Abstract
Calcifying Tendinitis (CT) shoulder a self limiting disorder characterized by deposition of calcium salts in rotator cuff muscles. The main symptom being pain followed by activity restriction resolving on its own in most cases. Symptomatic patients are initially managed by NSAIDs, Physiotherapy, Corticosteroid injections. ESWT involves acoustic waves causing fragmentation of deposits with pain releif. Ultrasound guided needling barbotage have shown promising results. Arthroscopic excision remains the definitive management for patients associated with complications as cuff tear and for uncomplicated patients. In calcifying tendinitis the initial evaluation, maintenance of function and appropriate choice of treatment modalities determines the prognosis
Keywords: Calcifying tendinitis, Natural history, Etiopathogenesis, Extracorporeal shock wave therapy, USG therapy, Surgical Resection
1. Introduction
Calcifying Tendinitis (CT) Shoulder a self limiting disorder of shoulder characterized by deposition of calcium salts in rotator cuff muscles. Names synonymous are Calcific periarthritis, Calcifying Tendinitis (CT) Shoulder a self limiting disorder of shoulder characterized by deposition of calcium salts in rotator cuff muscles. Names synonymous are Calcific periarthritis1. The etiology still remains unclear with many proposed theories of etiopathogenesis. The presenting symptom often is pain associated with activity persisting for months with spontaneous regression in most of the cases. Some have persistent pain & edema requiring active intervention. This article focus about the overview of calcifying tendinitis of shoulder and multiple management options for symptomatic cases.
2. History and demographics
Calcifying tendinitis was first described by Duplay in 1872 as painful periarthritis of the shoulder.2 In 1934 Codman described the calcification occuring in tendons instead of Subacromial bursa as thought earlier.3 In 1952 Plenk coined the term Calcifying Tendinitis.4
In a series of 6061 asymptomatic patients Bosworth et al. reported an incidence of 2.7%.1 Documented incidence by different authors varies from 2.7 to 22% more in women compared to men.1,5 Bilateral incidence in about 10–20% of cases.6 The common age group affected is between 30–50 years.5,7 It commonly involves Supraspinatus(80%) Tendon followed by Infraspinatus rarely affecting Teres minor and Subscapularis.
2.1. Natural history of disease
The Calcifying tendinitis is hypothesized to occur in following stages8:
-
(I)
Precalcific Stage
-
(II)ICalcific Stage
-
a)Formative Phase b) Resting Phase c) Resorptive Phase.
-
a)
-
(III)
Repair Stage
The deposits of Calcium are amorphous to semisolid in texture. The deposits consist of Calcium carbonate hydroxyapatite identified by means of Spectroscopy and X-ray diffraction techniques.9 The hydroxyapatite salts consists of two forms Type A and Type B. Chiou et al. stated that the proportion of Type B hydroxyapatite increases with decrease in Type A during the process of progressive calcification.9 Based on USG findings deposits are classified into following morphological shapes and their correlation with clinical features are described as follows.9
Formative Phase associated with arc or fragmented/punctuate deposits and mild pain; Resting phase with nodular deposits associated with moderate to severe pain. Resorptive phase with cystic deposits associated with severe pain.
2.2. Complications
The progression of natural course of untreated disease leads to following complications10
Adhesive Capsulitis
Rotator cuff tear
Greater Tuberosity osteolysis
Osssifying Tendinitis
2.3. Etiology and pathogenesis
2.3.1. Etio pathogenesis
Etiopathogenesis of CT remains a debatable topic with multiple theories proposed. Two broad groups of theories attempt to explain CT. A group proposing Degenerative changes or minor trauma of the tendons predisposes to Calcification which is basically Dystropic type of calcification. Sandstorm proposed that Vascular ischemia of tendons leading to tendon necrosis that promotes dystropic Calcification.11
Bishop and Bosworth individually came out with a different theory of repetitive trauma of tendons that in-turn leads to tendon degeneration followed by calcification.1,12
Mohr again emphasized the theory of tendon necrosis predisposing to intracellular calcium accumulation as micro spheroliths and Psammomas.13
Other group of theories describes the process as an active process mediated by chondrocytes that arise from metaplasia which inturn causes calcium deposition in the Matrix.
Uhtoff identified that cartilage metaplasia of tendons predisposing f
or calcification of tendons as an active cell mediated process.14
Benjamin also proposed that cartilage metaplasia leading to Enchondral ossification of fibrocartilage.15
Rui came out with new theory proposing erroneous differentiation of tendon-derived stem cells (TDSCs) leading to chondral metaplasia.16
Recent theories involving role of BMP-2, BMP-4 and BMP-7 in metaplasia of tendon cells leading to calcification are also proposed.17
In a cadaveric study18 by Riley et al. the chemical composition of deposits found to have amorphous calcium phosphate and Hydroxyapatite predominantly unlike degenerative tendon which contains many forms of calcium salts including calcium triphosphate, pyrophosphate, carbonate and Hydroxyapatite which is predominantly ‘Dystrophic calcification’. Degenerate tendons have increased Type III collagen but in CT no significant increase in these concentrations. Resorption of Calcific deposits is evaluated and involvement of Multinucleated Giant cell has been identified.19 TRAP positive Giant cells contain Cathepsin K which confirms the Osteoclastic lineage and its involvement in Resorption.19,20
2.4. Clinical features
Clinical manifestations include pain in shoulder with or without restriction of movements. Symptoms commonly resolve on its own, except for some cases where they persist. Bosworth described resolution rate of 6.4% of deposits per year, with 9.3% of deposits resolving within 3 years.1,21 Wolk and Wittenberg described resolution of calcification and symptoms in about 70% of the patients within a period of 49 months with spontaneous resolution of 82% within 8.6 years22. Benno et al. in study of 63 patients described association of calcifying tendinitis with renal lithiasis in 33% of individuals in comparison with 9% in control group.23
Pain being the major clinical symptom Neer described different causes of pain occurring Calcifying tendinitis24 (Table 1).
Table 1.
Causes of Pain occurring in calcifying tendinitis.
| Causes of Pain | |
|---|---|
| 1. | Calcium causing chemical irritation of tissues |
| 2. | Tissue edema causing pressure |
| 3. | Bursal thickening due to irritation causing impingement |
| 4. | Pain caused by chronic stiffening of Glenohumeral joint |
The clinical features are documented by several scoring systems among which a commonly used scoring system includes Constant Murley score with Total 100 points distributed as
Subjective : Pain-15 points ; Ability to perform ADLS- 20 points
Objective : ROM- 40 points ; Muscle power- 25 points
Strength is measured in 90 ° abducted arm with 30 ° flexion with extended elbow.
3. Imaging evaluation
3.1. Xray
Radiographic evaluation of shoulder is done by 2 views mainly
-
•
Rockwood View: It is a true AP view with 30 ° caudal tilt focusing on subacromial space.
-
•
Shoulder Outlet View
The commonly used radiographic classifications for CT are Gartner Hayer classification and SFA classification as in Table 2.
Table 2.
Radiologic classifications of Calcifying Tendinitis.
| Gartner and Hayer25 | Type I | Dense calcifications with well defined border |
| Type II | Dense with Indefinite borders | |
| Type III | Transparent with indistinct border | |
| SFA Classification (French Society of Arthroscopy)26 | Type A | Dense, well Defined, Circumscribed |
| Type B | Dense, Well Defined,Segmented | |
| Type C | Transparent and nonhomogenous | |
| Type D | Dystropic deposit at tendon origin. |
The locations of deposits are described based on True AP and shoulder outlet view using Quadrant technique.6 In AP view (Fig. 1A) a perpendicular reference line drawn from Lateral border of Acromion the distance between medial border of the deposit and the reference line measured in millimeters and noted with minus if it is medial and noted with plus if it is lateral to the line.
Fig. 1.
(A) True AP view (B) Outlet view of Shoulder for Localization of Deposits.
In outlet view (Fig. 1B) 5 sectors are defined Sector 0 anterior to the Anterior border of acromion, Length of Acromion divided into 3 sectors from anterior to posterior as Sectors 1–3, Sector 4 lies posterior to the Posterior border of acromion.
The radiographic volume of the deposits determined by the product of Length (l), Breadth (b) Obtained from AP view, and depth (d) from outlet view.
Radiographic volume (V) = l*b*d
3.2. Ultrasonogram
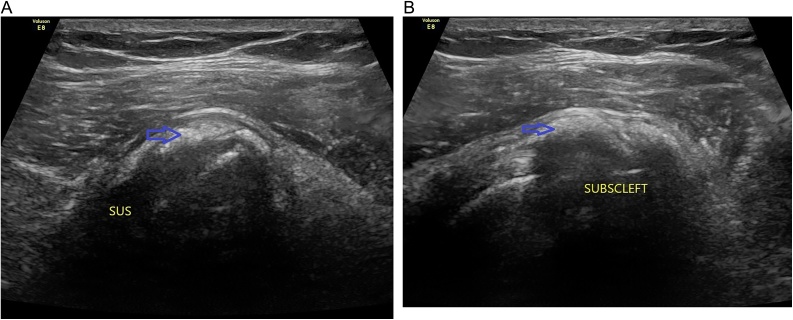
Standard USG imaging using longitudinal and transverse views determines size, localization, echogenicity of the deposits (Fig. 2). Based on these characteristics Farin and Jaroma27 classified them as in Table 3.
Fig. 2.
Ultrasonogram images of calcification deposits in (A) Supraspinatus tendon (B) Subscapularis tendon.
Table 3.
Ultrasound imaging and Findings.
| Standard USG | HRUS | CDUS |
|---|---|---|
| Type I – Large deposits or Deposits in bursa & cuff | (i) Arc shaped | Grade 0 (no color flow signal) |
| Type II – Several small Scattered calcifications | (ii) Punctuate or fragmented | Grade 1 (<3 color spots or a short line) |
| Type III – Few small deposits | (iii) Nodular | Grade 2 (3–6 color spots or short lines) |
| (iv) Cystic. | Grade 3(>6 color spots or color lines) |
High Resolution Ultrasound (HRUS) is useful in determining the calcification, morphology and presence of associated Rotator cuff tear. HRUS with color Doppler is more descriptive about the calcification and their vascularity. Chiou et al.28 classified the deposits based on findings on HRUS into 4 types as in Table 3.
The non arc shaped deposits described by HRUS are usually associated with Resorptive phase.28
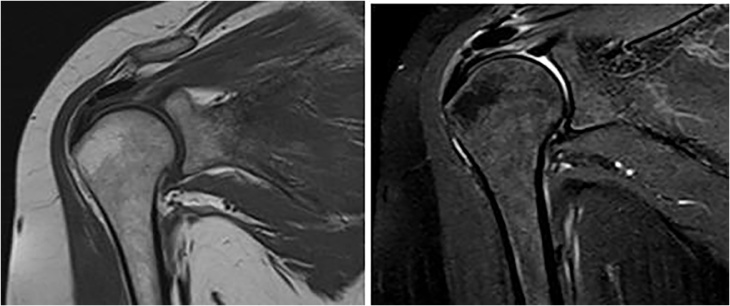
3.3. MRI imaging
It plays an important role in identification and localization of calcific deposits within the cuff, surrounding edema, associated pathologies of rotator cuff as complete or partial tears, presence of sub acromial bursitis (Fig. 3).
Fig. 3.
MRI images of Calcifying tendinitis.
4. Management
4.1. Conservative treatment
The initial line of management of Calcifying Tendinitis is Non operative. It includes symptomatic management using systematic NSAIDs in acute phase, physical therapy using cold or heat & manual therapy with exercises improving Range of movements. In a study of 125 patients Noel observed good clinical results in 50.4% with non operative treatment at the end of 6 months.29 Wolk and Wittenberg described in their study about 70% patients turned asymptomatic after a mean period 49 months.22 Ogon et al. in a study of 420 patients with 488 shoulders analyzed the prognostic factors (Table 4) in calcifying tendnitis with non operative treatment.6 He described failure of Non operative treatment for a period of 6 months in 27% with mean duration of symptoms 3.9years.
Table 4.
Predictive factors of outcome in calcifying tendinitis.
| Positive Factors | Negative Factors |
|---|---|
| Gartner Type III Deposits | Anterior Subacromial localisaiton |
| Lack of sonographic sound extinction | Large size deposits (>1500 mm3) |
| Medial localization of deposits | |
| Bilateral occurrence of deposits |
Deposits that are multifocal don’t have a statistically significant prognostic value. Therapeutic effect of administration of local steroids is debatable without enough studies supporting them.
4.2. Extracorporeal shock wave therapy
It involves treatment of calcific deposits using Acoustic waves generated from Peizoelectric, Electrohydraulic and Electromagnetic devices30, 31, 32, 33 The Flux density of waves are measured by the energy delivered per square area (mJ/mm2).
The waves are graded based on Flux Density into 3 Groups31
-
(i)
Low energy < 0.08 mJ/mm2 (ii) Medium Energy 0.08-0.28 mJ/mm2 (iii) High Energy > 0.28 mJ/mm2
Low Energy and High energy waves are used in treatment of calcifying tendinitis. ESWT involves 3 mechanisms in treating CT as described by Loew et al.32
-
(1)
Fragmentation of deposits by mechanical impact (2) Phagocytosis of deposits with molecular effect (3) Denervation of pain with analgesic effect.
4.2.1. Technique30,33
Patient in Supine position with Adducted arm Calcific deposits are localized under fluoroscopy and gel applied to the skin across which the shock waves are administered. ESWT is given with 1500–2500 impulses per session in 2 sessions 12–16 days apart. During therapy first 200 impulses administered at 1 HZ followed by impulses at 2 HZ frequency.
Vavken et al.34 in 2009 in one of the largest Meta analysis of Calcifying tendinitis involving 14 studies including 995 patients evaluated in terms of Constant-Murley score (CMS), Visual Analogue scale (VAS) and radiographic evaluation. Results shows better outcome as pain scores and resolution among High Energy group compared other non-operative treatment modalities. High Energy group have superior outcomes in pain reduction and CMS compared with Low energy group (P < 0.001).
The local skin changes and pain during the procedure remains the limiting factor in High energy ESWT compared with other 2 groups.
Verstraelen et al.35 in a meta-analysis of 5 RCTs with 359 participants compares High Energy ESWT Vs Low energy ESWT and found out significant improvement in CMS with High energy group 25.82 compared with 15.94 in low energy group. Rate of resorption of calcific deposits with high energy group is higher by 10%–35 % than Low Energy group. Disadvantages of High Energy group are being painful requiring anaesthesia, need to be performed on inpatient basis, expensive and local skin changes.
Bionka et al.36 in a systematic review of 11 RCTs with Calcific tendinitis found High energy ESWT gives superior results compared to low energy in short term follow-up. High energy ESWT also have better results against placebo in short, middle and long term follow-up. Moreover, High energy ESWT was more effective (moderate evidence) with focus on calcific deposit versus focus on tuberculum major in short- and long-term. Multiple randomized control trials conducted showing good results with ESWT (Table 5).
Table 5.
Studies involving ESWT in management of calcifying tendinitis.
| Author and year | Study | No of patients | OUtcome measure | Results |
|---|---|---|---|---|
| Albert et al.30 | RCT High energy ESWT vs low energy ESWT | 80 | Constant Murley score | Mean CMS improvement for ESWT High Energy – 27.3 Low Energy – 11.3 |
| Gerdesmeyer et al.33 | Double blinded RCT13 HIGH Energy ESWT vs Low Energy vs Sham treatment | 144 | Constant Murley score | Mean CMS improvement for ESWT High Energy – 31.6 Low Energy – 17.7 Sham Treatment - 13.7 |
| Ioppolo et al. 37 | RCT High Energy(0.2 /mm2) ESWT vs Low Energy (0.1/mm2)ESWT | 46 | Constant Murley score, Visual Analogue Scale | Mean CMS improvement for ESWT High Energy – 30.17 (61%) Low Energy- 10.21 (21%) |
Extracorporeal Shock wave therapy being non invasive method with good functional outcome is a potential management option with early recovery. The High energy ESWT capable of showing better results in short term and Long term basis compared with Low energy. Early recovery, as patients are not on any functional restrictions during the therapy. ESWT is Cost effective by 5–7 times compared with surgery.38 Study by Rompe et al. showed superior results with surgery compared to ESWT.39
4.3. Ultrasound guided needling barbotage
Barbotage involves image guided irrigation of deposits followed by aspiration. It is minimally invasive, cost effective, procedure that can be done in outpatient basis.
4.3.1. Technique40
Patient made to sit with hand behind the back and for needling of subscapularis or patient is made to lie prone with arm abducted and externally rotated. Procedure done using 18, 20 G needle with syringe filled with lidocaine.
Deposits are identified with USG and entry made into the deposit after anaesthetizing the pathway. Some amount of lidocaine injected into it and the calcium dissolved re-enters syringe passively. Similarly repeated injections done until removal of deposits. Further procedure continued with saline. The puncture and aspiration can be done with 2 different needles.
The criteria41 for correct placement of needle within the deposits include
-
1
Aspiration of calcium particles in syringe 2. Calcium deposits in lumen of needle 3. Decreased flow due to needle block with calcium
Krasny et al.41 in a randomized control trial including 80 patients compared ESWT with USG guided needling (Group I) Vs ESWT (Group II). The clinical results showed Group I patients having better outcome with 62.5% of patients showing excellent results in comparison with 32.5% of Group II patients. Constant score improvement of 30.5 points and resolution of deposits in 60% of Group I patients while 32.5% of Group II patients. 26 patients of 80(32.5%) does not show clinical improvement and underwent surgery after mean 4.1 months follow up. It includes 8 from Group I and 18 from Group II. The study also showed that there is no significant correlation between resolution of deposits and clinical improvement.
4.4. Surgical removal
The Calcium deposits are surgically removed only if the conservative treatment fails due to the self limiting nature of the disease. This can be carried out by open surgery or Arthroscopy. Arthroscopy being less aggressive on tissues is associated with less surgical morbity and early recovery. There are number of studies reporting excellent results with arthroscopic excision of deposits.42, 43, 44, 45
Ogon et al. described the negative prognostic factors that may require early surgical intervention.6
4.4.1. Technique42, 43, 44
The procedure is carried out with patient lying down in a beach chair position at edge of the table. Standard posterior portal is established and glenohumeral joint evaluation done. The site of calcification identified by hypervascularity of cuff tendon. A needle is inserted into the calcified cuff under scopy guidance and localisation of calcification done by observing calcium in needle tip. The site is marked with sutures. Lateral portal established and Sub acromial space identified. Multiple punctures done on area of calcification and calcium release in sub acromial space identified. bursectomy done. Debridement carried out using Soft tissue shaver and calcareous material aspirated. Peroperative fluroscopy can be done to confirm extent of removal of deposits. The resulting defect of the tendon is repaired end to end or end to bone insertion.
The sub acromial decompression is done only in patient with signs of impingement. Richard et al.45 in a study including 50 patients described that routine use of Subacromial decompression is not beneficial and it delays the recovery and return to work. The affected arm immobilized in arm sling for 3–5 days followed by rehabilitation and exercises to improve Range of movements initiated. Patients are reviewed periodically evaluated using functional score.
Enrico R et al. in a comparative study44 of 50 patients treated with Arthroscopy and Low energy ESWT reported Excellent results in 81.8% Patients treated with Arthroscopy and 70.83% of patients treated with low ESWT with complete or partial resolution of the deposits. Patients associated with fair or poor results had no evidence of resolution of deposits. Arthroscopy group patients returned to work after a mean 8 ± 3 weeks while ESWT group patients continued working during the course of treatment. Many studies have shown promising results with surgical excision of calcification (Table 6).
Table 6.
Studies with results of Surgical treatment of calcifying tendinitis.
| Author and year | Study | No of patients | Outcome measure | Results |
|---|---|---|---|---|
| Neto et al.42 | Retrospective study | 14 | University of California Los Angeles UCLA scale |
Mean UCLA Preoperative - 20.9 points Postoperative – 33.9 points Excellent Results- 71.4% Good Results- 28.5% Poor results – 7% |
| Ranaletta et al.43 | Retrospective study Arthroscopy vs Conservative measures | 26 | Constant Murley score UCLA scale Visual Analogue Scale |
Mean CMS improvement – 61.4 UCLA scale improvement- 16.4 VAS decrease- 7.9 DASH score decrease- 59.4 Post operative frozen shoulder – 2 patients 7.6% |
| Enrico et al.44 | Arthroscopic Excision vs Low energy ESWT | 46 Arthroscopy- 22 ESWT – 24 |
UCLA scale | Mean UCLA improvement for arthroscopy 20.94 points Excellent to good results- 81.81% Low energy ESWT- 15.75 points Excellent to good results- 70.83% Statistically no significant difference. |
| Alessandro et al.46 | Prospective comparative Study of Needling with Arthroscopic lavage vs Arthroscopic excision |
40 patients Needling with Lavage - 28 patients Arthroscopic removal- 12patients |
Constant Murley score UCLA scale Visual Analogue Scale |
Needling with Lavage Mean CMS improvement – 33.2 UCLA scale improvement- 12.7 VAS decrease- 5.4 points Arthroscopic excision Mean CMS improvement – 34 points UCLA scale improvement- 11.5 points VAS decrease- 5.5 points |
Wittenberg et al.22 in a study of 100 patients treated with Surgery and conservative measures reported better outcome in patients treated surgery with Patte Score 90.9 in surgery group compared to 81 in conservative Group. This study also shows that surgery group of patients have early recovery within 5 months average for ADLS with less frequency of partial or complete tears in contrast with 20 months in Conservative group. Studies comparing Needling & Arthoscopic lavage Vs Arthroscopic removal42 shows both having better similar functional outcome and early recovery.
The complete removal of calcium deposits is debatable as some studies show better outcome with complete removal.
Newer modalities such as Ultrasound therapy,4 Radial shock wave therapy47 have shown positive results but they have to be studied further for their efficacy.
5. Summary
Calcifying tendinitis of shoulder is usually asymptomatic condition which requires understanding of the disease nature for management. Initially can be treated by conservative measures due to self limiting and resolving nature of the disease. Specific prognostic factors to be identified at an early stage to decide about the course of disease progression and active management. This decreases the agony and complications. With multiple options in hand the management to be decided as per individual patient and associated complications. USG Guided needling done as a day care procedure shows better outcomes compared to sham treatment. But limited literature comparing them with surgical management makes it difficult to comment on their preference over surgery.
Both ESWT and surgery have shown promising results in many studies. Surgery being the definitive procedure have proven to have good outcomes especially in patients with associated Rotator cuff tears which can be addressed simultaneously. The role of Subacromial decompression is controversial and indicated in patients with symptoms of impingement as they delay the recovery. Surgery seem to decrease the duration of symptoms considerably if intervened at appropriate timing. Studies comparing surgery and ESWT have shown variable results. ESWT being non invasive, cost effective procedure with similar outcomes as surgery, especially in uncomplicated cases. The important advantage over surgery is time to return to work significantly less compared to surgery. It can be used in carefully selected patients.
In calcifying tendinitis the initial evaluation, maintanence of function and appropriate choice of treatment modalities determines the prognosis.
Conflict of interest
None.
Contributor Information
Balaji Umamahesvaran, Email: baluddoc@gmail.com.
Senthil Nathan Sambandam, Email: sambandamortho@gmail.com.
Varatharaj Mounasamy, Email: vmounasa@yahoo.com.
Ponnusami Pillai Gokulakrishnan, Email: pgokulakrishnan24@gmail.com.
Munis Ashraf, Email: munis6@gmail.com.
References
- 1.Bosworth B.M. Calcium deposits in the shoulder and subacromial bursitis: a survey of 12,122 shoulders. JAMA. 1941;116(22):2477–2482. [Google Scholar]
- 2.Duplay S. De la peri-arthrite scapulo-humerale et des raideurs de l'epaule qui en sont la consequence. Arch Gen Med. 1872;20:513–542. [Google Scholar]
- 3.Codman E.A. Thomas Todd Co; Boston: 1934. The shoulder: rupture of the supraspinatus tendon and other lesions in or about the subacromial bursa. [Google Scholar]
- 4.Ebenbichler G.R., Erdogmus C.B., Resch K.L. Ultrasound therapy For calcific tendinitis of the shoulder ultrasound therapy For calcific tendinitis of the shoulder. N Engl J Med. 1999;340(May (20)):1533–1538. doi: 10.1056/NEJM199905203402002. [DOI] [PubMed] [Google Scholar]
- 5.DePalma A.F., Kruper J.S. Long term study of shoulder joints afflicted and treated for calcific tendinitis. Clin Orthop. 1961;20:61–72. PMID: 13721957 [PubMed] [PubMed] [Google Scholar]
- 6.Ogon P., Suedkamp N.P., Jaeger M., Izadpanah K., Koestler W., Maier D. Prognostic factors in nonoperative therapy for chronic symptomatic calcific tendinitis of the shoulder. Arthritis Rheum. 2009;60(10):2978–2984. doi: 10.1002/art.24845. [DOI] [PubMed] [Google Scholar]
- 7.Welfling J., Kahn M.F., Desroy M., Paolaggi J.B., de Sèze S. Calcifications of the shoulder. II. The disease of multiple tendinous calcifications. Rev Rhum Mal Osteoartic. 1965;32(6):325–334. [PubMed] [PubMed] [Google Scholar]
- 8.Hamada J., Tamai K., Ono W., Saotome K. Does the nature of deposited basic calcium phosphate crystals determine clinical course in calcific periarthritis of the shoulder? J Rheumathol. 2006;33:326–332. [PubMed] [Google Scholar]
- 9.Chiou H.J., Hung S.C., in S.Y., Wei Y.S., Li M.J. Correlations among mineral components, progressive calcification process and clinical symptoms of calcific tendonitis. Rheumatology. 2010;49:548–555. doi: 10.1093/rheumatology/kep359. CrossRefPubMed. [DOI] [PubMed] [Google Scholar]
- 10.Merolla G., Bhat M.G., Paladini P., Porcellini G. Complications of calcific tendinitis of the shoulder: a concise review. J Orthop Traumatol. 2015;16(3):175–183. doi: 10.1007/s10195-015-0339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandstrom C. Peridentinis calcarea. A common disease of middle life. Its diagnosis pathology and treatment. AJR. 1938;40:1–21. [Google Scholar]
- 12.Bishop W.A. Calcification of the supraspinatus tendon: cause, pathologic picture and relation to the scalenus anticus syndrome. Arch Surg. 1939;39:231–246. [Google Scholar]
- 13.Mohr W., Bilger S. Morphologische Grundstrukturen der kalzifizierten Tendopathie und ihre Bedeutung fur die Pathogenese. Z Rheumatol. 1990;49:346–355. PubMed. [PubMed] [Google Scholar]
- 14.Uhthoff H.K., Loehr J.W. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis and management. J AM Acad Orthop Surg. 1997;5:183–191. doi: 10.5435/00124635-199707000-00001. PubMed. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin M., Rufai A., Ralphs J.R. The mechanism of formation of bonny spurs (enthesophytes) in the Achilles tendon. Arthritis Rheum. 2000;43:576–583. doi: 10.1002/1529-0131(200003)43:3<576::AID-ANR14>3.0.CO;2-A. CrossRef. [DOI] [PubMed] [Google Scholar]
- 16.Rui Y.F., Lui P.P.Y., Chan L.S., Chan K.M., Fu S.C., Li G. Does erroneous differentiation of tendon-derived stem cells contribute to the pathogenesis of calcifying tendinopathy? Chin Med J. 2011;124(4):606–610. [PubMed] [Google Scholar]
- 17.Lui P.P. Histopathological changes in tendinopathy—potential roles of BMPs? Rheumatology. 2013;52(12):2116–2126. doi: 10.1093/rheumatology/ket165. [DOI] [PubMed] [Google Scholar]
- 18.Riley G.P., Harrall R.L., Constant C.R., Cawston T.E., Hazleman B.L. Prevalence and possible pathological significance of calcium phosphate salt accumulation in tendon matrix degeneration. Ann Rheum Dis. 1996;55:109–115. doi: 10.1136/ard.55.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakase T., Takeuchi E., Sugamoto K. Involvement of multinucleated giant cells synthesizing cathepsin K in calcified tendinitis of the rotator cuff tendons. Rheumatology (Oxford, England) 2000;39(10):1074–1077. doi: 10.1093/rheumatology/39.10.1074. [DOI] [PubMed] [Google Scholar]
- 20.Oliva F., Barisani D., Grasso A., Maffulli N. Gene expression analysis in calcific tendinopathy of the rotator cuff. Eur Cell Mater. 2011;21:548–557. doi: 10.22203/ecm.v021a41. [DOI] [PubMed] [Google Scholar]
- 21.Bosworth B.M. Examination of the shoulder for calcium deposits. Technique of fluoroscopy and spot film roentgenography. J Bone Jt Surg. 1941;23:567–577. [Google Scholar]
- 22.Wolk T., Wittenberg R.H. Calcifying subacromial syndrome—clinical and ultrasound outcome of non-surgical therapy. Z Orthop Ihre Grenzgeb. 1997;135:451–457. doi: 10.1055/s-2008-1039415. [DOI] [PubMed] [Google Scholar]
- 23.Ejnisman B., Andreoli C.V., Monteiro G.C. Calcifying tendinopathy: a local or a systemic condition? Revista Brasileira de Ortopedia (English Edition) 2012;47(4):479–482. doi: 10.1016/S2255-4971(15)30132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neer C.S. II, editor. Shoulder reconstruction. WB Saunders; Philadelphia: 1990. Less frequent procedures; pp. 421–485. [Google Scholar]
- 25.Gartner J., Heyer A. Calcific tendinitis of the shoulder. Orthopade. 1995;24:284–302. In German. [PubMed] [Google Scholar]
- 26.Mole D., Kempf J.F., Gleyze P., Rio B., Bonnomet F., Walch G. Results of endoscopic treatment of non-broken tendinopathies of the rotator cuff. 2. Calcifications of the rotator cuff. Rev Chir Orthop Reparatrice Appar Mot. 1993;79:532–541. In French. [PubMed] [Google Scholar]
- 27.Farin P.U., Jaroma H. Sonographic findings of rotator cuff calcifications. J Ultrasound Med. 1995;14:7–14. doi: 10.7863/jum.1995.14.1.7. [DOI] [PubMed] [Google Scholar]
- 28.Chiou H.J., Chou Y.H., Wu J.J., Hsu C.C., Huang D.Y., Chang C.Y. Evaluation of calcific tendonitis of the rotator cuff: role of color Doppler ultrasonography. J Ultrasound Med. 2002;21(3):287–289. doi: 10.7863/jum.2002.21.3.289. [DOI] [PubMed] [Google Scholar]
- 29.Noel E. Treatment of calcific tendinitis and adhesive capsulitis of the shoulder. Rev Rhum Engl Ed. 1997;64:619–628. [PubMed] [Google Scholar]
- 30.Albert J.-D., Meadeb J., Guggenbuhl P. High-energy extracorporeal shock-wave therapy for calcifying tendinitis of the rotator cuff: a randomised trial. J Bone Jt Surg. Br Vol. 2007;89(3):335–341. doi: 10.1302/0301-620X.89B3.18249. [DOI] [PubMed] [Google Scholar]
- 31.Rompe J.D., Kirkpatrick C.J., Kullmer K., Schwitalle M., Krischek O. Dose-related effects of shock waves on rabbit tendo Achillis: a sonographic and histological study. J Bone Jt Surg [Br] 1998;80-B:546–552. doi: 10.1302/0301-620x.80b3.8434. [DOI] [PubMed] [Google Scholar]
- 32.Loew M., Daecke W., Kusnierczak D., Rahmanzadeh M., Ewerbeck V. Shock-wave therapy is effective for chronic calcifying tendinitis of the shoulder. J Bone Jt Surg Br. 1999;81:863–867. doi: 10.1302/0301-620x.81b5.9374. [DOI] [PubMed] [Google Scholar]
- 33.Gerdesmeyer L., Wegenpfeil S., Hakke M. Extracorporeal shock wave therapy for treatment of chronic calcifying tendonitis. A randomized control trial. JAMA. 2003;290(November (19)):2573. doi: 10.1001/jama.290.19.2573. [DOI] [PubMed] [Google Scholar]
- 34.Vavken P., Holinka J., Rompe J.D., Dorotka R. Focused extracorporeal shock wave therapy in calcifying tendinitis of the shoulder: a meta-analysis. Sports Health: Multidiscip Approach. 2009;1(2):137–144. doi: 10.1177/1941738108331197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verstraelen F.U., Den Kleef N.J.H.M., Jansen L., Morrenhof J.W. High-energy versus low-energy extracorporeal shock wave therapy for calcifying tendinitis of the shoulder: which is superior? A meta-analysis. Clin Orthop Relat Res. 2014;472(9):2816–2825. doi: 10.1007/s11999-014-3680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huisstede B.M.A., Gebremariam L., van der Sande R., Hay E.M., Koes B.W. Evidence for effectiveness of extracorporal shock-wave therapy (ESWT) to treat calcific and non-calcific rotator cuff tendinosis – a systematic review. Man Ther. 2011;16(5):419–433. doi: 10.1016/j.math.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Ioppolo F., Tattoli M., Di Sante L. Extracorporeal shock-wave therapy for supraspinatus calcifying tendinitis: a randomized clinical trial comparing two different energy levels. Phys Ther. 2012;92:1376–1385. doi: 10.2522/ptj.20110252. [DOI] [PubMed] [Google Scholar]
- 38.Haake M., Rautmann M., Wirth T. Assessment of the treatment costs of extracorporeal shock wave therapy versus surgical treatment for shoulder disease. Int J Technol Assess Health Care. 2001;17(Fall (4)):612–617. [PubMed] [Google Scholar]
- 39.Rompe J.D., Zoellner J., Nafe B. Shock wave therapy versus conventional surgery in the treatment of calcifying tendinitis of the shoulder. Clin Orthop Relat Res. 2001;387:72–82. doi: 10.1097/00003086-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Del Cura J.L. Ultrasound-guided therapeutic procedures in the musculoskeletal system. Curr Probl Diagn Radiol. 2008;37(5):203–218. doi: 10.1067/j.cpradiol.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Krasny C., Enenkel M., Aigner N., Wlk M., Landsiedl F. Ultrasound-guided needling combined with shock-wave therapy for the treatment of calcifying tendonitis of the shoulder. J Bone Jt Surg Br. 2005;87(4):501–507. doi: 10.1302/0301-620X.87B4.15769. [DOI] [PubMed] [Google Scholar]
- 42.Neto A.A.F., Trevizani C.S., Benegas E. Arthroscopic treatment of calcifying tendinitis of the rotator cuff. Revista Brasileira de Ortopedia (English Edition) 2010;45(5):432–436. doi: 10.1016/S2255-4971(15)30432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ranalletta M., Rossi L.a., Bongiovanni S.L., Tanoira I., Piuzzi N., Maignon G. Arthroscopic removal and rotator cuff repair without acromioplasty for the treatment of symptomatic calcifying tendinitis of the supraspinatus tendon. Orthop J Sports Med. 2015;3(4):1–5. doi: 10.1177/2325967115577957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rebuzzi E., Coletti N., Schiavetti S., Giusto F. Arthroscopy surgery versus shock wave therapy for chronic calcifying tendinitis of the shoulder. J Orthop Traumatol. 2008;9(4):179–185. doi: 10.1007/s10195-008-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marder Richard A., Heiden Eric A., Kim Sunny. PhDa calcific tendonitis of the shoulder: is subacromial decompression in combination with removal of the calcific deposit beneficial? J Shoulder Elbow Surg. 2011;20:955–960. doi: 10.1016/j.jse.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 46.Castagna A., Giorgi S.D., Garofalo R., Conti M., Tafuri S., Moretti B. Calcifying tendinitis of the shoulder: arthroscopic needling versus complete calcium removal and rotator cuff repair. A prospective comparative study. Joints. 2015;3(October–December (4)):166–172. doi: 10.11138/jts/2015.3.4.166. Published online 2016 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cacchio A., Paoloni M., Barile A. Effectiveness of radial shock-wave therapy for calcific tendinitis of the shoulder: single-blind, randomized clinical study. Phys Ther. 2006;86(5):672–682. http://www.ncbi.nlm.nih.gov/pubmed/16649891 Retrieved from. [PubMed] [Google Scholar]