Abstract
Background
Children exposed to gestational diabetes mellitus (GDM) in utero are at increased risk of neurodevelopmental difficulties, including autism and impaired motor control. However, the underlying neurophysiology is unknown.
Methods
Using transcranial magnetic stimulation, we assessed cortical excitability, long-term depression (LTD)-like neuroplasticity in 45 GDM-exposed and 12 control children aged 11–13 years. Data were analysed against salivary cortisol and maternal diabetes severity and treatment (insulin [N = 22] or metformin [N = 23]) during pregnancy.
Findings
GDM-exposed children had reduced cortical excitability (p = .003), LTD-like neuroplasticity (p = .005), and salivary cortisol (p < .001) when compared with control children. Higher maternal insulin resistance (IR) before and during GDM treatment was associated with a blunted neuroplastic response in children (p = .014) and this was not accounted for by maternal BMI. Additional maternal and neonatal measures, including fasting plasma glucose and inflammatory markers, predicted neurophysiological outcomes. The metformin and insulin treatment groups had similar outcomes.
Interpretation
These results suggest that GDM can contribute to subtle differences in child neurophysiology, and possibly cortisol secretion, persisting into early adolescence. Importantly, these effects appear to occur during second trimester, before pharmacologic treatment typically commences, and can be predicted by maternal insulin resistance. Therefore, earlier detection and treatment of GDM may be warranted. Metformin appears to be safe for these aspects of neurodevelopment.
Abbreviations: AMT, active motor threshold; cTBS, continuous theta burst stimulation; EMG, electromyogram; GA, gestational age; GDM, gestational diabetes mellitus; HOMA-IR, homeostatic model assessment for insulin resistance; HPA, hypothalamic-pituitary-adrenal; SI1mV, stimulus intensity to evoke 1 mV response; TMS, transcranial magnetic stimulation; IR, insulin resistance; LTD, long term depression; MEP, motor evoked potential; MiG, Metformin in Gestational diabetes trial; NMDAR, N-methyl-d-aspartate receptor; nmol/L, nano-moles per litre; RMT, resting motor threshold
Keywords: Transcranial magnetic stimulation, Hypothalamic-pituitary-adrenal axis, Neurodevelopment, Hyperglycaemia, Metformin, Insulin
Highlights
-
•
Children exposed to gestational diabetes (GDM) had lower cortical excitability, measured as higher resting motor thresholds.
-
•
The GDM group also exhibited smaller LTD-like neuroplastic responses to repetitive brain stimulation.
-
•
These were associated with lower salivary cortisol and with maternal diabetes severity, especially insulin resistance.
Our results suggest that gestational diabetes, even when detected and treated as per clinical guidelines, has subtle effects on important neurophysiological processes persisting into early adolescence, including neuroplasticity and possibly cortisol secretion. The extent of these effects is related to the severity of maternal diabetes, particularly insulin resistance, during mid pregnancy. These results may aid in understanding how insulin resistance and hyperglycemia affect the developing brain. Further, our results indicate that earlier detection and pharmacologic treatment of gestational diabetes may attenuate or prevent these changes to neurodevelopment, and that maternal metformin treatment does not influence these aspects of development.
1. Introduction
Gestational diabetes mellitus (GDM) affects 5–10% of pregnancies, with a higher prevalence in obese women (WHO, 2014). Emerging evidence indicates that children exposed in utero to GDM are at higher risk of neurodevelopmental difficulties, including attention deficit hyperactivity disorder (Nomura et al., 2012), autism spectrum disorders (Xu et al., 2014), and impaired motor development (Ornoy et al., 1999). Additionally, maternal obesity has independently been associated with a range of neurodevelopmental and psychiatric disorders in the offspring (Edlow, 2016). Animal research underpins the hypothesis that oxidative stress and inflammation associated with maternal hyperglycemia are major drivers of altered neurodevelopment in GDM-affected fetuses (Sullivan et al., 2014), while obesity is associated with a chronic, low-grade, metabolically-induced inflammatory state (Pantham et al., 2015). Placental inflammation is observed in obesity- and GDM-affected pregnancies (Saloman et al., 2016), and intrauterine inflammation can evoke fetal brain injury (Elovitz et al., 2011). Further, maternal hyperglycemia can retard dendritic development in the fetal brain (Jing et al., 2014). Taken together, these findings suggest that GDM-exposed fetuses experience an adverse environment in utero that contributes to abnormal neurodevelopment. However, there are currently no neurophysiological data in children exposed to GDM (or maternal obesity), so the mechanisms underlying these neurodevelopmental disturbances are unknown.
Evidence for a link between inflammation and suboptimal neurodevelopment also comes from studies of children born preterm. While the aetiology of preterm birth is multifactorial, the only established pathological, causal factor is infection and/or inflammation (Romero et al., 2007), the exposure to which significantly increases the risk of alterations in cortical microstructure and functional connectivity (Counsell et al., 2008), and poor neurodevelopmental outcomes postnatally (Leviton et al., 2013). These subtle but significant changes are believed to underlie the high incidence of low severity neurodevelopmental impairments commonly reported in children born preterm, including cognitive, motor and behavioural impairments (Mwaniki et al., 2012) and altered neuroplasticity (i.e. the brain's ability to alter the strength of its synaptic connections) (Pitcher et al., 2012a). While GDM-exposed children born at term exhibit some of the same neurodevelopmental impairments as children born preterm, it is currently unknown if they exhibit similar abnormalities in neuroplasticity. Since neuroplasticity is widely accepted as a key mechanism underlying learning and memory, abnormalities in neuroplasticity may help to explain the physiological processes responsible for adverse neurodevelopmental outcomes in children exposed to GDM in utero.
Here we used transcranial magnetic stimulation (TMS) techniques to investigate cortical excitability and the capacity for long-term depression (LTD)-like neuroplasticity in children born to women enrolled in the Metformin in Gestational diabetes (MiG) randomised controlled trial (Rowan et al., 2008) to explore potential associations between maternal hyperglycemia and cortical function in the offspring. The MiG trial examined the safety and efficacy of the oral anti-hyperglycemic agent metformin versus insulin to treat GDM, and demonstrated that metformin is equally effective and safe as insulin for both mother and child (Barrett et al., 2013; Battin et al., 2015; Rowan et al., 2011; Wouldes et al., 2016). A secondary aim of the present study was to compare the neurophysiologic outcomes of metformin- versus insulin-exposed children, as there remains a lack of long-term and neurophysiological data assessing the potential impact of metformin. This is important because, unlike exogenous insulin, metformin crosses the placenta (Charles et al., 2006) and interacts with the fetus in largely unknown ways. Reassuringly, the available evidence suggests that the likely effects of metformin in the fetus are anti-inflammatory (Scheen et al., 2015) and neuroprotective (Chung et al., 2015). Thus, metformin may benefit the fetal brain in GDM-exposed pregnancies beyond its role in maternal glycemic control.
2. Methods
2.1. Ethical Approval
All procedures were approved by the Women's and Children's Health Network and University of Adelaide Human Research Ethics Committees, and conducted in accordance with the Declaration of Helsinki (2008 revision). Participants were pre-screened for contraindications to TMS (Rossi et al., 2009). Parents provided written, informed consent and accompanied their children to the experimental session.
2.2. Subjects
45 GDM-exposed (age: 11.8 ± 0.7 years [mean ± SD], 20 females) were recruited from the Adelaide arm of the MiG trial (Rowan et al., 2008). Their mothers had been treated for GDM not responding to lifestyle alteration, with a 1:1 random allocation at study entry to receive either insulin or metformin treatment (at 30 ± 2.6 weeks gestation). Eight of the metformin-treated women in the current study had required supplemental insulin to achieve euglycemia, but there was no difference in demographic or clinical characteristics of these women compared with the metformin-only treatment women, and these subjects are included in the metformin group. Twelve control children not exposed to GDM (age: 12.8 ± 0.8 years, 8 females) were recruited from labour ward records and matched as closely as possible for gestational age at birth (GA). Mothers in the GDM group had higher body mass indices (BMIs) (34.1 ± 6.8) than control group mothers (23.7 ± 4.6; p < .001).
2.3. Maternal Measures
Insulin resistance was measured in mothers in the MiG trial using the homeostatic model assessment of insulin resistance at trial entry (HOMA-IR-Trial) at 30 ± 2.6 weeks gestation (mean ± SD) and at 36 weeks gestation (HOMA-IR-36). Additional perinatal records were obtained.
2.4. Recording Procedures
Children were seated with their hands and forearms supported. Adhesive Ag/AgCl bipolar surface electrodes were applied over the right first dorsal interosseous (FDI) hand muscle to obtain surface electromyography (EMG) recordings. EMG signals were amplified (×1000; 1902 amplifier; CED), bandpass filtered (20 Hz–1 kHz), and digitized at 5 kHz (1401 interface; CED), and were stored offline for later analysis. Researchers were blinded to the treatment status of the participant's mother when collecting and analysing data.
2.5. Transcranial Magnetic Stimulation (TMS)
TMS is a non-invasive brain stimulation technique in which the motor cortex is electromagnetically stimulated to produce a motor evoked potential (MEP) recorded in a contralateral muscle using EMG (Di Lazzaro and Rothwell, 2014). Motor cortical excitability was assessed with single-pulse TMS applied to the left primary motor cortex (M1) representation of the right FDI muscle using a 70 mm figure-of-eight coil connected to a monophasic Magstim 2002 stimulator (Magstim Co, Whitland, UK). The coil was oriented with the handle pointing postero-laterally at a 45° angle to the sagittal plane, producing a posterior-anterior current flow across M1. The optimal site for consistently evoking MEPs in the FDI was determined and marked on the scalp. Resting motor threshold (RMT) was determined as the lowest TMS intensity required to evoke MEPs of at least 50 μV peak-to-peak amplitude in the resting FDI in at least five of ten consecutive trials. Active motor threshold (AMT) was assessed while the subject maintained a voluntary contraction of approximately 10% of their maximum for FDI, and determined as the lowest TMS intensity required to evoke MEPs of at least 200 μV peak-to-peak amplitude in at least five of ten consecutive trials. The TMS intensity that evoked MEPs of ~1 mV peak-to-peak amplitude (SI1mV) was also determined, and used throughout the experiment for evoking test MEPs (Pitcher et al., 2015).
2.6. LTD-like Neuroplasticity Induction With cTBS
Continuous theta burst stimulation (cTBS; a repetitive TMS protocol) over M1 was used to induce LTD-like suppression of MEP amplitudes. Pharmacological studies indicate cTBS-induced MEP suppression is NMDA-receptor-dependent and similar mechanistically to LTD (Huang et al., 2007). An air-cooled figure-of-eight coil connected to a Magstim Super Rapid stimulator (Magstim, Whitland, UK) was used to apply repetitive TMS to the optimal site for stimulating the right FDI. The cTBS protocol consists of 600 pulses applied in bursts of three pulses at 50 Hz, repeated at 5 Hz for a total of 40 s (Huang et al., 2005). Stimulation intensity was set to 80% of AMT. MEPs were recorded in blocks of 15 trials prior to cTBS (i.e. baseline) and at 0, 5, 10, 20 and 30 min following cTBS. The peak-to-peak amplitudes of the 15 MEPs in each block were measured and a mean amplitude calculated. Changes in MEP amplitude relative to baseline MEP amplitude were used as an index of LTD-like plasticity (Pitcher et al., 2012a). All MEPs were recorded at high gain and any with obvious EMG activity in the 100 ms before the TMS stimulus were discarded. Mean variability (coefficient of variation, COV) of MEP amplitudes was also calculated to determine correlation with plasticity induction (Hordacre et al., 2017).
2.7. Salivary Cortisol
Saliva samples were obtained from each child immediately before TMS baseline measures (13:22 ± 0.25 h) using a Salivette (Sarstedt) and standardized collection procedures. Salivettes were centrifuged to obtain saliva, which was stored at −20 °C until assayed. Twenty-five microliter aliquots of saliva were assayed in duplicate for cortisol by enzyme-linked immunosorbent assay (ELISA) according to manufacturer's instructions (HS-Cortisol; Salimetrics).
2.8. Statistical Analysis
Data were analysed using SPSS (Version 24; IBM). Repeated-measures ANCOVAs (rmANCOVAs) with polynomial contrasts were performed with Bonferroni's correction for multiple comparisons. Between-subject factors were GDM group (GDM or Control) and Treatment Group (Control, Insulin, or Metformin). The within-subject factor was Time (0, 5, 10, 20, or 30 minute post-cTBS). All potential covariates were assessed for statistical significance. Gestational age at birth (days) was found significant and remained as a covariate. Separate rmANOVAs for each treatment and GDM group were conducted on the raw MEP data with the factor Time (baseline, 0, 5, 10, 20, and 30 minute post-cTBS). Post-hoc comparisons were conducted where indicated to compare the post-cTBS and baseline MEP amplitudes. Linear regression analyses were used to examine the relations between cortisol, maternal characteristics, and mean and peak response to cTBS (Table 1). Mediation analyses, using the PROCESSING macro for SPSS (see Hayes and Rockwood, 2017), were performed using cortisol as a potential mediator. All associations were checked statistically and visually for the presence of potential outliers and to check for normality of data.
Table 1.
Associations between maternal and neonatal markers and child neurophysiological outcomes in GDM-exposed children.
| Child physiological outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Maternal and neonatal characteristics | RMT | SI1mV | Peak LTD | Mean LTD | Response Direction | MEP COV | Cortisol | |
| Insulin resistance – rand | # | |||||||
| ↓ | ||||||||
| Insulin resistance – 36 wks | # | ↓ ⁎ | ↑ ⁎⁎ | |||||
| ↓ | n = 26 | n = 26 | ||||||
| Fasting plasma glucose – rand | ↓ ⁎ | |||||||
| n = 28 | ||||||||
| Fasting plasma glucose – 36 wks | ↓ ⁎⁎ | |||||||
| n = 28 | ||||||||
| Insulin – 36 wks | # | ↓ ⁎⁎ | ↑ ⁎⁎ | |||||
| ↓ | n = 26 | n = 26 | ||||||
| C-peptide – rand | ↓ ⁎ | # | ||||||
| n = 25 | ||||||||
| Cholesterol – 36 wks | ↓ ⁎ | ↓ ⁎ | ||||||
| n = 27 | n = 27 | |||||||
| Maternal BMI – rand | # | |||||||
| Previous GDM diagnosis | ↑ ⁎ | ↑ ⁎⁎ | # | # | ||||
| n = 41 | n = 32 | ↑ | ↑ | |||||
| Cord C-peptide | ↓ ⁎ | |||||||
| n = 14 | ||||||||
| Cord C-reactive protein | ↑ ⁎⁎ | |||||||
| n = 29 | ||||||||
| Cord triglycerides | ↓ ⁎ | ↓ ⁎ | ↑ ⁎⁎ | |||||
| n = 25 | n = 25 | n = 25 | ||||||
| Neonatal hypoglycemia | # | ↓ ⁎ | ↑ ⁎ | ↓ ⁎ | ||||
| n = 33 | n = 33 | n = 38 | ||||||
| Apgar-5 | ↓ ⁎ | |||||||
| n = 35 | ||||||||
| Baby weight (g) | ↑ ⁎ | ↓ ⁎ | ||||||
| n = 34 | n = 37 | |||||||
Significant correlations are indicated with an arrow. “Rand” denotes measurements at trial entry (randomisation); ‘36 wks’ denotes measurements at 36 weeks gestation. Arrows indicate direction of relationship.
p < .05.
p < .01.
Denotes a correlation where p < .08 but >0.05.
3. Results
3.1. Baseline Characteristics and Cortical Excitability
RMTs (% maximum stimulator output) were higher in the GDM group (57.9 ± 12.3% [mean ± SD], N = 42) than in the Control group (44.9 ± 5.7%, N = 12) when corrected for age (F(1,51) = 6.41, p = .003, ηp2 = 0.199) (Fig. 1), but did not differ between the Metformin (58.8 ± 12.7%, N = 22) and Insulin (56.9 ± 12.1%, N = 21) groups (p = .743). Similarly, the stimulator intensity (% maximum stimulator output) required to evoke test MEPs (SI1mV), AMT and cTBS intensity was higher in the GDM group (SI1mV, 70.0 ± 14.3%; AMT, 52.6 ± 7.9%; cTBSi, 42.2 ± 5.8%; all p < .001) compared with controls (SI1mV, 57.3 ± 13.8%; AMT, 43.9 ± 7.1%; cTBSi, 35.2 ± 5.5%), but did not differ between the Metformin and Insulin groups (all p > .364). Children in the Control group were older than those in the Metformin and Insulin groups, but there was no effect of age on any primary analyses when correcting for salivary cortisol concentration or gestational age at birth. There were no effects of child sex or level of maternal education on any of the primary outcomes.
Fig. 1.
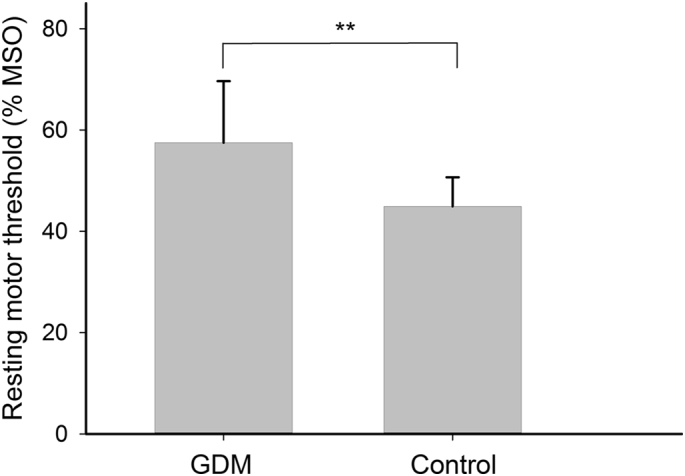
Cortical excitability. Resting motor threshold (RMT) was higher in the GDM group compared with Controls, indicating lower cortical excitability. **p < .001.
Ten of the GDM-exposed participants (22%) were unsuitable for the neuroplasticity analyses; 3 had RMTs which exceeded the maximum stimulator output, and 7 had assessable RMTs (M = 75.9) but were not able to produce supra-threshold test MEPs approaching 1 mV (SI1mV) required for the neuroplasticity induction protocol. The 12 Control participants were all able to be assessed (difference, χ2 = 3.23, φ = 0.238, p = .072).
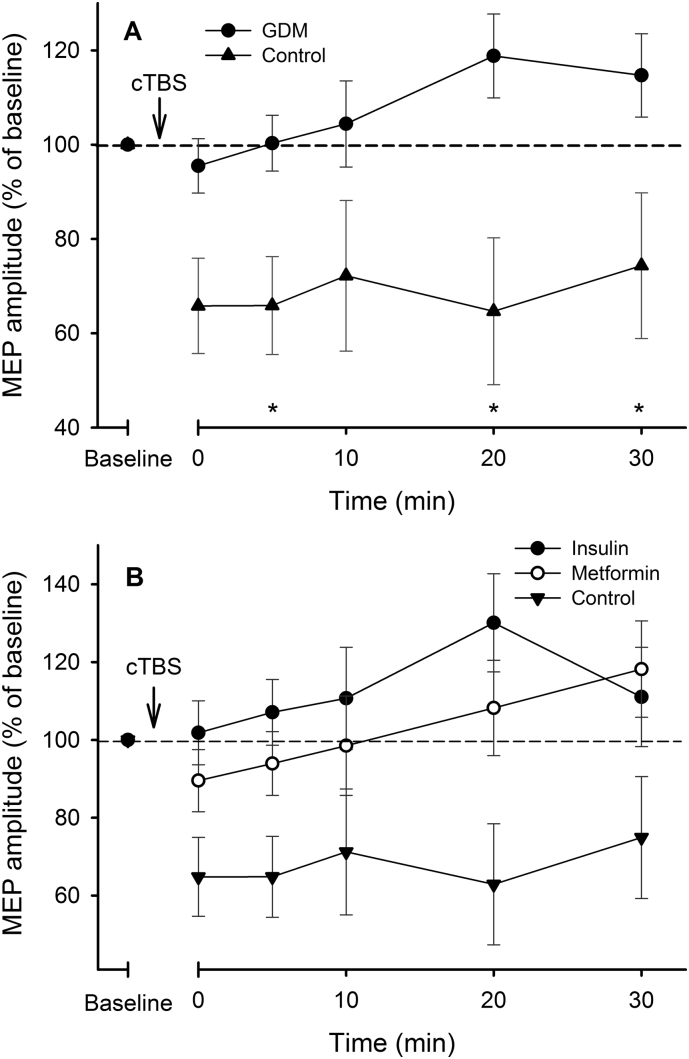
3.2. LTD-like Neuroplasticity is Reduced in Children Exposed to GDM
The neuroplastic response to cTBS was blunted or absent in the GDM-exposed group compared with the Control group after correcting for GA (F(1,42) = 8.63, p = .005, ηp2 = 0.170) (Fig. 2A). There was a main effect of Time for the Control participants (p = .008), with reductions in MEP amplitudes from baseline at all post-cTBS time-points (all p ≤ .038). There was no effect of Time for GDM-exposed participants (p = .092), and no change in MEP amplitudes from baseline at any time point (all p ≥ .163). There was no effect of time in the Insulin (F(5) = 1.94, p = .097) or Metformin (F(5) = 1.02, p = .369) groups (Fig. 2B). When correcting for GA, the mean (F(1,45) = 8.86, p = .005, ηp2 = 0.171) and peak (F(1,45) = 4.96, p = .031, ηp2 = 0.106) post-cTBS MEP suppression were greater in Control children than in GDM-exposed children. This was not accounted for by maternal BMI. Post-hoc analysis revealed that Control children demonstrated a greater LTD-like response to cTBS than insulin-exposed children (p = .047, 95% CI [−55.01 – −0.31]). Control and Metformin (p = .153, 95% CI [−50.02–5.38]) and Metformin and Insulin groups (p = 1.00, 95% CI [−30.61–19.92]) did not differ in response to cTBS.
Fig. 2.
MEP amplitudes following cTBS. A) The Control group exhibited significantly greater suppression of MEP amplitudes following cTBS, indicating a greater LTD-like neuroplastic response. B) Metformin and Insulin groups exhibited similar, non-significant changes in MEP amplitudes following cTBS, in contrast to the Control group. *p < .05 between groups. Error bars indicate SEMs.
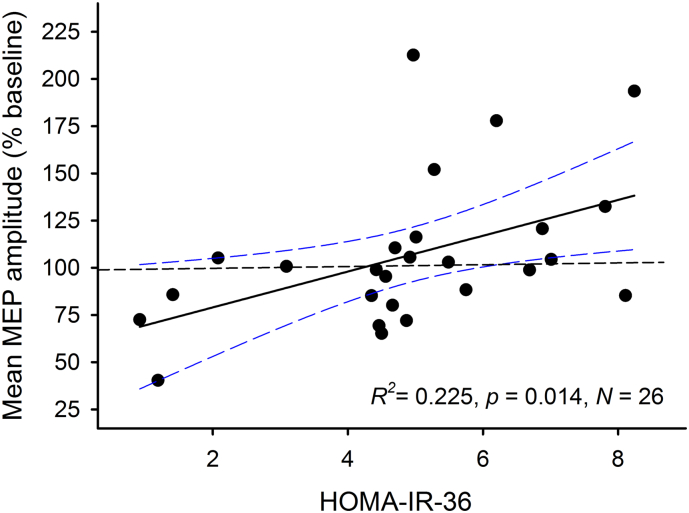
3.3. Maternal Insulin Resistance and Metabolic Markers Are Associated with Neurophysiological Outcomes
Higher maternal insulin resistance at 36 weeks GA was associated with reduced mean (F(1,25) = 6.97, p = .014, R2 = 0.225; Fig. 3) and peak (F(1,25) = 4.20, p = .052, R2 = 0.149) MEP suppression following cTBS in GDM-exposed children. HOMA-IR-Trial (30 ± 2.6 weeks GA) also explained a near-significant proportion of variation in mean MEP suppression (F(1,25) = 3.91, p = .061, R2 = 0.157). Further analysis of HOMA-IR-36 revealed an association with mean MEP amplitude variability throughout the experiments (coefficient of variation, COV), whereby higher maternal HOMA-IR-36 was associated with lower MEP COV (p = .045, R2 = 0.160) when corrected for child age. Table 1 shows a number of additional associations that were observed between child neurophysiology and maternal and neonatal metabolic and inflammatory markers.
Fig. 3.
Maternal IR and child neuroplasticity. Maternal insulin resistance at 36 weeks gestation (HOMA-IR-36) was associated with a reduced LTD-like neuroplastic response to cTBS in GDM-exposed children, as indicated by larger post-cTBS MEP amplitudes. N = 26.
Within the GDM group, a comparison was made to determine whether a previous GDM diagnosis is associated with different outcomes in children, as a previous diagnosis would place a woman in “high risk” status and potentially lead to earlier detection and treatment of GDM. Indeed, women with a previous diagnosis of GDM were assigned to treatment earlier (N = 8, 28.7 ± 3.2 weeks GA) than both women in their first pregnancy (N = 13, 31.5 ± 1.1 weeks GA, p = .007) and primiparous women without a prior GDM diagnosis (N = 11, 30.9 ± 2.7 weeks GA, p = .025). Children of women with a previous diagnosis of GDM (% baseline; 51.7 ± 26.8) had stronger peak LTD-like responses to cTBS than did first-born children (87.9 ± 32.3, p = .004), while first-born children had stronger responses than children of women with a previous pregnancy not complicated by GDM (64.4 ± 14.1, p = .035).
In the GDM group, children who were heavier (in grams) at birth had lower cortical excitability (p = .016, R2 = 0.168) and salivary cortisol (p = .049, R2 = 0.106) than did children who were lighter and this was associated with greater cord C-peptide, glucose, and triglycerides. Similar observations were made with birth weight centiles.
3.4. Low Salivary Cortisol is Associated with a Reduced LTD-like Neuroplastic Response
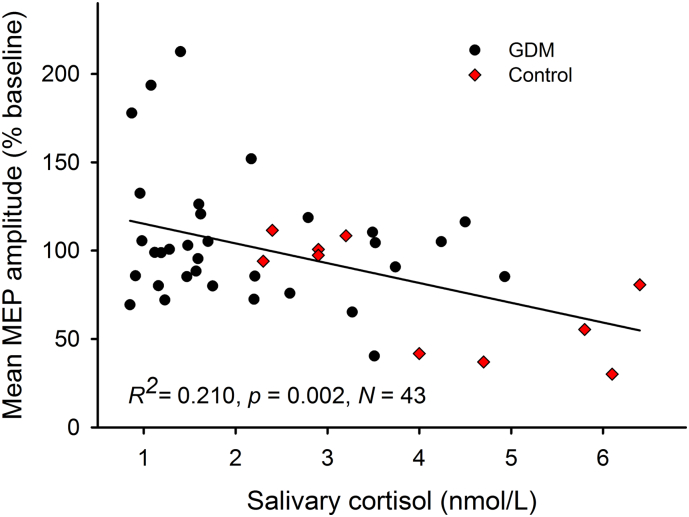
Diurnal variations in human salivary cortisol concentrations are well characterised, and affect neuroplasticity induction depending on time of day of the test (Sale et al., 2008; Pitcher et al., 2012a). Here, we found no group difference in the time of day (p = .953; mean = 13:22 h ± 1.9 h) at which the cTBS experiments were performed and no effect of time of day on any of the analyses. Immediately before cTBS, cortisol concentration ranged between 2.3 and 6.4 nmol/L in the Control group, 0.8–4.2 nmol/L in the Insulin group, and 0.9–4.9 nmol/L in the Metformin group. Mean (mean ± SD) concentrations differed between the Control group (4.1 ± 1.6 nmol/L) and both the Metformin (2.1 ± 1.2 nmol/L; p < .001) and Insulin (1.9 ± 1.1 nmol/L; p < .001) groups. The Metformin and Insulin groups did not differ from each other (p = .690). Normal salivary cortisol concentrations in children are reported to be approximately 4 nmol/L at the corresponding time of day (McCarthy et al., 2009), suggesting that the values in our GDM-exposed subjects were low.
Cortisol explained over 20% of the variation in the mean response to cTBS (F(1,42) = 10.91, p = .002, R2 = 0.210) (Fig. 4). Cortisol also was negatively associated with both RMT (F(1,46) = 4.92, p = .032, R2 = 0.099) and SI1mV (F(1,46) = 5.48, p = .024, R2 = 0.115), indicating that higher cortisol concentration was associated with greater cortical excitability. Mediation analysis did not detect a mediation effect of cortisol on the relationship between GDM group and cortical excitability (indirect effect; SE = 2.70, 95% CI: −7.23, 3.52), suggesting GDM influences excitability directly or through other mechanisms. Conversely, cortisol was found to mediate the relationship between GDM group and mean neuroplastic response (indirect effect; SE = 9.06, 95% CI: −40.02, −4.37) with a strong direct effect on plasticity (B = −9.67, SE = 4.12, p = .024).
Fig. 4.
Cortisol and neuroplasticity. Salivary cortisol concentration (nmol/L) was associated with an increased LTD-like neuroplastic response to cTBS as indicated by smaller post-cTBS MEP amplitudes.
Higher mean MEP COV was also associated with stronger peak MEP suppression following cTBS (p = .011, R2 = 0.142), and with higher cortisol concentration (p = .010, R2 = 0.149). Group analysis revealed lower mean MEP COV in the Insulin group (M = 0.45) compared with the Control group (M = 0.60; p = .015), and while the metformin group (M = 0.54) had values similar to controls (p = .258) and not significantly different from the insulin group (p = .060).
4. Discussion
To our knowledge, this is the first study to provide neurophysiological evidence that exposure to GDM in utero is associated with reductions in cortical excitability and neuroplasticity, and an associated reduction in basal salivary cortisol, at 11–13 years of age in the offspring. Child neurophysiological responses were associated with a number of maternal perinatal factors – most prominently maternal insulin resistance and inflammation. Importantly, these effects were present in children whose mothers were diagnosed and treated per the Australian clinical guidelines current at the time of the trial, and who were monitored closely and treated within a randomised controlled trial. Neurophysiological outcomes in children exposed to metformin were comparable with those whose mothers were treated with insulin.
Children in our GDM-exposed sample exhibited reduced cortical excitability, possibly reflecting suboptimal or delayed neurodevelopment of underlying brain structures. Pharmacological studies show that the RMT reflects the properties of neuronal membrane voltage-gated sodium channels. Additionally, imaging studies show that the RMT reflects white matter maturation, myelination, and structural integrity, and individuals with lower RMTs have better white matter development and neurodevelopmental outcomes (Ziemann et al., 2015). For example, many children born preterm can be shown to have compromised development of both white and grey matter, microstructural alterations, and altered neural connectivity remaining into adolescence (Counsell et al., 2008), which are believed to underlie the decreased cortical excitability and LTD-like plasticity observed in adolescents born preterm (Pitcher et al., 2012a,b). While RMT is high in very young children and gradually reduces to near adult values by 12–14 years of age (Garvey and Mall, 2008), the relatively high RMTs seen in the GDM-exposed children were not explained by age at assessment, GA at birth, or cortisol independently. Additionally, RMTs could not be acquired in three GDM-exposed children, and their data are not included in the RMT comparison, such that the effect size is likely to be underestimated. Given the association between cortical excitability and motor skills – particularly manual dexterity (Pitcher et al., 2012b) – these results suggest GDM may impact motor function. This should be explored in future studies.
Overall, in children exposed to GDM the neuroplastic response to cTBS was absent or reduced when compared with their non-GDM-exposed peers, suggesting that GDM contributes to altered development of the neural mechanisms underlying LTD-like synaptic plasticity. Altered neurophysiological responses in children were most strongly predicted by maternal IR measured both before commencement of pharmacologic treatment in late second to early third trimester, as well as at 36 weeks gestation following 1–2 months of metformin or insulin treatment. Interestingly, maternal plasma glucose was not found to be associated directly with neuroplasticity, though this finding was based on two single measures of fasting plasma glucose, which may not reflect overall maternal glycemia. However, a number of other maternal and neonatal metabolic markers were related. Of note, higher cord blood triglyceride and C-reactive protein concentrations were associated with poorer neuroplastic responses. Thus, the relationship between GDM and cortical development may be complex and involve, primarily, inflammatory mechanisms. As maternal BMI did not account for the effect of IR, IR measurements may capture these effects, possibly by measuring the underlying pathology of GDM and thus a range of its consequences, including inflammation, altered lipid metabolism, and altered fetal growth. Indeed, maternal IR was associated with maternal glucose, BMI, inflammatory markers, and cord triglycerides, and may act as a composite measure in this context. Similar observations have been made in animal models, whereby maternal inflammation and hyperglycemia alter fetal insulin signalling and, consequently, neurodevelopment, for example by retarding dendritic development (Jing et al., 2014).
Neonatal hypoglycemia also strongly predicted both plasticity and salivary cortisol in children, and this was not associated with the maternal factors mentioned above. Thus, alteration to the response to TMS in children is likely complicated further by neonatal metabolic factors and the immediate post-natal environment. Although GDM increases the risk of neonatal hypoglycemia, we had limited access to neonatal records for the control group, so we cannot determine if neonatal hypoglycemia similarly affects children not exposed to GDM.
Conversely, prior maternal diagnosis of GDM appeared to result in better outcomes in children. Children whose mothers had a previous pregnancy complicated by GDM had stronger neuroplastic responses than those whose mothers either did not have GDM previously or who had not given birth previously. Interestingly, the only systematic, observable difference between these groups was the time at which mothers were assigned a treatment, with those with a previous GDM diagnosis treated around two weeks earlier, likely due to their increased risk status. Thus, earlier treatment may be responsible for better outcomes in children. However, this too is likely more complex, and may involve metabolic differences, as time of treatment allocation (weeks GA) did not predict neuroplasticity independently.
Although the importance of the neuroplastic response to TMS to functional outcomes is unclear, it is known to involve mechanisms which underlie synaptic plasticity, learning, and memory, which includes activation and modulation of the NMDA receptor at excitatory synapses (Ziemann et al., 2015). Additionally, theta burst stimulation is known to influence motor learning (Teo et al., 2011). Thus, the response to plasticity induction is assumed to reflect the activity of the underlying synaptic machinery and therefore be of importance to the associated functional domains. The extent to which the outcomes here relate to, for example, cognition and motor skills, should be explored further in our cohort and others.
Low afternoon salivary cortisol observed in the GDM group represents one possible explanation for the altered neuroplastic responses exhibited by GDM-exposed children. Indeed, cortisol was found to be a mediator between GDM group and mean plasticity. However, cortisol cannot explain the entirety of the variation in plasticity, and did not influence or mediate the relationship between IR and plasticity response. Thus, it may be most likely that reduced neuroplastic response is influenced both by a direct neurophysiological consequence of GDM on cortical development and function, and an indirect consequence of sub-normal circulating cortisol, as corticosteroids alter both the function and subunit composition of NMDA-type glutamate receptors and hence synaptic plasticity (Tse et al., 2012), including the response to cTBS (Pitcher et al., 2012a).
It is important to note that we collected only a single measurement of cortisol. Given the importance of circadian patterns of cortisol release to cognitive (Gibson et al., 2010) and neuropsychiatric functioning (Wulff et al., 2010), we plan to explore further this relationship by assessing the cortisol awakening response in our sample to determine whether circadian or stress-induced patterns of cortisol secretion are altered.
To conclude, we found that gestational diabetes is associated with altered cortical and neuroendocrine development in offspring, but the extent to which these effects are causally linked, or are separate manifestations of an altered perinatal environment, is unclear. However, the severity of maternal insulin resistance prior to and during treatment, in addition to maternal and cord metabolic markers, appears to predict these outcomes in offspring. Given that hyperglycemia in these women was strictly controlled as per clinical guidelines for the remainder of their pregnancy, the data presented here suggest that the fetal brain is susceptible to mild to moderate maternal insulin resistance and inflammation earlier in pregnancy, regardless of subsequent treatment, and that this represents a risk for impaired cortical development and HPA function into childhood and adolescence. While some “high-risk” patients are diagnosed earlier, GDM is usually not diagnosed before the 24th–28th weeks of gestation. However, our findings (and others) suggest that by this time the neural pathology of fetuses exposed to GDM may already be established, and that prevention or reversal of associated adverse perinatal outcomes may be limited (Georgiou et al., 2011). Although our study is relatively small, the convergence of a number of characteristics of both mother and child on several outcomes strengthens our conclusions. Nevertheless, these data should be replicated and extended, and we plan to conduct more comprehensive neurodevelopment and neuroendocrine assessments of this cohort in the future. Given our results, we suggest that methods for early prediction of such outcomes, such as HOMA-IR, metabolic and proteomic markers, and modelling approaches, may be an important step in developing more sensitive indices of risk (Ozgu-Erdinc et al., 2015). Early pharmacologic interventions, particularly in pregnancies at high risk or in which lifestyle advice is unlikely to suffice, may help to prevent the potential abnormalities in cortical physiology and neuroendocrine function identified in this study. Reassuringly, metformin appears safe with respect to those aspects of cortical development assessed here, and may be an appropriate treatment for early GDM.
Role of Funding Source
An intramural grant funded this study. The authors had sole responsibility for the study design, writing of the manuscript and decision to submit it for publication.
Conflicts of Interest
The authors have nothing to declare.
Contributions of Authors
JBP, JMVD, and LAS designed the study. JMVD and JBP performed the literature search. JMVD, AJG, LAS, SC, NAH, and MRG recruited participants, collected and analysed the data. JMVD, JBP, and LAS interpreted the data. WMH, JR and SC provided access to and interpretation of the MiG clinical trial data. JMVD prepared the figures and wrote the manuscript with JBP, and all other authors contributed to editing of drafts.
Ethics Committee Approval
Ethical Approval for all protocols and procedures was given by the University of Adelaide, and Women's and Children's Health Network Human Research Ethics Committees (I.D. number: HREC/16/WCHN/50).
Acknowledgements
The authors would like to thank all of the mothers and children of the MiG trial who have dedicated their time to the study, and without whom this research would not be possible.
References
- Barrett H.L., Gatford K.L., Houda C.M., De Blasio M.J., Mcintyre H.D., Callaway L.K., Dekker Nitert M., Coat S., Owens J.A., Hague W.M., Rowan J.A. Maternal and neonatal circulating markers of metabolic and cardiovascular risk in the metformin in gestational diabetes (MiG) trial: responses to maternal metformin versus insulin treatment. Diabetes Care. 2013;36:529–536. doi: 10.2337/dc12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battin M.R., Obolonkin V., Rush E., Hague W., Coat S., Rowan J. Blood pressure measurement at two years in offspring of women randomized to a trial of metformin for GDM: follow up data from the MiG trial. BMC Pediatr. 2015;15:54. doi: 10.1186/s12887-015-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles B., Norris R., Xiao X., Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther. Drug Monit. 2006;28:67–72. doi: 10.1097/01.ftd.0000184161.52573.0e. [DOI] [PubMed] [Google Scholar]
- Chung M.M., Chen Y.L., Pei D., Cheng Y.C., Sun B., Nicol C.J., Yen C.H., Chen H.M., Liang Y.J., Chiang M.C. The neuroprotective role of metformin in advanced glycation end product treated human neural stem cells is AMPK-dependent. Biochim. Biophys. Acta. 2015;1852:720–731. doi: 10.1016/j.bbadis.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Counsell S.J., Edwards A.D., Chew A.T., Anjari M., Dyet L.E., Srinivasan L., Boardman J.P., Allsop J.M., Hajnal J.V., Rutherford M.A., Cowan F.M. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- Edlow A.G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 2016;37(1):95–110. doi: 10.1002/pd.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovitz M.A., Brown A.G., Breen K., Anton L., Maubert M., Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int. J. Dev. Neurosci. 2011;29:663–671. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey M.A., Mall V. Transcranial magnetic stimulation in children. Clin. Neurophysiol. 2008;119:973–984. doi: 10.1016/j.clinph.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou H.M., Lopez S.I., Rice G.E. Novel screening approaches for the early detection of gestational diabetes mellitus. In: Radenkovic P.M., editor. Gestational Diabetes. INTECH; 2011. [Google Scholar]
- Gibson E.M., Wang C., Tjho S., Khattar N., Kriegsfeld L.J. Experimental 'jet lag' inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F., Rockwood N.J. Regression-based statistical mediation and moderation analysis in clinical research: observations, recommendations, and implementation. Behav. Res. Ther. 2017;98:39–57. doi: 10.1016/j.brat.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Hordacre B., Goldsworthy M.R., Vallence A.M., Darvishi S., Moezzi B., Hamada M., Rothwell J.C., Ridding M.C. Variability in neural excitability and plasticity induction in the human cortex: a brain stimulation study. Brain Stimul. 2017;10:588–595. doi: 10.1016/j.brs.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Huang Y.Z., Edwards M.J., Rounis E., Bhatia K.P., Rothwell J.C. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang Y.Z., Chen R.S., Rothwell J.C., Wen H.Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin. Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Jing Y.H., Song Y.F., Yao Y.M., Yin J., Wang D.G., Gao L.P. Retardation of fetal dendritic development induced by gestational hyperglycemia is associated with brain insulin/IGF-I signals. Int. J. Dev. Neurosci. 2014;37:15–20. doi: 10.1016/j.ijdevneu.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Rothwell J.C. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex. J. Physiol. 2014;592:4115–4128. doi: 10.1113/jphysiol.2014.274316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton A., Fichorova R.N., O'shea T.M., Kuban K., Paneth N., Dammann O., Allred E.N., Investigators E.S. Two-hit model of brain damage in the very preterm newborn: small for gestational age and postnatal systemic inflammation. Pediatr. Res. 2013;73:362–370. doi: 10.1038/pr.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccarthy A.M., Hanrahan K., Kleiber C., Zimmerman M.B., Lutgendorf S., Tsalikian E. Normative salivary cortisol values and responsivity in children. Appl. Nurs. Res. 2009;22:54–62. doi: 10.1016/j.apnr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwaniki M.K., Atieno M., Lawn J.E., Newton C.R. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y., Marks D.J., Grossman B., Yoon M., Loudon H., Stone J., Halperin J.M. Exposure to gestational diabetes mellitus and low socioeconomic status: effects on neurocognitive development and risk of attention-deficit/hyperactivity disorder in offspring. Arch. Pediatr. Adolesc. Med. 2012;166:337–343. doi: 10.1001/archpediatrics.2011.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A., Wolf A., Ratzon N., Greenbaum C., Dulitzky M. Neurodevelopmental outcome at early school age of children born to mothers with gestational diabetes. Arch. Dis. Child. Fetal Neonatal Ed. 1999;81:F10–4. doi: 10.1136/fn.81.1.f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgu-Erdinc A.S., Yilmaz S., Yeral M.I., Seckin K.D., Erkaya S., Danisman A.N. Prediction of gestational diabetes mellitus in the first trimester: comparison of C-reactive protein, fasting plasma glucose, insulin and insulin sensitivity indices. J. Matern. Fetal Neonatal Med. 2015;28:1957–1962. doi: 10.3109/14767058.2014.973397. [DOI] [PubMed] [Google Scholar]
- Pantham P., Aye I.L., Powell T.L. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36:709–715. doi: 10.1016/j.placenta.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher J.B., Riley A.M., Doeltgen S.H., Kurylowicz L., Rothwell J.C., Mcallister S.M., Smith A.E., Clow A., Kennaway D.J., Ridding M.C. Physiological evidence consistent with reduced neuroplasticity in human adolescents born preterm. J. Neurosci. 2012;32:16410–16416. doi: 10.1523/JNEUROSCI.3079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher J.B., Schneider L.A., Burns N.R., Drysdale J.L., Higgins R.D., Ridding M.C., Nettelbeck T.J., Haslam R.R., Robinson J.S. Reduced corticomotor excitability and motor skills development in children born preterm. J. Physiol. 2012;590:5827–5844. doi: 10.1113/jphysiol.2012.239269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher J.B., Doeltgen S.H., Goldsworthy M.R., Schneider L.A., Vallence A.M., Smith A.E., Semmler J.G., Mcdonnell M.N., Ridding M.C. A comparison of two methods for estimating 50% of the maximal motor evoked potential. Clin. Neurophysiol. 2015;126:2337–2341. doi: 10.1016/j.clinph.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Romero R., Espinoza J., Goncalves L.F., Kusanovic J.P., Friel L., Hassan S. The role of inflammation and infection in preterm birth. Semin. Reprod. Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P.M., Pascual-Leone A., Safety of, T. M. S. C. G Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan J.A., Hague W.M., Gao W., Battin M.R., Moore M.P., Mi G.T.I. Metformin versus insulin for the treatment of gestational diabetes. N. Engl. J. Med. 2008;358:2003–2015. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- Rowan J.A., Rush E.C., Obolonkin V., Battin M., Wouldes T., Hague W.M. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition at 2 years of age. Diabetes Care. 2011;34:2279–2284. doi: 10.2337/dc11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale M.V., Ridding M.C., Nordstrom M.A. Cortisol inhibits neuroplasticity induction in human motor cortex. J. Neurosci. 2008;28:8285–8293. doi: 10.1523/JNEUROSCI.1963-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloman C., Scholz-Romero K., Sarker S., Sweeney E., Kobayashi M., Correa P., Longo S., Duncombe G., Mitchell M.D., Rice G.E., Illanes S.E. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes. 2016;65:598–609. doi: 10.2337/db15-0966. [DOI] [PubMed] [Google Scholar]
- Scheen A.J., Esser N., Paquot N. Antidiabetic agents: potential anti-inflammatory activity beyond glucose control. Diabete Metab. 2015;41:183–194. doi: 10.1016/j.diabet.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Sullivan E.L., Nousen E.K., Chamlou K.A. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol. Behav. 2014;123:236–242. doi: 10.1016/j.physbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo J.T., Swayne O.B., Cheeran B., Greenwood R.J., Rothwell J.C. Human theta burst stimulation enhances subsequent motor learning and increases performance variability. Cereb. Cortex. 2011;21:1627–1638. doi: 10.1093/cercor/bhq231. [DOI] [PubMed] [Google Scholar]
- Tse Y.C., Bagot R.C., Wong T.P. Dynamic regulation of NMDAR function in the adult brain by the stress hormone corticosterone. Front. Cell. Neurosci. 2012;6:9. doi: 10.3389/fncel.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization guideline. Diabetes Res. Clin. Pract. 2014;103:341–363. doi: 10.1016/j.diabres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Wouldes T.A., Battin M., Coat S., Rush E.C., Hague W.M., Rowan J.A. Neurodevelopmental outcome at 2 years in offspring of women randomised to metformin or insulin treatment for gestational diabetes. Arch. Dis. Child. Fetal Neonatal Ed. 2016;101:F488–F493. doi: 10.1136/archdischild-2015-309602. [DOI] [PubMed] [Google Scholar]
- Wulff K., Gatti S., Wettstein J.G., Foster R.G. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- Xu G., Jing J., Bowers K., Liu B., Bao W. Maternal diabetes and the risk of autism spectrum disorders in the offspring: a systematic review and meta-analysis. J. Autism Dev. Disord. 2014;44:766–775. doi: 10.1007/s10803-013-1928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U., Reis J., Schwenkreis P., Rosanova M., Strafella A., Badawy R., Muller-Dahlhaus F. TMS and drugs revisited 2014. Clin. Neurophysiol. 2015;126:1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]