Abstract
The early to mid-1980s were an inflection point in the history of type 1 diabetes research. Two landmark events occurred: the initiation of immune-based interventions seeking to prevent type 1 diabetes and the presentation of an innovative model describing the disorder’s natural history. Both formed the basis for hundreds of subsequent studies designed to achieve a dramatic therapeutic goal—a means to prevent and/or reverse type 1 diabetes. However, the need to screen large numbers of individuals and prospectively monitor them using immunologic and metabolic tests for extended periods of time suggested such efforts would require a large collaborative network. Hence, the National Institutes of Health formed the landmark Diabetes Prevention Trial-Type 1 (DPT-1) in the mid-1990s, an effort that led to Type 1 Diabetes TrialNet. TrialNet studies have helped identify novel biomarkers; delineate type 1 diabetes progression, resulting in identification of highly predictable stages defined by the accumulation of autoantibodies (stage 1), dysglycemia (stage 2), and disease meeting clinical criteria for diagnosis (stage 3); and oversee numerous clinical trials aimed at preventing disease progression. Such efforts pave the way for stage-specific intervention trials with improved hope that a means to effectively disrupt the disorder’s development will be identified.
Introduction
One of the most preeminent dogmas regarding type 1 diabetes is that the disorder results from a chronic progressive autoimmune destruction of the insulin-producing pancreatic β-cells (reviewed in refs. 1 and 2). Indeed, an abundant body of clinical and preclinical literature supports this claim. However, recent studies of individuals at varying levels of risk for developing the disease highlight the need for modification of this model. This includes several emerging notions: β-cells themselves play an important role in pathogenesis (3), the rate of β-cell dysfunction is not linear, and there is heterogeneity in disease among those with type 1 diabetes.
Indeed, these and other findings have precipitated recent discussions of how to define type 1 diabetes as a clinical entity, given the potential for various pathways to disease development and, importantly, the likelihood that more precise therapeutic targeting will be necessary to effectively intervene in this disease for purposes of prevention and/or reversal. Hence, the goal of this Perspective is to convey an emerging body of information emanating from studies of the disorder’s progression combined with outcomes from trials of immunotherapy as disease-modifying agents, with a focus on the efforts in Type 1 Diabetes TrialNet that are leading to key changes in thoughts regarding the disorder.
What Do We Know and, More Importantly, What Do We Not Know Regarding the Natural History of Type 1 Diabetes?
The most fundamental concept of the Eisenbarth model—that autoimmunity and β-cell dysfunction precede clinical disease—has been repeatedly demonstrated (4) and has served as a remarkable road map for research investigation and clinical trials over the decades. However, certain components of the model have also been difficult to prove, too simplistic in their original thought, or were unfortunately with time shown to be erroneous (1). As a result, concepts from this model that have stood the test of time (i.e., what we know) have been modified to what may represent reality (i.e., what we think we know) or to what needs additional information (i.e., what we don’t know), facets of which are summarized in Table 1.
Table 1.
What we know, what we think we know, and what we do not know regarding the natural history of type 1 diabetes, relative to the classic model for the disorder’s development (1,4)
| Initial concept | Current view | Outstanding questions | |
|---|---|---|---|
| “Type 1A” diabetes |
The previous classification of diabetes reflected the distinction between the absence (T1) or presence (T2) of endogenous insulin secretion. The frame of immune (type 1A) and nonimmune (type 1B) forms of type 1 diabetes refers to whether or not there is evidence of immune-mediated disease. |
There are variable degrees of β-cell dysfunction and evidence of autoimmunity among all those with diabetes. Neither of the previous categories describe the unique pathophysiology, and the terms type 1A and type 1B are not commonly used. |
• Is autoimmune type 1 diabetes a “syndrome” of disorders having different pathogenic features, disease requirements, and natural histories?
• Are there new classifications that will help identify ideal candidates for certain therapies? |
| Precipitating event (in a window) |
One environmental exposure (e.g., viral infection) at one time point initiates type 1A diabetes. |
Many candidate environmental agents have been implicated but none with certainty; they may act throughout natural history of disease and likely will vary by geographic region. |
• What are the environmental agents (if any) that are associated with the disease?
• How many are required and do they interact?
• When are they in operation? |
| Linear loss in β-cell mass |
The loss in β-cell mass in type 1A diabetes occurs in a progressive/constant form. |
Growing evidence suggests that β-cell loss occurs in a nonlinear pattern, with acute changes in the peri-diagnostic period. |
• Can biomarkers or technologies (e.g., β-cell imaging) be developed that would monitor β-cell loss?
• If true, what drives the periods of exacerbation/remission in β-cell loss?
• What tools could be used to distinguish between β-cell loss and function? |
| Progressive loss of insulin release |
With increasing loss of β-cells, insulin release is also lost. |
Staged functional defects are evident. For much of the natural history of type 1 diabetes prior to disease onset, insulin levels do not decrease proportionally with β-cell loss. Impaired hormone processing may reflect β-cell stress throughout disease progression. |
• How does the stress produced by increasing demands on residual β-cells influence their rate of demise? |
| Glucose levels at overt (i.e., symptomatic) onset |
Blood glucose values remain in normal range until immediately prior to symptoms. |
TrialNet data demonstrate that glycemic dysregulation starts with postprandial elevations before abnormal fasting glucose or HbA1c and that symptomatic disease occurs only when fasting is elevated, commonly associated with increased HbA1c. The diagnostic threshold for glucose may be wrong. |
• What factors influence the time between initial dysglycemia and symptomatic onset? |
| β-Cell mass at time of overt onset |
Symptomatic onset occurs when ∼85% of β-cells are lost. |
The quantity of β-cells varies in healthy populations and with age. At diabetes onset, significant β-cell function can be detected with values that even can overlap healthy population. |
• What is the percentage of “functional” β-cell mass versus that which is “dysfunctional and potentially viable” at onset? |
| C-peptide |
Within a brief period, the lack of β-cells leads to no C-peptide production. |
The vast majority of patients with type 1 diabetes continue to produce small quantities of C-peptide for many years after onset. |
• What is the functional significance (if any) for low-level C-peptide production? Why does it remain in certain individuals? |
| Eventual β-cell mass | At or shortly after disease onset, all β-cells are lost. | Consistent with the notions of residual C-peptide secretion, many patients with type 1 diabetes retain β-cells (insulin-positive cells) for decades after disease onset. | • Why does the destruction of β-cells not continue to completion? • Are there properties of the β-cells or of the immune response that render them resistant to loss? |
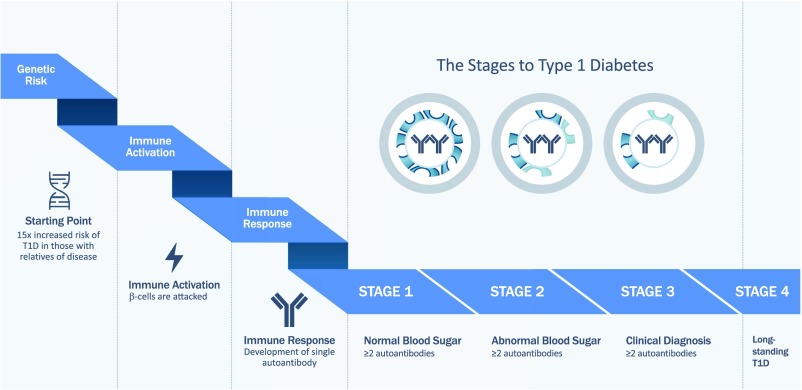
Irrespective of the model’s original accuracy, many noteworthy accomplishments have resulted from its testing and subsequent refinement. Among the most noteworthy of the notions that will be stressed throughout this Perspective is that type 1 diabetes is largely a predictable disease, which was discovered through long-term prospective analyses of individuals without type 1 diabetes but who had a series of genetic, immunologic, and metabolic markers associated with the disorder (5–7). Indeed, the degree of prediction has become so sound that the natural history of type 1 diabetes can now be divided into a series of stages (Fig. 1) (8) that serve as a useful guide for future therapeutic interventions.
Figure 1.
Stages of diabetes progression (8,62). Genetic risk of type 1 diabetes (T1D) progression precedes immune activation and seroconversion. The appearance of two or more autoantibodies defines stage 1 disease. Type 1 diabetes progression and loss of β-cell mass/function (indicated by loss of cells depicted in the circles) occurs as subjects develop abnormal blood glucose levels (stage 2) and, finally, meet American Diabetes Association criteria for clinical diagnosis (stage 3).
Type 1 Diabetes Prediction—What’s New and Where Have We Fallen Short?
As an international network of physicians and research scientists at academic institutions and hospitals, TrialNet offers risk screening for relatives of patients with type 1 diabetes to facilitate natural history studies of the disease as well as to identify suitable individuals for entry into clinical trials aimed at preserving insulin production at all stages of disease. Unique opportunities are made possible by large programs (such as TrialNet) that identify autoantibody-positive relatives through screening across the age spectrum of 1–45 years (9,10), as well as those testing baby cohorts, such as Diabetes Autoimmunity Study in the Young (DAISY) (11,12), BABYDIAB (13), Type 1 Diabetes Prediction and Prevention (DIPP) (14), and The Environmental Determinants of Diabetes in the Young (TEDDY) (15,16). Each afford the opportunity to follow individuals over many years based on genetic risk or from the first development of autoantibodies to overt hyperglycemia and clinical disease, providing groundbreaking information to better understand type 1 diabetes from inception and throughout progression, which was not possible only a short time ago. The insights from these clinical studies are complemented by the ever-growing number of novel observations using pathologic specimens provided by the Network for Pancreatic Organ Donors with Diabetes (nPOD) (17,18).
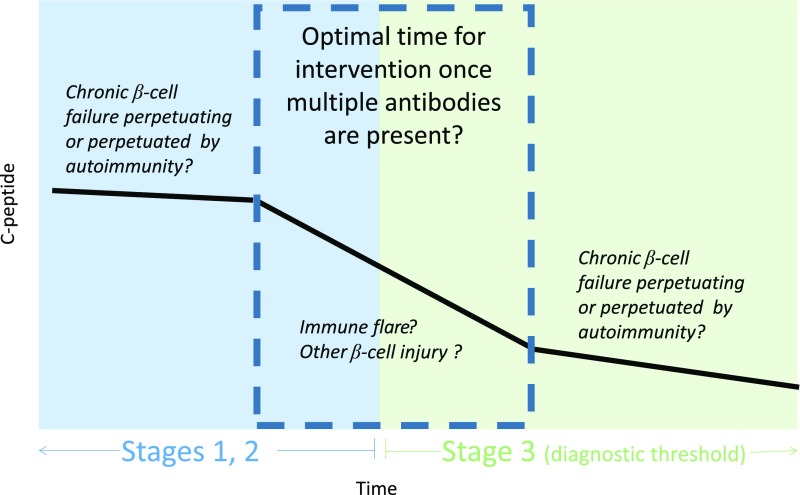
Collectively, these studies suggest that there are inflection points in disease progression. First, it is increasingly apparent that the factors predicting the initiation of autoimmunity (defined as seroconversion to autoantibody positivity) (8,19) may be different from those associated with progression from autoantibody positivity to clinical disease (20–26). Similarly, it is well known that family members of patients with type 1 diabetes have a 15-fold increased risk compared with the general population (27–29), yet the increased risk for family members is associated with the initiation of autoimmunity (i.e., seroconversion) and not necessarily subsequent progression to clinical type 1 diabetes (8). This raises the question of whether stochastic or environmental events may precipitate disease progression after initial seroconversion, with external factors perhaps playing a role in type 1 diabetes etiopathogenesis at later stages than previously hypothesized. A second inflection point is the time after autoimmunity when glucose dysregulation occurs. Data regarding this transition are robust enough that abnormal glucose tolerance is already used as a clinical trial end point of disease-modifying therapy. Evaluation of longitudinal insulin secretion data suggests the possibility of other inflection points in disease progression. In contrast to the Eisenbarth model, which posited a steady and linear change in secretion over time, data suggest relative stability in β-cell function and β-cell glucose sensitivity (slope of correlation between insulin secretion and glucose values) until a rapid fall 6–12 months prior to clinical diagnosis (30). Analysis of β-cell functional data and measurements of β-cell killing with an assay that measures β-cell–derived INS DNA in individuals enrolled in TrialNet clinical trials after disease onset also illustrated a biphasic fall in secretion with more rapid changes closer to clinical diagnosis (31,32). Preliminary analysis (unpublished data, Type 1 Diabetes TrialNet Clinical Study Group) from the Long-Term Investigative Follow-Up in TrialNet (LIFT) study suggests that the 6–12 months on either side of clinical diagnosis constitute a period of rapid change in β-cell function, with implications both for the timing of clinical trials to interrupt this process and the identification of another gap in our mechanistic understanding during this period of disease progression. Importantly, recent data from TrialNet ancillary studies found an increase of both β-cell death (32,33) and abnormal insulin processing (26) during this period of significant functional changes, offering the possibility of using unmethylated INS DNA or fasting serum proinsulin–to–C-peptide ratio as biomarkers to determine timing for intervention or as outcomes of trials of disease-modifying therapy. These data also suggest a hypothesis (Fig. 2) whereby there are two processes occurring in disease progression—underlying chronic β-cell failure upon which periodic flares result in an acute wave of destruction.
Figure 2.
Proposed model suggesting the hypothesis that the peri-diagnostic period is the optimal time for intervention. The model posits acute flares of disease superimposed upon chronic underlying tissue (i.e., β-cell) injury.
Improved disease prediction is an iterative process; the availability of longitudinal data and samples allow for refinement of models as new data emerge. For example, TrialNet now incorporates screening for ZnT8 autoantibodies (34). TrialNet samples have been used to evaluate the contribution of electrochemiluminescence assays, which detect autoantibody affinity, to predict risk of progression. TrialNet also continues to screen for islet cell antibodies (ICA), measured by immunofluorescence in pancreatic islets, as the risk conferred by ICA positivity is additive to antibodies to known autoantigens (35,36), such as insulin, ZnT8, GAD, and the tyrosine phosphatase, IA-2. Understanding what other antigens contribute to ICA formation is an important scientific gap and will likely improve risk prediction in the future.
Previously, multiple autoantibody–positive relatives with normal glucose tolerance were considered to have a 3- to 5-year symptomatic disease risk of 35–50%, and those with abnormal glucose tolerance, about 60–80%. Combined efforts from multiple longitudinal studies now demonstrate that once multiple autoantibodies are present there appears to be a relentless cumulative progression to disease, particularly in younger patients. For individuals with or without a family history of type 1 diabetes who are identified to have multiple (i.e., two or more) islet cell autoantibodies, it is no longer a question of if they will develop disease but only when it will occur (7). This realization has resulted in a consensus paper, with support from the major entities associated with diabetes care (including the American Diabetes Association), that suggested a new definition of stages of type 1 diabetes that better reflect the autoimmune nature of the disorder (8) and hopefully will provide a theoretical framework for us to intervene early in the disease when we can do the most good (Fig. 1).
Within this effort, stage 1 disease is defined as having two or more autoantibodies (8). It is expected that interventions aimed at this stage would be particularly effective as they would prevent the development of impaired glucose tolerance and clinical diabetes. Stage 2 reflects the metabolic dysfunction that begins to become evident and the continuing destruction of pancreatic islet β-cells. Stage 3 represents overt hyperglycemia and what previously would be defined as the beginning of type 1 diabetes by long-standing criteria (37). Perhaps one reason our clinical trials in patients with recent-onset type 1 diabetes (discussed below) have not been as effective as originally hoped is simply that interventions were begun too late in the disease course to fully restore both immune balance and metabolic function. Stage 4 is the postdiagnosis period of long-standing disease, a period that still represents a time to intervene as many individuals continue to maintain detectable levels of C-peptide for many years after diagnosis (38,39). This paradigm shift involving staging affords us a unique opportunity to move therapeutic efforts into the treatment of autoimmunity rather than the reversal of clinically overt disease, which, as noted, likely represents a more difficult problem.
Moving Beyond Stages of Diabetes
Defining stages of diabetes has provided firm ground for understanding of the disease and for the design of clinical trials, yet this approach pays little attention to heterogeneity in clinical course. Not all individuals follow the sequence described above, and there is wide variation in the rate of progression. For example, some infants followed from birth have multiple autoantibodies at their first assessment (40–42). Later in the disease course, there are some autoantibody-positive individuals who have significantly impaired first-phase insulin secretion before abnormalities are seen in glucose tolerance. Recent work highlights significant variation in functional β-cell mass, even among those with multiple autoantibodies and low first-phase insulin response (43). The heterogeneity seen in disease progression is also highlighted in studies from nPOD, where organ donor specimens exhibit wide variation in residual β-cell mass and immune infiltration (44–47).
As the only large-scale longitudinal study of autoantibody-positive individuals across a wide age range, TrialNet data clearly demonstrate that age is an important, yet unexplained factor in heterogeneity of disease progression at every stage (8,10,48,49). However, age itself cannot fully explain heterogeneity as illustrated in studies exploring the transition from a single to multiple autoantibodies. Overall, within a 5-year period, the majority of individuals with a single autoantibody do not progress to diabetes, but about 20% develop stage 1 diabetes (multiple autoantibodies) (22). Not unexpectedly, these individuals tend to be younger children. Recent analyses emphasized the complexity: for those with single insulin autoantibodies, the risk for progression is greatest in those <8 years of age, whereas for those with single GAD autoantibodies, the risk continues among older age-groups (50). The interaction of age, type of autoantibody, and HLA type was also found in the analysis of baby cohorts. The order of autoantibody first appearance (16,51) and age at which autoantibodies first appear (42) are both HLA associated. Efforts to understand mechanisms underlying autoantigen/epitope spreading must take this heterogeneity into account. Similarly, although there is little effect of increased BMI on disease progression overall, a greater risk was observed among obese teenagers in the years surrounding pubertal transition (23), raising the possibility of targeted intervention in this cohort.
It is also important to note that the concept of stages of diabetes came from studies of individuals with genetic risk (either through genetic screening in infancy or by virtue of being a family member of a patient with type 1 diabetes) and autoantibodies. Factors associated with progression in these populations have a high positive predictive value (i.e., proportion of individuals with a particular characteristic who will develop autoimmunity or clinically diagnosed type 1 diabetes), which is useful in the design of clinical trials to provide a better risk–benefit ratio for therapy and a smaller target sample size. It is important to note that there is little information regarding the natural history of individuals who develop type 1 diabetes without these characteristics. Whether or not type 1 diabetes in individuals without known genetic risk or autoantibodies or in much older populations represents a fundamentally different disease is unknown.
Although We Can Reliably Describe the Natural History of Disease, There Remain Questions Regarding Mechanistic Factors That Determine the Rate of Disease Progression
TrialNet has provided ancillary samples from its studies that have resulted in observations pointing to changes seen at various time points during development of clinical disease. Differences in inflammatory pathways, miRNA (52), innate immunity (53), β-cell death (32), and anergic (54) and autoreactive B lymphocyte populations (55) as well as alterations in invariant (56), regulatory (Treg), and effector T-cell number and function (57) have each been reported in autoantibody-positive as compared with control individuals, yet the variance observed within these groups highlights that a given individual could exhibit all or only a few of these features. Specifically, IL-1β and IL-6 responses to Toll-like receptor ligation were dysregulated in younger subject cohorts but not in adults with diabetes-related autoantibodies (53). Similarly, a subset of individuals with autoantibodies and new-onset type 1 diabetes exhibited low frequencies of polyreactive anergic B cells, indicating a potential causative role for impaired B-cell tolerance in certain individuals (54). Data from human donor tissues suggest that developmental, morphologic, functional, and molecular heterogeneity exists among β-cell subpopulations (reviewed in ref. 58) and that specific subpopulations may have enhanced resistance to autoimmune killing (59). Beyond this, impaired Treg signaling and function and aberrant coexpression of FOXP3 and IL-17 are detectable prior to type 1 diabetes onset and driven by specific genetic polymorphisms in a subset of autoantibody-positive subjects (60). Other genetic studies have also provided mechanistic clues. It has long been known that HLA genes drive a great deal of the genetic risk for type 1 diabetes and that much of the effect of HLA class II genotype on the risk for diabetes involves the initiation of autoimmunity; the hierarchal risk conferred by these genes for development of autoantibodies mimic the risk for type 1 diabetes. TrialNet data confirmed that the protection afforded by HLA-DRB1*15:01-DQA1*01:02-DQB1*06:02 is largely due to markedly less autoimmunity. However, protection from disease progression was also evident among autoantibody-positive relatives of patients with type 1 diabetes (21), and as previously noted, HLA has a role in risk of progression from one to multiple autoantibodies. It is unknown whether the mechanism by which HLA type influences autoimmunity is different than its role in further disease progression. A recent key observation demonstrates that much of the genetic risk for progression from autoimmunity to clinical disease may be due to non-HLA single nucleotide polymorphisms (61): how and why this is the case are unknown.
These mechanistic studies point toward new directions for targeted therapies to alter disease course. Yet, for each of these immune, metabolic, or β-cell measures, there is significant variance within the at-risk population, emphasizing mechanistic heterogeneity between individuals and thus the challenges to date in translating these findings to clinical trial. Moreover, as yet each mechanistic study has been conducted and analyzed independently; significant efforts are now being directed toward combining data from multiple assays together to determine if individuals cluster around specific mechanistic pathways (62). It is also expected that observations from natural history studies will complement mechanistic insights gained from intervention studies as described below.
Immune Therapies Can Alter Disease Course
In the past decade, TrialNet alone, or in cooperation with the National Institutes of Health Immune Tolerance Network (ITN), has conducted more than 11 clinical trials with the aim of altering the natural history of β-cell dysfunction/destruction in individuals recently diagnosed with type 1 diabetes. Four of these trials, with different putative mechanisms of action, have demonstrated a delay in the fall of β-cell function. Importantly, mechanistic studies from these trials have provided insights into changes in immune cell subsets that might be targeted in future therapies.
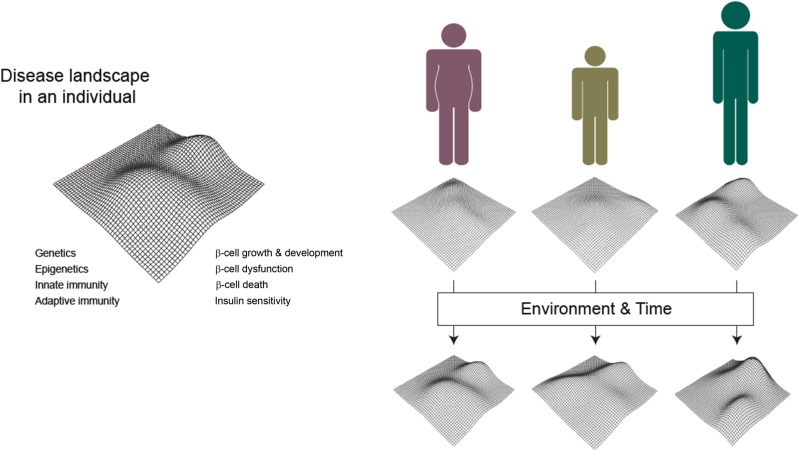
Among such insights, the TrialNet Abatacept Prevention Study was undertaken with the notion that the agent selectively binds to CD80 and CD86 blocking costimulation of T cells. Abatacept-treated patients with type 1 diabetes showed a statistically significant delay of approximately 9.6 months in the decline of C-peptide over the first 2 years of the disease. There was a reduction in the CD4+CD45RO+CD62L+ central memory (CD4+CM) T-cell population and a compensatory increase in naive CD4+ T cells, whereas in the placebo-treated group, an increase in CD4+CM T cells preceded β-cell functional decline (63). However, there was a broad range of metabolic and immunologic responses to the biologic therapy, with the greatest therapeutic benefits observed in younger subjects (i.e., those <18 years of age), consistent with the notion of heterogeneity in disease mechanisms and in therapeutic response/nonresponse among individual patients (Fig. 3). These efforts indicate that future studies need to address whether therapeutic targeting of specific CD4+ T-cell subsets will alter disease course in type 1 diabetes.
Figure 3.
Schematic of heterogeneity in clinical presentation and disease course. Left: Theoretical 3D landscape depicting the risk of type 1 diabetes in an individual (vertical dimension) driven by the complex relationships between both known and unknown factors, including genetics, immunity, and metabolic and β-cell parameters (horizontal dimensions). Right: Individual risk landscapes are modified by sources of variation such as age and BMI. Each individual’s risk continues to vary due to environmental exposures and the passage of time, increasing the overall heterogeneity of subject cohorts.
TrialNet’s The Effects of Rituximab on the Progression of Type 1 Diabetes in New Onset Subjects study provided a window into the potential role of B cells in type 1 diabetes. Failures of B-cell tolerance, both in central and peripheral checkpoints as well as in loss of anergic B cells, have been associated with progression to diabetes using samples from TrialNet studies (64,65). Rituximab treatment depletes circulating CD20+ B cells, with a recovery initially dominated by the appearance of transitional and naive B cells. In the TrialNet rituximab study, C-peptide in patients with type 1 diabetes was significantly improved at 1 year after study entry, but the effect was not maintained at the 2-year mark. Importantly, the B cells that arose after therapy were newly derived and, as before treatment, contained an increased percentage of autoreactive B cells relative to those in healthy control subjects (55). Thus, a single course of rituximab therapy did not durably modify the defect in B-cell central tolerance seen in type 1 diabetes. B-cell depletion did, however, prevent the development of new antibody responses to exogenous insulin. Primary and secondary responses were absent immediately after B-cell depletion when subjects were given PhiX or hepatitis A vaccines. Yet, response to vaccination recovered with B-cell repopulation, demonstrating that exposure to the antigen during B-cell depletion did not induce tolerance or lead to a permanent defect in response to vaccines (66). Compared with effects on insulin antibodies, the differences in titer of protective IgG mounted to prior vaccines and GAD65, IA-2, and ZnT8 autoantibodies were modest (67). With respect to T-cell responses, neither T-cell populations nor the proliferative response to control antigens changed with rituximab therapy as compared with placebo-treated individuals. However, subgroups of rituximab-treated individuals had an increased response to islet antigens and T-cell gene expression (68,69). These data suggest several possibilities involving interactions between T cells and recovering B cells after anti-CD20 monoclonal antibody treatment. Importantly, they also suggest that therapy with both B- and T-cell targeting agents could be synergistic.
The rituximab study also identified differences in responders that highlight the importance of considering heterogeneity between subjects in evaluating type 1 diabetes therapies. In a subgroup analysis, individuals <18 years of age (particularly those between 13 and 17 years) showed the most robust responses to the drug, as did those in whom HLADR3/DQ2 was absent. Those with intermediate levels of C-peptide (0.54 to 0.81 pmol/mL) showed better responses than those with higher or lower levels, despite findings from other settings in which those with the highest levels of C-peptide at baseline showed the most robust responses. Subjects with multiple autoantibodies at the time of entry also exhibited better responses than those with a single autoantibody, supporting a requirement for seropositivity in enrollment criteria for future B-cell directed trials at early stages of disease.
The AbATE (Autoimmunity-Blocking Antibody for Tolerance in Recently Diagnosed Type 1 Diabetes) trial of the non-FcR binding anti-CD3 monoclonal antibody teplizumab, also performed in collaboration between TrialNet and ITN, noted a significant improvement in C-peptide and a delay in its decline by 15.9 months (70). A post hoc analysis was used to identify responders based on a comparison with the control group in which all subjects lost at least 40% of their baseline C-peptide levels. With this, 45% of the drug-treated group were designated as responders. Long et al. (71) found that responders had an increased frequency of CD8+EOMES+TIGIT+KLRG1+ cells following drug treatment. These cells, with features of partially “exhausted” CD8+ T cells, showed induction of inhibitory molecules when activated in vitro, suggesting that teplizumab treatment had likely reprogrammed the cells. The observation that exhausted cells are important in disease progression and response to therapy is consistent with elegant studies by McKinney et al. (72) of chronic viral infections and other autoimmune diseases. Challenges remain in finding ways to augment and sustain these beneficial exhaustion phenotypes.
In a stage 4 pilot study, patients with established type 1 diabetes received antithymocyte globulin (ATG; thymoglobulin), a polyclonal immunodepleting agent, in combination with granulocyte-colony stimulating factor (G-CSF), which promotes hematopoietic mobilization (73,74). Combination therapy preserved C-peptide secretion and depleted CD4+ T cells with compensatory increases in CD19+ B-cell and CD8+ T-cell frequencies. Treatment also increased FOXP3+Helios+ Treg frequency. Importantly, these immunologic effects remained detectable 12–24 months following therapy. A lower dose ATG plus G-CSF trial is ongoing in patients with recent-onset disease to expand on these findings, with results anxiously expected.
The most recent effort to prevent or delay disease progression in those with stage 1 disease (multiple autoantibodies, normal glucose tolerance) via treatment with oral insulin proved to be ineffective in the primary study cohort. However, disease progression to stage 3 (clinical onset) was significantly delayed in a secondary cohort of individuals with normal glucose tolerance and low first-phase insulin response at the time of randomization (75). Efforts are ongoing to investigate the mechanisms underlying the apparent beneficial outcome seen by this latter group of study participants.
The Pathway Forward
TrialNet’s mission is to slow or even reverse disease progression by protecting β-cells through the course of disease. Advances in disease prediction and insights from mechanistic analyses hopefully will provide direction for future studies. Success in the mission, however, will require directly tackling disease heterogeneity. Each dimension associated with disease progression—demographic, clinical, genetic, metabolic, and immunologic—has varying contributions to β-cell dysfunction and death in a given group of individuals (Fig. 3). Thus, multidimensional data analysis is key to future studies. At the same time, existing analysis of natural history, clinical trial, and mechanistic data already supports next steps for trials.
Heterogeneity in therapeutic responses observed in past trials suggests that identification of potential responders prior to trial initiation might greatly improve the efficiency and safety of clinical studies. An important advance capitalizes on cross-trial data; given an individual’s baseline C-peptide value and their age, we can predict their expected C-peptide response (76) and define responders as those whose observed values are above expected values. This approach can be used to explore whether common mechanisms underlie responses to different therapies and whether there are common sets of immunologic measures at baseline that can predict response. A robust biomarker of the initial responses to treatment may enable the identification of likely responders at an early time point in the treatment course. In this way, a “test dose” of drug and prespecified analyses might enable investigators to avoid long-term treatment of individuals who are not likely to benefit from therapy. The robust and reproducible results from multiple trials can also allow for design and evaluation of single-arm studies to select those most promising for randomized trials.
Another primary consideration is the emerging ability to rationally design combination therapies. Mechanistic data from the four trials highlighted above suggest that the effects of single agents are not sufficient to avoid recurrence of disease. In the case of rituximab, T-cell responses were largely unaffected. With abatacept alone, changes in T-cell compartments were transient, even with continuous administration of the study drug. The failure to affect the autoreactive T-cell compartment with rituximab would suggest that combination with an agent that blocks T-cell responses (e.g., abatacept), potentially sequentially, might control resurgence of autoimmune responses when B lymphocytes recover. In addition, the analyses of T cells from anti-CD3 monoclonal antibody–treated subjects showed that changes induced in cellular subsets were not permanent and therefore suggest maintenance of the immunologic effects of that therapy will require either repeated dosing or use of another agent. Therefore, combinations that can inhibit recovery of autoreactive T-cell function, which may occur in response to inflammatory mediators or other cell growth factors, such as IL-7, form a potential combination to consider.
Concluding Thoughts
In summary, studies of the natural history of type 1 diabetes from TrialNet and other sources have identified disease heterogeneity in individuals based on demographic, clinical, metabolic, genetic, and immunologic factors. This heterogeneity initially made clinical trials more complicated as the disease course and responses to therapies are not uniform. At the same time, insights into the diversity of responses have provided an opportunity to develop new clinical studies that, moving forward, will combine therapies with synergistic mechanisms and select subjects who are most likely to respond to the interventions. With this information, an approach of precision medicine can be instituted that will improve efficacy and safety. In this way, development of treatments to prevent and stop progression of the disease can be hastened.
Article Information
Acknowledgments. The authors would like to thank the trial participants and their families, without whom the studies reviewed herein would not have been possible. They also thank Sam Skinner, Benaroya Research Institute, for illustrating Fig. 3. They acknowledge the significant contributions of Dr. Jay Skyler (University of Miami) to the operations of TrialNet.
Funding. The Type 1 Diabetes TrialNet Study Group is a clinical trials network currently funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085461, U01 DK085465, U01 DK085466, U01 DK085476, U01 DK085499, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, and UC4 DK106993, and JDRF.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or JDRF.
Duality of Interest. M.A.A. holds a patent on ATG plus G-CSF for treatment of type 1 diabetes. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010;464:1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson MA, Bluestone JA, Eisenbarth GS, et al. How does type 1 diabetes develop?: the notion of homicide or β-cell suicide revisited. Diabetes 2011;60:1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med 1986;314:1360–1368 [DOI] [PubMed] [Google Scholar]
- 5.Redondo MJ, Babu S, Zeidler A, et al.; Diabetes Prevention Trial Type 1 Study Group . Specific human leukocyte antigen DQ influence on expression of antiislet autoantibodies and progression to type 1 diabetes. J Clin Endocrinol Metab 2006;91:1705–1713 [DOI] [PubMed] [Google Scholar]
- 6.Greenbaum CJ, Buckingham B, Chase HP, Krischer J; Diabetes Prevention Trial-Type 1 Diabetes (DPT-1) Study Group . Metabolic tests to determine risk for type 1 diabetes in clinical trials. Diabetes Metab Res Rev 2011;27:584–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skyler JS, Greenbaum CJ, Lachin JM, et al.; Type 1 Diabetes TrialNet Study Group . Type 1 Diabetes TrialNet--an international collaborative clinical trials network. Ann N Y Acad Sci 2008;1150:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vehik K, Beam CA, Mahon JL, et al.; TrialNet Natural History Study Group . Development of autoantibodies in the TrialNet Natural History Study. Diabetes Care 2011;34:1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 1996;39:807–812 [DOI] [PubMed] [Google Scholar]
- 12.Barker JM, Barriga KJ, Yu L, et al.; Diabetes Autoimmunity Study in the Young . Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004;89:3896–3902 [DOI] [PubMed] [Google Scholar]
- 13.Hummel S, Ziegler AG. Early determinants of type 1 diabetes: experience from the BABYDIAB and BABYDIET studies. Am J Clin Nutr 2011;94(Suppl.):1821S–1823S [DOI] [PubMed] [Google Scholar]
- 14.Haller MJ, Schatz DA. The DIPP project: 20 years of discovery in type 1 diabetes. Pediatr Diabetes 2016;17(Suppl. 22):5–7 [DOI] [PubMed] [Google Scholar]
- 15.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci 2008;1150:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krischer JP, Lynch KF, Schatz DA, et al.; TEDDY Study Group . The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015;58:980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell-Thompson M. Organ donor specimens: What can they tell us about type 1 diabetes? Pediatr Diabetes 2015;16:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaddis JS, Pugliese A, Atkinson MA. A run on the biobank: what have we learned about type 1 diabetes from the nPOD tissue repository? Curr Opin Endocrinol Diabetes Obes 2015;22:290–295 [DOI] [PubMed] [Google Scholar]
- 19.Ziegler AG, Bonifacio E, Powers AC, Todd JA, Harrison LC, Atkinson MA. Type 1 diabetes prevention: a goal dependent on accepting a diagnosis of an asymptomatic disease. Diabetes 2016;65:3233–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sosenko JM, Skyler JS, Krischer JP, et al.; Diabetes Prevention Trial-Type 1 Study Group . Glucose excursions between states of glycemia with progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1 (DPT-1). Diabetes 2010;59:2386–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pugliese A, Boulware D, Yu L, et al.; Type 1 Diabetes TrialNet Study Group . HLA-DRB1*15:01-DQA1*01:02-DQB1*06:02 haplotype protects autoantibody-positive relatives from type 1 diabetes throughout the stages of disease progression. Diabetes 2016;65:1109–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bingley PJ, Boulware DC, Krischer JP; Type 1 Diabetes TrialNet Study Group . The implications of autoantibodies to a single islet antigen in relatives with normal glucose tolerance: development of other autoantibodies and progression to type 1 diabetes. Diabetologia 2016;59:542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meah FA, DiMeglio LA, Greenbaum CJ, et al.; Type 1 Diabetes TrialNet Study Group . The relationship between BMI and insulin resistance and progression from single to multiple autoantibody positivity and type 1 diabetes among TrialNet Pathway to Prevention participants. Diabetologia 2016;59:1186–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sosenko JM, Yu L, Skyler JS, et al. The use of electrochemiluminescence assays to predict autoantibody and glycemic progression toward type 1 diabetes in individuals with single autoantibodies. Diabetes Technol Ther 2017;19:183–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu P, Krischer JP; Type 1 Diabetes TrialNet Study Group . Prognostic classification factors associated with development of multiple autoantibodies, dysglycemia, and type 1 diabetes-a recursive partitioning analysis. Diabetes Care 2016;39:1036–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sims EK, Chaudhry Z, Watkins R, et al. Elevations in the fasting serum proinsulin-to-c-peptide ratio precede the onset of type 1 diabetes. Diabetes Care 2016;39:1519–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuomilehto J, Podar T, Tuomilehto-Wolf E, Virtala E. Evidence for importance of gender and birth cohort for risk of IDDM in offspring of IDDM parents. Diabetologia 1995;38:975–982 [DOI] [PubMed] [Google Scholar]
- 28.Harjutsalo V, Reunanen A, Tuomilehto J. Differential transmission of type 1 diabetes from diabetic fathers and mothers to their offspring. Diabetes 2006;55:1517–1524 [DOI] [PubMed] [Google Scholar]
- 29.Parkkola A, Härkönen T, Ryhänen SJ, Ilonen J, Knip M; Finnish Pediatric Diabetes Register . Extended family history of type 1 diabetes and phenotype and genotype of newly diagnosed children. Diabetes Care 2013;36:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS; DPT-1 Study Group . Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes 2010;59:679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenbaum CJ, Beam CA, Boulware D, et al.; Type 1 Diabetes TrialNet Study Group . Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes 2012;61:2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herold KC, Usmani-Brown S, Ghazi T, et al.; Type 1 Diabetes TrialNet Study Group . β cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest 2015;125:1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usmani-Brown S, Lebastchi J, Steck AK, Beam C, Herold KC, Ledizet M. Analysis of β-cell death in type 1 diabetes by droplet digital PCR. Endocrinology 2014;155:3694–3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu L, Boulware DC, Beam CA, et al.; Type 1 Diabetes TrialNet Study Group . Zinc transporter-8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care 2012;35:1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verge CF, Stenger D, Bonifacio E, et al. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: Combinatorial Islet Autoantibody Workshop. Diabetes 1998;47:1857–1866 [DOI] [PubMed] [Google Scholar]
- 36.Krischer JP, Cuthbertson DD, Yu L, et al. Screening strategies for the identification of multiple antibody-positive relatives of individuals with type 1 diabetes. J Clin Endocrinol Metab 2003;88:103–108 [DOI] [PubMed] [Google Scholar]
- 37.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl. 1):S81–S90 [DOI] [PubMed] [Google Scholar]
- 38.Oram RA, Jones AG, Besser RE, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014;57:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis AK, DuBose SN, Haller MJ, et al.; T1D Exchange Clinic Network . Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care 2015;38:476–481 [DOI] [PubMed] [Google Scholar]
- 40.Steck AK, Vehik K, Bonifacio E, et al.; TEDDY Study Group . Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015;38:808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ilonen J, Lempainen J, Hammais A, et al.; Finnish Pediatric Diabetes Register. Primary islet autoantibody at initial seroconversion and autoantibodies at diagnosis of type 1 diabetes as markers of disease heterogeneity. Pediatr Diabetes 2018;19:284–292 [DOI] [PubMed] [Google Scholar]
- 42.Steck AK, Johnson K, Barriga KJ, et al. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: Diabetes Autoimmunity Study in the Young. Diabetes Care 2011;34:1397–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hao W, Wookwyk A, Beam C, Bahnson T, Palmer JP, Greenbaum CJ. Assessment of beta cell mass and function by AIRmax and IVGTT in high risk subjects for type 1 diabetes. J Clin Endocrinol Metab 2017;102:4428–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 2016;65:719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diedisheim M, Mallone R, Boitard C, Larger E. Beta-cell mass in non-diabetic autoantibody-positive subjects: an analysis based on the Network for Pancreatic Organ Donors database. J Clin Endocrinol Metab 2016;101:1390–1397 [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Calvo T, Zapardiel-Gonzalo J, Amirian N, et al. Increase in pancreatic proinsulin and preservation of β-cell mass in autoantibody-positive donors prior to type 1 diabetes onset. Diabetes 2017;66:1334–1345 [DOI] [PMC free article] [PubMed]
- 47.Wasserfall C, Nick HS, Campbell-Thompson M, et al. Persistence of pancreatic insulin mRNA expression and proinsulin protein in type 1 diabetes pancreata. Cell Metab 2017;26:568–575.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziegler AG, Bonifacio E; BABYDIAB-BABYDIET Study Group . Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia 2012;55:1937–1943 [DOI] [PubMed] [Google Scholar]
- 49.Vehik K, Haller MJ, Beam CA, et al.; DPT-1 Study Group . Islet autoantibody seroconversion in the DPT-1 study: justification for repeat screening throughout childhood. Diabetes Care 2011;34:358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bosi E, Boulware DC, Becker DJ, et al.; Type 1 Diabetes TrialNet Study Group . Impact of age and antibody type on progression from single to multiple autoantibodies in type 1 diabetes relatives. J Clin Endocrinol Metab 2017;102:2881–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giannopoulou EZ, Winkler C, Chmiel R, et al. Islet autoantibody phenotypes and incidence in children at increased risk for type 1 diabetes. Diabetologia 2015;58:2317–2323 [DOI] [PubMed] [Google Scholar]
- 52.Snowhite IV, Allende G, Sosenko J, Pastori RL, Messinger Cayetano S, Pugliese A. Association of serum microRNAs with islet autoimmunity, disease progression and metabolic impairment in relatives at risk of type 1 diabetes. Diabetologia 2017;60:1409–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alkanani AK, Rewers M, Dong F, Waugh K, Gottlieb PA, Zipris D. Dysregulated Toll-like receptor-induced interleukin-1β and interleukin-6 responses in subjects at risk for the development of type 1 diabetes. Diabetes 2012;61:2525–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith MJ, Packard TA, O’Neill SK, et al. Loss of anergic B cells in prediabetic and new-onset type 1 diabetic patients. Diabetes 2015;64:1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chamberlain N, Massad C, Oe T, Cantaert T, Herold KC, Meffre E. Rituximab does not reset defective early B cell tolerance checkpoints. J Clin Invest 2016;126:282–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rouxel O, Da Silva J, Beaudoin L, et al. Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat Immunol 2017;18:1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arif S, Leete P, Nguyen V, et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes 2014;63:3835–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gutierrez GD, Gromada J, Sussel L. Heterogeneity of the pancreatic beta cell. Front Genet 2017;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rui J, Deng S, Arazi A, Perdigoto AL, Liu Z, Herold KC. β cells that resist immunological attack develop during progression of autoimmune diabetes in NOD mice. Cell Metab 2017;25:727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marwaha AK, Panagiotopoulos C, Biggs CM, et al. Pre-diagnostic genotyping identifies T1D subjects with impaired Treg IL-2 signaling and an elevated proportion of FOXP3+IL-17+ cells. Genes Immun 2017;18:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steck AK, Xu P, Geyer S, et al.; Type 1 Diabetes TrialNet Study Group . Can Non-HLA single nucleotide polymorphisms help stratify risk in TrialNet relatives at risk for type 1 diabetes? J Clin Endocrinol Metab 2017;102:2873–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Battaglia M, Anderson MS, Buckner JH, et al. Understanding and preventing type 1 diabetes through the unique working model of TrialNet [published correction appears in Diabetologia 2017;60:2540]. Diabetologia 2017;60:2139–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orban T, Beam CA, Xu P, et al.; Type 1 Diabetes TrialNet Abatacept Study Group . Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline. Diabetes 2014;63:3449–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Habib T, Funk A, Rieck M, et al. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J Immunol 2012;188:487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menard L, Saadoun D, Isnardi I, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest 2011;121:3635–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pescovitz MD, Torgerson TR, Ochs HD, et al.; Type 1 Diabetes TrialNet Study Group . Effect of rituximab on human in vivo antibody immune responses. J Allergy Clin Immunol 2011;128:1295–1302.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu L, Herold K, Krause-Steinrauf H, et al.; Type 1 Diabetes TrialNet Anti-CD20 Study Group . Rituximab selectively suppresses specific islet antibodies. Diabetes 2011;60:2560–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herold KC, Pescovitz MD, McGee P, et al.; Type 1 Diabetes TrialNet Anti-CD20 Study Group . Increased T cell proliferative responses to islet antigens identify clinical responders to anti-CD20 monoclonal antibody (rituximab) therapy in type 1 diabetes. J Immunol 2011;187:1998–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Linsley P, Greenbaum C, Rosasco M, et al. Cell levels in peripheral blood predict poor clinical response following rituximab treatment in new-onset type 1 diabetes. Genes Immun. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herold KC, Gitelman SE, Ehlers MR, et al.; AbATE Study Team . Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 2013;62:3766–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long SA, Thorpe J, DeBerg HA, et al. Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Sci Immunol 2016;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 2015;523:612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haller MJ, Gitelman SE, Gottlieb PA, et al. Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest 2015;125:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haller MJ, Gitelman SE, Gottlieb PA, et al. Antithymocyte globulin plus G-CSF combination therapy leads to sustained immunomodulatory and metabolic effects in a subset of responders with established type 1 diabetes. Diabetes 2016;65:3765–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ; Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group . Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 2017;318:1891–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bundy BN, Krischer JP; Type 1 Diabetes TrialNet Study Group . A model-based approach to sample size estimation in recent onset type 1 diabetes. Diabetes Metab Res Rev 2016;32:827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]