Abstract
We observed that a 4-h morning (AM) duodenal infusion of glucose versus saline doubled hepatic glucose uptake (HGU) and storage during a hyperinsulinemic–hyperglycemic (HIHG) clamp that afternoon (PM). To separate the effects of AM hyperglycemia versus AM hyperinsulinemia on the PM response, we used hepatic balance and tracer ([3-3H]glucose) techniques in conscious dogs. From 0 to 240 min, dogs underwent a euinsulinemic-hyperglycemic (GLC; n = 7) or hyperinsulinemic-euglycemic (INS; n = 8) clamp. Tracer equilibration and basal sampling occurred from 240 to 360 min, followed by an HIHG clamp (360–600 min; four times basal insulin, two times basal glycemia) with portal glucose infusion (4 mg ⋅ kg−1 ⋅ min−1). In the HIHG clamp, HGU (5.8 ± 0.9 vs. 3.3 ± 0.3 mg ⋅ kg−1 ⋅ min−1) and net glycogen storage (6.0 ± 0.8 vs. 2.9 ± 0.5 mg ⋅ kg−1 ⋅ min−1) were approximately twofold greater in INS than in GLC. PM hepatic glycogen content (1.9 ± 0.2 vs. 1.3 ± 0.2 g/kg body weight) and glycogen synthase (GS) activity were also greater in INS versus GLC, whereas glycogen phosphorylase (GP) activity was reduced. Thus AM hyperinsulinemia, but not AM hyperglycemia, enhanced the HGU response to a PM HIHG clamp by augmenting GS and reducing GP activity. AM hyperinsulinemia can prime the liver to extract and store glucose more effectively during subsequent same-day meals, potentially providing a tool to improve glucose control.
Introduction
The second-meal phenomenon, or Staub-Traugott effect (the reduced glycemic excursion during disposal of the second meal of the day compared with that of the first meal), has been observed in normal individuals and those with type 2 diabetes (1–5). Increased muscle glucose uptake (4) and, conversely, increased hepatic glucose disposal (1) have been reported in response to the second meal. We recently demonstrated in conscious dogs that a 4-h duodenal glucose infusion in the morning (AM), compared with an AM duodenal saline infusion, stimulated hepatic glucose uptake (HGU) and glycogen storage during a hyperinsulinemic-hyperglycemic (HIHG) clamp, mimicking a meal response, later in the same day (6). In contrast to our findings in regard to the liver, there was no difference between groups in nonhepatic (primarily muscle) glucose disposal during the afternoon (PM) clamp (6). Use of clamp conditions allowed us to control the PM pancreatic hormone and glucose levels so that we could quantify organ glucose disposal in response to the “second meal” without the complicating factors of varying concentrations of insulin, glucagon, and glucose. Given the importance of the liver in storing glucose in response to a meal (7,8) and the significant role that hepatic glycogen reserves play in maintaining glucose homeostasis and improving the response to hypoglycemia (9–12), the priming function of insulin could be helpful in developing new approaches to improve the care of those with insulin-treated diabetes. In the studies described in the current report, we explored whether it was the AM increase in glycemia, insulinemia, or both, that stimulated the liver to take up and store more glucose during the clamp period later in the same day.
Research Design and Methods
Animal Care and Surgical Procedures
Adult mongrel dogs of both sexes purchased from a U.S. Department of Agriculture–licensed vendor were studied. The protocol was approved by the Vanderbilt University Institutional Animal Care and Use Committee, and the animals were housed and cared for according to American Association for Laboratory Animal Care guidelines. The dogs were fed once daily a chow and meat diet in amounts calculated to be weight-maintaining, and they were kept on a 12 h light:12 h dark cycle with lights on at 0600 h.
Approximately 16 days before the study, surgery was performed under general anesthesia in each dog to insert sampling catheters in the femoral artery, hepatic portal vein, and left common hepatic vein; blood flow probes around the portal vein and hepatic artery; a splenic and a jejunal vein catheter to allow infusion into the hepatic portal circulation; and an infusion catheter into the inferior vena cava (IVC). The free ends of the splenic, jejunal vein, and IVC catheters were tunneled subcutaneously to a pocket at the back of the neck, and the flow probes and portal and hepatic vein catheters were secured in pockets in the abdominal area. On the day before the study, all dogs were fed their regular diet at noon, and their food bowls were removed within an hour; all dogs consumed at least 75% of the food served.
Experimental Design
Clamp 1
At the beginning of each experiment, the arterial sampling catheter and the two infusion catheters were removed from their subcutaneous pockets. Two groups of dogs (GLC, n = 7, 22.0 ± 0.9 kg; and INS, n = 8, 22.5 ± 1.0 kg) began an AM clamp (clamp 1) at 0 min (0630 h), with the clamp continuing until 240 min (Fig. 1). In both groups, clamp 1 began with the start of a somatostatin infusion (0.8 µg ⋅ kg−1 ⋅ min−1; Bachem, Torrance, CA) into the IVC. Glucagon (basal replacement at 0.5 ng ⋅ kg−1 ⋅ min−1; GlucaGen, Boehringer Ingelheim, Ridgefield, CT) was delivered intraportally via the splenic and jejunal catheters. In the GLC group, regular insulin (Novolin R; Novo Nordisk, Bagsværd, Denmark) was infused into the splenic and jejunal vein catheters at a basal rate (0.3 mU ⋅ kg−1 ⋅ min−1) throughout the 4 h AM clamp, and 20% dextrose (Baxter, Deerfield, IL) was infused intraportally via the splenic and jejunal catheters. The infusion rate was adjusted as necessary to raise the arterial blood glucose level from 77 to 120 mg/dL (to reproduce the glucose excursion observed in our previous study [6]). In the INS group, regular insulin was infused intraportally at 2.1 mU ⋅ kg−1 ⋅ min−1 from 0 to 30 min, 2.4 mU ⋅ kg−1 ⋅ min−1 from 30 to 60 min, and 1.5 mU ⋅ kg−1 ⋅ min−1 from 60 to 240 min. This infusion pattern was designed to mimic the insulin concentrations observed in our previous study in which there was no AM clamp but in which there was a continuous duodenal infusion of glucose for 4 h (6). In the INS group, 50% dextrose (Baxter) was infused into the IVC as necessary to maintain euglycemia.
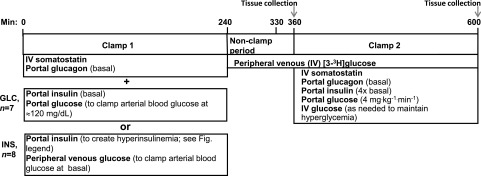
Figure 1.
Study design. Eleven dogs underwent two insulin and glucose clamp periods, beginning at 0 min. Clamp conditions were as described in the figure. In the INS group, insulin was infused intraportally at a rate (mU ⋅ kg−1 ⋅ min−1) of 2.1 from 0 to 30 min, 2.4 from 30 to 60 min, and 1.5 from 60 to 240 min. At 240 min, the first clamp ceased, and a primed continuous infusion of [3H]glucose began. After a 90-min equilibration period (240–330 min), there was a 30-min period of sampling under nonclamp conditions. These 11 dogs subsequently underwent an HIHG clamp in the presence of the portal glucose signal from 360 to 600 min (clamp 2). Hepatic tissue was collected from these dogs at 600 min. In addition to the dogs that underwent both clamps, two dogs that received the INS treatment and two that received the GLC treatment were euthanized at 360 min, with tissue biopsy samples obtained at that time to provide an indication of glycogen stores, enzyme activities, and protein expression before the start of clamp 2. IV, intravenous.
At the end of the first clamp period, the hormone infusions were stopped, arterial glucose concentrations were monitored, and glucose was infused via the IVC if necessary until the animals were able to maintain euglycemia. At that point, glucose infusions ceased, and the portal and hepatic sampling catheters and blood flow probes were exteriorized under local anesthesia. In all respects, the last 6 h of the study (the nonclamp period and clamp 2) were identical in the GLC and INS groups as well as with the groups contained in our previous report (6).
Nonclamp Period
A primed (38 µCi) continuous infusion of [3-3H]glucose (0.38 μCi/min) was administered via peripheral vein beginning at 240 min. After a 90-min equilibration period, nonclamp period blood samples were collected from the sampling catheters between 330 and 360 min. At the end of the nonclamp period, two INS and two GLC dogs were anesthetized, and hepatic tissue was rapidly collected from each of three liver lobes, freeze clamped, and stored at −80°C, as previously described (6). These four dogs were euthanized at the end of the tissue collection and are referred to as the amINS and amGLC dogs.
Clamp 2
From 360 to 600 min, all remaining dogs underwent a HIHG clamp with portal glucose infusion to mimic the conditions existing during absorption of a midday meal. During the clamp, somatostatin was infused as described above, and regular insulin (four times basal; 1.2 mU ⋅ kg−1 ⋅ min−1) and glucagon (basal) were replaced via intraportal infusion. Glucose (20%) was infused continuously at 4 mg ⋅ kg−1 ⋅ min−1 into the portal circulation. A primed, continuous infusion of 50% glucose was administered via a peripheral vein to create and maintain hyperglycemia, with the infusion rate adjusted as necessary to clamp arterial blood glucose at ∼150 mg/dL. Samples were taken every 15–30 min from the artery, portal vein, and hepatic vein catheters to allow measurement of hormones and substrates. At the end of the study, hepatic tissue was harvested as described above. The sample sizes were calculated to yield a 90% likelihood of detecting a difference of at least 2 mg ⋅ kg−1 ⋅ min−1 in net HGU between groups, with α = 0.05, based on data from our previous report (6).
Analyses
Western Blotting
Western blotting procedures were performed on liver tissue as described previously (13,14). Glycogen synthase (GS), glucokinase (GK), and Akt antibodies were purchased from Cell Signaling, Danvers, MA. CLOCK and Per1 antibodies were obtained from Santa Cruz Biotechnology, Dallas, TX. ImageJ software (http://rsb.info.nih.gov/ij/) was used for quantification. For reference (control) samples, we included tissues obtained from the SALno-clamp group in our previous publication (6); the dogs in that group received a duodenal saline infusion from 0 to 240 min and underwent tissue collection at 360 min, the starting time for the PM clamp period in the GLC and INS groups in the current report. Western blotting was done on 26 lane gels (SDS-PAGE; Bio-Rad, Hercules, CA) and expressed relative to cyclophilin B (for GK, CLOCK, and Per1) or total Akt or GS protein (for phosphorylated [p]Akt and pGS).
Enzyme Activity and Biochemical Analyses
Hepatic GK, GS, and glycogen phosphorylase (GP) activities were determined as previously described (15). Plasma glucose, [3H]glucose, glucagon, insulin, and nonesterified fatty acid (NEFA) levels; blood lactate and glycerol concentrations; and hepatic glycogen concentrations were measured using standard methods as described previously (16,17).
Calculations
Net hepatic substrate balances, net hepatic carbon retention, and net HGU were calculated with the arteriovenous difference method (18). Net hepatic glucose balance was calculated with an indirect method, as previously described (19), to reduce any error introduced by streaming of infusate in the portal vein. Unidirectional HGU, using [3H]glucose measurements) and hepatic sinusoidal plasma insulin and glucagon concentrations were calculated as previously described (18). Glycogen synthesis via the direct pathway was calculated by dividing hepatic [3H]-labeled glycogen at the end of the study by the average inflowing plasma [3H]glucose-specific radioactivity (19). Net hepatic carbon retention was calculated from the sum of the balance data for glucose and its metabolites. We have previously demonstrated that this approach provides a useful index of glycogen synthesis (19).
Statistical Analyses
Data are expressed as means ± SEM, except in Figs. 5 and 6, where individual data and medians are shown. Two-way ANOVA with repeated-measures design was used (SigmaStat; Systat, Richmond, CA), and post hoc analysis was performed using the Student-Newman-Keuls multiple comparisons test. A P value of <0.05 was considered significant. The amGLC and amINS dogs were not compared statistically with other groups because of their small numbers.
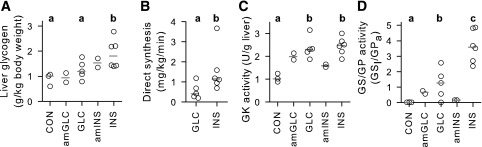
Figure 5.
Hepatic tissue analyses: glycogen concentrations (A), glycogen synthesis via the direct pathway (B), GK activity (C), and GS activity relative to that of GP (D). Individual data are depicted by unfilled circles, with dark gray lines indicating the median for each treatment. Control (CON) samples (n = 3) were taken from the SALno-clamp group described in our previous publication (6); see research design and methods (n = 5 for GLC and n = 6 for INS). The amGLC and amINS data were derived from the two dogs in each treatment group that underwent the AM clamp and the subsequent nonclamp period, with tissue collection occurring at 360 min. Statistical analyses were done only where treatments reflected at least n = 3. Groups marked with the same lower case letter were not significantly different from one another, and those marked with different letters differed significantly (P < 0.05).
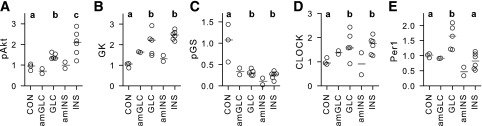
Figure 6.
Hepatic tissue protein analyses: pAkt (A), GK (B), pGS (C), CLOCK (D), and Per1 (E) proteins expressed relative to total Akt (for pAkt), cyclophilin B (for GK, CLOCK, and Per1), or total GS (for pGS). Individual data are depicted by unfilled circles, with dark gray lines indicating the median for each treatment. Control (CON) samples (n = 3) were taken from the SALno-clamp dogs described in our previous publication (6); see research design and methods (n = 5 for GLC and n = 6 for INS). The amGLC and amINS data were derived from the two dogs in each treatment group that underwent the AM clamp and the subsequent nonclamp period, with tissue collection occurring at 360 min. Statistical analyses were done only where treatments included at least n = 3. Groups marked with different letters differed significantly (P < 0.05).
Results
Clamp 1 Data
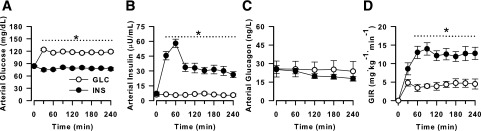
Glucose concentrations before clamp 1 were indistinguishable between the groups (Fig. 2A). During clamp 1, arterial blood glucose concentrations remained basal in the INS group (79 ± 1 mg/dL), and were ∼50% greater in the GLC group (P < 0.05), by design. The preclamp arterial insulin concentrations did not differ between groups, but the concentrations were higher in INS versus GLC throughout clamp 1, as designed (P < 0.05) (Fig. 2B). The plasma insulin concentrations in INS were highest during the 1st h of the clamp, with a peak concentration more than eightfold basal. This mimicked the pattern of hyperinsulinemia seen in our previous study, in which intraduodenal glucose was administered in the AM in the absence of a pancreatic clamp (6). There were no significant differences in glucagon concentrations between groups during clamp 1, with the concentrations in both groups remaining at basal levels (Fig. 2C). Cortisol concentrations in both groups were basal at the start of the AM clamp and did not change during the clamp in either group (data not shown). As a consequence of the hyperinsulinemia, the glucose infusion rates (GIRs) in INS were higher than those in GLC during clamp 1 (area under the curve from 0 to 240 min was 2,835 ± 419 vs. 1,078 ± 263 mg/kg in INS vs. GLC, respectively; P < 0.05) (Fig. 2D).
Figure 2.
Data from fasting samples taken before the intervention (0 min) and clamp 1 (beginning after the 0-min sample and continuing through 240 min). During clamp 1, euinsulinemic hyperglycemia was maintained in the GLC group (n = 7), and hyperinsulinemic euglycemia existed in the INS group (n = 8). Arterial blood glucose (A), arterial plasma insulin (B), arterial plasma glucagon (C), and GIR (D). *P < 0.05 between groups.
Nonclamp and Clamp 2 Data
Hormone and Hepatic Blood Flow Data
The arterial and hepatic sinusoidal plasma insulin concentrations during the midday nonclamp period were 35% and 42% less in the INS versus the GLC group, respectively, although the hepatic sinusoidal concentrations did not differ significantly between the groups (Table 1). During clamp 2, arterial and hepatic sinusoidal insulin concentrations were elevated in both groups to levels mimicking postprandial conditions (8,20) (P > 0.05 between groups) (Table 1). The arterial and hepatic sinusoidal plasma glucagon concentrations remained at basal levels throughout clamp 2 and were not significantly different between the groups at any time (Table 1). In addition, the hepatic blood flow did not differ significantly between groups (Table 1).
Table 1.
Arterial and hepatic sinusoidal plasma insulin and glucagon concentrations and hepatic blood flow during the nonclamp period and clamp 2
| Parameter and group | Nonclamp (330–360 min) | Clamp 2 (360–600 min) |
|---|---|---|
| Insulin (µU/mL) | ||
| Arterial | ||
| GLC | 6 ± 1 | 22 ± 2 |
| INS | 4 ± 1* | 25 ± 2 |
| Hepatic sinusoidal | ||
| GLC | 12 ± 3 | 76 ± 6 |
| INS | 7 ± 2 | 72 ± 4 |
| Glucagon (ng/L) | ||
| Arterial | ||
| GLC | 29 ± 3 | 24 ± 7 |
| INS | 20 ± 3 | 19 ± 2 |
| Hepatic sinusoidal | ||
| GLC | 36 ± 6 | 30 ± 7 |
| INS | 26 ± 3 | 25 ± 3 |
| Total hepatic blood flow (mL ⋅ kg−1 ⋅ min−1) | ||
| GLC | 35 ± 3 | 28 ± 2 |
| INS | 35 ± 3 | 28 ± 3 |
Data are mean ± SEM for all time points during the indicated period; n = 5 and 6 for the GLC and INS groups, respectively.
*P < 0.05 between groups.
Glucose Data
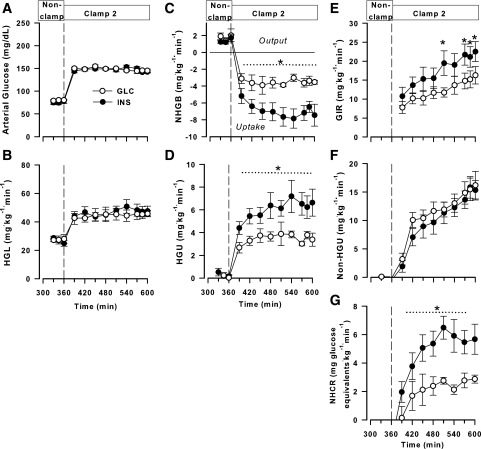
Blood glucose concentrations were similar in both groups during the midday nonclamp period (80 ± 1 and 75 ± 4 mg/dL in GLC and INS, respectively; P = 0.54) and during clamp 2 (148 ± 1 and 147 ± 1 mg/dL, respectively; P = 0.82) (Fig. 3A). In keeping with this, the hepatic glucose loads were virtually identical between the groups during the clamp (Fig. 3B). All animals were in a state of net hepatic glucose output during the midday nonclamp period (1.9 ± 0.5 and 1.5 ± 0.2 mg ⋅ kg−1 ⋅ min−1 in GLC and INS, respectively; P = 0.72). With the onset of the HIHG clamp including portal glucose infusion, both groups rapidly switched to net HGU (Fig. 3C), but the rate in the INS group was approximately double that in GLC (3.4 ± 0.4 and 7.1 ± 0.9 mg ⋅ kg−1 ⋅ min−1 in GLC and INS, respectively; P < 0.05). Consistent with this, the rate of unidirectional (tracer-determined) HGU was ∼70% greater (P < 0.05) in the INS than in the GLC group (clamp 2 areas under the curve: 886 ± 84 and 1,513 ± 207 mg/kg in GLC and INS, respectively) (Fig. 3D).
Figure 3.
Data from the midday nonclamp period and clamp 2 (an HIHG clamp with intraportal glucose infusion; 360–600 min). Arterial blood glucose (A), hepatic glucose load (HGL) (B), net hepatic glucose balance (NHGB) (C), unidirectional HGU (D), GIR (E), net HGU (F), and net hepatic glycogen synthesis (NHCR) (i.e., carbon retention) (G) in the GLC (n = 5) and INS (n = 6) groups. *P < 0.05 between groups.
The total GIR, including the fixed-rate portal vein infusion plus the variable peripheral glucose infusion required to maintain equivalent hyperglycemia, was >40% higher (P < 0.05) in INS than in GLC (3,623 ± 545 and 2,524 ± 358 mg/kg, respectively) (Fig. 3E). Net HGU increased throughout the clamp period in both groups, with the rates not significantly different between the groups at any time (Fig. 3F). Net hepatic carbon retention (glycogen synthesis) was >2.5-fold greater (P < 0.05) during the clamp period in the INS than in the GLC group (Fig. 3G).
Lactate, Glycerol, and NEFA
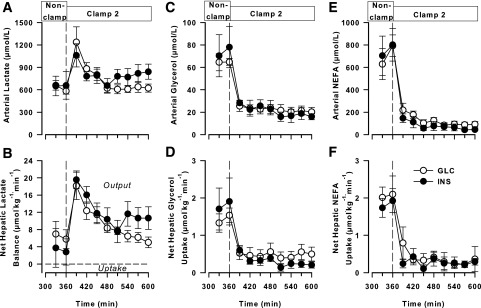
Arterial blood lactate concentrations and net hepatic lactate output (NHLO) in the nonclamp period did not differ between the INS and GLC groups. With the onset of clamp 2, both groups exhibited an increase in lactate concentrations and NHLO, with these parameters then declining toward baseline (Fig. 4A and B). Although the lactate concentrations and NHLO were both numerically higher in the INS than in the GLC group during the latter part of clamp 2, this did not reach statistical significance.
Figure 4.
Data from the midday nonclamp period and clamp 2 (an HIHG clamp with intraportal glucose infusion; 360–600 min). Arterial blood lactate (A), net hepatic lactate balance (B), arterial blood glycerol (C), net hepatic glycerol uptake (D), arterial plasma NEFA (E), and net hepatic NEFA uptake (F) in the GLC (n = 5) and INS (n = 6) groups. There were no significant differences between groups.
Neither the arterial glycerol concentration nor net hepatic glycerol uptake differed between the groups at any time (Fig. 4C and D). The concentration and net hepatic uptake both fell in both groups with the onset of clamp 2 and remained suppressed throughout the clamp. The arterial concentrations and net hepatic uptakes of NEFA followed a pattern similar to that of glycerol, with no differences between groups at any time (Fig. 4E and F).
Liver Tissue Analyses
Terminal liver glycogen concentrations in the pair of amGLC dogs appeared little different from those in the control dogs from our earlier study (that underwent no clamp but received duodenal saline during the 4-h AM treatment period [6]), whereas the concentrations in the GLC group tended to be greater (P = 0.09) than those in the controls (Fig. 5A). The median of the amINS dogs was intermediate between those of the GLC and INS groups, whereas the concentrations in the INS group were significantly greater than those in both the control and GLC groups (P < 0.05). Moreover, the rate of glycogen synthesis via the direct pathway in the INS group was >2.5 times higher than the rate in the GLC group (P < 0.05) (Fig. 5B). GK activity was ∼2.5-fold greater (P < 0.05) in the GLC and INS groups than in the control group but did not differ between the GLC and INS groups (Fig. 5C). GK activities in the amGLC and amINS dogs were intermediate between the control dogs versus the GLC and INS groups. GS activity relative to that of GP was enhanced (P < 0.05) in the INS and GLC groups, relative to the control group, and GS/GP activity in the INS group was more than twofold greater than that in the GLC group (P < 0.05) (Fig. 5D). The GS/GP activity in the amGLC and amINS dogs appeared little different from that in the control group.
Relative pAkt in both the GLC and INS groups was greater than in the control group (∼40% and 100%, respectively; P < 0.05 for both groups vs. control and P < 0.05 between GLC and INS) (Fig. 6A). The pAkt-to-Akt ratio in the amGLC and amINS dogs was similar to that in the control group. The median GK protein expression in the GLC and INS groups was similar, and both were twofold or greater than that of the control group (P < 0.05) (Fig. 6B), with amGLC and amINS GK protein expression being intermediate between the control versus the GLC and INS values. Relative pGS protein was reduced >70% in the GLC and INS groups versus the control group (P < 0.05) and tended to be lower in the INS versus GLC group (P = 0.11) (Fig. 6C). The amGLC and amINS dogs also showed a marked reduction from the control group in pGS expression. There were no significant differences in expression of the CLOCK gene between the GLC and INS groups, and expression in both groups was ∼70% greater than that in the control samples (P < 0.05) (Fig. 6D). CLOCK gene expression in the amGLC dogs was intermediate between that of the GLC and INS groups and that of the control group, whereas expression in the two amINS dogs was too disparate to suggest any trend. Per1 expression was increased (P < 0.05) in the GLC versus the INS and control groups and was numerically higher in the amGLC versus amINS animals (Fig. 6E).
Discussion
We previously demonstrated that a 4-h AM duodenal glucose versus saline infusion markedly enhanced HGU and glycogen storage during an HIHG clamp later in the same day (6). The current data make it evident that a 4-h period of hyperinsulinemic euglycemia in the AM can replicate the results observed with duodenal glucose delivery, but a 4-h period of euinsulinemic hyperglycemia has no such effect. The enhancement of HGU in the INS group in the current study was accompanied by augmentation of hepatic glycogen synthesis, as indicated both by increased hepatic carbon retention during the PM clamp and by larger hepatic glycogen stores at the end of study. Data from healthy normal weight and obese adults and those with type 2 diabetes demonstrate that breakfast can have a “priming” effect on glucose metabolism during the remainder of the day (3,21–23), such that, when a substantial breakfast is consumed, insulin sensitivity improves and glucose excursions are reduced at meal times later in the day. Although increased muscle glucose uptake (4) has been suggested to account for the increase in insulin sensitivity, our current and previous data (6), as well as data from other investigators (1,24), indicate that enhanced hepatic glucose disposal largely explains the phenomenon. Moreover, after an initial increase in glycolysis, reflected in the increase in NHLO early in clamp 2, much of the glucose taken up by the liver was deposited in glycogen (Fig. 3G). We have observed this early increase in glycolysis in response to a rapid change from NHLO to net uptake under a number of different study conditions (6,25,26) and determined that it reflects the time required for insulin to fully activate GS and inhibit GP.
Analysis of terminal hepatic tissue samples demonstrated that the differences in HGU and liver glycogen storage between the study groups was most closely related to their relative impacts on hepatic GS and GP. The effect of the prolonged period of AM hyperinsulinemia was to reduce pGS protein, stimulate GS activity, and reduce GP activity during the PM clamp. Although the GLC group exhibited reduced pGS protein relative to the control, the reduction tended to be more modest than in the INS group. Moreover, GS/GP activation was significantly enhanced in the INS versus the GLC group, consistent with dephosphorylation of liver GP being an upstream event in GS activation (27). pAkt expression was stimulated more in the INS versus the GLC group, in keeping with its role in enhancement of glycogen synthesis, at least partially via stimulating the phosphorylation of glycogen synthase kinase 3 (28). GK protein expression, GK activity, and CLOCK gene expression were very similar between the INS and GLC groups, suggesting that these were not primary contributors to the differences in liver glucose uptake and glycogen storage. The greater expression of Per1 in the GLC versus the INS group is of note, however, because Per1 is involved in feedback inhibition of CLOCK activity (29,30). Future studies will be required to examine these impacts more thoroughly and determine their metabolic effects. Alterations in clock gene expression have been noted to be associated with altered glucose metabolism in both humans and animal models (31–34).
The results of the current investigation are remarkably consistent with our previous results in the dogs receiving AM duodenal infusions of glucose versus saline (6). Thus, these data indicate that a tailored period of hyperinsulinemia in the AM can have potent effects on glucose disposal later in the day. This underlines the importance of hyperinsulinemia in bringing about the second-meal phenomenon, which has major effects on glucose disposal over the course of the day. Despite its having been identified a century ago, intriguing new information regarding the physiology underlying the phenomenon and its potential clinical applications continues to be obtained (35). The dog provides a valuable model for examination of its effect on the liver, because it allows quantification of HGU, and our data clearly show an enhancement of hepatic glycogen stores in the group experiencing hyperinsulinemia during the AM clamp. The current findings could have significant implications for individuals with insulin-treated diabetes. Despite the positive impacts of new insulin analogs, hypoglycemia continues to occur, creating a significant impediment to optimal care in insulin-treated individuals (36–38). Those with insulin-dependent diabetes have been found in a number of investigations, although not all, to have lower postprandial levels of hepatic glycogen than normal control subjects (9,39–42). A difference in liver glycogen levels between subjects with diabetes without complications and individuals without diabetes is most evident after the third meal of the day (40,43), and this may explain why some studies quantifying only the postbreakfast glycogen levels may not observe a difference between subjects with diabetes compared with control subjects without diabetes. It is noteworthy that normal dogs with enhanced hepatic glycogen reserves (achieved via fructose administration during an AM period of hyperinsulinemia and hyperglycemia) exhibited a markedly improved counterregulatory response (enhanced glucagon and epinephrine secretion, coupled with a stimulation of net hepatic glucose release) during a PM period of hypoglycemia in comparison with animals under the same hypoglycemic conditions but in the absence of the AM fructose infusion (10). Together, these findings suggest that measures to stimulate hepatic glycogen accumulation have the potential to reduce hypoglycemia risk. Combined with these findings, our current data suggest that the insulin regimen used in the AM, coupled with an adequate AM energy intake, may be important in developing optimal hepatic glycogen reserves daily and that this, in turn, can reduce the risk of hypoglycemia later in the day or night.
The question arises about whether hepatic glycogen content might have been saturated in the INS group because there was an increase in net hepatic lactate release and circulating lactate concentrations during the latter portion of the second clamp period in that group. Based on previous work from our laboratory, where an intraportal fructose infusion was used to enhance net HGU, hepatic glycogen stores in the INS group were unlikely to be completely saturated (44,45). Nevertheless, it is possible that glycogen storage was slowing as levels increased. Certainly it appeared that the rate of net glycogen synthesis (Fig. 3G) had plateaued in both groups during the latter part of clamp 2. This further highlights the potential for AM hyperinsulinemia to maximize hepatic glycogen reserves.
Growth hormone (GH), which would have been suppressed by somatostatin infusion, was not replaced in these studies. GH does not display a circadian rhythm in dogs (46), and thus, the study periods are unlikely to have coincided with any peak or trough in normal secretion. Moreover, although GH has well-established roles in glucose metabolism, many are chronic in nature (47). On an acute basis, GH is known to stimulate lipolysis, which could have affected glucose disposal (48). Glycerol and NEFA concentrations declined during the AM clamp with both treatments (data not shown), indicating that a differential effect between groups was unlikely. Moreover, the direct effect of insulin on the liver, and not its effect on fat metabolism, is the key factor in its regulation of hepatic glucose metabolism (19,49,50).
In conclusion, a tailored period of hyperinsulinemia during the AM stimulates hepatic glycogen accretion in response to a HIHG later in the same day. It remains to be seen whether patterned delivery of the AM insulin dose in those with insulin-dependent diabetes could enhance hepatic glycogen storage and subsequently improve the response to hypoglycemia later in the same day, particularly during the nocturnal period.
Article Information
Funding. This work was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK-18243 to A.D.C. and DK-020593 to the Metabolic Physiology Shared Resource and the Hormone Assay & Analytical Services Core. The Hormone Assay & Analytical Services Core also receives support from National Institutes of Health grant DK-059637. A.D.C. is the Jacquelyn A. Turner and Dr. Dorothy J. Turner Chair in Diabetes Research.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.C.M. directed all experiments, collected and interpreted data, and drafted and revised the manuscript. M.C.M., P.E.W., and A.D.C. participated in the design of experiments. M.S.S., B.F., and G.K. participated in the experiments. M.S.S., K.C.C., and M.S. performed biochemical and tissue analysis. B.F. and P.E.W. were responsible for surgical preparation and oversight of animal care. A.D.C. was involved in all aspects of the study, including review of the data. All authors provided input during the preparation of the manuscript. A.D.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016, and at the 52nd Annual Meeting of the European Association for the Study of Diabetes, Munich, Germany, 12–16 September 2016.
References
- 1.Abraira C, Buchanan B, Hodges L. Modification of glycogen deposition by priming glucose loads: the second-meal phenomenon. Am J Clin Nutr 1987;45:952–957 [DOI] [PubMed] [Google Scholar]
- 2.Chen MJ, Jovanovic A, Taylor R. Utilizing the second-meal effect in type 2 diabetes: practical use of a soya-yogurt snack. Diabetes Care 2010;33:2552–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jovanovic A, Gerrard J, Taylor R. The second-meal phenomenon in type 2 diabetes. Diabetes Care 2009;32:1199–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jovanovic A, Leverton E, Solanky B, et al. The second-meal phenomenon is associated with enhanced muscle glycogen storage in humans. Clin Sci (Lond) 2009;117:119–127 [DOI] [PubMed] [Google Scholar]
- 5.Staub H. Untersuchungen über den Zuckerstoffwechsel des Menschen. I Mitteilung Z Klin Med 1921;91:44–60 [Google Scholar]
- 6.Moore MC, Smith MS, Farmer B, et al. Priming effect of a morning meal on hepatic glucose disposition later in the day. Diabetes 2017;66:1136–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis MA, Williams PE, Cherrington AD. Effect of a mixed meal on hepatic lactate and gluconeogenic precursor metabolism in dogs. Am J Physiol 1984;247:E362–E369 [DOI] [PubMed] [Google Scholar]
- 8.Moore MC, Pagliassotti MJ, Swift LL, et al. Disposition of a mixed meal by the conscious dog. Am J Physiol 1994;266:E666–E675 [DOI] [PubMed] [Google Scholar]
- 9.Krssak M, Brehm A, Bernroider E, et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 2004;53:3048–3056 [DOI] [PubMed] [Google Scholar]
- 10.Winnick JJ, Kraft G, Gregory JM, et al. Hepatic glycogen can regulate hypoglycemic counterregulation via a liver-brain axis. J Clin Invest 2016;126:2236–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langhans W. Role of the liver in the control of glucose-lipid utilization and body weight. Curr Opin Clin Nutr Metab Care 2003;6:449–455 [DOI] [PubMed] [Google Scholar]
- 12.Rines AK, Sharabi K, Tavares CD, Puigserver P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat Rev Drug Discov 2016;15:786–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramnanan CJ, Edgerton DS, Rivera N, et al. Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes 2010;59:1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgerton DS, Ramnanan CJ, Grueter CA, et al. Effects of insulin on the metabolic control of hepatic gluconeogenesis in vivo. Diabetes 2009;58:2766–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres TP, Fujimoto Y, Donahue EP, et al. Defective glycogenesis contributes toward the inability to suppress hepatic glucose production in response to hyperglycemia and hyperinsulinemia in Zucker diabetic fatty rats. Diabetes 2011;60:2225–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore MC, Rossetti L, Pagliassotti MJ, et al. Neural and pancreatic influences on net hepatic glucose uptake and glycogen synthesis. Am J Physiol 1996;271:E215–E222 [DOI] [PubMed] [Google Scholar]
- 17.Keppler D, Decker K. Glycogen: determination with amyloglycosidase. In Methods of Enzymatic Analysis. 2nd ed. Bergmeyer HU, Ed. New York, Verlag Chemie Weinheim, Academic Press, 1974, p. 1127–1131 [Google Scholar]
- 18.Coate KC, Kraft G, Irimia JM, et al. Portal vein glucose entry triggers a coordinated cellular response that potentiates hepatic glucose uptake and storage in normal but not high-fat/high-fructose-fed dogs. Diabetes 2013;62:392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satake S, Moore MC, Igawa K, et al. Direct and indirect effects of insulin on glucose uptake and storage by the liver. Diabetes 2002;51:1663–1671 [DOI] [PubMed] [Google Scholar]
- 20.Moore MC, Werner U, Smith MS, Farmer TD, Cherrington AD. Effect of the glucagon-like peptide-1 receptor agonist lixisenatide on postprandial hepatic glucose metabolism in the conscious dog. Am J Physiol Endocrinol Metab 2013;305:E1473–E1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013;21:2504–2512 [DOI] [PubMed] [Google Scholar]
- 22.Jakubowicz D, Wainstein J, Ahren B, Landau Z, Bar-Dayan Y, Froy O. Fasting until noon triggers increased postprandial hyperglycemia and impaired insulin response after lunch and dinner in individuals with type 2 diabetes: a randomized clinical trial. Diabetes Care 2015;38:1820–1826 [DOI] [PubMed] [Google Scholar]
- 23.Saad A, Dalla Man C, Nandy DK, et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012;61:2691–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonuccelli S, Muscelli E, Gastaldelli A, et al. Improved tolerance to sequential glucose loading (Staub-Traugott effect): size and mechanisms. Am J Physiol Endocrinol Metab 2009;297:E532–E537 [DOI] [PubMed] [Google Scholar]
- 25.Coate KC, Kraft G, Shiota M, et al. Chronic overeating impairs hepatic glucose uptake and disposition. Am J Physiol Endocrinol Metab 2015;308:E860–E867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore MC, Hsieh PS, Neal DW, Cherrington AD. Nonhepatic response to portal glucose delivery in conscious dogs. Am J Physiol Endocrinol Metab 2000;279:E1271–E1277 [DOI] [PubMed] [Google Scholar]
- 27.Agius L. Role of glycogen phosphorylase in liver glycogen metabolism. Mol Aspects Med 2015;46:34–45 [DOI] [PubMed] [Google Scholar]
- 28.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther 2015;148:114–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng D, Lazar MA. Clocks, metabolism, and the epigenome. Mol Cell 2012;47:158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilorz V, Helfrich-Förster C, Oster H. The role of the circadian clock system in physiology. Pflugers Arch 2018;470:227–239 [DOI] [PubMed] [Google Scholar]
- 31.Kim H, Zheng Z, Walker PD, Kapatos G, Zhang K. CREBH maintains circadian glucose homeostasis by regulating hepatic glycogenolysis and gluconeogenesis. Mol Cell Biol 2017;37:e00048–e00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sen S, Raingard H, Dumont S, Kalsbeek A, Vuillez P, Challet E. Ultradian feeding in mice not only affects the peripheral clock in the liver, but also the master clock in the brain. Chronobiol Int 2017;34:17–36 [DOI] [PubMed] [Google Scholar]
- 33.Udoh US, Valcin JA, Swain TM, et al. Genetic deletion of the circadian clock transcription factor BMAL1 and chronic alcohol consumption differentially alter hepatic glycogen in mice. Am J Physiol Gastrointest Liver Physiol 2018;314:G431–G447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin E, Kuo PH, Liu YL, Yang AC, Kao CF, Tsai SJ. Effects of circadian clock genes and health-related behavior on metabolic syndrome in a Taiwanese population: evidence from association and interaction analysis. PLoS One 2017;12:e0173861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakubowicz D, Wainstein J, Landau Z, et al. Influences of breakfast on clock gene expression and postprandial glycemia in healthy individuals and individuals with diabetes: a randomized clinical trial. Diabetes Care 2017;40:1573–1579 [DOI] [PubMed] [Google Scholar]
- 36.Bolli GB, Andreoli AM, Lucidi P. Optimizing the replacement of basal insulin in type 1 diabetes mellitus: no longer an elusive goal in the post-NPH era. Diabetes Technol Ther 2011;13(Suppl. 1):S43–S52 [DOI] [PubMed] [Google Scholar]
- 37.Tsujino D, Nishimura R, Onda Y, et al. The relationship between HbA1c values and the occurrence of hypoglycemia as assessed by continuous glucose monitoring in patients with type 1 diabetes. Diabetol Metab Syndr 2016;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gubitosi-Klug RA, Braffett BH, White NH, et al.; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Risk of severe hypoglycemia in type 1 diabetes over 30 years of follow-up in the DCCT/EDIC study. Diabetes Care 2017;40:1010–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bischof MG, Bernroider E, Krssak M, et al. Hepatic glycogen metabolism in type 1 diabetes after long-term near normoglycemia. Diabetes 2002;51:49–54 [DOI] [PubMed] [Google Scholar]
- 40.Hwang JH, Perseghin G, Rothman DL, et al. Impaired net hepatic glycogen synthesis in insulin-dependent diabetic subjects during mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy study. J Clin Invest 1995;95:783–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bally L, Buehler T, Dokumaci AS, Boesch C, Stettler C. Hepatic and intramyocellular glycogen stores in adults with type 1 diabetes and healthy controls. Diabetes Res Clin Pract 2015;109:e1–e3 [DOI] [PubMed] [Google Scholar]
- 42.Matyka K, Dixon RM, Mohn A, et al. Daytime liver glycogen accumulation, measured by 13C magnetic resonance spectroscopy, in young children with type 1 diabetes mellitus. Diabet Med 2001;18:659–662 [DOI] [PubMed] [Google Scholar]
- 43.Stadler M, Krššák M, Jankovic D, et al. Fasting and postprandial liver glycogen content in patients with type 1 diabetes mellitus after successful pancreas-kidney transplantation with systemic venous insulin delivery. Clin Endocrinol (Oxf) 2014;80:208–213 [DOI] [PubMed] [Google Scholar]
- 44.Winnick JJ, An Z, Moore MC, et al. A physiological increase in the hepatic glycogen level does not affect the response of net hepatic glucose uptake to insulin. Am J Physiol Endocrinol Metab 2009;297:E358–E366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winnick JJ, An Z, Ramnanan CJ, et al. Hepatic glycogen supercompensation activates AMP-activated protein kinase, impairs insulin signaling, and reduces glycogen deposition in the liver. Diabetes 2011;60:398–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gobello C, Corrada YA, Castex GL, de la Sota RL, Goya RG. Secretory patterns of growth hormone in dogs: circannual, circadian, and ultradian rhythms. Can J Vet Res 2002;66:108–111 [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SH, Park MJ. Effects of growth hormone on glucose metabolism and insulin resistance in human. Ann Pediatr Endocrinol Metab 2017;22:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 2009;30:152–177 [DOI] [PubMed] [Google Scholar]
- 49.Edgerton DS, Kraft G, Smith M, et al. Insulin’s direct hepatic effect explains the inhibition of glucose production caused by insulin secretion. JCI Insight 2017;2:e91863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore MC, Smith MS, Sinha VP, et al. Novel PEGylated basal insulin LY2605541 has a preferential hepatic effect on glucose metabolism. Diabetes 2014;63:494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]