Abstract
Identification of sequence variants robustly associated with predisposition to diabetic kidney disease (DKD) has the potential to provide insights into the pathophysiological mechanisms responsible. We conducted a genome-wide association study (GWAS) of DKD in type 2 diabetes (T2D) using eight complementary dichotomous and quantitative DKD phenotypes: the principal dichotomous analysis involved 5,717 T2D subjects, 3,345 with DKD. Promising association signals were evaluated in up to 26,827 subjects with T2D (12,710 with DKD). A combined T1D+T2D GWAS was performed using complementary data available for subjects with T1D, which, with replication samples, involved up to 40,340 subjects with diabetes (18,582 with DKD). Analysis of specific DKD phenotypes identified a novel signal near GABRR1 (rs9942471, P = 4.5 × 10−8) associated with microalbuminuria in European T2D case subjects. However, no replication of this signal was observed in Asian subjects with T2D or in the equivalent T1D analysis. There was only limited support, in this substantially enlarged analysis, for association at previously reported DKD signals, except for those at UMOD and PRKAG2, both associated with estimated glomerular filtration rate. We conclude that, despite challenges in addressing phenotypic heterogeneity, access to increased sample sizes will continue to provide more robust inference regarding risk variant discovery for DKD.
Introduction
Progressive loss of renal function represents one of the most serious complications of diabetes, yet strategies for prevention and management are suboptimal. One of the principal obstacles to improved clinical interventions remains rudimentary understanding of the processes whereby sustained exposure to elevated levels of glucose (and/or other manifestations of the diabetic state) leads to progressive disturbance of renal morphology and function (1).
There is considerable variation in the progression and severity of renal complications of diabetes (collectively, diabetic kidney disease [DKD]). The prevalence of DKD in subjects with type 2 diabetes (T2D) is ∼30–50%: some patients experience a relatively rapid decline in renal function, whereas others maintain normal renal function despite decades of suboptimal glycemic control (2). The factors influencing this variation in outcome have not been fully characterized, but substantial evidence supports a genetic contribution. As in type 1 diabetes (T1D), DKD in those with T2D aggregates in families (3,4), and the prevalence of DKD in T2D differs considerably between ethnic groups (5–7).
These observations indicate that the identification of genetic variants influencing DKD predisposition should accelerate characterization of the biological basis of DKD. In contrast with most complex multifactorial traits, efforts to apply candidate gene and genome-wide association study (GWAS) approaches to DKD have met with limited success (8–11). Many genetic associations have been reported, but few robustly replicated loci have emerged. This likely reflects the comparatively small sample sizes of previous studies, such that power would have been limited to detection of common loci of unusually large effect. In the case of DKD in T2D, this is likely to have been compounded by the heterogeneity of the phenotype: autopsy studies indicate that only ∼50% of chronic kidney disease (CKD) in T2D can be attributed to classic diabetic nephropathy (12). The success of equivalent GWAS efforts for CKD (for which several replicated loci have been described) provides reassurance that it is possible to identify variants with broad impact on the progression of renal disease, irrespective of the dominant pathology (13).
Reduced kidney function, reflected by the estimated glomerular filtration rate (eGFR) and end-stage renal disease (ESRD), and dysfunction of the glomerular filtration barrier, reflected by albuminuria, can develop independently. This suggests that the two cardinal features of DKD involve distinct disease mechanisms and may be subject to different genetic effects. Albuminuria is known to be a poor predictor of diabetes-related ESRD, especially in the early stages, and regression to normoalbuminuria is common in patients with microalbuminuria (14).
These observations provide confidence that the combination of increased sample size and improved definition of DKD phenotypes should enable risk variant detection and uncover mechanisms that contribute to renal dysfunction in diabetes. In particular, the separation of case subjects into phenotypic classes based on disease stage and/or phenotype manifestations, incorporating information on both albumin excretion and eGFR, can be expected to increase etiological homogeneity and augment power for locus identification (14–16).
The SUrrogate markers for Micro- and Macrovascular hard endpoints for Innovative diabetes Tools (SUMMIT) Consortium adopted such a strategy to perform a GWAS for DKD in subjects with T1D (17). Here, we report on equivalent analyses conducted in the context of T2D, as well as those from a combined T1D+T2D analysis involving up to 40,340 subjects.
Research Design and Methods
DKD Phenotype Definitions
Not all patients with DKD will develop every form of the disease or progress to the most severe stage of ESRD. Dysfunction of the glomerular barrier, represented by albuminuria, and reduced kidney function, represented by eGFR, can develop independently. To explore the disease severity spectrum and the different disease processes represented by eGFR and albuminuria, we defined seven binary phenotypes using clinical measures of albumin-to-creatinine ratio (ACR), albumin excretion rate (AER), and eGFR (Table 1 [T2D only] and Supplementary Table 8 [T1D+T2D]). The phenotype definitions were aligned to other large-scale genetic studies of T1D DKD in SUMMIT (17) and the Diabetic Nephropathy Collaborative Research Initiative (DNCRI) (18). The definition of CKD was also aligned to that used by the CKDGen Consortium (eGFR <60 mL/min/1.73 m2), although we restricted case and control subjects to those with diabetes (13).
Table 1.
GWAS characteristics by DKD phenotypes in subjects with T2D and T1D
| Analysis | Case definition | Control definition | Subjects with T2D |
Subjects with T1D |
||
|---|---|---|---|---|---|---|
| Case, n | Control, n | Case, n | Control, n | |||
| All DKD |
Microalbuminuria OR Late DKD OR ESRD |
Normoalbuminuria (AER <20 µg/min OR AER <30 mg/24 h OR ACR <2.5/3.5 mg/mmol for men/women) AND duration of T2D >10 years‡ |
3,345 |
2,372 |
2,563 |
2,593 |
| Microalbuminuria* |
At least 2 out of 3 consecutive measurements with AER ≥20 AND <200 µg/min OR AER ≥30 AND <300 mg/24 h OR ACR ≥2.5/3.5 AND <25/35 mg/mmol for men/women |
Normoalbuminuria AND duration of T2D >10 years‡ |
1,989 |
2,238† |
806 |
2,593 |
| Late DKD |
At least one measurement with AER ≥200 µg/min OR AER ≥300 mg/24 h OR ACR ≥25/35 mg/mmol for men/women OR ESRD (eGFR <15 mL/min/1.73 m2 OR kidney transplantation OR dialysis) |
Normoalbuminuria AND duration of T2D >10 years‡ |
1,339 |
2,372 |
1,757 |
2,593 |
| ESRD vs. control subjects |
ESRD: eGFR <15 mL/min/1.73 m2 OR renal dialysis OR kidney transplant |
No DKD AND duration of T2D >10 years‡ |
371 |
2,076 |
813 |
2,398 |
| ESRD vs. no ESRD |
ESRD (see above) |
No ESRD AND duration of T2D >10 years‡ |
371 |
4,471 |
813 |
3,995 |
| CKD |
eGFR <60 mL/min/1.73 m2 |
No CKD AND duration of T2D >10 years‡ |
3,094 |
2,906 |
2,460 |
774 |
| CKD and DKD |
eGFR <45 mL/min/1.73 m2 AND all DKD |
No CKD AND no ESRD AND normoalbuminuria AND duration of T2D >10 years‡ |
897 |
1,610 |
1,750 |
1,385 |
| eGFR | 32,788 × serum creatinine (μmol/L)−1.154 × age−0.203 × [0.742 if female] (mL/min/1.73 m2) | 9,197 | 3,961 | |||
*Equivalent to the early DKD phenotype from Sandholm et al. (17).
†Not all studies were able to define microalbuminuria (due to limited information on microalbuminuric status) and thus the case and control group number is smaller than all DKD and late DKD.
‡The duration of diabetes for subjects with T1D was >15 years.
We used AER measured overnight (µg/min), during 24 h (mg/24 h), or as a spot measurement of ACR (mg/mmol) or eGFR calculated using the Modification of Diet Renal Disease Study (MDRD) formula (eGFR = 32,788 × serum creatinine (μmol/L)−1.154 × age−0.203 × [0.742 if female]) to classify disease stage and severity. We based the control definition on either AER or ACR, as most studies had measured either. In the studies that had measured both, two of the three measures for AER and ACR had to meet the control criteria (Table 1). We were unable to exclude albuminuric patients that presented as normoalbuminuric due to prescribed renin-angiotensin system blockers. As reduced kidney function (reflected by eGFR) and dysfunction of the glomerular filtration barrier (reflected by albuminuria) can develop independently, we did not exclude individuals with albuminuria from the control subjects for the eGFR-defined phenotypes and vice versa. In subjects with T2D, ∼46% of normoalbuminuric control subjects had an eGFR <60 mL/min/1.73 m2 (1,098/2,372).
In all, we defined seven dichotomous phenotypes:
The “all DKD” phenotype, our primary phenotype, designed to capture the broadest set of DKD phenotypes.
The “microalbuminuria” phenotype (equivalent to early DKD from Sandholm et al. [17]) to identify variants that contribute to early dysfunction of the glomerular barrier.
The “late DKD” phenotype to identify variants that contribute to severe glomerular barrier dysfunction.
Two ESRD-related phenotypes focused on identification of variants associated with end-stage renal failure, comparing those with ESRD either to control subjects without any DKD (“ESRD vs. control subjects”) or to control subjects without ESRD (“ESRD vs. no ESRD”).
The “CKD” phenotype to identify variants that contribute to reduced kidney function (eGFR).
The “CKD and DKD” phenotype to identify any variants that may contribute to the development of kidney disease irrespective of glomerular barrier dysfunction or reduced kidney function.
The “eGFR” phenotype, a continuous phenotype, to identify variants that play a role in kidney function that may not be detected by the analysis of the binary DKD phenotypes. The eGFR measures were not transformed as they approximated a normal distribution (Supplementary Fig. 1).
Study Populations
We identified DKD case and control subjects with T2D from the Scania Diabetes Registry (SDR) (19), Genetics of Diabetes Audit and Research in Tayside Scotland (GoDARTS) study (20), Steno Diabetes Centre (21), and Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) A and B studies (22). We identified independent replication studies in populations of European descent (Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe [DECODE], Family Investigation of Nephropathy and Diabetes [FIND], Diabetes Register Vaasa [DIREVA], Diagnostic Optimization and Treatment of Diabetes and Its Complications in the Chernihiv Region [DOLCE], Malmö Diet and Cancer [MDC], Inter99, Vejle Diabetes Biobank, and the Anglo-Danish-Dutch Study of Intensive Treatment In People with Screen Detected Diabetes in Primary Care [ADDITION] studies) and Asian descent (RIKEN, the Singapore Diabetic Cohort Study [SDCS], the Hong Kong Diabetes Registry [HKDR], and the Singapore Study of Macro-angiopathy and Microvascular Reactivity in Type 2 Diabetes [SMART2D] study) (Supplementary Table 1).
We combined the subjects with T2D with nonoverlapping samples from the study of DKD in subjects with T1D (17). Replication studies (of DKD in subjects with T1D) (17) were also used for replication in the combined analysis of T1D+T2D (Supplementary Table 1). None of these studies overlapped with samples included in the analysis of eGFR and CKD by the CKDGen Consortium (13).
Genome-Wide Genotyping and Imputation
The T2D discovery cohorts were genotyped on the Affymetrix SNP 6.0, the Illumina Omni express array, and the Illumina 610Quad arrays (Supplementary Table 4). Individual study centers excluded single nucleotide polymorphisms (SNPs) for minor allele frequency (MAF) <1%. SNPs with a MAF 1–5% were excluded if the Hardy-Weinberg equilibrium test P < 1 × 10−4 or the call rate <99%. SNPs with MAF ≥5% were excluded if the Hardy-Weinberg equilibrium test P < 5.7 × 10−7 or the call rate <95% (23). Samples were excluded if their call rate was <95%, genotype heterozygosity was >3 SD from the study sample mean, or they failed sex checks. Based on principal component analysis, population outliers were removed if they were not of European descent (compared with the 1000 Genomes Project [1000G] populations) or fell >3 SD away from the population means of the first two principal components for samples of European descent. Duplicates were removed but related individuals were retained for genotype imputation.
Genotypes were prephased using SHAPE-IT (v2) (24) and imputed using IMPUTEv2 (25) against the March 2013 1000G version 1 reference panel using standard protocols and recommended settings.
Replication Genotyping
Direct typing of twelve SNPs (rs11622435, rs12917707, rs17421627, rs1989248, rs2194025, rs2206136, rs4977388, rs61277444, rs6865390, rs7222331, rs9939609, and rs9942471) was performed in DIREVA samples using TaqMan allelic discrimination assays, according to the manufacturer’s protocol (Applied Biosystems, Carlsbad, CA). Sequenom multiplex genotyping was performed for the same SNPs in DOLCE, using the standard protocol (26).
Statistical Analysis
Heritability of DKD Phenotypes
Narrow sense heritability was estimated by GCTA (v1.26) (27) from 4.5 million directly typed and imputed markers (info >0.75) in GoDARTS (Supplementary Table 1) for all DKD, CKD, and eGFR. The sample size for these phenotypes exceeded the recommended threshold for reliable heritability estimates (N = 3,160 based on SE ≤0.1) (28).
Genome-Wide Association Analysis
Genome-wide association analyses were performed by individual study centers using an additive model while correcting for age, sex, and duration of diabetes. We estimated allelic effects using the score test from SNPTESTv2 in unrelated samples for dichotomous traits (29). Association P values were calculated using EMMAX from a larger sample of related individuals while correcting for a kinship matrix (30). For eGFR phenotype, we estimated allelic effects and association P values using EMMAX (30).
Power Calculations
We performed power calculations for dichotomous traits based on a MAF of 8%, an allelic odds ratio (OR) range of 1.05–2.00, and α = 5 × 10−8 (genome-wide significance). The power calculations were performed for the discovery meta-analysis of all DKD and separately for the meta-analyses of T2D only (3,345 DKD case and 2,372 DKD control subjects) and the combined T1D+T2D (5,908 DKD case and 4,965 DKD control subjects).
At α ≤ 5 × 10−8, we had >80% power to detect an allelic OR >1.40 in the T2D-only discovery analysis (Supplementary Fig. 2C) and an allelic OR >1.25 in the T1D+T2D discovery analysis (Supplementary Fig. 2B). We also performed power calculations for the reported DKD loci, as above, but using α = 9 × 10−4 (this α accounts for the number of loci tested but not the number of phenotypes analyzed). In T1D+T2D analysis, we had >80% power to detect variants with an allelic OR >1.20 (Supplementary Fig. 2A).
Discovery Meta-analysis
Two discovery meta-analyses were performed: one that included summary statistics estimated from subjects with T2D only and a second that combined T2D-only analyses with equivalent analyses in subjects with T1D (17). Individual study summary statistics were centrally filtered for a minor allele count in either case or control subjects <10 and an info score <0.4 for imputed variants.
EMMAX P values were combined in a sample size weighted z-statistic meta-analysis using METAL (version 25/03/2011) (31). Effect estimates were combined in a fixed-effect inverse-variance weighted meta-analysis using GWAMA (v2.1) (32). Meta-analysis results were restricted to allelic effects estimated in two or more studies. For binary traits, independent variants (>100 kb apart) were selected for replication from the T2D-only analysis based on association P ≤ 5 × 10−6 and from the T1D+T2D analysis based on P ≤ 1 × 10−6. For eGFR phenotype, SNPs were chosen for replication based on association P ≤ 5 × 10−6 in subjects with T2D or P < 1 × 10−6 in the T1D+T2D analysis. SNPs associated with eGFR at P ≤ 5 × 10−4 in either eGFR analysis (T2D only or T1D+T2D) that had also been reported at P ≤ 5 × 10−8 with eGFR by the CKDGen Consortium were also included in the list of SNPs for replication (13).
Replication
We sought replication for 164 lead variants in 13 studies of T2D DKD for which it was possible to obtain in silico replication from available GWAS data or replication from de novo genotyping (DIREVA and DOLCE) (Fig. 1). Replication studies aligned their DKD phenotypes with those used in the SUMMIT GWAS. Although association results for the lead variants were recovered for all compatible DKD phenotypes available in the replication samples (Supplementary Table 1), joint meta-analysis results were reported for those phenotypes where the primary GWAS associations exceeded the thresholds above.
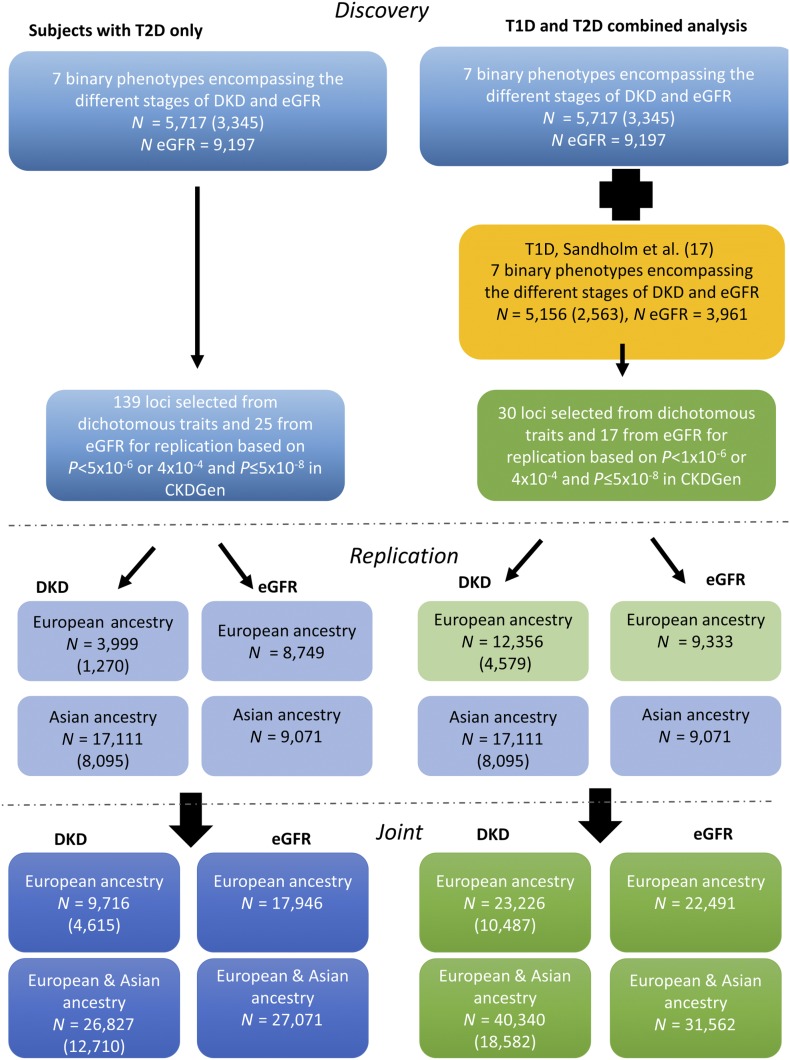
Figure 1.
Eight DKD phenotypes were analyzed in subjects with T2D (blue boxes) and in a combined (green boxes) analysis of subjects with T2D or T1D (yellow box). N indicates the total sample count for either the all DKD (number of case subjects is given in parentheses) or the eGFR phenotype and may vary by variant as well as by DKD phenotype. Replication was sought for 164 loci and 47 loci from each analysis, respectively, in subjects of European and Asian ancestry with either T1D or T2D.
As with the discovery, meta-analysis effect estimates from replication studies were combined using GWAMA (v2.1) (32), and EMMAX P values, using METAL (version 25/03/2011) (31).
Known DKD Variants
We examined the literature for variants that have been associated with DKD from candidate gene (P < 0.05) and GWA (P ≤ 5 × 10−8) studies. Sixty-one variants were identified and aligned to the reported risk allele for binary traits (or the trait-raising allele for quantitative traits). We assessed both direction of effect and strength of association in the current study for those phenotypes that most closely matched the original report (but irrespective of type of diabetes).
Genetic Risk Score Analysis
We included variants (P ≤ 5 × 10−8) from GWAS to generate genetic risk scores (GRS) for coronary artery disease (33), BMI (34), waist-to-hip ratio adjusted for BMI (35), LDL cholesterol, triglycerides, HDL cholesterol (36), fasting insulin, insulin resistance (37–39), fasting glucose (38), T1D (40), T2D (41), and systolic blood pressure (42). The relationship between the GRS and DKD phenotype was calculated using an inverse-variance weighted method described in Ehret et al. (42).
Results
DKD Definitions
We considered seven dichotomous phenotypes designed to capture the spectrum of DKD (see research design and methods) and eGFR. We aimed to identify variants that influence multiple stages in DKD progression, as well as those that have more stage-specific effects. The principal definition (all DKD) included 3,345 T2D subjects with any form of DKD (ranging from microalbuminuria to ESRD) as case subjects and 2,372 T2D subjects, normoalbuminuric despite >10 years duration of diabetes, as control subjects. The other six dichotomous phenotypic comparisons are described in Table 1 (see research design and methods).
Contribution of Genetic Variants to DKD
The genetic variation, explained by the SNPs on the genotyping array and estimated using GCTA (v1.26) (30) in up to 6,335 subjects with T2D from GoDARTS, was highest in CKD (h2 = 0.12) and similar for all DKD (h2 = 0.08) and eGFR (h2 = 0.07) (Supplementary Table 2). We restricted analyses to phenotypes with sample sizes deemed sufficient for accurate estimation of heritability (N ≥3,160 to obtain SE ≤0.1) (28).
GWAS for DKD in T2D
The DKD discovery analysis combined GWAS data from four studies of European descent: GoDARTS (20), SDR (19), Steno (21), and BENEDICT (phases A and B) (22) (Table 1 and Supplementary Table 3). For the principal (all DKD) analysis, the sample size of the discovery T2D-only meta-analysis had >80% power to detect variants with MAF ≥8% and allelic OR >1.40 (Supplementary Fig. 2C). The number of variants meta-analyzed for each DKD phenotype varied between 5,864,445 in the ESRD vs. no ESRD phenotype and 9,263,264 in the all DKD phenotype (Supplementary Table 4). These differences reflect the minor allele count exclusion filter.
Manhattan and quantile-quantile plots of discovery P values for each of the eight DKD phenotypes were well calibrated, and several showed a modest excess of significant associations (Supplementary Fig. 3). In the discovery GWAS, only one locus reached genome-wide significance: PLCB4 (encoding 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase β-4) on chromosome 20. The lead variant rs2206136 was associated with the CKD phenotype (effect allele frequency [EAF] 42%, OR 1.20 [95% CI 1.08, 1.34]; P = 2.1 × 10−8) (Table 2 and Supplementary Fig. 3A).
Table 2.
Five loci were associated (P ≤ 5 × 10−8) with CKD, microalbuminuria, and eGFR phenotypes in T2D or T1D+T2D subjects
| CHR:BP | Phenotype | SNP (locus) | Discovery |
Replication |
Joint analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EA/NEA (Info), EAF | OR/β (95% CI) | P | Ancestry | OR/β (95% CI) | P | OR/β (95% CI) | P | N | |||
| 20: 9351150 |
T2D CKD |
rs2206136 (PLCB4) |
A/T (0.98), 0.42 |
OR 1.20 (1.08, 1.34) |
2.1 × 10−8 |
European |
OR 1.02 (0.91, 1.15) |
0.69 |
OR 1.13 (1.05, 1.21) |
9.0 × 10−5 |
11,900 |
| Asian and European |
OR 1.03 (0.94, 1.13) |
0.68 |
OR 1.12 (1.05, 1.19) |
2.1 × 10−4 |
13,813 |
||||||
| 6: 89948232 |
T2D microalbu-minuria |
rs9942471 (GABRR1) |
A/C (0.99), 0.64 |
OR 1.24 (1.15, 1.34) |
2.1 × 10−7 |
European |
OR 1.32 (0.99, 1.75) |
0.06 |
OR 1.25 (1.16, 1.34) |
4.5 × 10−8 |
4,801 |
| Asian and European |
OR 1.11 (0.99, 1.23) |
0.12 |
OR 1.15 (1.08, 1.23) |
1.2 × 10−5 |
5,559 |
||||||
| 16: 20400839 |
T2D eGFR |
rs11864909 (UMOD) |
T/C (1.00), 0.28 |
β 2.42 (1.28, 3.56) |
2.7 × 10−5 |
European |
β 2.22 (1.16, 3.28) |
4.1 × 10−5 |
β 2.31 (1.54, 3.09) |
4.6 × 10−9 |
12,343 |
| Asian and European |
β 2.30 (1.48, 3.12) |
3.6 × 10−8 |
β 2.34 (1.68, 3.00) |
4.4 × 10−12 |
19,747 |
||||||
| 2: 170646916 |
T1D+T2D eGFR |
rs1974990* (SSB) |
G/T (0.98), 0.08 |
β 4.07 (2.61, 5.52) |
4.8 × 10−8 |
European |
No replication available |
β 4.07 (2.61, 5.52) |
4.8 × 10−8 |
13,158 |
|
| Asian and European |
β 0.04 (−2.69, 2.76) |
0.98 |
β 3.17 (1.88, 4.45) |
1.4 × 10−6 |
14,828 |
||||||
| 7: 151415041 |
T1D+T2D eGFR |
rs10224002 (PRKAG2) |
A/G (0.92), 0.74 |
β 1.75 (0.85, 2.66) |
1.5 × 10−4 |
European |
β 2.15 (0.93, 3.37) |
5.8 × 10−4 |
β 1.89 (1.17, 2.62) |
3.4 × 10−7 |
20,495 |
| Asian and European |
β 2.42 (1.28, 3.56) |
3.2 × 10−5 |
β 2.01 (1.30, 2.72) |
2.7 × 10−8 |
22,165 |
||||||
| 16: 20400839 | T1D+T2D eGFR | rs11864909 (UMOD) | T/C (0.99), 0.29 | β 1.90 (1.05, 2.74) | 1.1 × 10−5 | European |
β 2.22 (1.16, 3.28) |
4.1 × 10−5 |
β 2.02 (1.36, 2.69) |
2.1 × 10−9 |
16,304 |
| Asian and European | β 2.30 (1.48, 3.12) | 3.6 × 10−8 | β 2.11 (1.52, 2.70) | 2.3 × 10−12 | 23,708 | ||||||
BP, base pair; CHR, chromosome; EA, effect allele; EAF, effect allele frequency; NEA, non-effect allele.
*rs1974490 was only available in the 1000G reference panel and was not imputed in the European studies used in the replications.
To extend power to detect associations of lesser effect and to replicate the PLCB4 association, we identified 139 loci with SNP associations exceeding P ≤ 5 × 10−6 in at least one of the seven dichotomous DKD analyses. We also identified 22 loci (25 lead variants) for replication from the eGFR analysis (based on either P < 5 × 10−6 in our eGFR analyses alone or P < 5 × 10−4 in our analysis and a genome-wide association [P < 5 × 10−8] reported by the CKDGen Consortium) (Supplementary Fig. 3Q) (13). We sought replication for 164 lead variants in 13 studies of T2D DKD (9 involving European subjects and 4 involving Asian subjects) for which it was possible to obtain association analyses based on either in silico (from existing GWAS) or de novo genotyping (Fig. 1). Replication studies recoded their DKD phenotypes to align definitions with those used in the SUMMIT GWAS. Although association results for the lead variants were recovered for all compatible DKD phenotypes available in the replication samples (Supplementary Table 1), joint meta-analysis results are reported for only those phenotypes where the primary GWAS associations exceeded the thresholds above (Supplementary Table 5). The replication samples available for the all DKD phenotype included up to 3,999 T2D subjects of European ancestry (1,270 case subjects) and 17,111 (8,095 case subjects) from Asia (Supplementary Table 1).
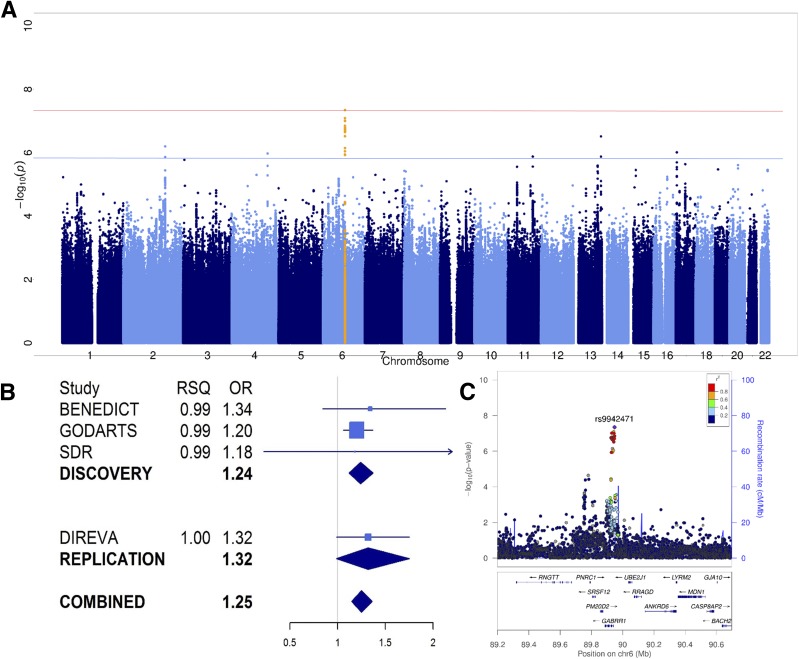
The CKD association near PLCB4 did not replicate in either European or Asian data (joint analysis, ORAsian+Euro 1.12 [95% CI 1.05, 1.19]; P = 2.1 × 10−4) (Table 2). Joint analysis of dichotomous DKD phenotypes identified one novel SNP association that marginally exceeded genome-wide significance (P = 5 × 10−8, without adjustment for the multiple GWAS we performed) (Table 2). This signal, on chromosome 6, is centered on rs9942471 and lies ∼7 kb upstream of GABRR1 (encoding the rho1 subunit of the GABA type a receptor). The major allele was associated with increased risk of microalbuminuria in subjects of European ancestry (joint analysis, EAF 64%, OREuro 1.25 [95% CI 1.16, 1.34]; P = 4.5 × 10−8) (Fig. 2 and Table 2). Associations of rs9942471 with other DKD phenotypes are given in Supplementary Table 6.
Figure 2.
A: Manhattan plot of P values from the meta-analysis of allelic effects on early DKD in subjects with T2D of European descent. The red line represents genome-wide significance (P < 5 × 10−8) and the blue line suggestive significance (P < 1 × 10−6). The peak represented by rs9942471 (P = 4.5 × 10−8) near GABRR1 is highlighted in orange. B: A forest plot of allelic OR and imputation information scores (RSQ) from individual studies that contributed to the discovery and replication analyses of rs9942471 in microalbuminuria phenotype; rs9942471 genotypes were not available in Steno. C: A LocusZoom plot of the signal near GABRR1 led by rs9942471 that was associated with microalbuminuria in European subjects with T2D.
rs9942471 is in high linkage disequilibrium (LD) (r2 >0.8) with the lead expression quantitative trait locus variant for GABRR1 expression in the artery, esophagus, and skin (P ≤ 4 × 10−8), and the major allele is associated with decreased expression (42). However, there was no evidence for replication of this SNP in T2D subjects of Asian ancestry only (EAF 90%, ORAsian 0.99 [95% CI 0.87, 1.13]; P = 0.91), although the higher frequency of the effect allele in Asians (90%) compared with Europeans (64%) reduces the power to detect an effect in subjects of Asian descent. Ethnic differences in regional LD could have contributed to failed replication: rs9942471 may be a better marker of the shared causal variant in subjects of European descent. However, this seems unlikely given broad similarity of LD patterns across subjects of European and Asian descent (estimated separately from the 1000G population).
Replication samples for the eGFR phenotype included 8,749 subjects of European and 9,071 subjects of Asian ancestry with T2D (Fig. 1). Joint analysis of discovery and replication results captured the well-established association with variants near UMOD (uromodulin), centered on rs11864909 (βAsian+Euro 2.34 [95% CI 1.68, 3.00] mL/min/1.73 m2; P = 4.4 × 10−12) (Table 2). There was no difference in effect by diabetes type: the effect estimate in subjects with T1D (βT1D 1.23 (−0.05, 2.51); P = 0.06) overlapped the effect size in subjects with T2D (17). We also compared the effects of variants associated with DKD phenotypes in subjects with T2D (Table 1) with their effects in equivalent DKD phenotypes in subjects with T1D (17) (Supplementary Table 7).
Combined T1D+T2D Analysis
To increase power to detect loci that contribute to processes involved in the development of DKD irrespective of diabetes subtype, we combined the results from the primary GWAS meta-analysis for T2D-DKD phenotypes with those for the corresponding T1D-DKD phenotypes (Supplementary Tables 4 and 9) (17). The combined discovery meta-analysis of all DKD included 10,873 subjects with diabetes of European descent (5,908 case subjects) and provided >80% power (α = 5 × 10−8) to detect a SNP association with an allelic OR >1.25 for variants with MAF >8% (Supplementary Fig. 2B). The number of variants meta-analyzed ranged from 7,959,015 for ESRD vs. no ESRD to 9,364,702 for the all DKD phenotype (Supplementary Table 4).
No significant associations were detected for dichotomous DKD phenotypes in the combined T1D+T2D meta-analysis (Supplementary Fig. 4 and Supplementary Table 9). The combined meta-analysis for eGFR highlighted a novel genome-wide significant association involving a cluster of variants on chromosome 2 led by rs1974990 (EAF 8%, β 4.07 [95% CI 2.61, 5.52] mL/min/1.73 m2; P = 4.8 × 10−8) and mapping near SSB (encoding Sjogren syndrome antigen B) (Table 2).
As in the T2D-only analysis, we selected 47 loci for replication (30 with P < 1 × 10−6 with at least one of the DKD phenotypes) from the combined T1D+T2D GWAS and an additional 17 loci from the equivalent analysis of eGFR. The combined association P value for rs9942471 (microalbuminuria, OR 1.10 [95% CI 1.02, 1.19]; P = 0.001) did not reach the threshold for replication. Lead variants at these 47 loci were tested for all DKD phenotypes available in the relevant replication samples in subjects with T1D or T2D (Supplementary Table 1). Meta-analysis results were only reported for those phenotypes that contributed to discovery-stage associations. This joint, combined T1D+T2D analysis generated a substantially enlarged data set for the all DKD phenotype (40,640 subjects [18,582 case subjects]) (Fig. 1). However, none of the variants selected for replication from the dichotomous phenotypes reached genome-wide significance (P ≤ 5 × 10−8).
The joint, combined analysis for eGFR in subjects of European and Asian descent included 31,562 subjects and replicated known associations near UMOD (rs11864909, βAsian+Euro 2.11 [95% CI 1.52, 2.70]; P = 2.3 × 10−12) and PRKAG2 (rs10224002, βAsian+Euro 2.01 [1.30, 2.72]; P = 2.7 × 10−8) (Table 2 and Supplementary Figs. 5 and 6). The PRKAG2 was nonsignificant (P ≤ 5 × 10−8) in individual analyses of eGFR in T2D-only (βEuro 2.13 [95% CI 1.28, 2.98]; P = 8.5 × 10−7) or T1D-only (βEuro 1.23 [−0.19, 2.65]; P = 0.09) analyses, and effect sizes did not differ by type of diabetes.
The association at SSB, detected in the combined eGFR analysis, did not replicate (rs1974990, β 0.04 [95% CI −2.69, 2.76] mL/min/1.73 m2; P = 0.98) and was no longer genome-wide significant in the joint, combined analysis (βAsian+Euro 3.17 [1.88, 4.45] mL/min/1.73 m2; P = 1.4 × 10−6) (Table 2).
Evaluating Previous Association Claims
Of the 61 published loci, for which there are published claims of association with T1D DKD or T2D DKD (8), 55 of these associations were represented by variants contributing to our meta-analyses of DKD phenotypes in either subjects with T2D only or T1D+T2D. Two of these, the eGFR associations at UMOD and PRKAG2, replicate at genome-wide significance in our data (Table 2). We tested the association of the remaining 53 lead variants in the T2D-only and combined analyses (Supplementary Fig. 6). Fourteen variants were associated with a DKD phenotype corresponding to the original report at nominal significance (P < 0.05), but only 10 of these were directionally consistent with previous reports (Supplementary Table 10). At a more stringent significance level (P < 9 × 10−4) that accounts for the 55 variants tested (but not the multiple phenotypic categories), only 2 variants were associated with a DKD phenotype that corresponded to the original report, both in the combined T1D+T2D analysis and both directionally consistent with previous reports. These two SNPs were rs2838302, near SIK1, associated with ESRD vs. no ESRD (EAF 8%, OR 1.39 [95% CI 1.12, 1.74]; P = 3.9 × 10−4), and rs7583877, near AFF3, associated with ESRD vs. no ESRD (OR 1.22 [95% CI 1.13, 1.32]; P = 4.8 × 10−4) (Supplementary Table 10). When we took account of the substantial participant overlap between the original reports and the samples in the current study, apparent replications failed to reach nominal (P < 0.05) significance (though, for these, the sample sizes available for independent replication were often small). Thus, other than the eGFR associations at UMOD and PRKAG2, we found limited evidence in this study to corroborate previously reported DKD associations, despite, for most variants, sample sizes considerably larger than those included in the original report. Validation of previously reported DKD associations could be complicated by differences in phenotype definitions and/or analytical methods between this study and published reports. We could not assess whether the UMOD or PRKAG2 allelic effects were different in this study compared with those reported by CKDGen Consortium as the allelic effects were not on the same scale (e.g., untransformed vs. log transformed).
Genetic Overlap With Risk Factors
Several exposures and diseases have been reported to increase DKD risk in epidemiological studies (1,2,43). To explore the extent to which these reflect shared genetic background, we constructed weighted GRS for 20 traits related to diabetes (37,39–41), insulin resistance (38), obesity (34,35), hypertension (42), coronary artery disease (33), and lipids (36). These GRS, constructed from signals identified (P < 5 × 10−8) in previously published GWAS, included between 10 and 96 SNPs per phenotype. We tested the association of these GRS with each of the DKD phenotypes from this study in both T2D-only and T1D+T2D data sets (42).
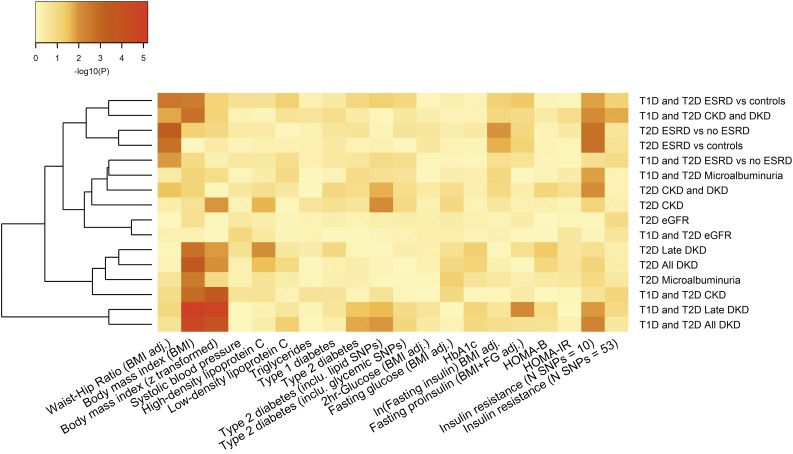
After Bonferroni correction (P ≤ 2.5 × 10−3, which accounts for the number of trait GRS but not the number of DKD phenotypes), a GRS for increased waist-to-hip ratio (P = 4.8 × 10−4) was associated with increased risk of ESRD vs. no ESRD phenotype and a GRS for increased BMI was associated with all DKD (P = 1.8 × 10−4) and late DKD (P = 1.8 × 10−3) phenotypes in subjects with T2D. A similar pattern of association for the BMI GRS was observed in the combined T1D+T2D all DKD analysis (P = 2.4 × 10−5) (Supplementary Table 11 and Fig. 3). This last result survives additional correction (α = 1.6 × 10−4) for the 16 DKD phenotypic comparisons considered.
Figure 3.
A heat map of GRS associations with DKD phenotypes in subjects with either T1D or T2D. A GRS for BMI was significant after correction for multiple testing, whereas other traits, including systolic blood pressure, were not associated with DKD phenotypes. adj., adjusted; FG, fasting glucose; In, insulin; IR, insulin resistance.
There is evidence implicating insulin resistance in the pathogenesis of DKD, and we wanted to understand whether the BMI GRS associations might reflect obesity-related insulin resistance (44,45). We focused on the effects of two alternative GRS for insulin resistance on DKD. The first, comprising lead variants (N = 10) associated with increased fasting insulin (BMI adjusted) (37), was associated with increased risk of ESRD in subjects with T2D (ESRD vs. no ESRD P = 1.6 × 10−3; ESRD vs. control subjects P = 1.7 × 10−3) (Supplementary Table 11 and Fig. 3). The second, comprising lead variants from 53 loci associated with high fasting insulin (BMI adjusted), low HDL cholesterol, and high triglycerides (39), failed to show any association with DKD phenotypes. These findings provide some support for the causal contribution of insulin resistance and obesity to DKD pathogenesis. However, there is potential that some of these effects reflect collider bias (46), and additional larger studies will be required to substantiate this inference.
Discussion
This study represents the largest study of the genetic basis of DKD in subjects with T2D to date, extending previous reports with respect to sample size and range of DKD phenotypes. We aimed to overcome some of the limitations of earlier studies in this area and to develop insights into the pathogenesis of DKD. Despite sample sizes that exceeded 40,000, the yield of novel discoveries was modest. There were no significant (P < 5 × 10−8) genetic associations with all DKD that was best-powered definition on sample size. The relatively large sample size came with increased phenotypic (and likely genetic) heterogeneity: it was for this reason that we examined a range of DKD phenotypes that might offer better power to detect genetic associations with more restricted phenotypic impacts.
This approach successfully identified a novel locus, GABRR1 (led by rs9942471), for a microalbuminuria phenotype in European subjects with T2D. The variants, near GABRR1, reached a level of significance (P < 5 × 10−8) that has typically been associated with robust, reproducible association in common disease GWAS. GABRR1 expression is upregulated in renal biopsies from DKD subjects (compared with control subjects) and in other non-DKD subjects characterized by glomerular scarring and inflammation (47). The variants were associated with GABRR1 expression in aorta, esophageal mucosa, and skin in the Genotype-Tissue Expression (GTEx) project. However, we found no replication of the GABRR1 association in subjects of European ancestry with T1D DKD or in subjects of Asian ancestry with T2D, though differences in risk allele frequencies between these two ancestries and the modest size of the replication data sets at this locus reduce the power of the latter analysis. Our overall assessment is that this association should be considered provisional until it is possible to undertake further rounds of adequately powered replication that could establish the definitive status of this variant and that this locus should also be assessed for effects on DKD progression in longitudinal studies.
Even in the absence of specific signals of association with DKD, it is possible to use the aggregate pattern of association across the genome to identify more subtle genetic effects. The GRS analyses described here provide genetic support for the causal contribution of obesity to the development of T2D DKD. This echoes strong epidemiological data, and mirrors equivalent analyses in T1D DKD (48,49). However, we cannot exclude that these associations may partly reflect collider bias (46): subjects with high BMI are likely to have a longer duration of diabetes and thus a higher chance of developing complications. Analyses using genetic instruments (GRS) for variation in insulin sensitivity produced variable results with respect to T2D DKD but indicate that the BMI effects may be partially mediated via obesity-related insulin resistance (37). There are substantial epidemiological data to support this link between insulin resistance and DKD risk (44,45).
The modest yield of association signals and the limited replication of previous claims of DKD association emphasizes challenges associated with the identification of DKD risk variants. For many complex traits, these have been overcome through a combination of increased sample size and phenotypic precision. Published genetic association studies of DKD have often used different definitions of DKD, which makes replication of previous findings difficult. In this study, we used phenotype definitions aligned to those used in the study of DKD in subjects with T1D (17). Standardizing the phenotype definitions in this way allowed for seamless combination of the GWAS data across the two studies and may streamline subsequent efforts to study the genetics of DKD. The phenotype definitions applied to this study address some of the challenges associated with increasing sample size while maintaining phenotype precision and should, in due course, support the identification of robust associations with DKD. It is clear that these phenotype definitions are not without limitations: in the absence of strong genetic signals, we have few clues to which particular diagnostic configurations will be most productive for genetic discovery. Targeting the phenotypes that show the greatest heritability may provide a guide (14).
Supplementary Material
Article Information
Funding. The research was supported by the European Union’s Seventh Framework Program (FP7/2007–2013) for the Innovative Medicine Initiative under grant agreement IMI/115006 (the SUMMIT Consortium); Academy of Finland (grants 263401 and 267882); Association Diabète Risque Vasculaire in Paris; Agency for Science, Technology and Research (A*STAR) in Singapore; Albert Påhlsson Foundation and Diabetesfonden; Alexandra Health (Private Limited) (SIGII/08005, SIGII/11001, SIG/11029, SIG/12024, SIG II/15205); The Chinese University of Hong Kong Focused Investment Scheme; Danish Diabetes Academy; DOLOrisk (European Union’s Horizon 2020 research and innovation program, grant 633491); European Research Council (grant 269045-GENE TARGET T2D); Ernhold Lundström; European Research Council (consolidator grant 649021, Orho-Melander); Finska Läkaresällskapet; Folkhälsan Research Foundation; French Ministry of Health; Heart Foundation of Jakobstad region; Helsinki University Central Hospital Research Funds (EVO); the Hong Kong Food and Health Bureau (01120796); Hong Kong Foundation for Research and Development in Diabetes; Hong Kong Government Research Grant Committee and Innovation and Technology Grant Committee; Hong Kong Research Grants Council Theme-based Research Scheme (T12-402/13N); Japan Agency for Medical Research and Development; JDRF (17-2012-542, 17-2013-7, 2-SRA-2014-276-Q-R, and 17-2013-9); Knut and Alice Wallenberg Foundation; Leading Project of Ministry of Education, Culture, Sports, Science and Technology in Japan; Liao Wun Yuk Memorial Fund; Linneus Foundation for the Lund University Diabetes Center; Liv och Hälsa Foundation; Ministry of Education, Culture, Sports, Science and Technology of the Japanese government; National Institute for Health Research Cambridge Biomedical Research Centre; National Medical Research Council in Singapore [PPG/AH(KTPH)/2011, CIRG13nov045]; Natural Sciences and Engineering Research Council of Canada; National Health Services Tayside; National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK105154); National Institutes for Health (R01-MH101814); Novo Nordisk Foundation (NNF14SA0003 and NNF15CC0018486); Påhlsson Foundation; Region Skåne; Rhodes Trust; Signe and Ane Gyllenberg Foundation; Sigrid Juselius Foundation; Skåne University Hospital; Société Francophone du Diabète; Swedish Diabetes Foundation; Swedish Heart and Lung Foundation; Swedish Research Council; Turku University Hospital Research Funds; University of Dundee; Vasa Hospital District; Wellcome (072960/Z/03/Z, 084726/Z/08/Z, 084727/Z/08/Z, 085475/Z/08/Z, 085475/B/08/Z, 098381, 090532, and 106310); and Wilhelm and Else Stockmann Foundation.
Duality of Interest. P.R. has given lectures for AstraZeneca, Bristol-Myers Squibb, and Boehringer Ingelheim and has served as a consultant for AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Boehringer Ingelheim, Astellas, Janssen, and Novo Nordisk. All fees from the aforementioned lectures were given to the Steno Diabetes Center that has equity interest in Novo Nordisk. D.Z. is an employee of Pfizer, Inc. E.F. is an employee of and owns stock in Pfizer, Inc. W.Y.S., J.C.N.C., and R.C.W.M. are co-founders of GemVCare, established under the Technology Start-up Support Scheme for Universities from the Hong Kong Government Innovation and Technology Commission. J.C.F. has received a consulting honorarium from Merck Sharp & Dohme. P.-H.G. has received lecture honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Genzyme, Merck Sharp & Dohme, Novartis, Novo Nordisk, and Sanofi and has received research grants from Eli Lilly and Roche. P.-H.G. is also an advisory board member for AbbVie, Boehringer Ingelheim, Eli Lilly, Janssen, Medscape, Merck Sharp & Dohme, Novartis, and Sanofi. M.I.M. serves on advisory panels for Pfizer and Novo Nordisk; has received honoraria from Eli Lilly, Pfizer, and Novo Nordisk; and has received research support from Eli Lilly, Pfizer, Novo Nordisk, Servier, Takeda, Roche, Merck Sharp & Dohme, Janssen, AbbVie, Boehringer Ingelheim, AstraZeneca, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. Central data analysis was performed by N.R.v.Z., E.A., N.S., N.W.R., D.Z., E.F., SUMMIT Consortium, and M.I.M. Data generation was performed by N.R.v.Z., E.A., H.D., C.L., Finnish Diabetic Nephropathy Study, M.K., J.L., G.J., A.O.Y.L., H.M.L., C.K.P.L., J.C.N.C., Hong Kong Diabetes Registry Theme-based Research Scheme Project Group, S.F.A., R.D., T.S.H.C., A.-J.M., Warren 3 and Genetics of Kidneys in Diabetes (GoKinD) Study Group, GENIE (GEnetics of Nephropathy an International Effort) Consortium, S.H., Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, T.S.A., M.O.-M., A.L., C.C., N.G., I.B., O.M., S.C.L., R.C.W.M., V.L., S.S.R., J.C.F., O.P., T.H., SUMMIT Consortium, C.N.A.P., P.-H.G., L.C.G., and M.I.M. Individual study design was performed by E.A., N.S., H.D., P.M.M., C.F., N.P., R.M.v.D., G.J., J.C.N.C., M.M., M.O.-M., A.L., C.C., D.R.W., I.B., S.C.L., R.C.W.M., E.S.T., S.M., T.T., J.N.H., O.P., T.H., G.R., SUMMIT Consortium, M.J.B., C.N.A.P., P.-H.G., H.M.C., and L.C.G. Local data analysis was performed by N.R.v.Z., E.A., N.S., H.D., N.W.R., C.L., N.R.R., P.M.M., E.V., A.P., R.P.I., R.M.S., N.P., M.I., A.T., X.S., J.L., G.J., J.C.N.C., Hong Kong Diabetes Registry Theme-based Research Scheme Project Group, S.F.A., R.D., L.W., A.-J.M., S.D., M.G.P., GENIE (GEnetics of Nephropathy an International Effort) Consortium, M.M., B.G., S.H., L.T.H., T.S.A., P.A., C.-A.S., O.M., A.D.P., D.T., A.P.M., S.C.L., R.C.W.M., V.L., S.S.R., J.C.F., G.R., SUMMIT Consortium, C.N.A.P., and H.M.C. The paper was prepared by N.R.v.Z., E.A., N.S., M.A., N.R.R., M.L., J.C.N.C., L.T.H., A.D.P., S.C.L., R.C.W.M., J.C.F., P.R., SUMMIT Consortium, H.M.C., L.C.G., and M.I.M. Sample collection was conducted by C.F., V.H., Finnish Diabetic Nephropathy Study, E.R., M.L.M., N.P., M.L., R.M.v.D., C.H.T.T., A.O.Y.L., C.K.P.L., C.C.S., W.Y.S., J.C.N.C., Hong Kong Diabetes Registry Theme-based Research Scheme Project Group, S.F.A., T.S.H.C., A.-J.M., Warren 3 and Genetics of Kidneys in Diabetes (GoKinD) Study Group, GENIE (GEnetics of Nephropathy an International Effort) Consortium, M.M., S.H., Diabetes Control and Complications Trial (DCCT)/ Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, M.O.-M., A.L., C.C., D.R.W., I.B., O.M., A.P.M., S.C.L., R.C.W.M., E.S.T., V.L., T.T., A.S.K., S.S.R., J.C.F., D.D., O.P., T.H., P.R., G.R., SUMMIT Consortium, C.N.A.P., P.-H.G., H.M.C., L.C.G., A.K., and G.J. N.R.v.Z and M.I.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0914/-/DC1.
A complete list of the members of the Finnish Diabetic Nephropathy Study (FinnDiane), Hong Kong Diabetes Registry Theme-based Research Scheme Project Group, Warren 3 and Genetics of Kidneys in Diabetes (GoKinD) Study Group, GENIE (GEnetics of Nephropathy an International Effort) Consortium, Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, and SUrrogate markers for Micro- and Macrovascular hard endpoints for Innovative diabetes Tools (SUMMIT) Consortium can be found in the Supplementary Data.
Contributor Information
Collaborators: Finnish Diabetic Nephropathy Study (FinnDiane), S. Koivula, T. Uggeldahl, T. Forslund, A. Halonen, A. Koistinen, P. Koskiaho, M. Laukkanen, J. Saltevo, M. Tiihonen, M. Forsen, H. Granlund, A.-C. Jonsson, B. Nyroos, P. Kinnunen, A. Orvola, T. Salonen, A. Vähänen, Kotka R. Paldanius, M. Riihelä, L. Ryysy, Kemi H. Laukkanen, P. Nyländen, A. Sademies, S. Anderson, B. Asplund, U. Byskata, P. Liedes, M. Kuusela, T. Virkkala, A. Nikkola, E. Ritola, Tapiola M. Niska, H. Saarinen, Samaria E. Oukko-Ruponen, T. Virtanen, Viherlaakso A. Lyytinen, Puistola H. Kari, T. Simonen, Suutarila A. Kaprio, J. Kärkkäinen, B. Rantaeskola, Toolo P. Kääriäinen, J. Haaga, A.-L. Pietiläinen, S. Klemetti, T. Nyandoto, E. Rontu, S. Satuli-Autere, Korso R. Toivonen, H. Virtanen Lansimaki, R. Ahonen, M. Ivaska-Suomela, A. Jauhiainen, Martinlaakso M. Laine, T. Pellonpää, R. Puranen, Myyrmaki A. Airas, J. Laakso, K. Rautavaara, Rekola M. Erola, E. Jatkola, Tikkurila R. Lönnblad, A. Malm, J. Mäkelä, E. Rautamo, P. Hentunen, J. Lagerstam, M. Feodoroff, D. Gordin, O. Heikkilä, K. Hietala, J. Fagerudd, M. Korolainen, L. Kyllönen, J. Kytö, S. Lindh, K. Pettersson-Fernholm, M. Rosengård-Bärlund, A. Sandelin, L. Thorn, J. Tuomikangas, T. Vesisenaho, J. Wadén, V. Sipilä, Forssa T. Kalliomäki, J. Koskelainen, R. Nikkanen, N. Savolainen, H. Sulonen, E. Valtonen, L. Norvio, A. Hämäläinen, E. Toivanen, Jamsa A. Parta, I. Pirttiniemi, S. Aranko, S. Ervasti, R. Kauppinen-Mäkelin, A. Kuusisto, T. Leppälä, K. Nikkilä, L. Pekkonen, Kajaani S. Jokelainen, K. Kananen, M. Karjalainen, P. Kemppainen, A.-M. Mankinen, A. Reponen, M. Sankari, P. Suominen, A. Lappalainen, M. Liimatainen, J. Santaholma, A. Aimolahti, E. Huovinen, V. Ilkka, M. Lehtimäki, E. Pälikkö-Kontinen, A. Vanhanen, E. Koskinen, T. Siitonen, E. Huttunen, R. Ikäheimo, P. Karhapää, P. Kekäläinen, M. Laakso, T. Lakka, E. Lampainen, L. Moilanen, S. Tanskanen, L. Niskanen, U. Tuovinen, I. Vauhkonen, E. Voutilainen, Hong Kong Diabetes Registry Theme-based Research Scheme Project Group, Ronald C.W. Ma, Juliana C.N. Chan, Yu Huang, Hui-yao Lan, Si Lok, Brian Tomlinson, Stephen K.W. Tsui, Weichuan Yu, Kevin Y.L. Yip, Ting Fung Chan, Xiaodan Fan, Wing Yee So, Cheuk Chun Szeto, Nelson Tang, Andrea O. Luk, Xiaoyu Tian, Guozhi Jiang, Claudia H.T. Tam, Heung Man Lee, Cadmon K.P. Lim, Katie K.H. Chan, Fangying Xie, Alex C.W. Ng, Grace P.Y. Cheung, Ming-wai Yeung, Shi Mai, Fei Xie, Sen Zhang, Pu Yu, Meng Weng, Warren 3 and Genetics of Kidneys in Diabetes (GoKinD) Study Group, A.P. Maxwell, A.J. McKnight, D.A. Savage, J. Walker, S. Thomas, G.C. Viberti, A.J.M. Boulton, S. Marshall, A.G. Demaine, B.A. Millward, S.C. Bain, GENIE (GEnetics of Nephropathy an International Effort) Consortium, Niina Sandholm, Carol Forsblom, Valma Harjutsalo, Ville-Petteri Mäkinen, Aila J. Ahola, Emma Dahlström, Daniel Gordin, Outi Heikkilä, Kustaa Hietala, Janne Kytö, Markku Lehto, Raija Lithovius, Nicolae Mircea Panduru, Maija Parkkonen, Milla Rosengård-Bärlund, Markku Saraheimo, Jenny Söderlund, Aino Soro-Paavonen, Anna Syreeni, Lena M. Thorn, Nina Tolonen, Johan Wadén, Per-Henrik Groop, Amy Jayne McKnight, Gareth J. McKay, Alexander P. Maxwell, Rany M. Salem, Tamara Isakova, Cameron Palmer, Candace Guiducci, Andrew Taylor, Daniel B. Mirel, Winfred W. Williams, Joel N. Hirschhorn, Jose C. Florez, Eoin P. Brennan, Denise M. Sadlier, Finian Martin, Catherine Godson, Diabetes Control and Complications Trial (DCCT)/ Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, Lynne Mayer, Rose Gubitosi-Klug, Patti Bourne, Mark Schutta, Mary Ellen Lackaye, Naina Sinha Gregory, Davida Kruger, J. Kimberly Jones, Arti Bhan, Ellen Golden, Lloyd Aiello, Mary Larkin, David Nathan, Georgia Ziegler, Susan Caulder, Clare Pittman, Louis Luttrell, Maria Lopes-Virella, Mary Johnson, Kimberly Gunyou, Richard Bergenstal, Brenda Vittetoe, William Sivitz, Nancy Flaherty, John Bantle, Susan Hitt, David Goldstein, Dean Hainsworth, Lori Cimino, Trevor Orchard, Christine Wigley, Samuel Dagogo-Jack, Suzanne Strowig, Philip Raskin, Annette Barnie, Bernard Zinman, Robyn Fahlstrom, Jerry Palmer, Judith Harth, Marsha Driscoll, Charlotte McDonald, Janie Lipps Hagan, Michael May, Lucy Levandoski, Neil White, Patricia Gatcomb, William Tamborlane, Daphne Adelman, Susan Colson, Mark Molitch, Gayle Lorenzi, Sunder Mudaliar, Sherry Johnsonbaugh, Ryan Miller, Janene Canady, David Schade, Maria Luisa Bernal, John Malone, Anthony Morrison, Catherine Martin, William Herman, Rodica Pop-Busui, Catherine Cowie, Ellen Leschek, Patricia Cleary, John Lachin, Bernie Zinman, Gayle Lorenzi, Barbara Braffett, Mike Steffes, Valerie Arends, Barbara Blodi, Ronald Danis, Daniel Lawrence, Hugh Wabers, Elsayed Soliman, Zhu-Ming Zhang, Charles Campbell, Susan Hensley, Lisa Keasler, SUrrogate markers for Micro- and Macrovascular hard endpoints for Innovative diabetes Tools (SUMMIT) Consortium, Michael Mark, Markus Albertini, Carine Boustany, Alexander Ehlgen, Martin Gerl, Jochen Huber, Corinna Schölch, Heike Zimdahl-Gelling, Leif Groop, Elisabet Agardh, Emma Ahlqvist, Tord Ajanki, Nibal Al Maghrabi, Peter Almgren, Jan Apelqvist, Eva Bengtsson, Lisa Berglund, Harry Björckbacka, Ulrika Blom-Nilsson, Mattias Borell, Agneta Burström, Corrado Cilio, Magnus Cinthio, Karl Dreja, Pontus Dunér, Daniel Engelbertsen, Joao Fadista, Maria Gomez, Isabel Goncalves, Bo Hedblad, Anna Hultgårdh, Martin E. Johansson, Cecilia Kennbäck, Jasmina Kravic, Claes Ladenvall, Åke Lernmark, Eero Lindholm, Charlotte Ling, Holger Luthman, Olle Melander, Malin Neptin, Jan Nilsson, Peter Nilsson, Tobias Nilsson, Gunilla Nordin, Marju Orho-Melander, Emilia Ottoson-Laakso, Annie Persson, Margaretha Persson, Mats-Åke Persson, Jacqueline Postma, Elisabeth Pranter, Sara Rattik, Gunnar Sterner, Lilian Tindberg, Maria Wigren, Anna Zetterqvist, Mikael Åkerlund, Gerd Ostling, Timo Kanninen, Anni Ahonen-Bishopp, Anita Eliasson, Timo Herrala, Paivi Tikka-Kleemola, Anders Hamsten, Christer Betsholtz, Ami Björkholm, Fariba Foroogh, Guillem Genové, Karl Gertow, Bruna Gigante, Bing He, Karin Leander, Olga McLeod, Maria Nastase-Mannila, Jaako Patrakka, Angela Silveira, Rona Strawbridge, Karl Tryggvason, Max Vikström, John Ohrvik, Anne-May Österholm, Barbara Thorand, Christian Gieger, Harald Grallert, Tonia Ludwig, Barbara Nitz, Andrea Schneider, Rui Wang-Sattler, Astrid Zierer, Giuseppe Remuzzi, Ariela Benigni, Roberta Donadelli, Maria Domenica Lesti, Marina Noris, Norberto Perico, Annalisa Perna, Rossella Piras, Piero Ruggenenti, Erica Rurali, David Dunger, Ludo Chassin, Neil Dalton, John Deanfield, Jane Horsford, Clare Rice, James Rudd, Neil Walker, Karen Whitehead, Max Wong, Helen Colhoun, Fiona Adams, Tahira Akbar, Jill Belch, Harshal Deshmukh, Fiona Dove, Angela Ellingford, Bassam Farran, Mike Ferguson, Gary Henderson, Graeme Houston, Faisel Khan, Graham Leese, Yiyuan Liu, Shona Livingstone, Helen Looker, Margaret McCann, Stuart McGurnaghan, Andrew Morris, David Newton, Colin Palmer, Ewan Pearson, Gillian Reekie, Natalie Smith, Angela Shore, Kuni Aizawa, Claire Ball, Nick Bellenger, Francesco Casanova, Tim Frayling, Phil Gates, Kim Gooding, Andrew Hattersley, Roland Ling, David Mawson, Robin Shandas, David Strain, Clare Thorn, Ulf Smith, Ann Hammarstedt, Hans Häring, Oluf Pedersen, Georgio Sesti, Per-Henrik Groop, Emma Fagerholm, Carol Forsblom, Valma Harjutsalo, Maikki Parkkonen, Niina Sandholm, Nina Tolonen, Iiro Toppila, Erkka Valo, Veikko Salomaa, Aki Havulinna, Kati Kristiansson, Pia Okamo, Tomi Peltola, Markus Perola, Arto Pietilä, Samuli Ripatti, Marketta Taimi, Seppo Ylä-Herttuala, Mohan Babu, Marike Dijkstra, Erika Gurzeler, Jenni Huusko, Ivana Kholová, Markku Laakso, Mari Merentie, Marja Poikolainen, Mark McCarthy, Chris Groves, Thorhildur Juliusdottir, Fredrik Karpe, Vasiliki Lagou, Andrew Morris, Will Rayner, Neil Robertson, Natalie van Zuydam, Claudio Cobelli, Barbara Di Camillo, Francesca Finotello, Francesco Sambo, Gianna Toffolo, Emanuele Trifoglio, Riccardo Bellazzi, Nicola Barbarini, Mauro Bucalo, Christiana Larizza, Paolo Magni, Alberto Malovini, Simone Marini, Francesca Mulas, Silvana Quaglini, Lucia Sacchi, Francesca Vitali, Ele Ferrannini, Beatrice Boldrini, Michaela Kozakova, Andrea Mari, Carmela Morizzo, Lucrecia Mota, Andrea Natali, Carlo Palombo, Elena Venturi, Mark Walker, Carlo Patrono, Francesca Pagliaccia, Bianca Rocca, Pirjo Nuutila, Johanna Haukkala, Juhani Knuuti, Anne Roivainen, Antti Saraste, Paul McKeague, Marco Colombo, Birgit Steckel-Hamann, Krister Bokvist, Sudha Shankar, Melissa Thomas, Li-ming Gan, Suvi Heinonen, Ann-Cathrine Jönsson-Rylander, Remi Momo, Volker Schnecke, Robert Unwin, Anna Walentinsson, Carl Whatling, Everson Nogoceke, Gonzalo Durán Pacheco, Ivan Formentini, Thomas Schindler, Piero Tortoli, Luca Bassi, Enrico Boni, Alessandro Dallai, Francesco Guidi, Matteo Lenge, Riccardo Matera, Alessandro Ramalli, Stefano Ricci, Jacopo Viti, Bernd Jablonka, Dan Crowther, Johan Gassenhuber, Sibylle Hess, Thomas Hubschle, Hans-Paul Juretschke, Hartmut Rutten, Thorsten Sadowski, Paulus Wohlfart, Julia Brosnan, Valerie Clerin, Eric Fauman, Craig Hyde, Anders Malarstig, Nick Pullen, Mera Tilley, Theresa Tuthill, Ciara Vangjeli, and Daniel Ziemek
References
- 1.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 2013;93:137–188 [DOI] [PubMed] [Google Scholar]
- 2.Krolewski AS, Skupien J, Rossing P, Warram JH. Fast renal decline to end-stage renal disease: an unrecognized feature of nephropathy in diabetes. Kidney Int 2017;91:1300–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 1989;320:1161–1165 [DOI] [PubMed] [Google Scholar]
- 4.Quinn M, Angelico MC, Warram JH, Krolewski AS. Familial factors determine the development of diabetic nephropathy in patients with IDDM. Diabetologia 1996;39:940–945 [DOI] [PubMed] [Google Scholar]
- 5.Vijay V, Snehalatha C, Shina K, Lalitha S, Ramachandran A. Familial aggregation of diabetic kidney disease in Type 2 diabetes in south India. Diabetes Res Clin Pract 1999;43:167–171 [DOI] [PubMed] [Google Scholar]
- 6.Pettitt DJ, Saad MF, Bennett PH, Nelson RG, Knowler WC. Familial predisposition to renal disease in two generations of Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1990;33:438–443 [DOI] [PubMed] [Google Scholar]
- 7.Freedman BI, Spray BJ, Tuttle AB, Buckalew VM Jr. The familial risk of end-stage renal disease in African Americans. Am J Kidney Dis 1993;21:387–393 [DOI] [PubMed] [Google Scholar]
- 8.Mooyaart AL, Valk EJ, van Es LA, et al. Genetic associations in diabetic nephropathy: a meta-analysis. Diabetologia 2011;54:544–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pezzolesi MG, Poznik GD, Mychaleckyj JC, et al.; DCCT/EDIC Research Group . Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 2009;58:1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKnight AJ, Currie D, Patterson CC, Maxwell AP, Fogarty DG; Warren 3/UK GoKinD Study Group . Targeted genome-wide investigation identifies novel SNPs associated with diabetic nephropathy. HUGO J 2009;3:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandholm N, Salem RM, McKnight AJ, et al.; DCCT/EDIC Research Group . New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet 2012;8:e1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pham TT, Sim JJ, Kujubu DA, Liu IL, Kumar VA. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol 2007;27:322–328 [DOI] [PubMed] [Google Scholar]
- 13.Pattaro C, Teumer A, Gorski M, et al.; ICBP Consortium; AGEN Consortium; CARDIOGRAM; CHARGe-Heart Failure Group; ECHOGen Consortium . Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat Commun 2016;7:10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Böger CA, Sedor JR. GWAS of diabetic nephropathy: is the GENIE out of the bottle? PLoS Genet 2012;8:e1002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Placha G, Canani LH, Warram JH, Krolewski AS. Evidence for different susceptibility genes for proteinuria and ESRD in type 2 diabetes. Adv Chronic Kidney Dis 2005;12:155–169 [DOI] [PubMed] [Google Scholar]
- 16.Ellis JW, Chen MH, Foster MC, et al.; CKDGen Consortium; CARe Renal Consortium . Validated SNPs for eGFR and their associations with albuminuria. Hum Mol Genet 2012;21:3293–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandholm N, Van Zuydam N, Ahlqvist E, et al.; FinnDiane Study Group; DCCT/EDIC Study Group; GENIE Consortium; SUMMIT Consortium . The genetic landscape of renal complications in type 1 diabetes. J Am Soc Nephrol 2017;28:557–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todd JN, Salem R, Sandholm N, et al. Novel genetic determinants of diabetic kidney disease. Diabetes 2016;65(Suppl. 1):A100 [Google Scholar]
- 19.Lindholm E, Agardh E, Tuomi T, Groop L, Agardh CD. Classifying diabetes according to the new WHO clinical stages. Eur J Epidemiol 2001;17:983–989 [DOI] [PubMed] [Google Scholar]
- 20.Morris AD, Boyle DI, MacAlpine R, et al.; DARTS/MEMO Collaboration . The Diabetes Audit and Research in Tayside Scotland (DARTS) study: electronic record linkage to create a diabetes register. BMJ 1997;315:524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care 2002;25:859–864 [DOI] [PubMed] [Google Scholar]
- 22.Ruggenenti P, Remuzzi G. Nephropathy of type 1 and type 2 diabetes: diverse pathophysiology, same treatment? Nephrol Dial Transplant 2000;15:1900–1902 [DOI] [PubMed] [Google Scholar]
- 23.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007;447:661–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delaneau O, Zagury JF, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods 2013;10:5–6 [DOI] [PubMed] [Google Scholar]
- 25.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet 2012;44:955–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradić M, Costa J, Chelo IM. Genotyping with Sequenom. Methods Mol Biol 2011;772:193–210 [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet 2011;88:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visscher PM, Hemani G, Vinkhuyzen AA, et al. Statistical power to detect genetic (co)variance of complex traits using SNP data in unrelated samples. PLoS Genet 2014;10:e1004269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 2007;39:906–913 [DOI] [PubMed] [Google Scholar]
- 30.Kang HM, Sul JH, Service SK, et al. Variance component model to account for sample structure in genome-wide association studies. Nat Genet 2010;42:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010;26:2190–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mägi R, Morris AP. GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics 2010;11:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deloukas P, Kanoni S, Willenborg C, et al.; CARDIoGRAMplusC4D Consortium; DIAGRAM Consortium; CARDIOGENICS Consortium; MuTHER Consortium; Wellcome Trust Case Control Consortium . Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet 2013;45:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Speliotes EK, Willer CJ, Berndt SI, et al.; MAGIC; Procardis Consortium . Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heid IM, Jackson AU, Randall JC, et al.; MAGIC . Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet 2010;42:949–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willer CJ, Sanna S, Jackson AU, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 2008;40:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott RA, Fall T, Pasko D, et al.; RISC Study Group; EPIC-InterAct Consortium . Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independent of obesity. Diabetes 2014;63:4378–4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott RA, Lagou V, Welch RP, et al.; DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium . Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet 2012;44:991–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lotta LA, Gulati P, Day FR, et al.; EPIC-InterAct Consortium; Cambridge FPLD1 Consortium . Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet 2017;49:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrett JC, Clayton DG, Concannon P, et al.; Type 1 Diabetes Genetics Consortium . Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009;41:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahajan A, Go MJ, Zhang W, et al.; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium; Asian Genetic Epidemiology Network Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; Mexican American Type 2 Diabetes (MAT2D) Consortium; Type 2 Diabetes Genetic Exploration by Nex-generation sequencing in muylti-Ethnic Samples (T2D-GENES) Consortium . Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet 2014;46:234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehret GB, Munroe PB, Rice KM, et al.; International Consortium for Blood Pressure Genome-Wide Association Studies; CARDIoGRAM Consortium; CKDGen Consortium; KidneyGen Consortium; EchoGen Consortium; CHARGE-HF Consortium . Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011;478:103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill CJ, Cardwell CR, Maxwell AP, et al. Obesity and kidney disease in type 1 and 2 diabetes: an analysis of the National Diabetes Audit. QJM 2013;106:933–942 [DOI] [PubMed] [Google Scholar]
- 44.Groop L, Ekstrand A, Forsblom C, et al. Insulin resistance, hypertension and microalbuminuria in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1993;36:642–647 [DOI] [PubMed] [Google Scholar]
- 45.Karalliedde J, Gnudi L. Diabetes mellitus, a complex and heterogeneous disease, and the role of insulin resistance as a determinant of diabetic kidney disease. Nephrol Dial Transplant 2016;31:206–213 [DOI] [PubMed] [Google Scholar]
- 46.Paternoster L, Tilling K, Davey Smith G. Genetic epidemiology and Mendelian randomization for informing disease therapeutics: conceptual and methodological challenges. PLoS Genet 2017;13:e1006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ju W, Greene CS, Eichinger F, et al. Defining cell-type specificity at the transcriptional level in human disease. Genome Res 2013;23:1862–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maric-Bilkan C. Obesity and diabetic kidney disease. Med Clin North Am 2013;97:59–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Todd JN, Dahlström EH, Salem RM, et al.; FinnDiane Study Group . Genetic Evidence for a causal role of obesity in diabetic kidney disease. Diabetes 2015;64:4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.