Abstract
Insulin-induced hypoglycemia in diabetes is associated with impaired glucagon secretion. In this study, we tested whether stimulation of GPR119, a G-protein–coupled receptor expressed in pancreatic islet as well as enteroendocrine cells and previously shown to stimulate insulin and incretin secretion, might enhance glucagon secretion during hypoglycemia. In the study, GPR119 agonists were applied to isolated islets or perfused pancreata to assess insulin and glucagon secretion during hypoglycemic or hyperglycemic conditions. Insulin infusion hypoglycemic clamps were performed with or without GPR119 agonist pretreatment to assess glucagon counterregulation in healthy and streptozotocin (STZ)-induced diabetic rats, including those exposed to recurrent bouts of insulin-induced hypoglycemia that leads to suppression of hypoglycemia-induced glucagon release. Hypoglycemic clamp studies were also conducted in GPR119 knockout (KO) mice to evaluate whether the pharmacological stimulatory actions of GPR119 agonists on glucagon secretion during hypoglycemia were an on-target effect. The results revealed that GPR119 agonist-treated pancreata or cultured islets had increased glucagon secretion during low glucose perfusion. In vivo, GPR119 agonists also significantly increased glucagon secretion during hypoglycemia in healthy and STZ-diabetic rats, a response that was absent in GPR119 KO mice. In addition, impaired glucagon counterregulatory responses were restored by a GPR119 agonist in STZ-diabetic rats that were exposed to antecedent bouts of hypoglycemia. Thus, GPR119 agonists have the ability to pharmacologically augment glucagon secretion, specifically in response to hypoglycemia in diabetic rodents. Whether this effect might serve to diminish the occurrence and severity of iatrogenic hypoglycemia during intensive insulin therapy in patients with diabetes remains to be established.
Introduction
GPR119 is an X-linked, class A (rhodopsin-like) Gαs receptor expressed on enteroendocrine cells and in pancreatic islets (1–3). Lysophospholipids and other lipid metabolites are endogenous ligands that, upon activating GPR119, stimulate insulin secretion, acting by direct action on β-cells and/or by their capacity to stimulate secretion of glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) (1–4). Recent studies, however, indicate that the predominant glucoregulatory effect of GPR119 is attributable to stimulation of incretin secretion and that a direct effect on β-cells is modest (5).
Small-molecule GPR119 agonists have been used to evaluate GPR119 as a novel target for treating type 2 diabetes mellitus (T2DM). However, unfortunately, they have had only a modest effect in reducing hyperglycemia (5–7), and, as a result, further development of GPR119 agonist for T2DM treatment has, for the most part, been discontinued. However, reports of these agonists increasing plasma glucagon (8,9) and of GPR119 receptor expression on glucagon-secreting α-cells (10) have given impetus to more investigation of the role of this receptor in governing α-cell physiology. Furthermore, single-cell transcriptome profiling of pancreatic cells in mouse and human islets indicates that the most robust expression of GPR119 is actually on α-cells rather than β-cells (11,12). In keeping with this, we have recently observed that in pancreas perfusion and islet incubation studies, structurally varied GPR119 agonist potentiated glucagon secretion during hypoglycemia perfusions.
A rise in glucagon secretion as plasma glucose levels decline to hypoglycemic thresholds is the major initial factor driving the counterregulatory response (11,13). Thus, the impaired glucagon secretory response to insulin-induced hypoglycemia that commonly develops in patients with type 1 diabetes mellitus (T1DM) serves a critical role in limiting the implementation of intensive insulin therapy (12). It is noteworthy that the molecular mechanisms driving the impaired glucagon secretory response during hypoglycemia seen in T1DM as well as in a subgroup of insulin-treated patients with T2DM remain uncertain (14–17). Several years ago, a salutary effect to bolster glucagon secretion was reported using a somatostatin receptor antagonist in a rat model of T1DM (18). However, there are no current approved therapeutics to redress the defect in glucagon secretion during severe hypoglycemia apart from the administration of exogenous glucagon that is used as an emergency treatment (19,20). The current studies were therefore undertaken to test the novel hypothesis that GPR119 small-molecule agonists could specifically act pharmacologically to increase glucagon secretion during hypoglycemia but not during euglycemic and hyperglycemic conditions.
Research Design and Methods
All animal procedures conducted at Merck Research Laboratories (MRL) were reviewed and approved by the Institutional Animal Care and Use Committee. The Guide for the Care and Use of Laboratory Animals was followed in the conduct of the animal studies. For the studies conducted in rats at Yale University, animal care and experimental protocols were reviewed and approved by the Yale Animal Care and Use Committee.
Islet Isolation and FACS Sorting Studies
Islets were isolated from age-matched wild-type (C57BL/6) mice and GcG-YFP transgenic mice that expressed YFP protein under control of the preproglucagon promoter (21). A total of 150–200 islets were dispersed into single cells in 1× trypsin-dispersing medium containing 1 mmol/L EGTA (1/500 vol and 100 mmol/L EGTA, pH 8). After centrifugation, pellets were resuspended in 0.5 mL PBS for FACS. Sorted cells were lysed for RNA purification and cDNA synthesis followed by TaqMan real-time RT-PCR gene expression analysis.
Insulin and Glucagon Secretion in Mouse and Human Islets
Three batches of islets (purity >85% and viability >90%) from normal subject cadaver organ donors (20, 58, and 62 years old) were obtained from the Islet Cell Resource Centers and the National Disease Resource Interchange (Philadelphia, PA). Selected human or isolated mouse islets were transferred to RPMI medium (10% FCS and 5.5 mmol/L glucose) and cultured for 24 h before insulin or glucagon secretion was determined by 1-h incubation in Krebs-Ringer bicarbonate (KRB) buffer in a 96-well format as previously described (15). Buffer was assayed for insulin (Insulin ELISA kit; ALPCO, Salem, NH) and glucagon (human, mouse, and rat; ELISA kit; ALPCO).
In Situ Hybridization Studies
Formalin-fixed paraffin-embedded mouse and human pancreas tissue sections were analyzed. Human pancreas was received from the Network for Pancreatic Organ Donors with Diabetes. Only tissues with RNA integrity numbers >7 were studied. Seven donors were chosen for the study (n = 2 for donors without diabetes, n = 4 for T1DM, and n = 1 for T2DM). Duplex in situ hybridization (ISH) and immunostaining were applied to analyze coexpression of GPR119 mRNA with glucagon, insulin, or somatostatin, corresponding to α-, β-, and δ-cells, respectively, using the RNAscope 2.0 Assay 2-plex kit and probes from Advanced Cellular Diagnostics (22).
Rat Pancreas Perfusion Study
Male Wistar Han rats were purchased from Charles River Laboratories Inc., and in situ pancreatic perfusions were performed when rats were 8 weeks old. Food was removed ∼4 to 5 h prior to surgery, rats were sedated using 100 mg/kg intraperitoneal Na-pentobarbital (Nembutal; Akorn Pharmaceuticals), the peritoneum was opened, and a 27-gauge cannula was inserted into the dorsally ligated celiac artery for perfusant afflux. The hepatic portal vein was ligated dorsally, and a 25-gauge cannula was inserted ventrally for perfusant efflux. Subsequently, rats were perfused with modified KRB buffers (23), supplemented with vehicle (0.1% DMSO) or GPR119 agonist compounds, respectively. The glucose concentration of the KRB buffer was initially at 2 mmol/L for 20 min, then increased to 12 mmol/L to create a hyperglycemic condition for 20 min, and finally changed back to 2 mmol/L glucose with the addition of 30 mmol/L L-arginine buffer for 8 min. An initial test revealed that at a 3-mL/min perfusion rate, it required at least 4–6 min for the perfusant solution to be completely exchanged in the whole perfusion loop. The perfusion chamber was kept warm at 37°C and saturated with an O2/CO2 mixture, and perfusant was collected each minute (with 90% buffer recovery) for later analysis of insulin and glucagon.
Rat Hyperinsulinemic-Hypoglycemic Clamp Studies
Rat hypoglycemia studies were conducted at MRL and Yale University using independent study designs and different GPR119 agonists. The studies at MRL were carried out in male Wistar rats (8–10 weeks of age and weight 200–250 g) that were surgically prepared with catheterization into a jugular and a femoral vein for drug delivery and blood sampling, respectively. After recovery for 1 week, rats were fasted overnight preceding clamp studies.
Baseline blood samples were obtained 75 min before the clamp for measurement of plasma glucose, glucagon, insulin, GLP-1, GIP, and other parameters. Subsequently, at 60 min prior to a clamp, rats were dosed orally with small-molecule GPR119 agonists or a vehicle (0.2% hydroxypropyl cellulose [SL grade]/0.01% sodium lauryl sulfate, 1 mL/kg). A primed (0.02 units/kg/min), continuous infusion (0.006 units/min) of human insulin (Humulin R U-100; Eli Lilly and Company) was administered to induce hypoglycemia (∼50 or ∼70 mg/dL), blood glucose was monitored every 10 min, and once hypoglycemia was achieved, this level was maintained using a variable 20% glucose infusion. The plasma glucagon profile during the 2-h clamp was used to determine the area under the curve (AUC) response.
The procedures used for conducting hypoglycemic clamps in rats at Yale University have been previously described in detail (17). Briefly, Sprague-Dawley male rats had surgical implantation of vascular catheters in a carotid artery for blood sampling and right jugular vein for infusions. Three studies were conducted, each of which evaluated the effect of a GPR119 small-molecule agonist on glucagon secretion in response to insulin-induced hypoglycemia. The first study was conducted in healthy rats, the second study used rats rendered diabetic by injection of streptozotocin (STZ; 45 mg/kg in saline buffer), and the third study used STZ-diabetic rats that were subjected to three bouts of hypoglycemia on successive days preceding the hypoglycemic clamp study. In study 1, healthy animals were orally dosed once with a GPR119 agonist (compound A; 1, 10, and 30 mg/kg) or vehicle preceding initiation of the clamp. Thereafter, a primed, constant infusion of insulin (25 mU/kg/min) was used to induce hypoglycemia, which was maintained at 50 mg/dL for 90 min using a variable rate of glucose infusion. In study 2, STZ-diabetic animals (and nondiabetic control rats) were given a 10 mg/kg dose of compound A (or vehicle) by oral gavage on the night preceding the hypoglycemic clamp. The dose was repeated 1 h before hypoglycemia clamp. During the primed-constant infusion of insulin (50 mU/kg/min), a period of euglycemia (∼110 mg/dL) was maintained for 90 min before hypoglycemia was induced. The third study used STZ-diabetic rats that were exposed to a 3-day recurrent hypoglycemia protocol to induce impaired glucagon secretion when they were subsequently challenged with an episode of insulin-induced hypoglycemia. Briefly, STZ-diabetic rats as well as nondiabetic rats received intraperitoneal injections of human regular insulin (30 units/kg in STZ rats and 10 units/kg in nondiabetic rats), and food was withheld so that animals would be subjected to 3 h of hypoglycemia (tail vein glucose ≤50 mg/dL).
Glucagon Release in Response to Levemir-Induced Hypoglycemia in Normal Lean and GPR119 Knockout Mice
Male C57BL/6N mice (13–17 weeks of age) and GPR119 knockout (KO) mice were obtained from Taconic Farms (Germantown, NY). On the morning of the study, mice were fasted for 2 h and subsequently given an intraperitoneal injection of Levemir (20 nmol/kg; Novo Nordisk) to induce hypoglycemia. Mice received oral administration of a GPR119 agonist (compound B; 1, 3, 10, or 30 mg/kg) or vehicle 30 min prior to insulin delivery. Another group of mice received vehicle treatment and an intraperitoneal injection of 0.9% saline instead of insulin. Blood samples were collected from retro-orbital bleeding at 0, 60, 120, and 150 min for determination of glucose and glucagon concentration.
Continuous Glucose Monitoring Study in Normal Wistar Han Rats
Rats were implanted with a glucose sensor (Data Sciences International) into the abdominal aorta at 8 weeks of age (24) according to the manufacturer’s instructions and then allowed to recover for 1 week. On the day of the experiment, rats were fasted from 8:00 a.m. to 5:00 p.m. and then refed after 5:00 p.m. At 9:30 a.m., the rats were orally administrated a vehicle or compound A (10 mg/kg), followed by a subcutaneous Levemir injection (3 units/kg) 1 h later. Blood glucose levels were automatically recorded via the continuous glucose monitoring (CGM) system connected to a glucose sensor every 10 min for 24 h.
Statistical Analysis
Results are shown as mean ± SE. Statistical analysis was performed using ANOVA or two-tailed t test, as appropriate, with significance defined at P < 0.05.
Results
Medicinal Chemistry
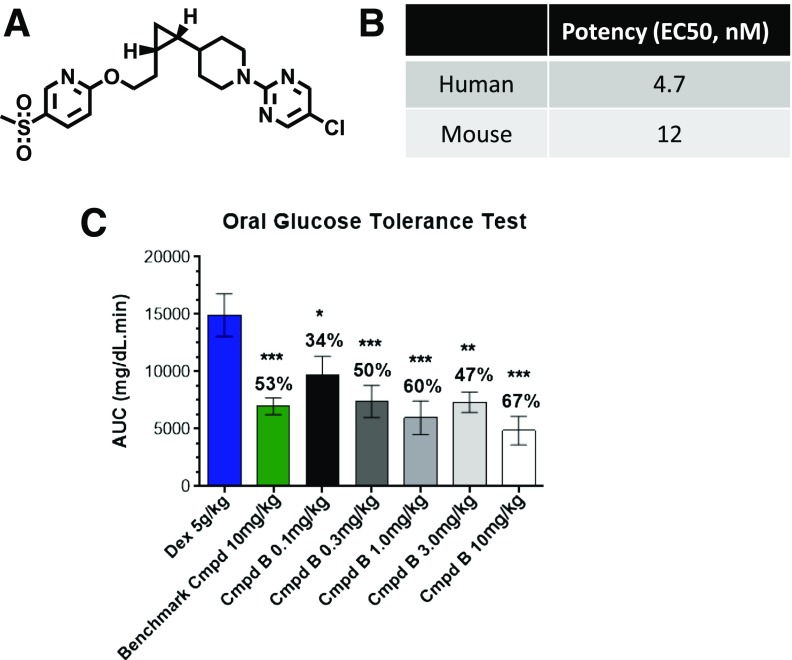
The chemistry efforts to identify and develop small-molecule GPR119 agonists at MRL have been previously described (7). For the current studies, three structurally distinct analogs were used, as described below, as well as other GPR119 compounds previously described (5,7). A key reason to use several compounds rather than just one was to address whether an effect on glucagon secretion was mechanism based or instead might solely be attributable to one compound. Compound A was used in the studies conducted at Yale and some studies at MRL; it is a potent GPR119 agonist selective for rodent and human GPR119 (half-maximal effective concentration at 0.2–0.3 nmol/L). When this compound is given via an oral gavage of 10 mg/kg to rats, it has sufficient half-life to sustain drug exposure at 24 h. Compound B was primarily used for the studies conducted at MRL, and its structure and in vitro potency are shown in Fig. 1, together with its efficacy to reduce glycemic excursion during an oral glucose tolerance test in high-fat–fed mice. The structure of compound C is shown in Fig. 2A, and it was used in some of the in vitro studies.
Figure 1.
A small-molecule GPR119 agonist. Compound B (Cmpd B) is a small-molecule agonist of GPR119 with a distinct structure (A) and robust potency (B) in activating mouse or human GPR119 tested in recombinant HEK293 cells. (C) High-fat–fed mice were pretreated with dextrose (Dex) or GPR119 agonist compounds at the indicated doses and then subjected to oral glucose tolerance tests. The AUC for the oral glucose tolerance test is presented, and the number over each bar represents the percentage of reduction of AUC compared with that of the control group administered Dex. Data are mean ± SE from 8–10 rats/group. *P < 0.05; **P < 0.01; ***P < 0.001 vs. Dex group. EC50, half-maximal effective concentration.
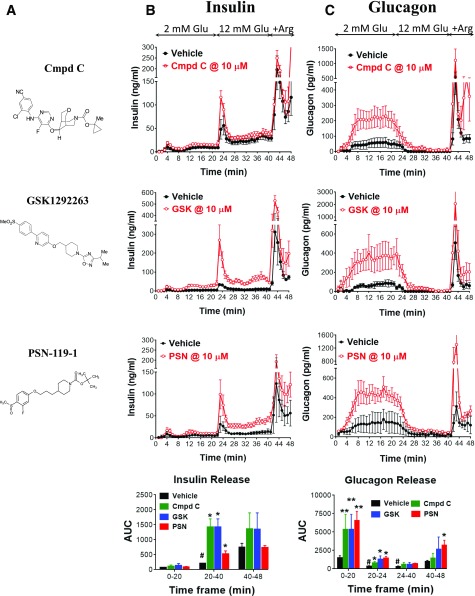
Figure 2.
Insulin and glucagon secretion from normal rat pancreata upon GPR119 agonist stimulation. A: Three structurally distinct GPR119 agonists developed by Merck (compound C [Cmpd C]), GlaxoSmithKline (GSK1292263 [GSK]), and Prosidion (PSN-119-1 [PSN]). Insulin (B) and glucagon (C) levels in perfusant collected from ex vivo rat pancreata perfused sequentially with 2 mmol/L and 12 mmol/L glucose (Glu), 2 mmol/L glucose plus 30 mmol/L L-arginine (+Arg), and without (vehicle) or with GPR119 agonists as described in research design and methods. The AUC was calculated for each group during the time periods of 0–20, 20–40, 20–24, 24–40, and 40–48 min, respectively, corresponding to different perfusion stages as indicated. Of note, all three GPR119 agonists enhanced insulin secretion during the 12 mmol/L perfusion and enhanced glucagon release during low glucose perfusion at 2 mmol/L. *P < 0.05; **P < 0.01 vs. vehicle at the corresponding time points; #P < 0.05 vs. vehicle at the time period of 0–20 min. Data are mean ± SE from five to six rats for each group.
GPR119 Effects on Glucagon Secretion in Pancreas Perfusion and Islet Cultures
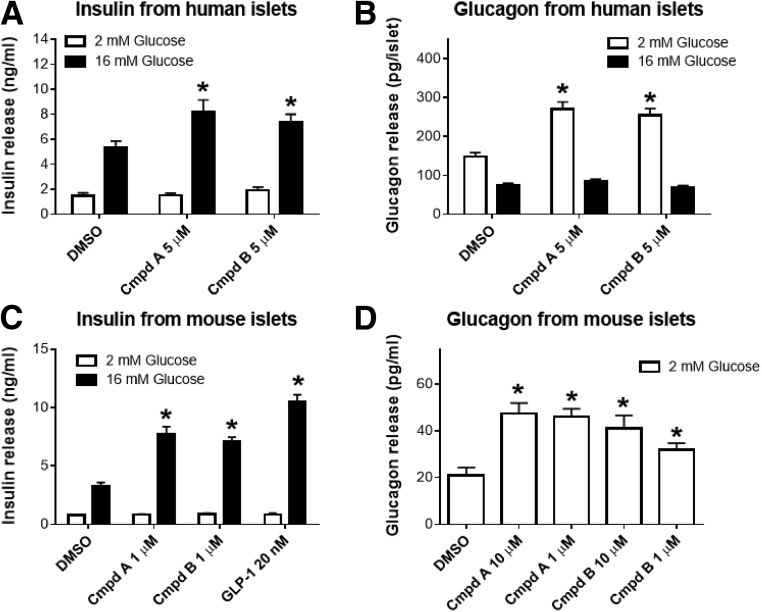
Perfusion of rat pancreata with each of three structurally distinct GPR119 agonists had no significant effect on insulin secretion when the perfusate contained 2 mmol/L glucose. However, when the glucose perfusion was increased to 12 mmol/L glucose, a significant increase in insulin secretion occurred (Fig. 2B). In addition, each of these three GPR119 agonist compounds produced a marked increase in glucagon secretion when the perfusate contained 2 mmol/L glucose, an effect that was sustained for an additional 4 min when the glucose perfusate concentration was increased from 2 to 12 mmol/L (Fig. 2C). From 24 to 40 min, when presumably the glucose concentration in the perfusate had reached a stable 12 mmol/L level, glucagon secretion returned to a comparable suppressed rate as during vehicle treatment. It is noteworthy that a subsequent perfusion of 2 mmol/L glucose plus L-arginine also augmented insulin and glucagon secretion (Fig. 2B and C), indicating that the perfused pancreata was physiologically functional throughout the preceding 40-min perfusion study. These glucagon secretion findings were confirmed in cultured islets isolated from humans without diabetes (Fig. 3A and B) and nondiabetic mouse (Fig. 3C and D), in which both compounds A and B significantly increased glucagon secretion by approximately twofold during hypoglycemic incubations.
Figure 3.
Insulin (A and C) and glucagon (B and D) secretion from cultured human without diabetes (A and B) or normal C56BL/6 mouse (C and D) islets. Batch islet incubation experiments were conducted for insulin or glucagon secretion as described in research design and methods. Both GPR119 agonists, compound A (Cmpd A) and compound B (Cmpd B), enhanced glucose-stimulated insulin secretion at 16 mmol/L glucose and augmented glucose release at 2 mmol/L glucose. Data are mean ± SE calculated from three to six independent experiments with triplicates for each experiment. *P < 0.01 by ANOVA.
In Vivo Effect of GPR119 Agonist on Glucagon Secretion
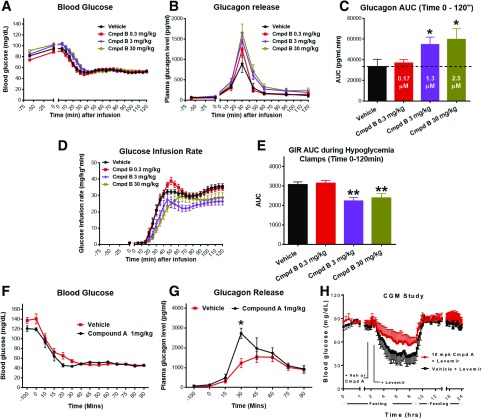
In healthy rats, hypoglycemia was induced and maintained at 50 mg/dL during a 2-h infusion of insulin (Fig. 4A). At the onset of hypoglycemia, when vehicle treatment was given, there was a prominent counterregulatory increase in glucagon secretion. However, preadministration of compound B at doses of 0.3, 3, and 30 mg/kg significantly augmented the glucagon response in a dose-responsive manner (Fig. 4B and C). At the highest dose of compound B, glucagon release was approximately twofold higher than with vehicle treatment. In association with augmentation of glucagon secretion, there was a significantly diminished requirement for delivery of exogenous glucose infusion at the 3 and 30 mg/kg doses of compound B (Fig. 4D and E). These findings of augmentation of glucagon secretion were corroborated in a hypoglycemic clamp study in healthy rats (Fig. 4F and G) performed at Yale University. In that study, compound A significantly increased glucagon secretion by approximately twofold during hypoglycemia as compared with vehicle treatment. In addition, a CGM study demonstrated that compound A was able to significantly attenuate Levemir-induced hypoglycemia in fasted healthy nondiabetic rats (Fig. 4H). Thus, GPR119 is able to pharmacologically bolster glucagon release during hypoglycemia and, in turn, reduce the need for exogenous glucose to prevent severe hypoglycemia.
Figure 4.
GPR119 agonists augment glucagon secretion in normal rats during hypoglycemic clamp. Normal Wistar rats were predosed with GPR119 agonist compound B (Cmpd B; A–E) or compound A (Cmpd A; F–H) and then subjected to an insulin-induced hypoglycemia clamp study (A–G) or CGM study (H), as described in the Research Design and Methods. Blood samples were collected at the indicated time points for measurement of plasma levels of glucose, glucagon, insulin, GLP-1, GIP, and other parameters. Blood glucose (A), glucagon (B), AUC of glucagon (C), glucose infusion rate (GIR) (D), and AUC of GIR from 0–120 min (E) in rats treated with vehicle or Cmpd B at the indicated doses. The numbers in white inside each bar of C represent the blood drug concentration of Cmpd B at the end of the clamp study at 120 min. Blood glucose (F) and plasma glucagon levels (G) in rats treated with vehicle or Cmpd A during the hypoglycemia clamp. Data are depicted as mean ± SE from 8–10 rats for each group. *P < 0.05; **P < 0.01 vs. vehicle at the indicated group or time point. H: CGM for 24 h of blood glucose levels in normal rats during fasting (0–9 h) and feeding (9–24 h) stages. Compared with vehicle (Veh), pretreatment with Cmpd A ameliorated Levemir-induced hypoglycemia. Data are mean ± SE from three rats for each group.
To explore whether the effect of GPR119 agonism might influence the glycemic threshold for glucagon release and whether augmentation of glucagon secretion is context specific for hypoglycemia, a mild hypoglycemic clamp study (Supplementary Fig. 1A–C) and a euglycemic clamp study (Supplementary Fig. 1D–F) were performed in normal rats. Compound B (30 mg/kg) administered 1 h preceding the mild hypoglycemia clamp (70 mg/dL) significantly increased glucagon secretion as compared with vehicle treatment, although the response at mild hypoglycemia was less pronounced than that during severe hypoglycemia (Supplementary Fig. 1B and C). In contrast, we failed to detect an increase in plasma glucagon levels following compound B pretreatment when blood glucose was clamped at normoglycemic levels (Supplementary Fig. 1D–F).
GPR119 Expression in Murine and Human Islets
It is well established that GPR119 activation can stimulate incretin secretion (2–4). Aligned with this, we observed that during hypoglycemia, pretreatment with compound B increased plasma concentrations of both GIP and GLP-1 (Supplementary Fig. 2), although an increase of GIP was not observed at the highest dose of compound B (30 mg/kg). Given that GIP has been reported to potentiate glucagon secretion during hypoglycemia (25), and this might contribute to the in vivo effects of GPR119 agonists to augment glucagon release during hypoglycemia, we examined glucagon secretion in isolated human and murine islets by structurally diverse GPR119 agonists. These experiments, however, suggest direct target engagement of α-cells (Fig. 3).
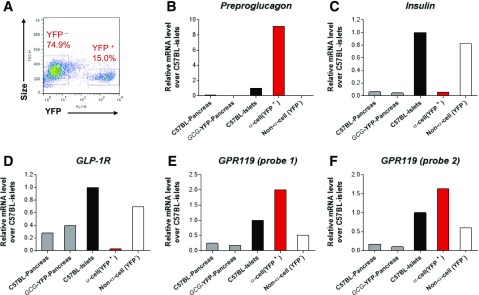
To confirm recent data indicating expression of GPR119 on individual islet α-cells (26,27), we also examined GPR119 expression in murine α-cells that were FACS sorted from Gcg-YFP transgenic mice by TaqMan real-time RT-PCR gene expression analysis. In agreement with previous reports (10), ∼15% of total islet cell population was YFP+, presumably α-cells, that exhibited a high level of preproglucagon mRNA, but low mRNA expression of insulin and GLP-1 receptor (Fig. 5A–D). Remarkably, those cells also demonstrated a two- to fourfold higher level of GPR119 mRNA when compared with YFP− cells (Fig. 5E and F), confirming that GPR119 is robustly expressed in α-cells, which concurs with single-cell transcriptional profiling data (26,27). This observation was further corroborated by ISH of GPR119 mRNA and immunostaining of islet hormones on pancreas sections from normal mice as well as human subjects without diabetes and in pancreatic tissues from donors with T1DM and T2DM. As shown in Supplementary Fig. 3, GPR119 mRNA was visualized in insulin- and glucagon-containing cells in normal murine or human islets. In the samples from donors with T1DM and T2DM, GPR119 mRNA was also evident in glucagon-containing α-cells (Supplementary Fig. 4), and a predominance of α-cells in pancreatic islets from donors with diabetes was notable.
Figure 5.
GPR119 is expressed in glucagon+ cells of mouse islets. Pooled islets or pancreata, isolated from C57BL/6 or Gcg-YFP transgenic mice, were dispersed into single cells and subjected to FACS sorting (A), followed by TaqMan real-time RT-PCR analyses of the genes (B–F) as indicated. Two sets of TaqMan probes (E and F) were used for GPR119 detection. Data are depicted as representative from three independent experiments with similar observations.
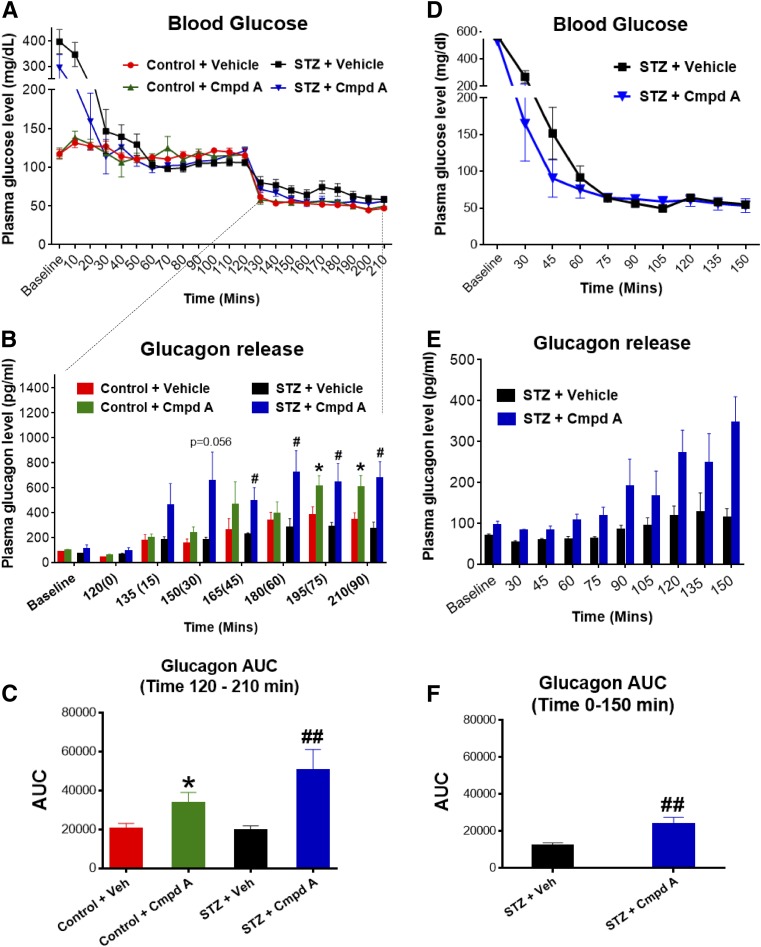
Effect of GPR119 Agonism on Glucagon Secretion in STZ-Induced Diabetic Rats
Two hypoglycemic clamp studies were conducted at Yale University using STZ-diabetic rats with marked baseline hyperglycemia (300–400 mg/dL), as shown in Fig. 6. The first study was a single exposure to hypoglycemia produced by an insulin clamp (Fig. 6A). Nondiabetic control rats that received compound A manifested an increased plasma glucagon response to hypoglycemia, a pharmacological effect that was even more pronounced in the STZ-diabetic rats that received compound A (Fig. 6A–C). The GPR119-mediated augmentation of glucagon secretion during hypoglycemia was sustained for 90 min. Plasma epinephrine increases in response to hypoglycemia, in contrast, were similar across groups. There was no effect of compound A pretreatment on plasma glucagon levels during hyperglycemia or the euglycemic run-in phase of phase of hypoglycemic experiment (Fig. 6A).
Figure 6.
GPR119 agonism enhanced glucagon secretion in STZ-diabetic rats during hypoglycemia. STZ-induced diabetic rats were exposed to single-episode (A–C) or multiple recurrent (D–F) hypoglycemia and preadministered with vehicle (Veh) or compound A (Cmpd A; 30 mg/kg) before hypoglycemic clamp studies as described in research design and methods. Blood samples were collected at the indicated time points for measurement of plasma levels of glucose (A and D) and glucagon (B and E) and calculation of the AUC of glucagon response (C and F). After Cmpd A treatment, diabetic rats responded to hypoglycemia more quickly and with an augmented glucagon response as compared with diabetic rats treated with vehicle only. Data are depicted as mean ± SE from 7–10 rats for each group. *P < 0.05 vs. control rats + vehicle (Veh); #P < 0.05; ##P < 0.01 vs. STZ + Veh at the indicated group.
The second study used STZ-diabetic rats to examine glucagon counterregulatory responses during a hypoglycemic clamp conducted after exposure to recurrent episodes of insulin-induced hypoglycemia for 3 successive days, a protocol known to severely impair glucagon release. During the hypoglycemia clamp, plasma glucose in STZ-diabetic rats was maintained at 50 mg/dL for 90 min (Fig. 6D). The STZ-diabetic rats with vehicle treatment that had experienced 3 days of antecedent hypoglycemia had a severely blunted glucagon response during the clamp-induced hypoglycemia (Fig. 6D–F). In marked contrast, the compound A–treated STZ rats exhibited a robust increase in glucagon counterregulation, manifested at onset of hypoglycemia and sustained throughout 90 min of hypoglycemia (Fig. 6E and F).
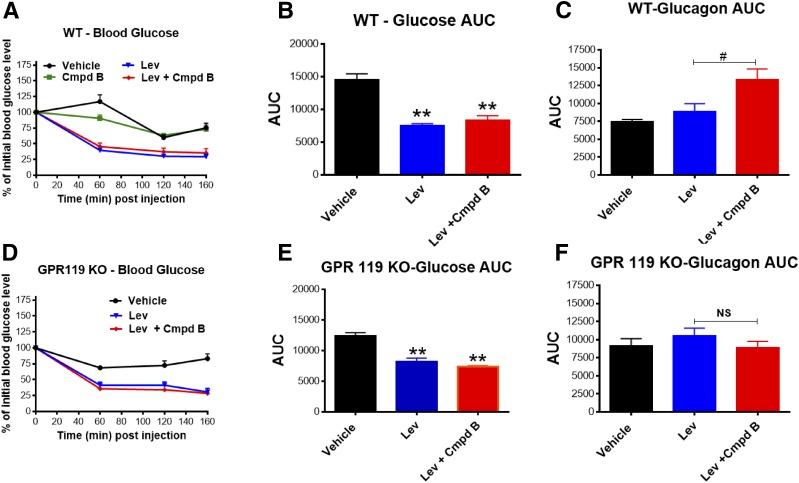
Effects of GPR119 Agonism on Glucagon-Stimulated Counterregulation in GPR119 KO Mice
A recent study has reported that the glucagon response to insulin-induced hypoglycemia appears to be intact in GPR119 KO mice (28). To test if the effect of compound B to augment hypoglycemia-related glucagon secretion represents an on-target pharmacological effect, GPR119 KO mice and their littermate wild-type controls were subjected to Levemir insulin–induced (20 nmol/kg) hypoglycemia with and without pretreatment of a GPR119 agonist. The Levemir injection produced an ∼50% reduction in fasting plasma glucose by 60 min that was sustained for an additional 100 min, when studies were halted (Fig. 7A, B, D and E). In wild-type control mice, administration of compound B prior to Levemir injection significantly increased the glucagon secretion response to hypoglycemia (Fig. 7C), a response that did not occur in GPR119 KO mice, indicating that the pharmacological action of compound B is most likely specifically mediated by the GPR119 receptor. In wild-type mice, despite a significant increase in glucagon release mediated by GPR119 agonist pretreatment, plasma glucose levels after Levemir insulin injection were not significantly different from vehicle treatment (Fig. 7B). However, as earlier noted (Fig. 4H), using a CGM system to more accurately and fully monitor blood glucose responses in healthy rats, compound A mitigated sustained hypoglycemia induced by Levemir injection.
Figure 7.
On-target effects of GPR119 agonist. GPR119 KO mice and littermate wild-type (WT) control mice were injected with Levemir insulin (Lev; 20 nmol/kg) to induce hypoglycemia followed by vehicle or compound B (Cmpd B; 100 mg/kg) treatment. Blood samples were collected at the indicated time points for measurement of blood glucose (A and D) and glucagon levels. AUCs were calculated for blood glucose (B and E) and glucagon (C and F). Note that Cmpd B (100 mg/kg) alone did not impact in vivo blood glucose level (A), but 20 mol/kg Levemir did induce hypoglycemia, although the corresponding glucagon response was modest. The combination of Cmpd B and Levemir insulin significantly augmented glucagon secretion in WT mice but not in GPR119 KO mice. Data are depicted as mean ± SE from six to eight mice for each group. **P < 0.01 vs. vehicle; #P < 0.05; NS, P > 0.05 vs. Lev.
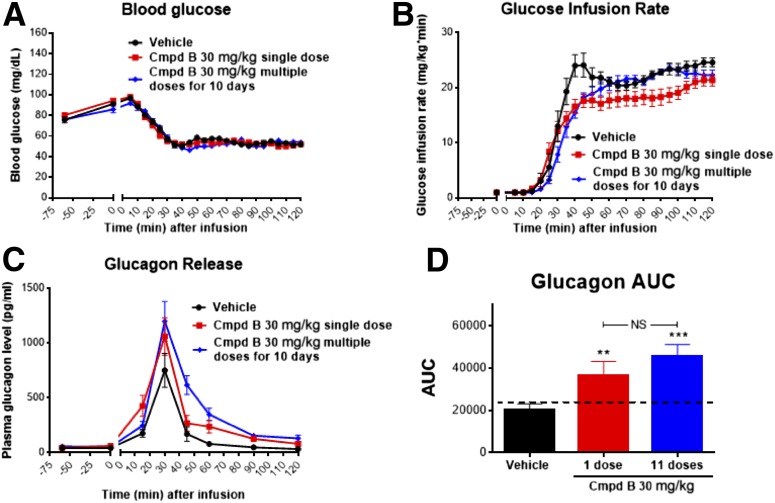
Subchronic Dosing of Compound B in Rats
GPR119 is a Gs-protein–coupled receptor, and a potential concern with chronic administration of a Gs-protein–coupled receptor agonist is a loss of sustained pharmacological activity due to an induction of tachyphylaxis, mediated by receptor downregulation or other mechanisms, such as recruitment of β-arrestin (24). To examine whether tachyphylaxis (for hypoglycemia-related glucagon secretion) might occur, one group of rats received compound B (30 mg/kg in feed for 10 days) prior to an insulin-induced hypoglycemic clamp, and the glucagon response was compared with those of vehicle-treated controls and of rats that received a single dose of compound B preceding the hypoglycemic clamp. Reflecting the long half-life of compound B, substantial drug exposure was obtained in the group receiving in-feed administration for 10 days; the plasma drug concentrations were 20 ± 5 μmol/L vs. 3 ± 1 μmol/L for the group given a single dose. As shown in Fig. 8, compound B–mediated increases of glucagon secretion during hypoglycemia were maintained after 10 days of dosing (i.e., not different from those of rats receiving a single dose of compound B).
Figure 8.
No tachyphylaxis effect of GPR119 agonist on hypoglycemia-related glucagon response. Normal Wistar rats were predosed without or with compound B (Cmpd B) at a single dose (30 mg/kg) or multiple doses (30 mg/kg each) for 10 days through in-feed drug administration prior to hypoglycemia clamp studies. Blood samples were collected at the indicated time points for measurement of plasma levels of glucose (A), glucose infusion rate (B), glucagon (C), and AUC of glucagon (D). As noted, the increases of glucagon secretion during hypoglycemia mediated by Cmpd B were not significantly different between the groups of rats receiving single vs. multiple doses of Cmpd B. Data are depicted as mean ± SE from 8–10 rats for each group. **P < 0.01; ***P < 0.001 vs. vehicle; NS, P > 0.05.
Discussion
The current studies were undertaken to test the hypothesis that activation of the GPR119 receptor by small-molecule agonists can act pharmacologically to increase secretion of glucagon in response to insulin-induced hypoglycemia. To the best of our knowledge, despite considerable interest during the past decade in GPR119 physiology and small-molecule drug discovery for engaging GPR119 as a treatment for T2DM, there have been no in vivo studies designed to test the hypothesis that GPR119 might play a key role in governing glucagon’s counterregulatory response to insulin-induced hypoglycemia. The concept was generated empirically by observations that diverse GPR119 agonists potentiated glucagon secretion from rodent pancreata perfused at hypoglycemia but not during hyperglycemia. Of note, these GPR119 agonists did modestly stimulate insulin secretion under hyperglycemic perfusion (but not hypoglycemic perfusion), consistent with other (though not all) prior observations (1–4,28,29). In vivo pharmacology, in contrast, supported our hypothesis that GPR119 agonists may stimulate glucagon secretion during insulin-induced hypoglycemia.
In healthy rats studied during hypoglycemic clamps that were given with vehicle treatment, there was, as expected, a robust increase in glucagon secretion at the onset of hypoglycemia. However, following pretreatment using two small-molecule GPR119 agonists (compounds A and B), the counterregulatory glucagon response to hypoglycemia was amplified significantly, and a reduced requirement for infusion of exogenous glucose was observed. Additional evidence supporting the physiological value of GPR119-mediated stimulation of glucagon release during hypoglycemia was evident from the use of CGM. Specifically, in rats that received a long-acting insulin injection that induced sustained hypoglycemia, GRP119 agonist pretreatment mitigated the effect. Hypoglycemic clamp studies were also conducted using STZ-induced diabetic rats, and again, it was observed that pretreatment with a GPR119 agonist significantly amplified glucagon secretion during hypoglycemia, whereas there was no effect to stimulate glucagon secretion in the same animals during hyperglycemia or euglycemia run-in periods before the hypoglycemic clamp. In addition, we observed in an STZ-diabetic rat model exposed to 3 days of antecedent insulin-induced hypoglycemia (done to markedly impair the glucagon response to a subsequent hypoglycemic challenge) that GPR119 agonism induced a strong and sustained restitution of glucagon secretion throughout hypoglycemia. Collateral studies in healthy rats indicated that pretreatment with a GPR119 agonist might lower the glycemic threshold at which glucagon counterregulation is evoked, a finding of potential importance, because this suggests that receptor engagement might prevent hypoglycemia, though more research in this area is warranted.
The incretin hormone GIP has been shown to bolster glucagon secretory response to hypoglycemia in T1DM with an indication that it alters the glycemic threshold for α-cell sensing and response (25). The positive findings in the perfused pancreata in isolated islets and the presence of GPR119 on α-cells indicate that the effect to increase glucagon secretion during hypoglycemia is at least partially a direct GPR119 receptor–mediated effect on α-cells and not solely dependent on GIP. It is noteworthy in this regard that recent single-cell transcriptional profiling studies of pancreatic islet cells indicate robust expression of GPR119 in α-cells, more so than its expression in β-cells (26,27). Taken together, the emerging findings on effects of GIP and GPR119 to heighten glucagon counterregulation to hypoglycemia could be mediated by enhancing glucose sensing in α-cells. This may offer exciting opportunities for the discovery of new therapeutics, including application of GPR119 agonists, to lessen the risk of hypoglycemia in insulin-treated patients with diabetes.
There were several lines of evidence indicating that stimulation of glucagon secretion during hypoglycemia is a mechanism-based effect of GPR119 agonism rather than a compound-specific effect. For example, the amplified glucagon responses to hypoglycemia following GPR119 agonist pretreatment were observed with structurally diverse small-molecule agonists that had demonstrated excellent in vitro selectivity and potency for GPR119. To further investigate the on-target effect, GPR119−/− homozygous KO mice were studied as compared with wild-type mice; of note, the KO mice did mount a normal glucagon response to insulin-induced hypoglycemia, which was also observed in a recent study (5). However, GPR119 agonist pretreatment of GPR119 KO mice using pharmacological doses failed to exhibit potentiation of the glucagon response to hypoglycemia. Thus, amplification of an otherwise normal glucagon counterregulatory response to insulin-induced hypoglycemia by GPR119 agonism may be of clinical value in T1DM, a condition in which there is a severely impaired glucagon response to hypoglycemia within a few years of a T1DM diagnosis. Accompanying the markedly deficient capacity for insulin secretion in T1DM, there is also a parallel markedly impaired secretion of glucagon during insulin-induced hypoglycemia, despite normal or more commonly elevated fasting and postprandial levels of glucagon (12,13). Moreover, the deficit of glucagon secretion in response to hypoglycemia is not rectified by tight glycemic control (30). When an impairment of glucagon secretion during insulin-induced hypoglycemia is also accompanied by hypoglycemic autonomic failure, patients with T1DM become particularly vulnerable to iatrogenic hypoglycemia, including frequent dangerous bouts of nocturnal hypoglycemia that may increase during efforts to attain strict glycemic control (20,31,32). These episodes of hypoglycemia also greatly increase the risk of another hypoglycemic event (33,34). It is noteworthy that impaired hypoglycemic counterregulatory responses have also been reported in patients with long-term T2DM who exhibit a marked deficit in β-cell function and require insulin therapy (35). These insulin-treated patients with T2DM display an increased risk of hypoglycemia, although typically not with equal frequency or severity as those with T1DM (32,36).
Unfortunately, the molecular mechanisms that cause a severely diminished glucagon secretion response during hypoglycemia in T1DM or T2DM are uncertain (14–17). Thus, it remains to be determined whether the findings obtained in rodent and murine studies will translate clinically. The presence of GPR119 on α-cells was demonstrated in this study in cadaveric human islets obtained from donors without diabetes and donors with T2DM and T1DM, although its functionality could not be evaluated. Furthermore, it remains uncertain how much pharmacological amplification of the impaired glucagon response to hypoglycemia in T1DM or T2DM will be needed to impact frequency and severity of hypoglycemic episodes. Recent clinical trials by Haymond et al. (37) indicate that glucagon mini-doses can be efficacious in treating hypoglycemia in T1DM. The advent of a dual-hormone (insulin and glucagon) closed-loop artificial pancreas is certain to provide more insight. If GPR119 agonism can enhance glucagon counterregulatory responses in humans, glucagon under these circumstances will be delivered directly to the liver via the portal circulation. In portal venous cannulated dog studies, as well as in clinical investigations using peripheral delivery, a potent dose-response stimulation of hepatic glucose production has been consistently observed (38–40). Therefore, use of a GPR119 small-molecule agonist in T1DM so as to restore glucagon secretion during hypoglycemia to levels described in healthy volunteers with intact counterregulation (as described in Gerich et al. [12]) would seem to be a reasonable threshold target for assessing clinical translation in early phase proof of pharmacology studies analogous to the proof of pharmacology studies in rodents presented in this report. However, the experimental conditions of a hypoglycemic clamp study, although a very useful dynamic challenge to appraise hormonal counterregulation, make it difficult due to the sustained infusion of insulin to fully evaluate the clinical potential of GPR119-mediated amplification of glucagon secretion to prevent hypoglycemia or speed recovery during an episode.
It seems most likely that GPR119 small-molecule agonist administration would not be used as an emergency treatment, but instead could be taken daily as an adjunct to insulin therapy, as a prophylaxis to reduce the frequency and severity of hypoglycemia. However, the clinical use of GPR119 agonist administration as an adjunctive treatment of T1DM (and potentially of insulin-treated T2DM) will require randomized clinical trials to demonstrate a significant reduction in the incidence of severe hypoglycemia in a real-life setting. An overall reduction in mild and moderate hypoglycemic episodes without causing deterioration in glycemic control would also support GPR119 agonist use as an axillary treatment in T1DM. It is noteworthy that numerous small-molecule GPR119 agonists have already entered the clinic, albeit in relatively short-duration studies, and in general have been shown to be safe and well tolerated (5). In summary, the current studies conducted in rodents indicate that GPR119 agonism can pharmacologically augment glucagon secretion during hypoglycemia. Clinical translation of these findings in patients with T1DM, most of whom have a severe impairment of glucagon secretion during hypoglycemia, as well in those with T2DM with a similar vulnerability, could have the potential to lessen the frequency and severity of insulin-induced hypoglycemia, one of the most challenging and frightening complications of insulin therapy of diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank Shu-Cheng Chen at MRL for help with the ISH work. Helpful insights and support were provided by Taro Akiyama of MRL and Aaron Kowalski of JDRF.
Duality of Interest. During the conduct of these studies, N.X.L., T.K., M.W., L.Y., G.D., A.P., L.W., M.E., and D.E.K. were full-time employees of and held stock in Merck & Co., Inc (Kenilworth, NJ), which was the sponsor of this research. S.B. and R.S. received funding for the research and compound A from Merck & Co., Inc. Additional support for the research conducted at Yale University was provided by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-020495 and P30-DK-045735) as well as the Yale Center for Clinical Investigation supported by the Clinical Translational Science Award (UL1-RR-024139).
Author Contributions. N.X.L., T.K., and D.E.K. designed the studies conducted at MRL. S.B. and R.S. designed the studies conducted at Yale University. N.X.L., S.B., T.K., M.W., L.Y., G.D., and A.P. researched data. N.X.L., S.B., L.W., M.E., R.S., and D.E.K. interpreted the data. N.X.L., S.B., R.S., and D.E.K. wrote the manuscript. H.B.W. led the medicinal chemistry research that discovered and synthesized the GPR119 agonists. D.E.K. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-0031/-/DC1.
References
- 1.Soga T, Ohishi T, Matsui T, et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor [published correction appears in Biochem Biophys Res Commun 2005;329:417]. Biochem Biophys Res Commun 2005;326:744–751 [DOI] [PubMed] [Google Scholar]
- 2.Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 2009;58:1058–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overton HA. Babbs AJ, Doel SM, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab 2006;3:167–175 [DOI] [PubMed] [Google Scholar]
- 4.Hansen HS, Rosenkilde MM, Holst JJ, Schwartz TW. GPR119 as a fat sensor. Trends Pharmacol Sci 2012;33:374–381 [DOI] [PubMed] [Google Scholar]
- 5.Ritter K, Buning C, Halland N, Pöverlein C, Schwink L. G protein-coupled receptor 119 (GPR119) agonists for the treatment of diabetes: recent progress and prevailing challenges. J Med Chem 2016;59:3579–3592 [DOI] [PubMed] [Google Scholar]
- 6.Semple G, Fioravanti B, Peteira G, et al. Discovery of the first potent and orally efficacious agonist of the orphan G-protein coupled receptor 119. J Med Chem 2008;51:5172–5175 [DOI] [PubMed] [Google Scholar]
- 7.Szewczyk JW, Acton J, Adams AD, et al. Design of potent and selective GPR119 agonists for type II diabetes. Bioorg Med Chem Lett 2011;21:2665–2669 [DOI] [PubMed] [Google Scholar]
- 8.Flock G, Holland D, Seino Y, Drucker DJ. GPR119 regulates murine glucose homeostasis through incretin responses receptor-dependent and independent mechanisms. Endocrinology 2011;152:374–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He YL, Davis L, Bhad P, et al. LEZ763, a novel GPR119 agonist, increases GLP-1, GIP, PYY, and glucagon, but has minimal effects on glucose in patients with type 2 diabetes (Abstract). Diabetes 2015;64(Suppl. 1A):122–LB [Google Scholar]
- 10.Odori S, Hosoda K, Tomita T, et al. GPR119 expression in normal human tissues and islet cell tumors: evidence for its islet-gastrointestinal distribution, expression in pancreatic beta and alpha cells, and involvement in islet function. Metabolism 2013;62:70–78 [DOI] [PubMed] [Google Scholar]
- 11.Rizza RA, Cryer P, Gerich JE. Role of glucagon, catecholamines, and growth hormone in human glucose counter-regulation. J Clin Invest 1979;64:62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 1973;182:171–173 [DOI] [PubMed] [Google Scholar]
- 13.Gerich JE. Lilly lecture 1988. Glucose counterregulation and its impact on diabetes mellitus. Diabetes 1988;37:1608–1617 [DOI] [PubMed] [Google Scholar]
- 14.Li J, Yu Q, Ahooghalandari P, et al. Submembrane ATP and Ca2+ kinetics in α-cells: unexpected signaling for glucagon secretion. FASEB J 2015;29:3379–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li XN, Herrington J, Petrov A, et al. The role of voltage-gated potassium channels Kv2.1 and Kv2.2 in the regulation of insulin and somatostatin release from pancreatic islets. J Pharmacol Exp Ther 2013;344:407–416. [DOI] [PubMed] [Google Scholar]
- 16.McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Diabetes 2010;59:2333–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paranjape SA, Chan O, Zhu W, et al. Influence of insulin in the ventromedial hypothalamus on pancreatic glucagon secretion in vivo. Diabetes 2010;59:1521–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue JT, Burdett E, Coy DH, Giacca A, Efendic S, Vranic M. Somatostatin receptor type 2 antagonism improves glucagon and corticosterone counterregulatory responses to hypoglycemia in streptozotocin-induced diabetic rats. Diabetes 2012;61:197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Hypoglycaemia Study Group Minimizing hypoglycemia in diabetes. Diabetes Care 2015;38:1583–1591 [DOI] [PubMed] [Google Scholar]
- 20.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab 2008;8:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 2012;14:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geisler K, Künzel J, Grundtner P, Müller A, Beckmann MW, Dittrich R. The perfused swine uterus model: long-term perfusion. Reprod Biol Endocrinol 2012;10:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol 2003;63:9–18 [DOI] [PubMed] [Google Scholar]
- 25.Christensen M, Calanna S, Sparre-Ulrich AH, et al. Glucose-dependent insulinotropic polypeptide augments glucagon responses to hypoglycemia in type 1 diabetes. Diabetes Care 2015;64:72–78 [DOI] [PubMed] [Google Scholar]
- 26.Adriaenssens AE, Svendsen B, Lam BY, et al. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 2016;59:2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segerstolpe Å, Palasantza A, Eliasson P, et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab 2016;24:593–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panaro BL, Flock G, Campbell JE, Beaudry JL, Cao X, Drucker DJ. β-cell inactivation of GPR119 unmasks incretin dependence of GPR119-mediated glucoregulation. Diabetes 2017;66:1626–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauffer L, Iakoubov R, Brubaker PL. GPR119: “double-dipping” for better glycemic control. Endocrinology 2008;149:2035–2037 [DOI] [PubMed] [Google Scholar]
- 30.Bolli G, Calabrese G, De Feo P, et al. Lack of glucagon response in glucose counter-regulation in type 1 (insulin-dependent) diabetics: absence of recovery after prolonged optimal insulin therapy. Diabetologia 1982;22:100–105 [DOI] [PubMed] [Google Scholar]
- 31.Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol 2014;10:711–722 [DOI] [PubMed] [Google Scholar]
- 32.UK Hypoglycaemia Study Group Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007;50:1140–1147 [DOI] [PubMed] [Google Scholar]
- 33.Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV. Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes 1988;37:901–907 [DOI] [PubMed] [Google Scholar]
- 34.Davis SN, Mann S, Briscoe VJ, Ertl AC, Tate DB. Effects of intensive therapy and antecedent hypoglycemia on counterregulatory responses to hypoglycemia in type 2 diabetes. Diabetes 2009;58:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Israelian Z, Szoke E, Woerle J, et al. Multiple defects in counterregulation of hypoglycemia in modestly advanced type 2 diabetes mellitus. Metabolism 2006;55:593–598 [DOI] [PubMed] [Google Scholar]
- 36.McCoy RG, Lipska KJ, Yao X, et al. Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med 2016;176:969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haymond MW, Redondo M, McKay S, et al. Nonaqueous, mini-dose glucagon for treatment of mild hypoglycemia in adults wtih tupe 1 diabetes: a dose-seeking study. Diabetes Care 2016;39:465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherrington AD, Lacy WW, Chiasson JL. Effect of glucagon on glucose production during insulin deficiency in the dog. J Clin Invest 1978;62:664–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda M, Defronzo RA, Glass L, et al. Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism 2002;51:1111–1119 [DOI] [PubMed] [Google Scholar]
- 40.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 2012;122:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.