Abstract
Background
Management of advanced/recurrent low-grade serous ovarian carcinoma (LGOSC) is often challenging. Effective treatment options remain limited for hormone and chemotherapy-resistant LGSOC.
CASE: A 65-year-old woman with recurrent widespread LGSOC harboring the KRAS-G12 V hotspot mutation experienced a dramatic clinical response to Binimetinib (MEK162), a mitogen-activated protein kinase (MEK) inhibitor, after failing multiple chemotherapy and hormonal treatments. An 81% reduction of target lesions by RECIST 1.1 over 31 months of response duration was confirmed with serial CT scans. Episodes of drug-related toxicity (pneumonitis) easily resolved without sequelae with the use of oral steroids.
Conclusion
Binimetinib may present a new treatment option for hormone- and chemotherapy-resistant LGSOC harboring KRAS mutations.
Keywords: Binimetinib, MEK162, Low-grade ovarian cancer, Serous, Recurrent, Treatment-resistant
Highlights
-
•
Low-grade serous ovarian carcinoma (LGSOC) is chemotherapy resistant.
-
•
RAS-RAF-MEK-ERK pathway activation by BRAF and KRAS mutations are common in LGSOC.
-
•
Binimetinib (MEK162) is a non-ATP-competitive MEK 1/2 inhibitor.
-
•
MEK162 may represent an effective treatment in LGSOC with BRAF and KRAS mutations.
1. Introduction
Low-grade-serous-ovarian-carcinoma(LGSOC) is a rare subtype of epithelial ovarian cancer (EOC) that accounts for 6–10% of serous ovarian cancer and 5–8% of all EOC (Oswald and Gourley, 2015). Multiple studies demonstrated it is histologically, molecularly, and clinically distinct from high-grade-serous-ovarian-cancer (HGSOC) (Oswald and Gourley, 2015; Vang et al., 2009). LGSOC is typically diagnosed at a younger age compared to HGSOC, has an indolent growth rate and is resistant to cytotoxic chemotherapy compared to HGSOC; the response rate to cytotoxic chemotherapy in both the neoadjuvant and recurrent setting is <4% (Gershenson et al., 2009; Schmeler et al., 2008).
Patients with recurrent LGSOC are generally treated via surgery with curative intent, while systemic chemotherapy or hormonal therapy is utilized in patients with non-resectable disease. The overall response rate to hormonal therapy in a recurrent setting is only 9% with median progression free survival (PFS) of 7.4 months (Gershenson et al., 2012). Novel effective treatment options for patients with recurrent/metastatic LGSOC are needed.
The mitogen-activated protein kinase (MAPK) pathway is a key signaling cascade, driving cell proliferation, differentiation, and survival (Friday and Adjei, 2008). Inappropriate activation of the MAPK pathway (RAS-RAF-MEK-ERK pathway) has been shown to occur in many cancers including LGSOC (Miller et al., 2014). The use of MEK inhibitors (CI-1040) in preclinical models of EOC has shown profound growth inhibition and apoptosis in EOC cells with mutations in either KRAS or BRAF compared with EOC cells containing wild-type sequences (8). A phase II clinical trial from the Gynecologic Oncology Group (GOG) showed encouraging data regarding the effect of MEK inhibition in recurrent LGSOC (Farley et al., 2013). Binimetinib (MEK162) is a non-ATP-competitive MEK 1/2 inhibitor that has proven activity in NRAS- and BRAF-mutant melanoma in phase II trials (Ascierto et al., 2013).
We present a case of a recurrent LGSOC patient with KRAS mutation who previously failed multiple lines of chemotherapy and hormonal therapy, who experienced an impressive and durable clinical response to MEK162.
2. Case
The patient is 65-year-old woman who was initially diagnosed with an advanced-stage Mullerian-Type serous cancer in April 2013. Treatment was initiated with neoadjuvant chemotherapy (NACT) using carboplatin/paclitaxel. After 3 cycles of NACT the tumor showed poor responsiveness, and the regimen was switched to pegylated-lipososomal-doxorubicin (PLD)/carboplatin. After receiving 3 more cycles of NACT, she underwent surgery (10/28/2013), and the final pathology revealed LGSOC with positive estrogen-receptor (ER) and negative progesterone-receptor. She received 3 cycles of adjuvant PLD/carboplatin, which was completed on 02/12/2014. Her serum cancer antigen 125 (CA125) was normalized, and there was no disease by computed-tomography (CT) imaging.
She remained disease free until January 2015 when her CA125 was found to be elevated to 88.1 U/mL. CT imaging showed no evidence of recurrence. However, the pelvic examination during the subsequent follow-up revealed a small mass on the vaginal vault, the biopsy of which confirmed recurrent LGSOC. She underwent secondary debulking surgery in April 2015, and letrozole was initiated given ER tumor-positivity. Letrozole was switched to exemestane shortly after the initial administration due to unbearable joint pain and hand stiffness. Unfortunately, a CT scan of the chest, abdomen, and pelvis 3 months after aromatase-inhibitor initiation revealed progression of disease with new lesions. She was referred to our institution for further treatment. She was counselled for enrollment in a Phase III clinical trial (clinicaltrial.gov, NCT01849874) investigating Binimetinib (MEK162), a MEK1/2 inhibitor, versus physician's choice chemotherapy and was randomized to receive MEK162 (45 mg, twice daily, orally) starting on 09/19/2015. Baseline CAT scans demonstrated multiple large metastatic lesions in both her chest and peritoneal cavity (Fig. 1 A and 1C). Within 8 weeks of MEK162 treatment, CA125 decreased to 32.7 U/mL (baseline of 76.4 U/mL), and a CT scan showed stable-disease (SD). Other than mild fatigue (grade 1), she tolerated the treatment well. As MEK162 treatment continued, her disease remained stable on a CT imaging and CA125 continued to decline. By the 24th weeks of treatment, CA125 decreased to 28.1 U/mL, and a CT imaging continued to show SD, but chest CT revealed ground glass opacity of the lung. As the patient developed dyspnea on exertion and worsening fatigue, MEK162 was then interrupted for drug-related pneumonitis (grade2) and worsening fatigue (grade3); by interrupting the medication her respiratory symptoms and fatigue improved rapidly. For persistent abnormalities on a subsequent chest CT scan, she was started on prednisone treatment by her pulmonologist. The respiratory symptom and the lung lesions on the CT were completely resolved after 3 weeks of steroid treatment. MEK162 was restarted at a reduced dose (30 mg, twice daily, orally) on 4/15/2016 (30th week since initial MEK162 treatment), but treatment was held for 2 additional weeks shortly after treatment re-initiation secondary to persistent fluid retention and electrolyte imbalance; MEK162 was then resumed again at 33rd week since initial MEK162. A follow up CT scan done on 6/23/2016 (39th week of MEK162) continued to show SD from the baseline by RECIST 1.1, and CA125 was 9.7 U/mL. As she remained on MEK162, her disease continued to respond with SD on CT imaging and normalized CA125. After 26 consecutive weeks of MEK162 treatment, she developed a 2nd episode of drug-related pneumonitis (12/20/2016) (65th week of MEK162). She was again treated with prednisone and MEK162 was held. A CT scan obtained on 02/10/2017 (72nd week of MEK162) demonstrated a partial response (PR) with 43.95% size reduction in the target lesions (Fig. 1B and D).
Fig. 1.
CT scans demonstrating activity of MEK162.
Upper panel: Representative right pleura metastatic lesion. A. Baseline measurement of pleura lesion. B. Regression of the lesion after 72 weeks of MEK162: partial response by RECIST 1.1.
Lower panel: Representative peritoneal metastatic deposit.
C. Baseline measurement of right peritoneal metastatic tumor deposit.
D. Regression of the lesion after 72 weeks of MEK162: partial response (overall 43.95% size reduction in target lesions by RECIST 1.1).
E. Further regression of the lesion after 125 weeks of MEK162: partial response (overall 81.05% size reduction in target lesions by RECIST 1.1).
With the PR on CT scan imaging, the strong desire of patient to continue oral treatment, and after consultation with her pulmonologist, the drug was re-initiated on 3/21/2017 (78th week of MEK162), and treatment continued for 8 weeks. Another CT scan on 5/22/2017, in the absence of respiratory symptoms, demonstrated glassy looking pneumonitis (86th week of MEK162). The CT scan also revealed continuously improving PR with a 64.75% size-reduction of the target lesions when compared to baseline. After resolution of pneumonitis, MEK162 was resumed in combination with a prophylactic low dose of prednisone on 7/7/2017 (93rd week of MEK162). She has remained on MEK162 without further episodes of drug-related pneumonitis. The patient's remarkable clinical response to the treatment continues with a striking 81.05% size-reduction in the target lesions from baseline by RECIST 1.1 on 2/12/2018 (the 125th week of MEK162) (Figure1E). The patient's disease course with treatments is illustrated in Fig. 2.
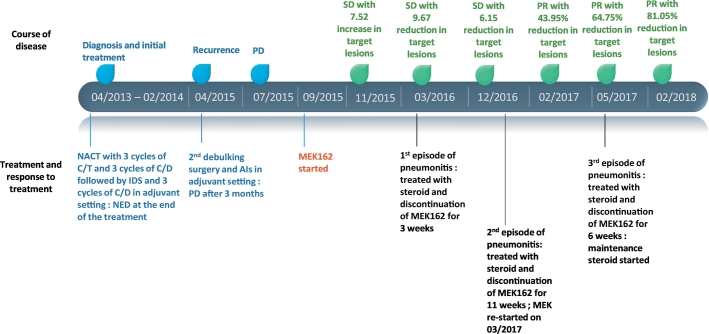
Fig. 2.
Timeline of patient's disease course with treatment: (NACT; neoadjuvant chemotherapy; C/T, carboplatin/paclitaxel; C/D, carboplatin/pegylated-lipososomal-doxorubicin; IDS, interval debulking surgery; AIs, aromatase inhibitors; PD, progression of disease; SD, stable disease; PR, partial response; NED, no evidence of disease).
3. Discussion
Due to its unresponsiveness to cytotoxic chemotherapy, treatment of unresectable LGSOC has been a challenge. Alternative treatment modalities including hormonal therapy and anti-angiogenic agent have been studied (Oswald and Gourley, 2015; Gershenson et al., 2009; Schmeler et al., 2008; Watanabe et al., 2016), however, treatment response to hormonal therapy is suboptimal (9%) and bevacizumab studies showed conflicting findings in terms of response-rate even though it did appear that anti-angiogenic treatment prolonged the duration of response (Oswald and Gourley, 2015).
The MAPK pathway has recently been identified as a potential target opportunity for treatment of LGSOC. Fifteen of 22 LGSOCs (68%) had mutation in either KRAS (35%) or BRAF (33%). None had mutations in both BRAF and KRAS (Miller et al., 2014). To gain knowledge in the genetic characteristics of the patient's tumor demonstrating such exquisite sensitivity to MEK-inhibition in our case, we performed next-generation-sequencing (NGS) testing using a commercially available platform (Foundation-Medicine, (FM) Inc. Cambridge, MA) (14). Our patient's tumor harbored a hotspot mutation in the KRAS gene (i.e. G12 V).
Clinical trials evaluating the efficacy of MEK inhibitors in LGSOC have utilized a number of MEK inhibitors targeting either MEK 1 or MEK 1/2. GOG239 evaluated the efficacy of selumetinib in an open-label, single-arm, phase II study. Fifty-two patients diagnosed with recurrent LGSOCs were enrolled and received selumetinib 50 mg twice daily. 8 patients (15.4%) had a complete (Oswald and Gourley, 2015) or partial response (Miller et al., 2014), and 34 (65%) had stable disease (SD) (Farley et al., 2013).
Binitimetinib (MEK162) is a highly selective inhibitor of MEK1/2 with demonstrated activity in-vitro and in-vivo. Recent clinical studies demonstrated the efficacy and safety of MEK162 (Ascierto et al., 2013; Watanabe et al., 2016; Bendell et al., 2017; Dummer et al., 2017). In a phase II study examining MEK162 in advanced melanoma, 71 patients with NRAS- or Val600 BRAF-mutated melanoma were given MEK162 45 mg twice daily. Six (20%) of 30 patients with NRAS mutation had partial response as did eight (20%) of 41 patients with BRAF mutation. A phase III study comparing binitimetinib to dacarbazine showed improved PFS with binitimetinib in patients with NRAS-mutant melanoma (median PFS 2.8 months vs. 1.5 months, P < .001) (Dummer et al., 2017). Another study examining MEK162 in advanced solid tumors such as lung, colorectal, biliary, pancreas, and thyroid tumors showed SD in 67% of patients with duration of response >180 days in 5 patients (Watanabe et al., 2016). The safety profile of MEK162 has been proven to be tolerable with 45 mg twice daily dosage (Ascierto et al., 2013; Watanabe et al., 2016; Bendell et al., 2017). It has been reported that a significant portion of patients treated with MEK162 may experience toxicities such as skin rash, dermatitis acneiform, visual/retinal events, nausea, vomiting, diarrhea, peripheral edema, fatigue, asymptomatic reversible elevation of creating kinase, and interstitial lung disease/pneumonitis. However, grade 3–4 adverse events, except for creatinine phosphokinase, occurred in <7% of patients treated with MEK162 45 mg twice daily dosage, and there was no treatment-related deaths. In our case report, our patient experienced fatigue and recurrent pneumonitis with MEK162; however, both these symptoms easily resolved without sequelae with the use of steroids; the patient is currently continuing MEK162 treatment with a persistent PR over 31 months. MEK162 can be used for prolonged periods of time with proper management of treatment-related adverse events.
MEK162 may represent a novel, highly effective treatment option for a subset of recurrent LGSOC patients with disease refractory to standard salvage treatment.
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of interest
There is no conflict of interest.
Author contributions
Chanhee Han, Stefania Bellone, Luca Zammataro, Peter E. Schwartz, and Alessandro D. Santin participated in drafting and revising this manuscript. Stefania Bellone and Luca Zammataro provided materials for the figures. All authors read and approved this manuscript to be submitted.
Acknowledgement
We thank the patient for participating in MILO study (NCT01849874) and allowing us to publish this care report.
References
- Ascierto P.A., Schadendorf D., Berking C., Agarwala S.S., van Herpen C.M., Queirolo P. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013 Mar;14(3):249–256. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]
- Bendell J.C., Javle M., Bekaii-Saab T.S., Finn R.S., Wainberg Z.A., Laheru D.A. A phase 1 dose-escalation and expansion study of binimetinib (MEK162), a potent and selective oral MEK1/2 inhibitor. Br. J. Cancer. 2017 Feb 28;116(5):575–583. doi: 10.1038/bjc.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer R., Schadendorf D., Ascierto P.A., Arance A., Dutriaux C., Di Giacomo A.M. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017 Apr;18(4):435–445. doi: 10.1016/S1470-2045(17)30180-8. [DOI] [PubMed] [Google Scholar]
- Farley J., Brady W.E., Vathipadiekal V., Lankes H.A., Coleman R., Morgan M.A. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013 Feb;14(2):134–140. doi: 10.1016/S1470-2045(12)70572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday B.B., Adjei A.A. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin. Cancer Res. 2008 Jan 15;14(2):342–346. doi: 10.1158/1078-0432.CCR-07-4790. [DOI] [PubMed] [Google Scholar]
- Gershenson D.M., Sun C.C., Bodurka D., Coleman R.L., Lu K.H., Sood A.K. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol. Oncol. 2009 Jul;114(1):48–52. doi: 10.1016/j.ygyno.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Gershenson D.M., Sun C.C., Iyer R.B., Malpica A.L., Kavanagh J.J., Bodurka D.C. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol. Oncol. 2012 Jun;125(3):661–666. doi: 10.1016/j.ygyno.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C.R., Oliver K.E., Farley J.H. MEK1/2 inhibitors in the treatment of gynecologic malignancies. Gynecol. Oncol. 2014 Apr;133(1):128–137. doi: 10.1016/j.ygyno.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Oswald A.J., Gourley C. Low-grade epithelial ovarian cancer: a number of distinct clinical entities? Curr. Opin. Oncol. 2015 Sep;27(5):412–419. doi: 10.1097/CCO.0000000000000216. [DOI] [PubMed] [Google Scholar]
- Schmeler K.M., Sun C.C., Bodurka D.C., Deavers M.T., Malpica A., Coleman R.L. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol. Oncol. 2008 Mar;108(3):510–514. doi: 10.1016/j.ygyno.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Vang R., Shih Ie M., Kurman R.J. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv. Anat. Pathol. 2009 Sep;16(5):267–282. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Otsu S., Hirashima Y., Morinaga R., Nishikawa K., Hisamatsu Y. A phase I study of binimetinib (MEK162) in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2016 Jun;77(6):1157–1164. doi: 10.1007/s00280-016-3019-5. [DOI] [PubMed] [Google Scholar]