating hormone (FSH) concentrations in men with type 2 diabetes were first described by us in 2004 (1,2). These patients had normal LH and FSH responses to gonadotropin-releasing hormone (GnRH) stimulation pointing to a hypothalamic defect. These abnormalities were independent of the duration and severity of diabetes (HbA1c). MRI in these hypogonadal patients showed no abnormality in brain or the pituitary (2). Free testosterone concentrations were inversely related to BMI. This association of HH with type 2 diabetes has now been confirmed in several studies and is present in 25–40% of these men (7,8). We also found that younger men with type 2 diabetes between the ages of 18 and 35 years have a similarly high prevalence of HH (9).
HH is relatively rare in type 1 diabetes, unless the patients are obese. Therefore, HH is not entirely a function of diabetes or hyperglycemia per se (10). Thus, in view of the inverse relationship between BMI and testosterone concentrations in both type 1 and type 2 diabetes, HH is probably related to insulin resistance (2,8,10). Improvement of systemic insulin resistance by rosiglitazone leads to a modest increase in testosterone concentrations in men with type 2 diabetes (11), but without the restoration of testosterone concentrations to normal.
Association of Obesity and Metabolic Syndrome With Low Free Testosterone
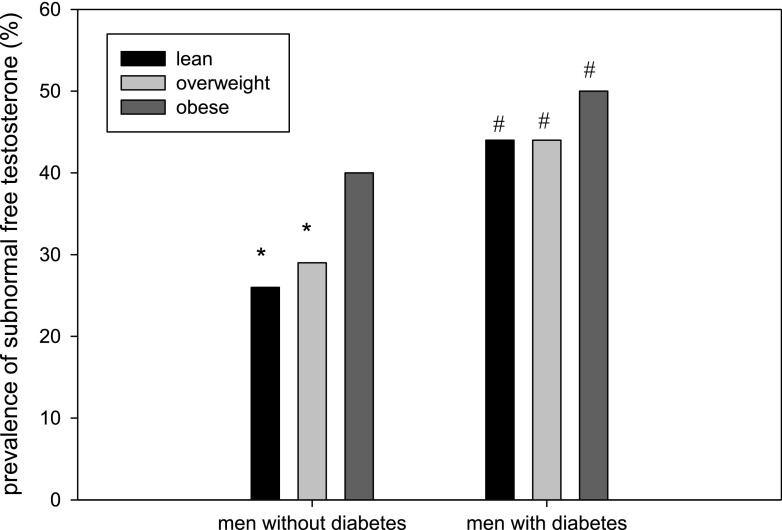
Many studies have shown free testosterone concentrations to be low in the obese, especially in those with a BMI ≥40 kg/m2 (12,13). Zumoff et al. (13) studied 48 healthy men with BMI ranging from 21 to 95 kg/m2 and collected blood samples every 20 min for a period of 24 h. They found that 24-h mean free testosterone and non-SHBG–bound testosterone were related inversely to BMI. Vermeulen et al. (12) compared 35 obese men (mean BMI 41.1 kg/m2) with 54 lean men. The free testosterone concentrations were 26% lower in the obese. The authors also compared LH pulsatility over 12 h in eight obese and lean men and found that the mean integrated LH levels over 12 h were significantly lower in obese men. Free testosterone concentrations correlated positively with the sum of LH pulse amplitudes in each individual (12). Some recent studies have examined the prevalence of hypogonadism in obesity in a much larger number of men. A study from the Netherlands in 160 obese men (mean age 58 years) found a 36% prevalence of HH (14). The largest study in this regard found that 40% of obese men and 50% of obese men with diabetes had subnormal free testosterone concentrations (Fig. 1) (3). Thus, obesity is associated with a high prevalence of hypogonadism, and the presence of diabetes adds to that risk only minimally (1).
Figure 1.
Prevalence of subnormal free testosterone concentrations in lean, overweight, and obese men (based on BMI) with and without diabetes. Mean age 60 years; range 45–96 years. A total of 44% of men with diabetes and 33% of age-matched men without diabetes had subnormal free testosterone concentrations, respectively. Sample size: 275 lean, 687 overweight, and 489 obese men without diabetes; 36 lean, 135 overweight, and 227 obese men with diabetes. *P < 0.05 vs. obese men in the same group (with diabetes or without diabetes); #P < 0.05 vs. men without diabetes. Adapted from data published in Dhindsa et al. (3).
The inverse relation of free testosterone concentrations with obesity is not restricted to middle-aged men (9,15,16). We compared the testosterone, LH, and FSH concentrations of 25 lean and 25 obese boys in Tanner stage 4 and 5 (aged 14–20 years) (16). The free testosterone concentrations (measured by equilibrium dialysis followed by mass spectrometry) of obese boys were 40% lower than those of lean boys. In addition, 40% of obese adolescents had subnormal free testosterone concentrations (defined as <5th percentile of free testosterone concentrations of lean adolescents). The gonadotropin concentrations of lean and obese boys were similar. The free testosterone concentrations were inversely related to BMI, HOMA of insulin resistance (HOMA-IR), and CRP concentrations. Another study has also shown lower free testosterone concentrations in obese postpubertal adolescents (17).
The association of diabesity with HH is not simply reflective of increased fat mass but also of adverse metabolic health. Some studies show that free testosterone concentrations are inversely related to HOMA-IR, triglyceride concentrations, inflammatory mediators, and subcutaneous adipocyte cell size, independently of BMI (16,18). In men with metabolic syndrome, free testosterone concentrations decrease proportionately with increasing number of components of metabolic syndrome (19).
Possible Pathophysiological Mechanisms Underlying HH in Diabesity
Estradiol
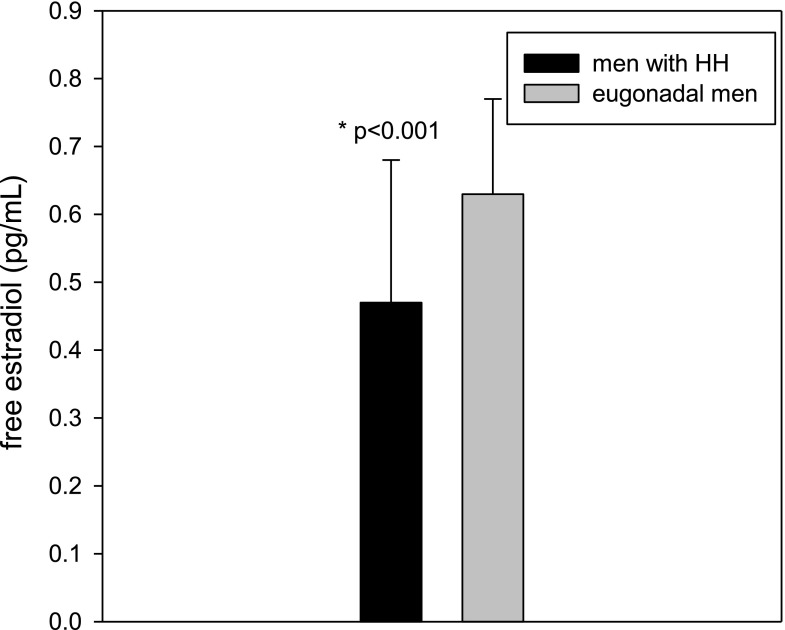
Because testosterone and androstenedione in the male can be converted to estradiol and estrone, respectively, through the action of aromatase in the mesenchymal cells and preadipocytes of adipose tissue, it has been suggested that excessive estrogen production due to aromatase activity in the obese may potentially suppress the hypothalamic secretion of GnRH (1,20). However, we have shown that this widely believed presumption is not true (21). Total and free estradiol concentrations in men with HH are significantly lower than in those without HH (21) (Fig. 2). Population-based studies such as the European Male Ageing Study also showed that estradiol concentrations are lower in hypogonadal men as compared with eugonadal men, regardless of whether the hypogonadism is primary or secondary (22). Clomiphene (estrogen antagonist) and aromatase inhibitors (which decrease estradiol concentrations) have been shown to increase testosterone concentrations in obese men with low testosterone. This, however, cannot be taken as evidence of estradiol as the cause of low testosterone in those men. Estradiol has an inhibitory effect upon gonadotropin secretion even in a normal physiological setting (23). Clearly, the suppression of LH, FSH, and testosterone in diabesity is not induced by circulating estradiol concentrations.
Figure 2.
Men with HH have lower free estradiol concentrations than eugonadal men. Estradiol concentrations in men with and without HH were 0.47 (0.35, 0.68) and 0.63 (0.46, 0.77) pg/mL, respectively. Adapted from Dhindsa et al. (21).
Obstructive Sleep Apnea
Serum testosterone concentrations begin to increase with the onset of sleep and in young men they peak at the first rapid eye movement sleep episode and remain at that level until waking. Testosterone production in men is related to rapid eye movement sleep, sleep duration, and sleep architecture (24). Obstructive sleep apnea is common in men with obesity and type 2 diabetes. A quarter of men with diabesity have severe sleep apnea, defined as apnea-hypopnea index of >30/h (25). However, sleep apnea does not seem to be a determinant of total, free, or bioavailable testosterone concentrations independently of BMI (24). Furthermore, positive airway pressure therapy for sleep apnea does not increase testosterone concentrations (26). Thus, obstructive sleep apnea per se is not a major contributor to the lowering of free testosterone concentrations in obesity.
Neuronal Insulin Resistance
Obesity is associated with decreased insulin signaling in the central nervous system (27). This association may be bidirectional because increasing insulin delivery to the central nervous system decreases body weight in men (28). Neuronal insulin receptor knockout mice become obese and insulin resistant (29). Interestingly, these mice also have a reduction in LH concentrations by 60–90% and low testosterone concentrations. These animals respond to GnRH challenge by normal or supranormal release of LH. In addition, it is known that the incubation of hypothalamic neurons with insulin results in the facilitation of secretion of GnRH (30). However, it is unlikely that the site of action of insulin on reproductive axis in vivo is the GnRH neuron. Isolated knockout of the insulin receptor in a GnRH neuron does not lead to a decrease in LH concentrations or in fertility in either male or female mice (31). Although the site (or sites) responsible for hypogonadotropism in neuronal insulin receptor knockout mice is not known, it is clear that insulin action and insulin responsiveness in the brain are necessary for the maintenance of the functional integrity of the hypothalamo-hypophyseal-gonadal axis.
Leptin
Leptin is known to play a permissive role in the regulation of reproductive axis. Leptin appears to serve as a signal of energy reserves to regulate the hypothalamo-pituitary-gonadal axis in relation to nutritional status. Men and women with anorexia nervosa have hypogonadotropism and low levels of leptin. Absence of the leptin gene or leptin signaling in humans results in HH (32). Although there are extremely uncommon forms of human obesity due to the lack of the leptin gene, almost all obesity in humans is associated with leptin resistance and high leptin concentrations. It is possible that leptin resistance in the hypothalamus or in some other neurons is responsible for the hypogonadotropism seen in obesity.
Kisspeptin
It is now known that kisspeptin, a hypothalamic neuropeptide encoded by the KISS1 gene, and the presence of kisspeptin receptors on the GnRH neurons (G protein–coupled receptor 54) is obligate for the release of GnRH (33). Intravenous injection of kisspeptin increases LH and testosterone concentrations in men with type 2 diabetes and HH (34). This suggests that the hypothalamic defect in men with HH and type 2 diabetes is either at kisspeptin level or proximal to it. Kisspeptin neurons express both leptin and insulin receptors, thus possibly accumulating evidence of metabolic health and translating it into reproductive health.
Inflammation
Tumor necrosis factor-α and interleukin-1β have been shown to suppress hypothalamic GnRH and LH secretion in experimental animals and in vitro (35). CRP concentrations are markedly increased in hypogonadal men as compared with men with normal testosterone concentrations (6.5 vs. 3.2 mg/L) (8,36). It is thus possible that inflammatory mediators may contribute to the suppression of the hypothalamo-hypophyseal axis and the syndrome of HH in type 2 diabetes. The presence of inflammation may also contribute to insulin resistance because inflammation-related mediators contribute to insulin resistance (37). These mediators are also known to be increased in obesity (38).
What Comes First: HH or Diabesity?
It is generally assumed that the association of low testosterone and obesity is bidirectional. Low testosterone predisposes to obesity and vice versa. Indeed, there are definite lines of evidence for both directions of this association in different clinical settings. Men who are rendered hypogonadal by GnRH agonist therapy for advanced prostate cancer increase their subcutaneous and visceral fat mass by 15–20%. Consistent with this, they develop insulin resistance, and their risk of incident type 2 diabetes increases by 30% (39). In the other direction, boys who are obese prepubertally do not achieve normal testosterone concentrations at the completion of puberty. As mentioned above, obese boys have lower free testosterone concentrations than lean boys (16). Furthermore, bariatric surgery in morbidly obese patients restores testosterone concentrations to normal after marked weight loss (40).
Is Testicular Function Directly Altered in Diabesity?
Human chorionic gonadotropin–induced testosterone secretion by Leydig cells is related inversely to insulin sensitivity among men with varying degrees of glucose tolerance (41). Decreased testosterone secretion in insulin-resistant men after pharmacological stimulation by high doses of human chorionic gonadotropin suggests that the lesion resulting in HH of diabesity may occur at several levels of the hypothalamic-pituitary-gonadal axis. The absence, however, of an increase in gonadotropin concentrations indicates that the primary major defect in diabesity is at the hypothalamo-hypophyseal level (1).
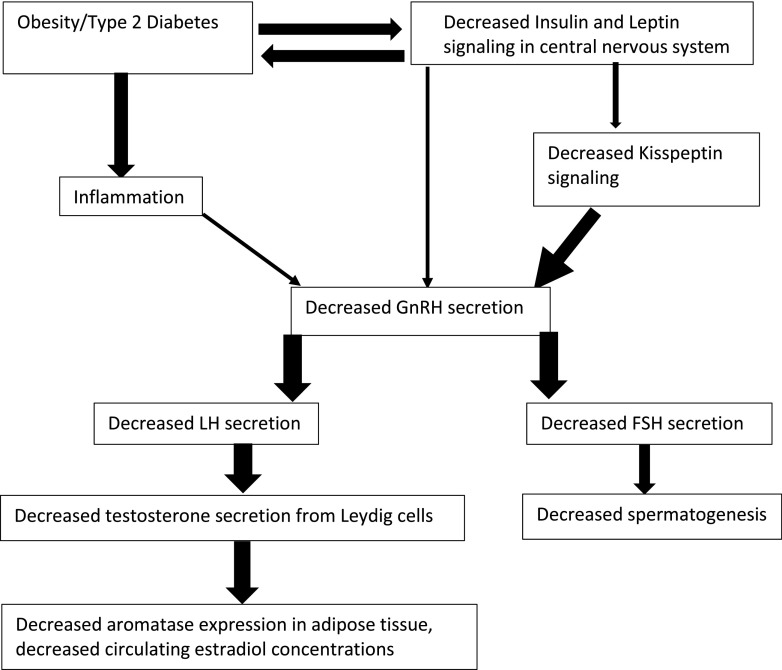
The specific metabolic insult in diabesity that results in hypogonadotropism is yet to be defined. It is very likely that the cause is multifactorial (Fig. 3). Decreased insulin and leptin signaling in the central nervous system appear to be the leading candidates. Direct evidence in humans supporting or disproving this hypothesis is, however, lacking.
Figure 3.
Interplay of different factors in obesity associated HH. The thickness of arrows is proportional to the strength of available evidence supporting the mechanism.
Effect of Weight Loss on Testosterone Concentrations
Many studies have shown that weight loss increases total testosterone and SHBG concentrations (summarized in Grossmann [42]). On average, 5% weight loss increases total testosterone concentrations by 50 ng/dL. Bariatric surgery in morbidly obese adults has been shown to induce a reversal of the hypogonadal state and the restoration of normal testosterone concentrations (40). A meta-analysis of 29 studies of men undergoing bariatric surgery showed that 64% of the men had subnormal total testosterone concentrations prior to surgery (40). The prevalence of subnormal free testosterone concentrations was not ascertained in the analysis. After bariatric surgery, total testosterone and SHBG concentrations increased by 233 ng/dL and 22 nmol/L, respectively, in a total of 439 men across 16 studies. Eight studies calculated free testosterone concentrations in a total of 259 men: there was an increase of 2.2 ng/dL on average. LH and FSH concentrations increased by 1.0 and 1.8 IU/L, respectively.
It is tempting to speculate that the reduction in aromatase activity due to fat loss is the major mechanism underlying the increase in testosterone concentrations following weight loss. However, as we have discussed before, elevated estrogen concentrations are not the cause of obesity-associated HH. Weight loss may restore neuronal leptin sensitivity and thus increase gonadotropin release. However, exercise, even in the absence of weight loss, increases testosterone concentrations (43). It is possible that insulin sensitization at the hypothalamic level mediates the increase in free testosterone concentrations following weight loss.
Clinical Characteristics of HH in Men With Diabesity and the Effects of Testosterone Treatment
Sexual Symptoms
It is well accepted that subnormal testosterone concentrations are associated with sexual symptoms in elderly men. Cross-sectional studies have found that approximately two-thirds of men with type 2 diabetes and HH have symptoms of low libido, erectile dysfunction, or fatigue (7). In clinical practice, it is often difficult to determine whether the etiology of symptoms is hypogonadism or one of the other comorbidities. However, the prevalence of sexual symptoms is higher in men with HH as compared with eugonadal men (44). In a randomized trial of transdermal testosterone gel or placebo gel for 1 year in patients with type 2 diabetes and hypogonadism, there was an improvement in sexual desire, but other symptoms did not change (45). Trials of testosterone replacement in hypogonadal men with type 2 diabetes have also shown an improvement in sexual desire (44–46). In contrast to consistent effect of testosterone replacement therapy (TRT) on improving libido, its effect on erectile dysfunction is modest and inconsistent among studies (44,45). Hypogonadal men with erectile dysfunction are much more likely to have symptomatic benefit from phosphodiesterase-5 inhibitors than from TRT. Furthermore, addition of TRT to phosphodiesterase-5 inhibitors does not improve the erectile response as compared with phosphodiesterase-5 inhibitors alone (47).
Body Composition
It is well known that muscle mass is inversely related to testosterone concentrations in men and that testosterone treatment results in an increase in muscle mass. We measured the fat mass and lean mass by DEXA scans in 138 men with type 2 diabetes. Men with HH had a higher BMI (by 3 to 4 kg/m2), 12% more subcutaneous fat mass, and higher waist-to-hip ratio (2,7,48) as compared with eugonadal men. Men with HH also have higher visceral and subcutaneous fat content as compared with eugonadal men (44). After testosterone replacement, there is a decrease in fat mass and increase in lean mass (Table 1). We randomized 44 men with type 2 diabetes and HH to intramuscular therapy with testosterone or placebo injections for 6 months (44). Men randomized to TRT lost ∼3 kg of subcutaneous fat and gained ∼3 kg of lean mass, without a net change in body weight. There was no change in visceral or hepatic fat mass after TRT.
Table 1.
Effect of TRT on nonsexual parameters in men with diabesity
| Parameter | Change | References |
|---|---|---|
| Fat mass | ↓ | (44,49–51,53,54, 56, 61) |
| Lean mass | ↑ | (44,53,56) |
| BMI | ↔ | (44,45,55,56) |
| Insulin sensitivity | ↑ | (44,49,50,54) |
| Glycemic control | ↔ | (44–46,55,56,61) |
| Hemoglobin | ↑ | (44,62) |
| Bone density | Not studied | |
| Lipids | ↓ in total cholesterol, LDL, and lipoprotein (a), minor decrease in HDL, ↔ in triglycerides | (44,45,56) |
| Cardiovascular disease | Not studied | |
| PSA | ↔ | (44,45,51,54) |
We focused on randomized controlled trials in men with obesity, type 2 diabetes, or metabolic syndrome. If there was disagreement among studies on a parameter, we chose to depict it as increased (↑), decreased (↓), or no change/variable results (↔) based on the totality of evidence.
Similar to the effects of TRT in men with type 2 diabetes, a decrease in subcutaneous fat and an increase in lean mass after TRT have been shown to occur in obese men without diabetes and men with metabolic syndrome (49–51). The loss of fat after testosterone therapy is attributed to aromatization of testosterone to estradiol (52). It is of interest that although caloric reduction induced weight loss is due to reduction of both fat mass and lean mass, weight loss due to TRT in combination with very low-calorie diet is exclusively due to fat loss (53). TRT also reduces circulating leptin concentrations (44).
Insulin Resistance
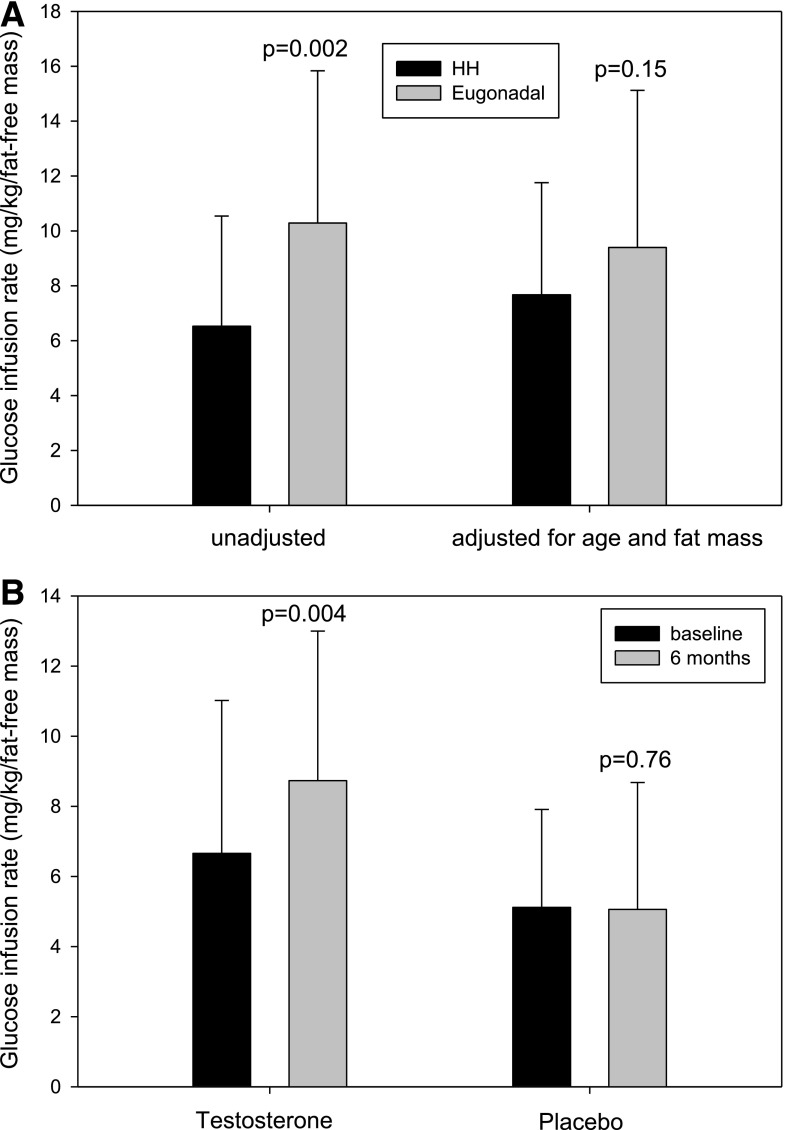
Many studies have shown that hypogonadism is associated with insulin resistance. Almost two decades ago, Mårin et al. (49,50,54) demonstrated an improvement in insulin sensitivity (as measured by euglycemic clamp) with oral and transdermal T treatment in obese men without diabetes. We have recently shown that men with type 2 diabetes and HH are less insulin sensitive than those without HH and that TRT increases insulin sensitivity (as measured by glucose infusion rate during hyperinsulinemic-euglycemic clamps) in men with HH (Fig. 4) (44). HOMA-IR also decreased by 40%. Jones et al. (45) showed a 16% decrease in HOMA-IR after 1 year of treatment with transdermal testosterone in 220 hypogonadal men with type 2 diabetes. However, two trials did not show a change in HOMA-IR after replacement with long-acting intramuscular testosterone given for 30–40 weeks in hypogonadal men with type 2 diabetes (55,56). HOMA-IR may be inadequate as an index of insulin resistance, especially in patients with type 2 diabetes, because β-cell loss and inadequate insulin secretion can lead to inappropriately low insulin concentrations and HOMA-IR. In contrast, HOMA-IR is assessed under fasting conditions and therefore a different index of insulin sensitivity than hyperinsulinemic clamp, which largely measures glucose uptake by muscle. Because TRT increases muscle mass, TRT may have a more prominent effect on muscle glucose uptake rather than HOMA-IR. In our study, there was no change in glucose uptake during clamps at an early time point of 3 weeks (44). Thus, it appears that the insulin sensitization of testosterone is not an immediate effect and may be mediated by changes in body composition. The results of TRT on insulin sensitization are inconsistent in hypogonadal men who are not obese (57). In addition, TRT replacement in trials composed largely of men with low normal testosterone (instead of subnormal) concentrations fail to show a change in insulin sensitivity (58).
Figure 4.
A: Insulin sensitivity measured by hyperinsulinemic-euglycemic insulin clamps in 94 men with type 2 diabetes (44 men had HH, and 50 were eugonadal). Men with HH had greater BMI (40 vs. 34 kg/m2; P < 0.001) but similar age (55 vs. 52 years; P = 0.08) and HbA1c (7.0 vs. 7.1%; P = 0.7) as compared with eugonadal men. The glucose infusion rate during hyperinsulinemic-euglycemic insulin clamps was 36% lower in men with HH as compared with eugonadal men. The glucose infusion rate between men with and without HH was, however, not different once adjusted for BMI difference in the two groups. This suggests that obesity is the predominant determinant of insulin resistance in men with HH. B: A total of 44 men with HH were randomized to intramuscular testosterone or placebo injections every 2 weeks for 6 months. Insulin sensitivity (glucose uptake during clamps) increased by 32% after 6 months. Data adapted from Dhindsa et al. (44).
In parallel with an increase in glucose uptake during clamps, we found a significant increase in the expression of insulin receptor β, insulin receptor substrate-1 (IRS-1), Akt-2, and GLUT-4 in adipose tissue. These data are consistent with similar effects found after TRT in mice (59). This provides a mechanistic explanation for the increase in insulin sensitivity. Testosterone administration also induced a reduction in free fatty acid concentrations, probably through the suppression of lipolysis. Free fatty acids are known to induce oxidative and inflammatory stress and interfere with insulin signal transduction (60). The improvement in insulin sensitivity following testosterone treatment was also associated with suppression of other inflammatory mediators that interfere with insulin signaling. These included IKKβ, SOCS-3, and PTEN in mononuclear cells and TLR-4 and PTP-1B in adipose tissue. Although SOCS-3 interferes with insulin signal transduction by causing the ubiquitination and proteasomal degradation of IRS-1, IKKβ induces serine phosphorylation of IRS-1 and thus prevents insulin signal transduction though IRS-1. PTP-1B dephosphorylates the insulin receptor after the autophosphorylation by tyrosine kinase and thus limits insulin signaling. The expression of PTEN, another protein that interferes with insulin signaling, was also suppressed in mononuclear cells following testosterone treatment (44). However, we cannot state whether they are the direct actions of testosterone or are indirectly mediated by changes in adiposity.
Glycemic Control
Some studies have evaluated glycemic control by measuring HbA1c and fasting glucose after testosterone therapy (Table 1). Kapoor et al. (46) showed a decrease in fasting glucose (28 mg/dL) and HbA1c (0.37%) as compared with placebo with 3 months of testosterone replacement in a small trial. A trial in men with new-onset type 2 diabetes with transdermal testosterone also showed a decrease in HbA1c from 7.5 to 6.3% over a period of 1 year (61). This was in conjunction with diet and exercise, but no hypoglycemic medications. However, some randomized trials did not show an effect of testosterone replacement on HbA1c (44,45,55,56). Furthermore, it is possible that increased erythropoiesis after testosterone replacement may affect HbA1c independently of changes in glycemia. Studies with careful collection of self-monitored glucose concentrations and measures of glycemia other than HbA1c should be designed to evaluate the effect of testosterone on HbA1c independently of glycemia.
Anemia
Men with HH have a higher prevalence of normocytic normochromic anemia (38%) as compared with eugonadal men (3%) (36). This anemia is associated with an increase in hepcidin and a decrease in ferroportin and transferrin receptor expression. Testosterone therapy increases erythropoiesis. We found that hemoglobin and hematocrit concentrations increased by 0.54 g/dL and 2.3%, respectively, after 6 months of testosterone therapy in men with type 2 diabetes (44). TRT in hypogonadal men with type 2 diabetes leads to a significant increase in plasma erythropoietin concentration and enhances the mobilization of iron from its stores by suppressing hepcidin and increasing the expression of ferroportin and transferrin on mononuclear cells (62).
Expression of Androgen Receptor in HH
It is intuitive to expect that the state of HH in diabesity would result in a compensatory increase in the expression of androgen receptor. However, we found a decreased expression of androgen receptor in mononuclear cells and adipose tissue in men with HH and diabetes as compared with eugonadal men (63). The expression of estrogen receptor-α and aromatase was also reduced in adipose tissue. Thus, rather than there being a compensatory increase, there is a decrease in androgen receptor, estrogen receptor, and aromatase expression. Thus, the hypogonadal state in diabetes is also associated with diminished responsiveness to testosterone and estradiol. TRT restored the expression of androgen receptor, estrogen receptor, and aromatase to normal.
Bone Density
In epidemiological studies, estradiol concentrations correlate more robustly with bone mineral density than testosterone concentrations in men (64). Free testosterone concentrations are positively associated with bone density in arms, ribs, and lumbar spine in men with type 2 diabetes (48). No study has evaluated the relation between free estradiol concentrations and bone density or fractures in men with HH and diabesity. The effect of TRT on bone density or fracture rates in men with diabesity has also not been studied.
Lipid Profile
It used to be believed that testosterone treatment adversely affects cardiovascular risk because it lowers HDL cholesterol concentration. However, that effect is restricted to treatment that achieves supranormal concentrations of testosterone (for example, during abuse of androgens for body building). Testosterone has not been shown to cause any meaningful change in HDL concentrations in studies in which testosterone is replaced to normal levels (5). Some studies have shown a reduction in LDL or total cholesterol concentrations after TRT in men with diabetes (45,56). Most studies find no change or a small decrease in HDL cholesterol and triglycerides after TRT (44,45,56).
Cardiovascular Disease
Epidemiological studies have shown that elderly men with low testosterone are more likely to die of a major cardiovascular event (65). Inverse association of mortality with endogenous testosterone concentrations has also been observed in men with diabetes (66). However, no randomized control trials have been conducted to examine the question: “Does TRT change cardiovascular outcomes in men?” Cardiovascular outcomes have been sporadically reported in randomized trials of TRT designed for other end points (such as muscle strength and glucose control), but these trials were underpowered to look at cardiac events. Meta-analyses of these trials do not find a consistent effect of TRT on cardiovascular events (67). A recently concluded randomized placebo-controlled trial in elderly men (≥65 years of age, 790 subjects) by Snyder et al. (68) found no difference in cardiovascular events between men who received TRT or placebo for 1 year. A prospective cohort study in an endocrine clinic investigated the effect of TRT in 238 hypogonadal men with HH and type 2 diabetes on all-cause mortality (66). Sixty-four hypogonadal men received testosterone (mean duration 42 ± 20 months), and 174 men were not treated. The mortality rate in untreated hypogonadal men was 20%, whereas hypogonadal men treated with testosterone had a mortality rate of 9.4% (P = 0.002). Most retrospective epidemiological studies have shown a benefit on cardiovascular events from long-term testosterone use in elderly men (69–71). In one of the largest studies conducted on this issue, Sharma et al. (70) showed a 56% reduction in total mortality and 24% reduction in myocardial infarction with the use of TRT. Some reports have shown evidence of harm with short-term testosterone use. However, these studies had a number of shortcomings (67). Large-scale prospective randomized controlled trials on testosterone therapy, focusing on cardiovascular benefits and risks, are clearly needed.
Prostate-Specific Antigen
Men with type 2 diabetes have 20% lower prostate-specific antigen (PSA) concentrations than men without diabetes. PSA concentrations are lower in hypogonadal than in eugonadal men with diabetes (0.89 vs. 1.1 ng/mL) (72). It is interesting that the incidence of prostatic carcinoma is lower in men with diabetes. This is in contrast to the increased incidence of cancer in diabetes in various organs including the colon, kidney, breast, endometrium, and pancreas. Similar to type 2 diabetes, obesity is also associated with 10–30% lower PSA concentrations and lower prostate cancer incidence (73). Obese men are half as likely to have PSA concentrations >4 mg/L. The lower PSA concentrations may not be a result of lower testosterone concentrations in obesity and type 2 diabetes, but may also be due to the larger plasma volumes and hence hemodilution (74). Prostate cancer progression and mortality, however, are increased in obese men, possibly related to later detection due to lower PSA concentrations (75).
The PSA concentrations are lower in hypogonadal men than in eugonadal men. TRT may result in a modest increase in PSA concentrations (∼30%). In most studies of TRT replacement in men with diabesity, there is no change in PSA concentrations after TRT (44,45,51,54). In this context, it is important that the replacement of testosterone in hypogonadal patients in general does not lead to an increased risk of prostatic carcinoma, although the trials have been too limited in duration and number of patients (5).
Spermatogenesis
Some (but not all) studies have shown that BMI is inversely related to sperm counts, sperm morphology, and sperm motility. Data are most consistent about the impact of morbid obesity on spermatogenesis. In a meta-analysis of 21 studies, BMI >40 kg/m2 was associated with a doubling of risk of oligospermia (<40 million spermatozoa per ejaculate) or azoospermia (76). The high prevalence of HH in obesity and type 2 diabetes, especially in young men, may partially explain the high prevalence of infertility or oligospermia in these men. Studies comparing sperm parameters of men who are obese or have diabetes with and without HH have not been conducted. TRT cannot be used in those who desire fertility because it decreases spermatogenesis.
Practical Considerations for a Clinician
Should Every Man With Diabesity Have His Testosterone Concentration Checked?
The answer to this question is yes. The high prevalence justifies screening for HH. Limiting testing only to men who report symptoms is likely to result in false negatives. Hypogonadal patients may slide gradually into this clinical state without any overt symptoms, which may be revealed through direct questioning. Asymptomatic men may realize that they had been symptomatic only after a trial with testosterone. However, a complete discussion of risks and benefits between the patient and physician should precede a trial of TRT, and the decision about treatment should be weighed carefully by the physician.
What Test Should Be Used to Make a Biochemical Diagnosis of Testosterone Deficiency in a Man With Diabesity?
Free testosterone should be checked in the morning (fasting) by accurate methodology, preferably equilibrium dialysis/mass spectrometry. Studies have shown that testosterone has a diurnal variation, and its concentrations also decline after eating. Total testosterone should not be used to make diagnostic or therapeutic decisions.
What Workup Should Be Undertaken in a Man With Diabesity and Subnormal Free Testosterone?
Subnormal free testosterone needs to be confirmed (at least once). Men with one normal value and one low value are not hypogonadal. Studies have generally shown a lack of benefit of TRT in men with low normal testosterone concentrations. A man with confirmed hypogonadism should have LH concentrations measured. The majority have HH. MRI of the pituitary is not beneficial in the majority of these men due to low incidence of anatomical pathology. MRI of the pituitary should be done if there are visual symptoms, headaches, or other pituitary hormone deficits. MRI is also advisable if the free testosterone is very low (<50% of the lower limit of normal).
How Should Men Receiving TRT Be Followed?
SHBG concentrations are decreased by TRT. Hence, free testosterone should be used to titrate testosterone dosing during TRT. Hemoglobin and prostate should be monitored as per guidelines (5).
These recommendations are opinions based on the authors’ clinical experience.
Conclusions
HH is found in 25–33% of men with diabesity. Although the underlying mechanism is not known, neuronal insulin and leptin resistance may play a role at the hypothalamic level. Low free and bioavailable testosterone concentrations in these men are associated with an increased prevalence of sexual symptoms, obesity, high CRP concentrations, mild anemia, insulin resistance, and decreased bone mineral density. In addition, these men may have an elevated risk of cardiovascular events and death. Short-term studies of testosterone therapy have demonstrated an increase in libido, insulin sensitivity, and lean body mass and a reduction in inflammation and fat mass. Men on TRT should be monitored for the development of polycythemia or prostate complications. Trials of a longer duration are clearly required to definitively establish the benefits and risks of TRT in these men.
Article Information
Funding. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant R01-DK-075877 to P.D.) and American Diabetes Association (Junior Faculty grant 110JF13 to S.D.).
Duality of Interest. P.D. is on the speaker panel for and provides research support to AbbVie. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.D. and P.D. wrote the manuscript and researched data. H.G. contributed to discussion and reviewed and edited the manuscript. M.B. reviewed and edited the manuscript.
References
- 1.Dandona P, Dhindsa S. Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab 2011;96:2643–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004;89:5462–5468 [DOI] [PubMed] [Google Scholar]
- 3.Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010;33:1186–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 2009;361:1152–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018;103:1715–1744 [DOI] [PubMed] [Google Scholar]
- 6.Barrett-Connor E, Khaw KT, Yen SS. Endogenous sex hormone levels in older adult men with diabetes mellitus. Am J Epidemiol 1990;132:895–901 [DOI] [PubMed] [Google Scholar]
- 7.Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007;30:911–917 [DOI] [PubMed] [Google Scholar]
- 8.Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008;93:1834–1840 [DOI] [PubMed] [Google Scholar]
- 9.Chandel A, Dhindsa S, Topiwala S, Chaudhuri A, Dandona P. Testosterone concentration in young patients with diabetes. Diabetes Care 2008;31:2013–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomar R, Dhindsa S, Chaudhuri A, Mohanty P, Garg R, Dandona P. Contrasting testosterone concentrations in type 1 and type 2 diabetes. Diabetes Care 2006;29:1120–1122 [DOI] [PubMed] [Google Scholar]
- 11.Kapoor D, Channer KS, Jones TH. Rosiglitazone increases bioactive testosterone and reduces waist circumference in hypogonadal men with type 2 diabetes. Diab Vasc Dis Res 2008;5:135–137 [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab 1993;76:1140–1146 [DOI] [PubMed] [Google Scholar]
- 13.Zumoff B, Strain GW, Miller LK, et al. Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab 1990;71:929–931 [DOI] [PubMed] [Google Scholar]
- 14.Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, de Boer H. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med 2008;66:103–109 [PubMed] [Google Scholar]
- 15.Nielsen TL, Hagen C, Wraae K, et al. Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J Clin Endocrinol Metab 2007;92:2696–2705 [DOI] [PubMed] [Google Scholar]
- 16.Mogri M, Dhindsa S, Quattrin T, Ghanim H, Dandona P. Testosterone concentrations in young pubertal and post-pubertal obese males. Clin Endocrinol (Oxf) 2013;78:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandewalle S, Taes Y, Fiers T, et al. Sex steroids in relation to sexual and skeletal maturation in obese male adolescents. J Clin Endocrinol Metab 2014;99:2977–2985 [DOI] [PubMed] [Google Scholar]
- 18.Bekaert M, Van Nieuwenhove Y, Calders P, et al. Determinants of testosterone levels in human male obesity. Endocrine 2015;50:202–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corona G, Mannucci E, Schulman C, et al. Psychobiologic correlates of the metabolic syndrome and associated sexual dysfunction. Eur Urol 2006;50:595–604; discussion 604 [DOI] [PubMed] [Google Scholar]
- 20.Pitteloud N, Dwyer AA, DeCruz S, et al. The relative role of gonadal sex steroids and gonadotropin-releasing hormone pulse frequency in the regulation of follicle-stimulating hormone secretion in men. J Clin Endocrinol Metab 2008;93:2686–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhindsa S, Furlanetto R, Vora M, Ghanim H, Chaudhuri A, Dandona P. Low estradiol concentrations in men with subnormal testosterone concentrations and type 2 diabetes. Diabetes Care 2011;34:1854–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tajar A, Forti G, O’Neill TW, et al.; EMAS Group . Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010;95:1810–1818 [DOI] [PubMed] [Google Scholar]
- 23.T’Sjoen GG, Giagulli VA, Delva H, Crabbe P, De Bacquer D, Kaufman JM. Comparative assessment in young and elderly men of the gonadotropin response to aromatase inhibition. J Clin Endocrinol Metab 2005;90:5717–5722 [DOI] [PubMed] [Google Scholar]
- 24.Wittert G. The relationship between sleep disorders and testosterone in men. Asian J Androl 2014;16:262–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster GD, Sanders MH, Millman R, et al.; Sleep AHEAD Research Group . Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 2009;32:1017–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang XB, Jiang XT, Du YP, Yuan YT, Chen B. Efficacy of continuous positive airway pressure on testosterone in men with obstructive sleep apnea: a meta-analysis. PLoS One 2014;9:e115033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev 2016;96:1169–1209 [DOI] [PubMed] [Google Scholar]
- 28.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes 2004;53:3024–3029 [DOI] [PubMed] [Google Scholar]
- 29.Brüning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science 2000;289:2122–2125 [DOI] [PubMed] [Google Scholar]
- 30.Salvi R, Castillo E, Voirol MJ, et al. Gonadotropin-releasing hormone-expressing neurons immortalized conditionally are activated by insulin: implication of the mitogen-activated protein kinase pathway. Endocrinology 2006;147:816–826 [DOI] [PubMed] [Google Scholar]
- 31.Divall SA, Williams TR, Carver SE, et al. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest 2010;120:2900–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 2002;110:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med 2003;349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 34.George JT, Veldhuis JD, Tena-Sempere M, Millar RP, Anderson RA. Exploring the pathophysiology of hypogonadism in men with type 2 diabetes: kisspeptin-10 stimulates serum testosterone and LH secretion in men with type 2 diabetes and mild biochemical hypogonadism. Clin Endocrinol (Oxf) 2013;79:100–104 [DOI] [PubMed] [Google Scholar]
- 35.Watanobe H, Hayakawa Y. Hypothalamic interleukin-1 beta and tumor necrosis factor-alpha, but not interleukin-6, mediate the endotoxin-induced suppression of the reproductive axis in rats. Endocrinology 2003;144:4868–4875 [DOI] [PubMed] [Google Scholar]
- 36.Bhatia V, Chaudhuri A, Tomar R, Dhindsa S, Ghanim H, Dandona P. Low testosterone and high C-reactive protein concentrations predict low hematocrit in type 2 diabetes. Diabetes Care 2006;29:2289–2294 [DOI] [PubMed] [Google Scholar]
- 37.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004;25:4–7 [DOI] [PubMed] [Google Scholar]
- 38.Ghanim H, Aljada A, Daoud N, Deopurkar R, Chaudhuri A, Dandona P. Role of inflammatory mediators in the suppression of insulin receptor phosphorylation in circulating mononuclear cells of obese subjects. Diabetologia 2007;50:278–285 [DOI] [PubMed] [Google Scholar]
- 39.Grossmann M, Cheung AS, Zajac JD. Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Pract Res Clin Endocrinol Metab 2013;27:603–616 [DOI] [PubMed] [Google Scholar]
- 40.Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 2017;23:390–408 [DOI] [PubMed] [Google Scholar]
- 41.Pitteloud N, Hardin M, Dwyer AA, et al. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab 2005;90:2636–2641 [DOI] [PubMed] [Google Scholar]
- 42.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab 2011;96:2341–2353 [DOI] [PubMed] [Google Scholar]
- 43.Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med 2005;35:339–361 [DOI] [PubMed] [Google Scholar]
- 44.Dhindsa S, Ghanim H, Batra M, et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care 2016;39:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones TH, Arver S, Behre HM, et al.; TIMES2 Investigators . Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 2011;34:828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006;154:899–906 [DOI] [PubMed] [Google Scholar]
- 47.Shabsigh R, Kaufman JM, Steidle C, Padma-Nathan H. Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol 2004;172:658–663 [DOI] [PubMed] [Google Scholar]
- 48.Dhindsa S, Bhatia V, Dhindsa G, Chaudhuri A, Gollapudi GM, Dandona P. The effects of hypogonadism on body composition and bone mineral density in type 2 diabetic patients. Diabetes Care 2007;30:1860–1861 [DOI] [PubMed] [Google Scholar]
- 49.Mårin P, Holmäng S, Jönsson L, et al. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord 1992;16:991–997 [PubMed] [Google Scholar]
- 50.Mårin P, Krotkiewski M, Björntorp P. Androgen treatment of middle-aged, obese men: effects on metabolism, muscle and adipose tissues. Eur J Med 1992;1:329–336 [PubMed] [Google Scholar]
- 51.Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010;73:602–612 [DOI] [PubMed] [Google Scholar]
- 52.Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 2013;369:1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng Tang Fui M, Prendergast LA, Dupuis P, et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med 2016;14:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mårin P, Holmäng S, Gustafsson C, et al. Androgen treatment of abdominally obese men. Obes Res 1993;1:245–251 [DOI] [PubMed] [Google Scholar]
- 55.Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P; BLAST Study Group . Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med 2014;11:840–856 [DOI] [PubMed] [Google Scholar]
- 56.Gianatti EJ, Dupuis P, Hoermann R, et al. Effect of testosterone treatment on glucose metabolism in men with type 2 diabetes: a randomized controlled trial. Diabetes Care 2014;37:2098–2107 [DOI] [PubMed] [Google Scholar]
- 57.Magnussen LV, Glintborg D, Hermann P, Hougaard DM, Højlund K, Andersen M. Effect of testosterone on insulin sensitivity, oxidative metabolism and body composition in aging men with type 2 diabetes on metformin monotherapy. Diabetes Obes Metab 2016;18:980–989 [DOI] [PubMed] [Google Scholar]
- 58.Huang G, Pencina KM, Li Z, et al. Long-term testosterone administration on insulin sensitivity in older men with low or low-normal testosterone levels. J Clin Endocrinol Metab. 24 January 2018 [Epub ahead of print]. DOI: 10.1210/jc.2017-02545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly DM, Jones TH. Testosterone and obesity. Obes Rev 2015;16:581–606 [DOI] [PubMed] [Google Scholar]
- 60.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003;52:2882–2887 [DOI] [PubMed] [Google Scholar]
- 61.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl 2009;30:726–733 [DOI] [PubMed] [Google Scholar]
- 62.Dhindsa S, Ghanim H, Batra M, et al. Effect of testosterone on hepcidin, ferroportin, ferritin and iron binding capacity in patients with hypogonadotropic hypogonadism and type 2 diabetes. Clin Endocrinol (Oxf) 2016;85:772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghanim H, Dhindsa S, Abuaysheh S, et al. Diminished androgen and estrogen receptors and aromatase levels in hypogonadal diabetic men: reversal with testosterone. Eur J Endocrinol 2018;178:277–283 [DOI] [PubMed] [Google Scholar]
- 64.Khosla S, Melton LJ III, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 1998;83:2266–2274 [DOI] [PubMed] [Google Scholar]
- 65.Araujo AB, Kupelian V, Page ST, Handelsman DJ, Bremner WJ, McKinlay JB. Sex steroids and all-cause and cause-specific mortality in men. Arch Intern Med 2007;167:1252–1260 [DOI] [PubMed] [Google Scholar]
- 66.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol 2013;169:725–733 [DOI] [PubMed] [Google Scholar]
- 67.Goodman N, Guay A, Dandona P, Dhindsa S, Faiman C, Cunningham GR; AACE Reproductive Endocrinology Scientific Committee . American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract 2015;21:1066–1073 [DOI] [PubMed] [Google Scholar]
- 68.Snyder PJ, Bhasin S, Cunningham GR, et al.; Testosterone Trials Investigators . Effects of testosterone treatment in older men. N Engl J Med 2016;374:611–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheetham TC, An J, Jacobsen SJ, et al. Association of testosterone replacement with cardiovascular outcomes among men with androgen deficiency. JAMA Intern Med 2017;177:491–499 [DOI] [PubMed] [Google Scholar]
- 70.Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J 2015;36:2706–2715 [DOI] [PubMed] [Google Scholar]
- 71.Corona G, Maseroli E, Rastrelli G, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf 2014;13:1327–1351 [DOI] [PubMed] [Google Scholar]
- 72.Dhindsa S, Upadhyay M, Viswanathan P, Howard S, Chaudhuri A, Dandona P. Relationship of prostate-specific antigen to age and testosterone in men with type 2 diabetes mellitus. Endocr Pract 2008;14:1000–1005 [DOI] [PubMed] [Google Scholar]
- 73.Werny DM, Thompson T, Saraiya M, et al. Obesity is negatively associated with prostate-specific antigen in U.S. men, 2001-2004. Cancer Epidemiol Biomarkers Prev 2007;16:70–76 [DOI] [PubMed] [Google Scholar]
- 74.Bañez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA 2007;298:2275–2280 [DOI] [PubMed] [Google Scholar]
- 75.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–1638 [DOI] [PubMed] [Google Scholar]
- 76.Sermondade N, Faure C, Fezeu L, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update 2013;19:221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]