Introduction
Over the years, researchers tried to explain cancer pathogenesis by taking into consideration different factors, such as immune system, the genetic predisposition and environmental factors [1]. In this context, the inflammation and oxidative stress response represent important topics in scientific debate. The inflammation is a normal defensal tissue response to factors that disturb omeostasis of human biological system [1]. Therefore, it needs a delicate regulation that ensures the correct reaction against damage and allows the organ and tissue reparation associated to the recovery of their functions. However, the correct operation of the “repairing machine in our body” is due to a functional balance that allows the immune system to activate itself after physical and/or chemical stimuli and then by determining its shutting down which ensures the return to the starting conditions, essential for biological function protection. Response of genetic factors to environmental stressors can complicate inflammation chronicization, as well as the possible instauration of a chronic inflammation for autoimmune pathologies, that represent the primum movens for cancer onset, just like inflammatory bowel disease (IBD) [2]. The role of primary order carried out by immune system was first hypothesized by Rudolf Ludwig Virchow in 1863, after leucocytes presence demonstration in neoplastic tissue. This concept was taken up by a large number of studies on the topic, demonstrating a clear interconnection among inflammation, oxidative stress, cytokine production, chemokines and tumoral growth, invasion and metastasis [3]. The correlation between inflammation and cancer is now very clear given the numerous scientific evidence especially in gastrointestinal tract cancer [4]. In this context, an inflammatory microenvironment is an essential assumption for the development of most of tumors. Indeed, only a small part of cancers is correlated to germline mutation, whereas the majority is the result of a cooperation between environmental factors and genetic somatic mutations [5]. The increase of metabolic pathologies, such as obesity and diabetes in the last years, has caused an increase of tumor incidence in this population. These diseases are associated to a higher risk of developing cancer due to a direct cancerogenous effect, as well as an immune dysregulation able to promote chronic inflammation and oxidative stress through antioxidant system depletion [6].
Additionally, also advanced age and cell senescence are important factor as they are able to generate per se a higher predisposition to tumoral onset even through a dysregulation of inflammatory cascade. These factors also make the immune response less efficient in fighting cancer, allowing it to grow and metastasize [7].
Moreover, growing scientific evidence has demonstrated a possible role of gastrointestinal bacterial community in the regulation of several inflammatory/immunitary processes involved in tumor initiation or progression. Gut microbiota represents the totality of bacteria, virus and fungi that are in our gastroenteric tract. It is a complex ecological system consisting of at least 500 different bacterial species. Its qualitative and quantitative composition is deeply different depending on the considered gastroenteric tract. In the stomach, a small number of bacteria have been found, mainly consisting of lactobacilli, streptococci, staphylococci, enterobacteriaceae and yeasts. In the subsequent gastrointestinal tracts, there is a quantitative increase from 0 to 105 colony-forming unit/g (CFU/g) in the duodenum to 108 CFU/g in the ileum and 1010 CFU/g in the colon. In the colon more than 99% of the microorganisms are strictly anaerobic, such as bifidobacteria, Bacteroides spp., Clostridium spp., Eubacterium spp., Fusobacterium spp., and peptostreptococci [8]. It varies from one person to another and is modified by age, diet, type of birth, breastfeeding, ileocecal valve efficiency, use of active drugs in both heartburn and gastrointestinal mobility [9], [10], [11], [12].
In order to understand the connection between gut microbiota and cancer, we have to bear in mind that some of the functions carried out by a eubiotic gut are resistance to intestinal colonization by pathogen bacteria able to cause dysbiosis, induction in IgA production and antimicrobial secretions, regulation of structural entirety of tight junctions and, above all, regulation of innate and adaptive immunity [13], [14], [15], [16].
However, despite the growing interest regarding this field, nowadays, a standardized protocol to study the relationship between gut microbiota and cancer, especially in the interpretation of study results, does not exist. The strongest scientific evidence concerning this relationship exists in relation to gastrointestinal tumors.
In this review, we analyze, from a particular perspective, the different ways to induce inflammation and oxidative stress in order to explain the mechanisms underlying the development of several cancers. Moreover, because of the strong scientific evidence in literature, we focused our attention to the analysis of the role that gut microbiota plays in gastrointestinal cancer development/promotion.
The Role of Tumoral Microenvironment
The major environmental factors responsible for induction of chronic inflammatory process and cancer genesis are represented by chronic infection (such as viral hepatitis for hepatocellular carcinoma development, Helicobacter pylori infection to gastric cancer and mucosa associated lymphoid tissue development, schistosoma and bacteroides infection to bladder and colon cancer), toxic exposition (e.g., tobacco, chemical products and asbestos) as well as metabolic pathologies able to alter regulatory balance of inflammation [17], [18], [19]. An important concept underlies the interconnection between inflammation and cancer: the lasting inflammation. It would not be the intensity of the inflammatory response but the maintenance of a low-grade and chronic inflammation that determines the neoplastic transformation of eukaryotic cells or the induction of genotoxic damage [20]. However, it is important to underline that not all chronic inflammations, although systemic, are able to promote carcinogenesis. The development of solid tumors is associated to an intrinsic tumoral inflammation supported by protumorigenic microenvironment [5]. The tumoral microenvironment is composed by macrophages, neutrophils, mast cells, myeloid-derived suppressor cells, dendritic cells, and natural killer cells; moreover, adaptive immune cells (T and B lymphocytes) in addition to the cancer cells and their surrounding stroma (fibroblasts, endothelial cells, pericytes, and mesenchymal cells). These cells control tumor growth communicating through the production of autocrine, paracrine and endocrine mediators, thus controlling tumoral growth. The different types of interconnection, the grade and the type of cellular activation are factors that conditionate the interplay between immune system and the tumor in terms of tumor-promoting or antitumor response [21], [22]. It is possible to hypothesize that the two types of tissue inflammation can coexist in natural history of tumoral pathology. The role that this inflammation would have in tumor would be dictated, in one way or another, by a specific composition and activation of the tumoral microenvironment [23].
Macrophages could have a controversial role in this field. In fact, in relation to the acquisition of a specific functional phenotype, they could have a tumor-promoting or antitumoral activity. They can range from proinflammatory, antitumorigenic M1-phenotype to anti-inflammatory, protumorigenic M2-phenotype macrophages (fundamental for the induction of tumoral angiogenesis and for invasion and metastatic processes) [24]. T lymphocytes could have a similar role. In fact, they are divided into several subtypes: CD8+ cytotoxic T cells (CTLs) and CD4+ helper T (Th) cells, which include Th1, Th2, Th17, and T regulatory (Treg) cells, as well as natural killer T (NKT) cells. An increase of CTLs and Th1 in tumoral microenvironment seems to be related to better prognosis as highlighted in several studies on colon and pancreatic cancer, and a decrease in their amount in tumoral microenvironment is associated with a greater chance of spontaneous cancer development or as a result of exposure to toxic environmental factors [25], [26], [27].
However, controversial results of a possible procancer role played by T lymphocytes (CTLs, Th1, Th2 and Th17), except NKTs, are present in the literature [28], [29], [30]. Some oncogenes such as RAS and MYC would be able to trigger a signal transduction cascade that leads to recruitment of additional leukocytes in the tumor region and the expression of inflammatory cytokines and chemokines, as well as the production of angiogenic factors [31], [32]. Often, the necrosis developed in the core region of a fast-growing solid tumor induces the release of proinflammatory mediators such as interleukin (IL)-1 and High Mobility Group Box 1 (HMGB1) [33], which in turn causes the release of angiogenetic factors, thus promoting cancer survival either directly, by the increase in oxygen supply and nutrients to tumor tissue, and indirectly, by recruitment of proinflammatory cells and cytokines release [34]. Other tumors are able to stimulate inflammation through the direct production of proinflammatory substances, some of which activate macrophages through interaction with Toll-like receptor (TLR)-2 [35]. Another type of inflammation associated with the tumor is that provoked by antitumor therapy. It can represent the result of tissue necrosis with the consequent release of proinflammatory cytokines that increase tumor growth or, on the other hand, can be the antitumor response elicited by an increased exposure of tumor antigens to an immune system by radio and/or chemotherapy [36], [37].
Dysregulation of the inflammatory system is also involved in the tumor initiation process. A mechanism associated with inflammation-induced tumorigenesis is the upregulation of activation-induced cytidine deaminase (AID) that is an enzyme able to induce the switching of immunoglobulin gene class [38]. The expression of this enzyme is related to an innumerable series of tumors and is linked to the activation of inflammatory signals associated with exposure to environmental factors such as NF-kB or TGF-β–dependent pathways [38]. The overactivation and overexpression of AID lead to the induction of genetic instability that is the basis of the acquisition of new DNA mutations responsible for the tumor initiation process, especially if loaded with critical genes such as Tp53, c-Myc and Bcl-6 [39]. In this scenario, epigenetic regulation of gene expression is also crucial. Epigenetic mechanisms including micro-RNA–based silencing and DNA methylation are influenced by the onset of chronic inflammation responsible for the silencing of oncosuppressive genes such as INK4a and APC [40], and in particular, aberrant CpG island methylation in tumors is related to an increase of cancer development. An important factor able to induce tumor process and to acquire uncontrolled replication capacity by stem clone cells is represented by inflammation-induced production of growth factors and cytokines. As demonstrated by Oguma et al., TNF-α would indeed induce penetration within the β-catenin nucleus in conjunction with the inflammation-associated gastric cancer genesis in the absence of any type of mutation of Wnt/β-Catenin pathway [41]. On the other hand, the same DNA damage would be able to induce inflammation, which in turn would carry out procancerogenic activity. In a diethylnitrosamine-induced hepatocarcinoma model (DEN), DNA damage has been shown to induce cell death and necrosis, responsive to the release of DAMPs and thus stimulation of Toll-like receptors, resulting in inflammation activation [42], [43]. The activity of different types of oncoproteins such as Ras, Myc and RET may also trigger signal activation that can trigger the production of proinflammatory cytokines and chemokines such as IL-6, IL-8, IL-1β, etc. [5]. From this evidence, it is clear that complex relationship between inflammation and cancer is the basis of the meccanism that allows tumorigenesis and sustains disease progression up to the acquisition of metastatic capacity.
The Role of Chronic Inflammatory Diseases
Epidemiological studies have shown that chronic inflammation predisposes individuals to various types of cancer. It is estimated that underlying infections and inflammatory responses are linked to 15% to 20% of all deaths from cancer worldwide [44]. There are many triggers of chronic inflammation that increase the risk of developing cancer. Such triggers include microbial infections (for example, infection with Helicobacter pylori is associated with gastric cancer and gastric mucosal lymphoma or HCV infection which is associated with liver carcinoma), autoimmune diseases (for example, intestinal bowel disease (IBD) is associated with colon cancer) and chronic inflammatory conditions such as asthma, chronic obstructive pulmonary disease (COPD). Accordingly, treatment with nonsteroidal anti-inflammatory agents decreases the incidence and the mortality from several tumor types [45], [46], [47]. In particular, nonsteroidal anti-inflammatory drug use decreased cancer incidence in healthy population [48], and celecoxib demonstrated an effect on colorectal polyps in patients affected from familiar adenomatous polyposis syndrome [49].
One of the best studied example is colon cancer on IBD, in which colon cells are continuously exposed to growth-promoting inflammatory cytokines [50]. The increased risk of cancer in patients with IBD may be associated with the chronic proliferation required to repair damage to the epithelial monolayer caused by constant inflammation. In chronic inflammation, cytokines secreted by immune cells stimulate pathways [51], [52], [53] which are essential for cancer proliferation.
Tumor necrosis factor α (TNFα) plays a fundamental role in inflammation in IBD and has been the target of biological treatments. TNFα provokes inflammation by stimulating the production of IL-1β and IL-6, inducing expression of adhesion molecules, proliferation of fibroblasts, activation of procoagulant factors, and cytotoxicity of the acute-phase response [54]. The binding of TNFα to its receptor causes the activation of mitogen-activated protein kinases (MAPKs) and of the NF-ĸB pathway, which may influence barrier permeability. NF-ĸB activation leads to increased transcription of proinflammatory cytokines, resulting in a continuous feeding of the inflammatory response, and increased expression of myosin light chain kinase (MLCK) [55], [56], which in turn stimulates permeabilization of the intestinal barrier. Additionally, TNFα seems to stimulate the COX2-derived PGE2 which may have a direct impact on carcinogenesis through regulation of the WNT signaling pathway [54].
Another cytokine involved in the evolution of cancer is TGFβ1 that plays an important role in the epithelial-mesenchymal transition (EMT) [57], [58], thus inducing a migratory and apoptosis-resistant phenotype of intestinal epithelial cells through SLUG induction and subsequent L1CAM gene expression.
Of interest, several reports have revealed that, in addition to the cytokine proinflammatory effects, also several molecular alterations are involved in the inflammation-induced carcinogenesis which involve inactivation of tumor-suppressor genes, oncogene mutations, loss of heterozygosity, and chromosomal and microsatellite instability (MSI).
The TP53 tumor-suppressor gene appears to be a key factor in the early stages of IBD-associated colorectal carcinogenesis, as it develops early in patients with IBD, whereas it occurs later in sporadic colorectal cancer (CRC) [58]. It seems that TP53 abnormalities are driven by inflammation, this hypothesis being supported by the presence of increased TP53 expression in inflamed, nondysplastic, noncancerous colonic mucosa in IBD [59].
A very early and gradually occurring event in cancer development is the genomic instability. Indeed, in the subset of patients with IBD, the colonic epithelium is damaged by reactive oxygen species (ROS) produced in the context of the inflammatory milieu as a consequence of the oxidative stress, leading to cellular damage in terms of oxidation of proteins and DNA. Failure to remove or repair ROS-initiated damage can be either mutagenic or lethal to cells [60].
In absence of coding-region mutations, often epigenetic changes, represented by aberrant promoter methylation, occurring in association with silencing of tumor-suppressor genes [e.g. TP53, Kruppel-like factor 6 (KLF6), APC, KRAS, and deleted in colorectal cancer (DCC)] have been detected in patients with IBD-associated cancer [61]. In the context of the progression of inflammation in IBD, DNA methylation is a key factor in a subset of tumors affected by the CpG island methylator phenotype, a pathway that emerges as a form of epigenetic instability [62]. In this scenario, an important aspect of IBD-associated neoplasms is the defective DNA mismatch repair, manifested as microsatellite instability (MSI) and promoter hypermethylation of the mismatch repair gene mutL homolog 1 (MLH1) [63]. Recently, epigenetic abnormalities and aberrant methylation have been demonstrated also to alter those signal pathways involved in the stem cell proliferation and differentiation capacity, such as WNT, NOTCH and HEDGEHOG pathways. In particular, methylation of the WNT-signaling genes has been shown in early-stage IBD and to gradually rise during progression of IBD-associated CRC.
Another inflammatory condition associated with cancer development is the COPD which represents an abnormal and chronic inflammatory response of the lungs to noxious particles and gases. Of note, approximately 30% of patients with mild to moderate COPD have been reported to die from lung cancer [64], which has traditionally been linked to a common etiological exposure, namely, tobacco smoke. The chronic injurious state of this lung microenvironment may facilitate tumor development progressing from metaplasia, dysplasia, carcinoma in situ and subsequent malignant transformation [65]. Morphological changes in the bronchial epithelium are accompanied by an increase in loss of heterozygosity and field cancerization involving the accumulation of mutations that eventually predispose the lung to cancer [66], [67], [68]. Similarly to the intestinal IBD-associated carcinogenesis, TP53 mutations characterize the smoker epithelium exhibiting squamous metaplasia and occur early during transformation [66]. Microsatellite instability (MSI) is frequent in the nonmalignant bronchial epithelium of COPD and is associated with EGFR amplification [69]. The increased EGFR expression [70] and EGFR transactivation, observed in COPD epithelium, augment and prolong inflammatory responses initiated by viral and bacterial infection in the bronchial epithelial cells [71], [72]. Likewise, dysregulation of the PIK3CA/PTEN/Akt/mTOR signaling, which coordinates tumor-promoting survival, metabolism, migration and angiogenesis, is also observed in the bronchial airway of smokers with dysplastic lesions, suggesting an early activation during carcinogenesis [73]. Indeed, amplification of PIK3CA has been detected in high frequency in squamous cell carcinoma (SCC), in contrast to gain-of-function mutations that are much less frequent [74], whereas loss of PTEN protein expression or PTEN promoter methylation has been evaluated as an independent poor prognostic factor for patients with non–small cell lung cancer (NSCLC) associated with a more aggressive subset of lung tumors [75], [76], [77].
These examples of inflammation inducing cancer can be separated from the tumor-elicited inflammation, which occurs when the invasive tumor is already established and drives invasion and metastatic processes. In fact, the connection between inflammation and cancer can be viewed as bidirectional: an extrinsic pathway, driven by inflammatory conditions that increase cancer risk (such as IBD and COPD); and an intrinsic pathway, driven by genetic alterations that cause inflammation (such as oncogenes). In the latter case, the genetic events that cause neoplasia are also responsible for generating an inflammatory environment.
The most frequently mutated oncogenes in human cancer are represented by both MYC and members of the RAS family, and in turn components of the RAS–RAF signaling pathway, which are able to induce the production of tumor-promoting inflammatory chemokines and cytokines [32], [78], [79] and additionally to remodel the extracellular microenvironment, such as inducing angiogenesis [31], [80].
An example in which to explore the connection between oncogenes and inflammatory microenvironment is human papillary thyroid carcinoma, where the rearrangement of the RET gene is a frequent early event in the pathogenesis of carcinoma and is a necessary and sufficient event for this cancer to develop. The activation of RET induces a transcriptional program that is similar to that which occurs during inflammation and includes colony-stimulating factors (CSFs), which promote the survival of leukocytes and their recruitment from the blood to the tissues; interleukin 1β (IL-1β); cyclooxygenase 2 (COX2); chemokines that can attract monocytes and dendritic cells (CC-chemokine ligand 2 (CCL2) and CCL20); chemokines that promote angiogenesis (such as IL-8; also known as CXC-chemokine ligand 8 (CXCL8)); the chemokine receptor CXC-chemokine receptor 4 (CXCR4), which binds to CXCL12; extracellular-matrix-degrading enzymes; and the adhesion molecule lymphocyte selectin (L-selectin). These results show that an early genetic event that is necessary and sufficient for the development of a human tumor cancer directly promotes the build-up of an inflammatory microenvironment.
Which pathway, extrinsic or intrinsic, between inflammation and cancer would be more significant may be dependent on tumor type, or maybe, both are both essential. This is the case of pancreatic cancer in a murine model, where both pancreatitis and K-RAS gene mutations are frequently found and both are required to induce pancreatic intraepithelial neoplasia and invasive ductal carcinoma [78]. Thus, although the RAS-RAF pathway [32], [79] can drive tumor-promoting inflammation, an extrinsic inflammatory condition (pancreatitis) is needed to drive carcinogenesis in mice and presumably in humans.
It is likely that all tumor-promoting inflammation, whether it precedes or follows tumor development, is part of the normal response to injury and infection that has been usurped by cancer cells to their own advantage.
The Role of Oxidative Stress as a Bridge to Link Inflammation and Cancer
The main known mediator of the link between inflammation and cancer is the imbalance of oxidative stress induced by inflammation in a normal tissue and sustained by microenviromental inflammation in a context of malignant tumor.
Reactive oxygen species derivates (ROS or intermediates ROI) are produced from molecular oxygen O2 that is normally unreactive but is reduced to water through step-by-step reactions generating partially reduced and very reactive intermediates with oxidizing potential: the superoxide radical (O2−), hydrogen peroxide (H2O2) and the hydroxyl radical (OH) [81]. Similarly, reactive nitrogen species (RNS or intermediate RNI) are derived from nitrogen metabolism: NO, synthesized by the enzyme NOS (nitric oxide synthase), nitrogen dioxide (NO2) and peroxynitrite (ONOO-), from interaction of NO and O2, activator of nitrosylation [82]. These free radicals react with other chemical or structural compounds of cells and also recruit other inflammatory cells with secondary amplification of damage.
Physiologically, the main sources of reactive species in all cells are mitochondria, cytochrome P450 and peroxisome. Under physiological conditions, there is a constant endogenous production of reactive intermediates of ROI and RNI that interact as “signaling” molecules for metabolism, cell cycle and intercellular transduction pathways. The production of ROI and RNI is balanced by a removal performed by a series of protective molecules and systems globally defined as “antioxidant defenses,” such as superoxide dismutase, catalase, glutathione peroxidase and glutathione-S-transferase. When the generation of free radicals and active intermediates exceeds the system's ability to neutralize and eliminate them, the oxidative stress occurs. In these conditions, ROI and RNI act as “toxic” substances that may react with proteins, carbohydrates and lipids, with consequent alteration in both the intracellular and intercellular homeostasis, leading to possible cell death and regeneration. In the context of chronic inflammation, the start of ROI and RNI-mediated carcinogenesis may be direct (oxidation, nitration, halogenation of nuclear DNA, RNA and lipids), or may be mediated by the products of ROI-RNI and proteins, lipids and carbohydrates that are capable of forming DNA adducts. Proteins are more susceptible to oxidation by free radicals, where the oxidation of SH groups of cysteine reduces the activity of various enzymes as well as the synthesis of GSH, which is the main intracellular free radical scavenger. The oxidation of lipids induces the formation of aldehydes and lipid peroxides that in high concentrations are considered the more damaging species as they easily react with proteins, DNA and phospholipids, generating a variety of intra- and intermolecular toxic covalent adducts leading to the propagation and amplification of oxidative stress. ROI can also increase the expression of transcriptional factors including c-fos and c-jun oncogenes involved in neoplastic transformation and are able to recruit other inflammatory cells, thus intensifying the possibility of DNA mutations in normal tissue, leading to cancer development and proliferation [83]. In particular, in this peculiar stress condition, intracellular pathways NF-KB, AP-1, p53 and caspases are activated in terms of proangiogenic and antiapoptotic signals [84], [85]. Collectively, these events lead to a state of a more complex oxidative and metabolic stress, with implications in the control of cell regeneration at various level of DNA gene expression: mitochondrial DNA (mtDNA) is very sensitive to oxidative stress because it lacks of histone proteins and contributes itself to amplify radicals production in mitochondrial electron transport chain [86], [87]. A proof of the relevance of role of oxygen and nitrogen species as endogenous proinflammatory and procarcinogenetic agents is the consistent number of experimental evidences that alterations in components of anti- and pro-oxidative cascades have an impact on cancer development [88]. Lung cancer represents itself the best example of chemical-induced oxidative stress in a context of chronic inflammation as COPD [89]. Continuous cigarette smoking exposes pulmonary cells to over 4700 different chemical compounds including 1015 oxidants and free radicals [90], and 69 carcinogenic like tobacco-specific nitrosamines, formaldehyde and benzene [91]. Elevated levels of ROS are produced normally by inflammatory and endothelial cells in response to tobacco and pathogens [92], concomitantly with inhibition of the transcription nuclear factor erythroid 2-like 2 (NFE2L2 or NRF2) [93] that encodes for cytoprotective and antioxidant genes [94]. ROS compounds interact with polyunsaturated fatty acids and generate reactive carbonyl species, able to suppress cellular PTEN, with subsequent constitutive activation of protumoral AKT signaling [95]. These data suggest that various biological mechanisms are associated with cigarette smoking–induced cancer [96] in different lung tumor histological subtypes, depending on modulation of DNA oxidative status.
The Role of Gut Microbiota in Gastrointestinal Cancer
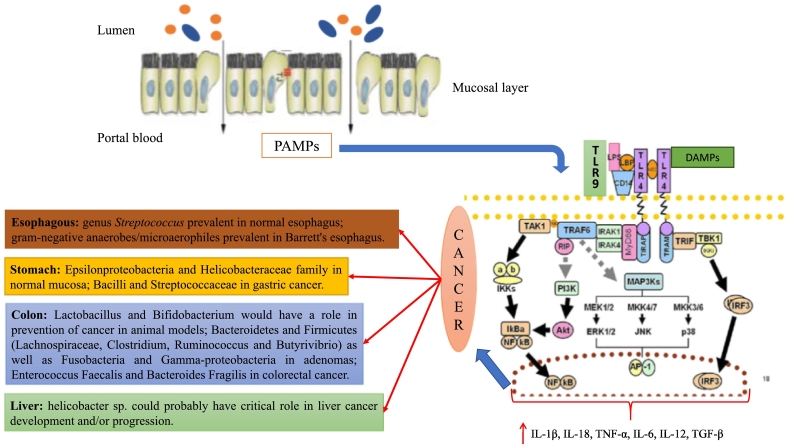
More than 90% of gut microbiota consists of two dominant phyla (Bacteroidetes and Firmicutes). The remaining part consists of five subdominant phyla: Actinobacteria, Proteobacteria, Fusobacteria, Cyanobacteria and Verrucomicrobia [97], [98], [99]. However, phyla evaluation in intestinal microbial composition is an approximation that does not consider the functional role carried out by the single bacterial species. For this reason, nowadays, the use of information deriving from outcome of scientific trials is scarcely applicable in routine clinical practice. Another strong limitation in the management of gut microbiota for the treatment of several pathologies is represented by the difficulty in modifying selectively the proportion of its microbial species, without modifying the balance able to maintain “the gut health” [100]. To maintain gut microbiota health means to maintain a correct proportion, which is different from one person to another, among different bacterial species. This allows defending metabolic, structural and immune functions of eubiotic microbiota, thus becoming essential for the host life [13], [14], [15], [16], [101], [102]. The communication between “gut microbiota system” and liver, brain, adipose tissue and other organs of human body is regulated by intestinal permeability (IP). Its increase, in relation to a dysbiotic gut, can be considered a key point for the comprehension of some pathogenetic mechanisms of systemic and liver disorders as well as cancer. In addition to food adsorbed by intestinal surface, bacterial products such as bacterial DNA, peptidoglycans (molecules belonging to the class of pathogen-associated molecular patterns-PAMPs) and, in some cases, intact bacteria can reach the liver in elevated quantities in relation to IP levels (Figure 1) [103]. The clear division between what is located in our intestine and portal blood is guaranteed by mechanisms able to regulate IP. Over time, the scientific findings in this field have led us to understood that IP is not a static phenomenon and mucosa intestinal is not a simple physical wall placed between intestine and portal blood. IP degree is very variable and is interconnected to several factors: type of diet, gene expression, intestinal/liver pathology, production of surface mucus, integrity of tight junctions, production of immunoglobulins (Ig)-A. Many of these factors are dependent from gut microbiota, which is the “lead” in IP control [16].
Figure 1.
Mechanism of bacteria-induced inflammation through inflammasome activation.
Bacterial products such as bacterial DNA and peptidoglycans (molecules belonging to the class of pathogen-associated molecular patterns [PAMPs]) reach the liver in a large amount in relation to the reduction of gut impermeability. They can activate innate immunity system through toll-like receptor binding and determine the recruitment of several transduction pathways such as Myeloid differentiation primary response 88 (MYD88) related pathways with the subsequent activation of Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), Interferon regulatory factor 3 (IRF3), Protein Kinase B (Akt) and Nuclear factor-kappa B (NF-kB). All these proteins are associated all together in a system of signal transduction called inflammasome that regulates the cellular reaction to several molecules identified through toll-like receptors (TLR) on cellular surface. The result is the activation of several types of cells in production of inflammatory cytokines: interleukin (IL)-1β, IL-18, IL-6, IL-12, transforming growth factor beta (TGF-β), and tumor necrosis factor alpha (TNF-α). A specific composition of gut microbiota is correlated with some types of gastrointestinal tract tumors through the activation of inflammasome involved in cancer development. Moreover, the scientific literature is also enriched of association studies that tried to link a specific composition of gut microbiota, in terms of prevalence of bacterial species, to oncologic tissues abnormalities.
As demonstrated by Cariello et al., there is a relationship between IP, portal hypertension, alcohol use, plasma levels of proinflammatory cytokines, and nitric oxide, expressed as nitrosothiols, and nitrite levels in patients with various types and degrees of chronic liver diseases. The assessment of IP degree through lactulose/mannitol test on 83 patients with chronic liver damage showed an increase directly proportional to the severity of liver disease. Independent factors from IP alteration were age, portal hypertension, alcohol use, and diabetes. Moreover, plasma levels of inflammatory cytokines and nitrosothiols were significantly higher in patients with altered IP [104]. Cancer represents the second cause of death worldwide, and particularly, gastrointestinal cancer is the leading cause of morbidity and mortality in the United States [105]. In recent years, there has been an increase in scientific research to investigate the role of microbiota and microbiota-linked inflammation in carcinogenesis [106], [107].
A possible strategy to explain the link between microbiota and cancer could be the “Molecular Pathological Epidemiology” which consists in a multidisciplinary study approach regarding the relationship between exogenous and endogenous factors (e.g., genetics) in the onset and progression of the tumor. Thanks to this view, it is possible to study the capability of a specific environmental factor to induce changes in the genetic expression that could influence cancer onset and its progression or prognosis [108], [109]. Certainly, this approach has allowed us to make a lot of progress regarding the understanding of the molecular mechanisms underlying neoplastic diseases such as colon cancer, allowing, moreover, to identify clusters of population with high risk to develop cancer and therefore deserving of more efficient screening surveillance [110]. A classic example of how the microbiota can promote or not the organization of the tumor by interacting with the environment could be its ability to exert an anti-inflammatory or proinflammatory power through the production of metabolic substances from the metabolism of diet components, inducing or not the generation of a tissue microenvironment favorable to cancer development [111]. Tumor progression is also associated with gene environment interactions. Recent advances in high-throughput technologies gave us the possibility to understand better the link between a specific microbial composition, the inflammation and the genetic modifications able to induce cancer [112].
The conduction of studies in this regard is enormously difficult because of the extreme degree of variability in the intestinal microbial composition between the analyzed individuals, and for this reason, there is, however, no univocal opinion to date, and future studies are needed in order to better understand the mechanisms implemented by specific microbial species in the induction of gastrointestinal cancer.
A large number of evidence have been produced in recent past years about the viability of the gut microbiota to influence the outcome of the therapy against different types of tumors. In this regard, the functional link between gut microbiota and immune system has been confirmed by the direct role of bacteria in influencing the efficacy of PD-1-based immunotherapy against epithelial tumors [113]. The alteration of the intestinal microbial composition induced by the use of antibiotic has been associated with a lower efficacy of the immunotherapeutic regimens against this category of tumors [113]. In particular, the relative proportional deficiency of Akkermansia muciniphila was correlated with a lower response to therapy [113]. Akkermansia muciniphila restored the efficacy of PD-1 blockade in an interleukin-12dependent manner by increasing the recruitment of CCR9 + CXCR3 + CD4+ T lymphocytes in tumoral microenvironment.
The proportion of Fusobacterium nucleatum in some recent studies has been found to be higher in patients with colorectal cancer [114], [115]. Its abundance also correlates with a higher rate of relapse after chemotherapy and would be associated with a worse prognosis of the disease. The relative abundance of this bacteria has also been correlated with a lower response to chemotherapy with 5-fluorouracil, capecitabine and oxaliplatin.
Such phenomena would be linked to the interaction that such beat would have in regulating the response to activation of TLR-4–mediated pathway and MyD88 inducing cellular autophagy with the subsequent reduction in the tumor microenvironment of immune cells able to fight the tumor [116], [117].
In the latter years, there was an increase in the incidence of esophageal adenocarcinoma (EA) and the gastric esophageal junction carcinoma that showed an inverse proportional relationship with the incidence of HP infection. This correlation has suggested that HP infection could play a protective role against EA due to the ability to induce hypochlorhydria as a result of the destruction of gastric parietal cells and the generation of atrophic gastritis [118], [119]. In itself, inflammation caused by esophageal gastrointestinal reflux on the distal part of the esophagus mucosa and the resulting metaplasia in the long run are able to altering the composition of the esophagus flora. In an interesting research, the microbial composition of biopsy samples of the esophagus was analyzed by bacterial 16S ribosomal RNA gene survey and showed two different types of microbiome in relation to the esophagus histology. In particular, the type I microbiome was dominated by the genus Streptococcus and was related to the phenotypically normal esophagus; on the contrary, the type II microbiome, composed for the greater part from gram-negative anaerobes/microaerophiles, was related to esophagitis and Barrett's esophagus [120]. However, further studies are necessary to understand the concrete role of EC induction variation. Gastric cancer represents the third cancer-related mortality cause worldwide, and its incidence has changed in the last years, becoming less frequent in the distal portion of the stomach (antrum and gastric body) and more frequent in the proximal region (esophagus-gastric junction) [121]. The most important and known risk factor for the development of gastric cancer is represented by Helicobacter pylori (HP) infection [122]. This evidence is confirmed by the higher rate of eradication associated to new therapies for HP infection that determined a clear reduction of gastric cancer (GC) incidence rate. Between 1% and 3% of patients with HP infection develop a gastric adenocarcinoma [123]. Among the factors able to trigger GC onset in patients with HP infection, an important role is carried out by HP-oncoprotein cytotoxin-associated gene A (CagA) in determining induction to progenitor cell proliferation in the gastric mucosa [123]. Other factors that correlated HP and CG directly are represented by secreted vacuolating cytotoxin A (Vac A) [124]. In particular, strains containing type s1, i1 or m1 alleles within the 5′ region of Vac A gene are mainly related to GC onset [125]. Moreover, HP could carry out an indirect activity in cancerogenesis process for CG. Gastric mucosa colonization by HP is able to cause a reduction of gastric mucosal flora richness: HP DNA represents about 93% to 97% of genetic sequence isolable from genomic analysis of mucosal gastric flora in HP-positive patients, and a total of 33 phylotypes were detected. By contrast, in HP-negative patient biopsy samples, it is possible to identify about 262 phylotypes [126]. This variation in gastric mucosal composition could have a role both in hypochlorhydria induction, which represents the key role for histological progression to intestinal-type GC, and in generating inflammation due to different microbic metabolism of nutrients ingested with diet that could have a role in cancer pathogenesis through inflammation [111], [127]. Gastric microbial composition modifies significantly itself in relation to mucosa damage from chronic gastritis to intestinal metaplasia and GC. Indeed, as the chronic gastritis passes from the GC, there is a marked reduction in the diversity of gastric mucosal flora: Bacilli and Streptococcaceae are mainly prevalent in GC samples compared with chronic gastritis and intestinal metaplasia samples. By contrast, Epsilonproteobacteria and Helicobacteraceae family are less represented in samples [128]. Some researchers highlighted a deep difference in terms of gastric mucosal composition among patients who live in high-risk areas and low-risk areas for GC development in Colombia. Two operational taxonomic units (OTUs), Leptotrichia wadei and a Veillonella sp., were more present among patients who live in high-risk geographic area for GC development, while 16 OTUs, including a Staphylococcus sp., were significantly more abundant in patients who live in low-risk geographic area for GC development [129]. However, researchers concluded that in order to consider a direct role related to these microbial composition variations in GC pathogenesis, further studies are necessary. The colorectal cancer (CRC) represents the third most frequent cause of cancer-related death in United States for both male and female, and its incidence has demonstrated a growing trend in the last few years [130]. Natural history of CRC considers a sequence of different anatomopathological entities that represent the stages through which the transformation of normal mucosa cells in tumoral ones occurs. This process is indissolubly related to genetic mutation acquisition, altered methylation of DNA, modification of chromatin or altered expression of microRNAs [131]. Universally accepted risk factors able to trigger this progression are age, type of diet (a diet rich in red meat and low in fibers and fruits), obesity, tobacco, metabolic syndrome and intestinal microbial composition modified by all the factors tested before [132]. A common point that explains the mechanism that links these factors to CRC development is represented by inflammation. In this regard, the role carried out by cyclo-oxygenase (COX)-2 and inducible nitric oxide synthase (iNOS) that normally are not expressed in epithelial cells and stromal cells is well known. COX-2 could have a role in determining the progression of colon polyps in cancer by acting as procarcinogenic enzyme, through the production of prostaglandin E2 (PGE2) that has proproliferative, proangiogenic, and antiapoptotic properties [133]. Furthermore, COX-2 would be able to induce the production of reactive aldehydes able to bind themselves and alter protein structure, damage DNA or inhibit the reparation and promote the acquisition of essential genetic mutations for disease progression [134]. In this intricate set of biological mechanisms, in recent years, there has been room for research aimed at clarifying the role that microbial intestinal flora could play in CRC despite the enormous difficulty in interpreting most data from in vitro or animal models [135]. The presence of microbial biofilm on the surface of colon adenomas has been recently found. It would be able to promote the penetration of products of bacterial derivation into the superficial epithelium through a decline of normal defenses of surface. The composition of microbial film composition associated to colon adenomas consists of Bacteroidetes and Firmicutes (Lachnospiraceae, Clostridium, Ruminococcus and Butyrivibrio) as well as Fusobacteria and Gamma-proteobacteria. Moreover, it is clear that adjacent healthy mucosa is lacking of typical biofilm found in correspondence of adenomas. According to the authors, the correlation between biofilm and adenoma is to be researched in production by polyamines bacteria that would be able to promote cellular growth and consequently CRC through the induction of genotoxic damage [136].
Other microbial species (Fusobacteria) would be able to promote CRC through sulfide production able to damage DNA. Fusobacterium nucleatum, for example, adheres, invades, and induces oncogenic and inflammatory responses to stimulate growth of CRC cells through its unique FadA adhesin. FadA binds to E-cadherin, activates β-catenin signaling, and differentially regulates the inflammatory and oncogenic responses [137]. From other studies, it was revealed that germ-free animals and animals treated with antibiotics able to sterilize intestine had a reduced rate of tumoral development, even if these results, in our opinion, complicate clinical evaluation given the impossibility to apply experimental model used to humans [138], [139]. Moreover, this great approximation, without an acute compositive evaluation of intestinal microbial species, on the role of microbiota in CRC pathogenesis could lead to an incorrect information about this topic.
Different microbial species carry out roles that are opposite in CRC. Just like Lactobacillus and Bifidobacterium would have a role in prevention of cancer in animal models, a manipulation of intestinal microbial composition carried out by nucleotide-binding oligomerization domain-like receptor family members (e.g., NOD-2, NLRP, and IPAF) would have a crucial role in bacterial eubiosis conservation [140], [141]. Loss of either Nod2 or RIP2 resulted in a proinflammatory microenvironment that enhanced epithelial dysplasia following chemically induced injury. The condition could be improved by treatment with antibiotics or an anti–interleukin-6 receptor-neutralizing antibody. Fecal microbiota transplantation from wild-type mouse to a knockout one for NOD-2 associates itself to a reduction of cancer risk, underlining a direct role linked to intestinal microbial composition in genesis of this pathology [142]. IP degree is another element to be taken into consideration in analysis of procancerogenous stimuli supported by gut microbiota. Normally, PAMPs are able to overcome the filter made up of surface mucus and cell tight junctions to reach submucosal level in contact with innate and specific immunity cells. This type of interaction provides physiological regulation of tolerance to microbial antigenes or their elimination through a process of microbial scavenger that predicts the onset of inflammation. As long as this inflammatory signal is activated in a “controlled” manner, it maintains bacterial eubiosis and normal tissue renewal through the classical cell turnover. However, in condition of IP increase as happens in pathological processes or particular diet habits, the dysregulation of inflammation causes the activation of signal pathway that leads to cell proliferation, as well as to be able to trigger DNA damage [143], [144].
In this regard, there are different studies in literature that demonstrate how an IP increase is related to reduced elaboration of surface mucus and alteration of structural/reduced expression of tight junctions is associated to a higher probability of developing colitis, dysplasia and cancer [145], [146], [147]. The mechanisms that support cholic cancerogenesis by gut microbiota are mainly related to induction of cell proliferation as well as apoptosis inhibition. Enterococcus faecalis and Bacteroides fragilis would be able, through COX-2 activation, to cause a proliferative effect related to superoxide and activation of nuclear factor kappa B (NF-kB), respectively [148], [149] Some receptors of innate immunity such as Toll-like receptors (TLRs) are able to recognize bacterial antigenes and activate cell translation pathways that hesitate in triggering inflammation and proliferative response. Normally, TLRs are insufficiently expressed in intestine, and this expression, even in the liver, is inducible in relation to their activation [150]. TLR-4 activation by lipopolysaccharides (LPS) induces proliferation through COX-2 activation and epidermal growth factor receptor (EGFR) just like demonstrated by a reduction of cell growth in TLR-4–deficient mice [151]. Moreover, TLR-4 activation is able to induce angiogenesis, which represents a further crucial step for cell growth and metastasis [152]. EGFR per se is able to promote cell proliferation through hydrogen peroxide formation. Enterococcus faecalis is able to increase EGFR expression, favoring this mechanism. Gefitinib use, EGFR inhibitor, is able to block Enterococcus faecalis–induced cell proliferation [153].
On the other hand, enterotoxigenic Bacteroides fragilis, in a study in vitro, was demonstrated to induce immediate apoptosis in HT-29 human colon cancer cells. However, in a second observational experimental phase, the toxin-induced activation of p38-MAPK and COX-2 was demonstrated to reduce apoptosis and promote cell proliferation [154]. Moreover, TNF-α activation, tumor necrosis factor receptor 2 (TNFR2) and NF-kB pathway by Enterococcus faecalis are able to reduce apoptosis through an increase of netrin-1, an important regulator of cell cycle [155]. Similar biological mechanisms able to promote cancerogenesis were also taken into consideration in hepatocellular carcinoma (HCC). It represents one of the most frequent neoplasias in the Western world since about 78,000 new cases in United States by 2020 are estimated [156]. About 80% to 90% of HCC cases are consecutive to a chronic inflammatory damage related to liver disease etiology (i.e., virus, alcohol, iron and copper accumulation), but it seems to be connected to PAMPs arrival through portal blood. It is well described in literature the role that gut microbiota plays in all phases of liver diseases. Indeed, it participates in mantaining an inflammatory state that acts as driving force for progression to fibrosis and cirrhosis [157]. The comprehension of connection between microbiota inflammation and HCC is based on a few observations: a close vascular connection between intestine and liver (gut-liver axis) exists. This axis receives nutrients not only by portal blood but also by toxins released by gut microbiota in relation to IP levels. The liver is rich in immunity cells (macrophages, lymphocytes, natural killer cells and dendritic cells) that are able to respond to stimulus generated by PAMPs, with the consequent induction of inflammation [158], [159]. The activation of inflammatory signal focused on the activation of NF-kB–dependent signal pathway involves the production of inflammatory cytokines such as TNF-α, IL-6, IL-1, ROS, etc., which, as we have seen, are able to stimulate cell proliferation and inhibit apoptosis, although their role is widely described in the promotion of the HCC rather than its induction [160], [161], [162]. Hepatocarcinogenesis, as demonstrated by Dapito et al., would be related to the activation by LPS of TLR-4 with the initiation of a chronic organ damage that comes out in the promotion of proliferation at the expense of apoptosis by the production of substances with mitogen activity like epiregulin [163]. The administration of antibiotics in this regard would be able to turn off the proliferative signal mediated by the activation of TLR-4, demonstrating an effective role of the microbiotic gut in the progression of the HCC [164]. Inflammation and hepatocellular damage resulting thereof are at the basis of the mechanisms for triggering a regenerative response. However, this response would occur in nonphysiological conditions, supported also by the activation and proliferation of stem cells (HSCs). They would contribute to the generation of fibrosis and then liver cirrhosis. The microenvironment that is created leads to a nonphysiological cell regeneration that is based on the deleterious damage-regeneration-repair cycle. Such vicious cycle would be the basis of the loss of proliferative control which may promote premalignant transformation and tumor growth [165]. In this regard, HSCs would play an important role in controlling cell proliferation by acting as true “mitotic controllers.” Such cells would be able to respond to stimuli by the presence of surface receptors such as TLR-4, which are typically activated by ligands such as LPS or desoxicolic acid [166]. The activation of these receptors involves the acquisition of a senescence-associated secretory phenotype that favors the production of epiregulin and therefore promotes cell proliferation and HCC progression. In a study by Yoshimoto et al. correlation was made between the acquisition of senescence-associated secretory phenotype of HSCs with the progression of HCC. Researchers have shown that mice with high-fat diet, compared to those with conventional diet, were more likely to develop HCC and had a greater density of senescent HSCs in peritumoral areas.
Enabling this functional profile to be acquired would be the inflammatory stimulus dictated by the production of IL-1β, in turn produced for the signal triggered by the high concentrations of desoxicolic acid and PAMPs in portal blood. In this sense, HFD would be able to induce a change in the intestinal microbial composition, favoring the survival of Firmicutes, especially Clostridium, which are capable of producing high levels of desoxicolic acid from the metabolism of colic acid. Such link ring explains the increased activation of NF-kB in mice fed with HFD and provides a basis for understanding the role of HSC in hepatocyte cancer [167].
Finally, pancreatic cancer is one of the most aggressive neoplasms, with a mortality rate that can reach over 90% of new cases within the first year of diagnosis [168]. For this reason, the understanding of the pathophysiologic mechanisms responsible for its onset and/or its progression is the basis, together with an appropriate renewal of therapies, to reduce the social impact of this disease. Again, chronic inflammation is the basis of the pathophysiological process that supports the formation of pancreatic cancer. Although the pancreas, unlike the liver, does not exhibit its intrinisc microbiome, according to a recent scientific theory, it could be reached by PAMPs both in the circulatory stream and through the biliary/pancreatic tract (transductal transmission). The arrival of such antigens leads to stimulation of innate immunity receptors, including TLR-4, resulting in proinflammatory cytokine production that would act along with other risk factors such as obesity and smoking for the onset of the disease [169]. The combination of the classic risk factors for pancreatic cancer and the penetration of PAMPs into the portal circle is evident in alcohol abuse. Alcohol is, in fact, a risk factor for pancreatic disease, from pancreatitis to cancer, but is also one of the best known causes of increased bowel permeability, leading to circulatory delivery of bacterial-derived products [104].
Such interesting and promising scientific discoveries are paving the way for a better understanding of pathogenetic mechanisms that support gastrointestinal cancer, enabling the scientific community to emerge on a new scenario that is promising to be exploited as a new therapeutic weapon against such diseases. However, nowadays, the results obtained from in vitro and experimental animals are not applicable to biology of human systems and therefore require further confirmations and in-depth studies. The key point for application to human therapy may be from the possibility in the near future of selective or superselective manipulation of intestinal microbial species, enabling a specific “microbial personalization” for the specific patient.
Conclusion
For several years, scientific evidence has shown the link between inflammation and cancer. In this context, it is clear that an inflammatory microenvironment is an essential assumption for the development of most tumors. Each cell and the proportion of specific inflammatory cell types in tumoral microenvironment could have a polarized role in tumor progression or tumor suppression according to the production of autocrine, paracrine and endocrine substances.
Moreover, inflammation is able to determine the production of angiogenetic factors and to promote the survival of tumor cells through a direct route, that is, the increase in oxygen supply and nutrients to tumor tissue, and an indirect route, which increases the recruitment of proinflammatory cells and releases cytokines that promote growth, invasion of tumor metastasis.
Several chronic inflammatory diseases are also able to determine an increase of cancer risk as happens in patients affected by IBD or COPD. In these chronic inflammations, in addition to the cytokine proinflammatory effects, several molecular alterations are also involved in the inflammation-induced carcinogenesis, such as inactivation of tumor-suppressor genes, oncogene mutations, loss of heterozygosity, and chromosomal and microsatellite instability.
A common mediator of carcinogenesis in inflamed tissues is the imbalance of oxidative stress induced by inflammation in a normal tissue and sustained by microenviromental inflammation in a context of malignant tumor.
Another factor involved in the generation of chronic inflammation that sustains cancer is represented by endotoxemia. The communication between “gut microbiota system” and liver, brain, adipose tissue and other organs of human body is regulated by intestinal permeability (IP). Its increase, in relation to a dysbiotic gut, can be considered a key point for the comprehension of some pathogenetic mechanisms of systemic and liver disorders as well as cancer. Indeed, there is a relationship between IP and plasma levels of proinflammatory cytokines, nitric oxide, and nitrite levels in patients with various types and degrees of chronic liver diseases.
However, despite the growing interest regarding this field, nowadays, a standardized protocol to study the relationship between gut microbiota and cancer especially in the interpretation of study results does not exist. The strongest scientific evidence of this relationship exists in relation to tumors of gastrointestinal tract. For this reason, we focused our attention on the pathogenetic mechanisms sustained by gut-derived inflammation in the development of gastrointestinal cancer.
Bearing in mind these mechanisms that link inflammation, oxidative stress and IP to cancer, it could be useful to plan the prophylaxis and the therapy of cancer in the next future.
References
- 1.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 2.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer — a population-based study. N Engl Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 3.David H. Rudolf Virchow and modern aspects of tumor pathology. Pathol Res Pract. 1988;183:356–364. doi: 10.1016/S0344-0338(88)80138-9. [DOI] [PubMed] [Google Scholar]
- 4.Cho CH, Yu J. World Scientific; Singapore: 2012. From inflammation to cancer: advances in diagnosis and therapy for gastrointestinal and hepatological diseases. [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Nishihara R, Zhang X, Ogino S, Qian ZR. Energy sensing pathways: bridging type 2 diabetes and colorectal cancer? J Diabetes Complications. 2017;31:1228–1236. doi: 10.1016/j.jdiacomp.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 9.Benson AK, Kelly SA, Legge R, Ma F, SJ, Low J, Kim M, Zhang PL Oh, Nehrenberg D, Hua K. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A. 2010;107:18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottman N, Smidt H, de Vos WM, Belzer C. The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol. 2012;2 doi: 10.3389/fcimb.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fändriks L. Roles of the gut in the metabolic syndrome: an overview. J Intern Med. 2017;281:319–336. doi: 10.1111/joim.12584. [DOI] [PubMed] [Google Scholar]
- 13.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2 doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiebiger U, Bereswill S, Heimesaat MM. Dissecting the interplay between intestinal microbiota and host immunity in health and disease: lessons learned from germfree and gnotobiotic animal models. Eur J Microbiol Immunol (Bp) 2016;6:253–271. doi: 10.1556/1886.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 16.Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, Theodorou V, Dekker J, Méheust A, de Wos WM. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312:G171–G193. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.S Wu, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long- term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogovskii VS. The linkage between inflammation and immune tolerance: interfering with inflammation in cancer. Curr Cancer Drug Targets. 2017;17:325–332. doi: 10.2174/1568009617666170109110816. [DOI] [PubMed] [Google Scholar]
- 21.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 23.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Jackaman C, Tomay F, Duong L, Abdol Razak NB, Pixley FJ, Metharom P, Nelson DJ. Aging and cancer: The role of macrophages and neutrophils. Ageing Res Rev. 2017;36:105–116. doi: 10.1016/j.arr.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 26.Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2003;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 27.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts SJ, Ng BY, Filler RB, Lewis J, Glusac EJ, Hayday AC, Tigelaar RE, Girardi M. Characterizing tumor-promoting T cells in chemically induced cutaneous carcinogenesis. Proc Natl Acad Sci U S A. 2007;104:6770–6775. doi: 10.1073/pnas.0604982104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 31.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 32.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 33.Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–648. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 34.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 35.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 37.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okazaki IM, Kotani A, Honjo T. Role of AID in tumorigenesis. Adv Immunol. 2007;94:245–273. doi: 10.1016/S0065-2776(06)94008-5. [DOI] [PubMed] [Google Scholar]
- 39.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 40.Cooper CS, Foster CS. Concepts of epigenetics in prostate cancer development. Br J Cancer. 2009;100:240–245. doi: 10.1038/sj.bjc.6604771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J. 2008;27:1671–1681. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai T, He G, Matsuzawa A, GY Yu, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 45.Koehne CH, Dubois RN. COX-2 inhibition and colorectal cancer. Semin Oncol. 2004;31:12–21. doi: 10.1053/j.seminoncol.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 46.Flossmann E, Rothwell PM. British Doctors Aspirin Trial and the UK-TIA Aspirin Trial. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 47.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 48.Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000;30:3–21. [PubMed] [Google Scholar]
- 49.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 50.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 51.Shigdar S, Li Y, Bhattacharya S, O'Connor M, Pu C, Lin J, Wang T, Xiang D, Kong L, Wei MQ. Inflammation and cancer stem cells. Cancer Lett. 2014;345:271–278. doi: 10.1016/j.canlet.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 52.Romano M, De Francesco F, Pirozzi G, Gringeri E, Boetto R, Di Domenico M, Zavan B, Ferraro GA, Cillo U. Expression of cancer stem cell biomarkers as a tool for a correct therapeutic approach to hepatocellular carcinoma. Oncoscience. 2015;2:443–456. doi: 10.18632/oncoscience.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romano M, De Francesco F, Gringeri E, Giordano A, GA Ferraro M, Domenico DI, Cillo U. Tumor microenvironment versus cancer stem cells in cholangiocarcinoma: synergistic effects? J Cell Physiol. 2016;231:768–776. doi: 10.1002/jcp.25190. [DOI] [PubMed] [Google Scholar]
- 54.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF- alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, Deuring J, Peppelenbosch MP, Kuipers EJ, de Haar C, van der Woude CJ. IL6-induced DNMT1 activity mediates SOCS3 promoter hypermethylation in ulcerative colitis-related colorectal cancer. Carcinogenesis. 2012;33:1889–1896. doi: 10.1093/carcin/bgs214. [DOI] [PubMed] [Google Scholar]
- 56.Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, Gores GJ. Sustained IL6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132:384–996. doi: 10.1053/j.gastro.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellenrieder V, Buck A, Gress TM. TGFbeta-regulated transcriptional mechanisms in cancer. Int J Gastrointest Cancer. 2002;31:61–69. doi: 10.1385/IJGC:31:1-3:61. [DOI] [PubMed] [Google Scholar]
- 58.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2009;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 59.Laurent C, Svrcek M, Flejou JF, Chenard MP, Duclos B, Freund JN, Reimund JM. Immunohistochemical expression of CDX2, β-catenin, and TP53 in inflammatory bowel disease- associated colorectal cancer. Inflamm Bowel Dis. 2011;17:232–240. doi: 10.1002/ibd.21451. [DOI] [PubMed] [Google Scholar]
- 60.Chang CL, Marra G, Chauhan DP, Ha HT, Chang DK, Ricciardiello L, Randolph A, Carethers JM, Boland CR. Oxidative stress inactivates the human DNA mismatch repair system. Am J Physiol Cell Physiol. 2002;283:C148–C154. doi: 10.1152/ajpcell.00422.2001. [DOI] [PubMed] [Google Scholar]
- 61.Gerrits MM, Chen M, Theeuwes M, van Dekken H, Sikkema M, Steyerberg EW, Lingsma HF, Siersema PD, Xia B, Kurster JG. Biomarker-based prediction of inflammatory bowel disease-related colorectal cancer: a case-control study. Cell Oncol (Dordr) 2011;34:107–117. doi: 10.1007/s13402-010-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toyota M, Ho C, Ahuja N, Jair KW, Li Q, Ohe-Toyota M, Baylin SB, Issa JP. Identification of differentially methylated sequences in colorectal cancer by methylated CpG island amplification. Cancer Res. 1999;59:2307–2312. [PubMed] [Google Scholar]
- 63.AS Fleisher M, Esteller N, Harpaz A, Leytin A, Rashid Y Xu, Liang J, Stine OC, Yin J, Zou TT. Microsatellite instability in inflammatory bowel disease- associated neoplastic lesions is associated with hypermethylation and diminished expression of the DNA mismatch repair gene, hMLH11. Cancer Res. 2000;60:4864–4868. [PubMed] [Google Scholar]
- 64.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Jr., Enright PL, Kanner RE, O'Hara P. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 65.Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, Macklem PT. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med. 1978;298:1277–1281. doi: 10.1056/NEJM197806082982303. [DOI] [PubMed] [Google Scholar]
- 66.Franklin WA, Gazdar AF, Haney J, Wistuba II, La Rosa FG, Kennedy T, Ritchey DM, Miller YE. Widely dispersed p 53 mutation in respiratory epithelium. A novel mechanism for field carcinogenesis. J Clin Invest. 1997;100:2133–2137. doi: 10.1172/JCI119748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minna JD, Fong K, Zochbauer-Muller S, Gazdar AF. Molecular pathogenesis of lung cancer and potential translational applications. Cancer J. 2002;8(Suppl. 1):S41–6. [PubMed] [Google Scholar]
- 68.Wistuba II, Mao L, Gazdar AF. Smoking molecular damage in bronchial epithelium. Oncogene. 2002;21:7298–7306. doi: 10.1038/sj.onc.1205806. [DOI] [PubMed] [Google Scholar]
- 69.Romeo MS, Sokolova IA, Morrison LE, Zeng C, Baron AE, Hirsch FR, Miller YE, Franklin WA, Varella-Garcia M. Chromosomal abnormalities in non-small cell lung carcinomas and in bronchial epithelia of high-risk smokers detected by multi-target interphase fluorescence in situ hybridization. J Mol Diagn. 2003;5:103–112. doi: 10.1016/s1525-1578(10)60459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Boer WI, Hau CM, van Schadewijk A, Stolk J, van Krieken JH, Hiemstra PS. Expression of epidermal growth factors and their receptors in the bronchial epithelium of subjects with chronic obstructive pulmonary disease. Am J Clin Pathol. 2006;125:184–192. doi: 10.1309/W1AX-KGT7-UA37-X257. [DOI] [PubMed] [Google Scholar]
- 71.Liu K, Anderson GP, Bozinovski S. DNA vector augments inflammation in epithelial cells via EGFR-dependent regulation of TLR4 and TLR2. Am J Respir Cell Mol Biol. 2008;39:305–311. doi: 10.1165/rcmb.2007-0458OC. [DOI] [PubMed] [Google Scholar]
- 72.Liu K, Gualano RC, Hibbs ML, Anderson GP, Bozinovski S. Epidermal growth factor receptor signaling to Erk 1/2 and STATs control the intensity of the epithelial inflammatory responses to rhinovirus infection. J Biol Chem. 2008;283:9977–9985. doi: 10.1074/jbc.M710257200. [DOI] [PubMed] [Google Scholar]
- 73.Gustafson AM, Soldi R, Anderlind C, Scholand MB, Qian J, Zhang X, Cooper K, Walker D, McWilliams A, Liu G. Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci Transl Med. 2010;2:26ra25d. doi: 10.1126/scitranslmed.3000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drilon A, Rekhtman N, Ladanyi M, Paik P. Squamous-cell carcinomas of the lung: emerging biology, controversies, and the promise of targeted therapy. Lancet Oncol. 2012;13:e418–e426. doi: 10.1016/S1470-2045(12)70291-7. [DOI] [PubMed] [Google Scholar]
- 75.Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51:181–191. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Lim WT, Zhang WH, Miller CR, Watters JW, Gao F, Viswanathan A, Govindan R, McLeod HL. PTEN and phosphorylated AKT expression and prognosis in early and late-stage non-–small cell lung cancer. Oncol Rep. 2007;17:853–857. [PubMed] [Google Scholar]
- 77.Soria JC, Lee HY, Lee JI, Wang L, Issa JP, Kemp BL, Liu DD, Kurie JM, Mao L, Khuri FR. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin Cancer Res. 2002;8:1178–1184. [PubMed] [Google Scholar]
- 78.Guerra C, Schumacher AJ, Canamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 79.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF–MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shchors K, Shchors E, Rostker F, Lawlor ER, Brown-Swigart L, Evan GI. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1β. Genes Dev. 2006;20:2527–2538. doi: 10.1101/gad.1455706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 83.Kroncke KD. Nitrosative stress and transcription. Biol Chem. 2003;384:1365–1377. doi: 10.1515/BC.2003.153. [DOI] [PubMed] [Google Scholar]
- 84.Baron JA, Sandler RS. Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu Rev Med. 2000;51:511–523. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- 85.Sata N, Klonowski-Stumpe H, Han B, Haussinger D, Niederau C. Cytotoxicity of peroxynitrite in rat pancreatic acinar AR4-2J cells. Pancreas. 1997;15:278–284. doi: 10.1097/00006676-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 86.Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991;350:427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- 87.Albring M, Griffith J, Attardi G. Association of a protein structure of probable membrane derivation with HeLa cell mitochondrial DNA near its origin of replication. Proc Natl Acad Sci U S A. 1977;74:1348–1352. doi: 10.1073/pnas.74.4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 89.Pastukh VM, Zhang L, Ruchko MV, Gorodnya O, Bardwell GC, Tuder RM, Gillespie MN. Oxidative DNA damage in lung tissue from patients with COPD is clustered in functionally significant sequences. Int J Chron Obstruct Pulmon Dis. 2011;6:209–217. doi: 10.2147/COPD.S15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rahman I. Pharmacological antioxidant strategies as therapeutic interventions for COPD. Biochim Biophys Acta. 2012;1822:714–728. doi: 10.1016/j.bbadis.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: a controversial issue. A tribute to Ernst L. Wynder. Chem Res Toxicol. 2001;14:767–790. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- 92.Dekhuijzen PN, Aben KK, Dekker I, Aarts LP, Wielders PL, van Herwaarden CL, Bast A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1991;154:813–816. doi: 10.1164/ajrccm.154.3.8810624. [DOI] [PubMed] [Google Scholar]
- 93.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, KH Yu, Yeo CJ, Calhoun ES. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Covey TM, Edes K, Coombs GS, Virshup DM, Fitzpatrick FA. Alkylation of the tumor suppressor PTEN activates Akt and beta-catenin signaling: a mechanism linking inflammation and oxidative stress with cancer. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garte S. Metabolic susceptibility genes as cancer risk factors: time for a reassessment? Cancer Epidemiol Biomarkers Prev. 2001;10:1233–1237. [PubMed] [Google Scholar]
- 97.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 98.Nadal I, Santacruz A, Marcos A, Warnberg J, Garagorri JM, Moreno LA, Martin-Matillas M, Campoy C, Martí A, Moleres A. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes. 2009;33:758–767. doi: 10.1038/ijo.2008.260. [DOI] [PubMed] [Google Scholar]
- 99.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 101.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 103.Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology. 2014;59:328–339. doi: 10.1002/hep.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cariello R, Federico A, Sapone A, Tuccillo C, Scialdone VR, Tiso A, Miranda A, Portincasa P, Carbonara V, Palasciano G. Intestinal permeability in patients with chronic liver diseases: Its relationship with the aetiology and the entity of liver damage. Dig Liver Dis. 2010;42:200–204. doi: 10.1016/j.dld.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 105.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 106.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10:324–335. doi: 10.1016/j.chom.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: The evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102:365–367. doi: 10.1093/jnci/djq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ogino S, Nosho K, Meyerhardt JA, Kirkner GJ, Chan AT, Kawasaki T, Giovannucci EL, Loda M, Fuchs CS. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol. 2008;26:5713–5720. doi: 10.1200/JCO.2008.18.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tilg H, Moschen AR. Food, immunity, and the microbiome. Gastroenterology. 2015;148:1107–1119. doi: 10.1053/j.gastro.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 112.Rajpoot M, Sharma AK, Sharma A, Gupta GK. Understanding the microbiome: emerging biomarkers for exploiting the microbiota for personalized medicine against cancer. Semin Cancer Biol. 2018 doi: 10.1016/j.semcancer.2018.02.003. [S1044-579X:30146-3] [DOI] [PubMed] [Google Scholar]
- 113.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaudone M, Rauber C, Roberti MP. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 114.Tahara T, Yamamoto E, Suzuki H, Maruyama R, Chung W, Garriga J, Jelinek J, Yamano HO, Sugai T, An B. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014;74:1311–1318. doi: 10.1158/0008-5472.CAN-13-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, Kurihara H, Kanno S, Yamamoto I, Ishigami K, Igarashi H. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016;22:557–566. doi: 10.3748/wjg.v22.i2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, Kim SA, Masuda A, Nowak JA, Nosho K. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015;1:653–661. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.T Yu, Guo F, Y Yu, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170:548–563. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abreu MT, Peek RM., Jr. Gastrointestinal malignancy and the microbiome. Gastroenterology. 2014;146:1534–1546. doi: 10.1053/j.gastro.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peek RM, Jr., Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 120.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality world-wide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 122.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 123.Amieva M, Peek RM., Jr. Pathobiology of Helicobacter pylori–induced gastric cancer. Gastroenterology. 2016;150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wroblewski LE, Peek RM., Jr. Helicobacter pylori in gastric carcinogenesis: mechanisms. Gastroenterol Clin North Am. 2013;42:285–298. doi: 10.1016/j.gtc.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miehlke S, Kirsch C, Agha-Amiri K, Gunther T, Lehn N, Malfertheiner P, Stolte M, Ehninger G, Bayerdörffer E. The Helicobacter pylori vacA s1, m1 genotype and cagA is associated with gastric carcinoma in Germany. Int J Cancer. 2000;87:322–327. [PubMed] [Google Scholar]
- 126.Andersson AF, Lindberg M, Jakobsson H, Bäckhed F, Nyrén P, Engstrand L. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 128.Eun CS, Kim BK, Han DS, Kim SY, Kim KM, Choi BY, Song KS, Kim YS, Kim JF. Differences in gastric mucosal microbiota profiling in patients with chronic gastritis, intestinal metaplasia, and gastric cancer using pyrosequencing methods. Helicobacter. 2014;19:407–416. doi: 10.1111/hel.12145. [DOI] [PubMed] [Google Scholar]
- 129.Yang I, Woltemate S, Piazuelo MB, Bravo LE, Yepez MC, Romero-Gallo J, Delgado AG, Wilson KT, Peek RM, Correa P. Different gastric microbiota compositions in two human populations with high and low gastric cancer risk in Colombia. Sci Rep. 2016;6:18594. doi: 10.1038/srep18594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 131.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr., Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hagland HR, Søreide K. Cellular metabolism in colorectal carcinogenesis: influence of lifestyle, gut microbiome and metabolic pathways. Cancer Lett. 2016;356:273–280. doi: 10.1016/j.canlet.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 133.Janakiram NB, Rao CV. The role of inflammation in colon cancer. Adv Exp Med Biol. 2014;816:25–52. doi: 10.1007/978-3-0348-0837-8_2. [DOI] [PubMed] [Google Scholar]
- 134.Speed N, Blair IA. Cyclooxygenase- and lipoxygenase-mediated DNA damage. Cancer Metastasis Rev. 2011;30:437–447. doi: 10.1007/s10555-011-9298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang X, Yang Y, Huycke MM. Microbiome-driven carcinogenesis in colorectal cancer: Models and mechanisms. Free Radic Biol Med. 2017;105:3–15. doi: 10.1016/j.freeradbiomed.2016.10.504. [DOI] [PubMed] [Google Scholar]
- 136.Johnson CH, Dejea CM, Edler D, Hoang LT, Santidrian AF, Felding BH. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015;21:891–897. doi: 10.1016/j.cmet.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesion. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Engle SJ, Ormsby I, Pawlowski S, Boivin GP, Croft J, Balish E, Doetschman T. Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Res. 2002;62:6362–6366. [PubMed] [Google Scholar]
- 139.Kado S, Uchida K, Funabashi H, Iwata S, Nagata Y, Ando M, Onoue M, Matsuoka Y, Ohwaki M, Morotomi M. Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor beta chain and p53 double-knockout mice. Cancer Res. 2001;61:2395–2398. [PubMed] [Google Scholar]
- 140.Zhan Y, Chen PJ, Sadler WD, Wang F, Poe S, Nuñez G, Eaton KA, Chen GY. Gut microbiota protects against gastrointestinal tumorigenesis caused by epithelial injury. Cancer Res. 2013;73:7199–7210. doi: 10.1158/0008-5472.CAN-13-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, Sharma D, Li L, Malireddi RK, Gurung P. Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell. 2015;162:45–58. doi: 10.1016/j.cell.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Investig. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Negroni A, Cucchiara S, Stronati L. Apoptosis, necrosis, and necroptosis in the gut and intestinal homeostasis. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/250762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Loktionov A. Cell exfoliation in the human colon: myth, reality and implications for colorectal cancer screening. Int J Cancer. 2007;120:2281–2289. doi: 10.1002/ijc.22647. [DOI] [PubMed] [Google Scholar]
- 145.Larsson JM, Karlsson H, Crespo JG, Johansson ME, Eklund L, Sjovall H, Hansson JC. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm Bowel Dis. 2011;17:2299–2307. doi: 10.1002/ibd.21625. [DOI] [PubMed] [Google Scholar]
- 146.Prorok-Hamon M, Friswell MK, Alswied A, Roberts CL, Song F, Flanagan PK, Knight P, Codling C, Marchesi JR, Winstanley C. Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut. 2014;63:761–770. doi: 10.1136/gutjnl-2013-304739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, Peterson SN, Snesrud EC, Borisy GG, Lazarev M. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A. 2014;111:18321–18326. doi: 10.1073/pnas.1406199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang X, Huycke MM. Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology. 2007;132:551–561. doi: 10.1053/j.gastro.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 149.Kim JM, Lee JY, Yoon YM, Oh YK, Kang JS, Kim YJ, Kim KH. Bacteroides fragilis enterotoxin induces cyclooxygenase-2 and fluid secretion in intestinal epithelial cells through NF-kappaB activation. Eur J Immunol. 2006;36:2446–2456. doi: 10.1002/eji.200535808. [DOI] [PubMed] [Google Scholar]
- 150.Fukata M, Abreu MT. Role of toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27:234–243. doi: 10.1038/sj.onc.1210908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, LS Thomas R Xu, Inoue H, Arditi M, Dannenberg AJ. Cox-2 is regulated by Toll-like Receptor-4 (TLR4) signaling: role in proliferation and Apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schirbel A, Kessler S, Rieder F, West G, Rebert N, Asosingh K, McDonald C, Fiocchi C. Pro-angiogenic activity of TLRs and NLRs: a novel link between gut microbiota and intestinal angiogenesis. Gastroenterology. 2013;144:613-623.e9. doi: 10.1053/j.gastro.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Boonanantanasarn K, Gill AL, Yap Y, Jayaprakash V, Sullivan MA, Gill SR. Enterococcus faecalis enhances cell proliferation through hydrogen peroxide-mediated epidermal growth factor receptor activation. Infect Immun. 2012;80:3545–3558. doi: 10.1128/IAI.00479-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim JM, Lee JY, Kim YJ. Inhibition of apoptosis in Bacteroides fragilis enterotoxin-stimulated intestinal epithelial cells through the induction of c-IAP-2. Eur J. 2008;38:2190–2199. doi: 10.1002/eji.200838191. [DOI] [PubMed] [Google Scholar]
- 155.Mehlen P, Delloye-Bourgeois C, Chédotal A. Novel roles for Slits and netrins: axon guidance cues as anticancer targets? Nat Rev Cancer. 2001;11:188–197. doi: 10.1038/nrc3005. [DOI] [PubMed] [Google Scholar]
- 156.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Lyon, France: International Agency for Research on Cancer; 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] http://globocan.iarc.fr [cited 2017 Dec 1]. Available from.
- 157.De Minicis S, Rychlicki C, Agostinelli L, Saccomanno S, Candelaresi C, Trozzi L, Mingarelli E, Facinelli B, Magi G, Palmieri C. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59:1738–1749. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 158.Son G, Kremer M, Hines IN. Contribution of gut bacteria to liver pathobiology. Gastroenterol Res Pract. 2010;2010 doi: 10.1155/2010/453563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liaskou E, Wilson DV, Oo YH. Innate immune cells in liver inflammation. Mediators Inflamm. 2012;2012:949157. doi: 10.1155/2012/949157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tsiaoussis GI, Assimakopoulos SF, Tsamandas AC, Triantos CK, Thomopoulos KC. Intestinal barrier dysfunction in cirrhosis: current concepts in pathophysiology and clinical implications. World J Hepatol. 2015;7:2058–2068. doi: 10.4254/wjh.v7.i17.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Darnaud M, Faivre J, Moniaux N. Targeting gut flora to prevent progression of hepatocellular carcinoma. J Hepatol. 2013;58:385–387. doi: 10.1016/j.jhep.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 162.Roh YS, Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J Gastroenterol Hepatol. 2013;28(Suppl. 1):38–42. doi: 10.1111/jgh.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 165.Stickel F, Hellerbrand C. Nonalcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59:1303–1307. doi: 10.1136/gut.2009.199661. [DOI] [PubMed] [Google Scholar]
- 166.Schnabl B, Purbeck CA, Choi YH, Hagedorn CH, Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–664. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- 167.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 168.Mitsuhashi K, Nosho K, Sukawa Y, Matsunaga Y, Ito M, Kurihara H, Kanno S, Igarashi H, Naito T, Adachi Y. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget. 2015;6:7209–7220. doi: 10.18632/oncotarget.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zambirinis CP, Pushalkar S, Saxena D, Miller G. Pancreatic cancer, inflammation, and microbiome. Cancer J. 2014;20:195–202. doi: 10.1097/PPO.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]