Abstract
Background
Pooled AB serum is often used as a media supplement for cell culture but it has the potential to transmit infectious diseases. To avoid this risk, we used autologous plasma as a media supplement for manufacturing dendritic cells (DCs) for cancer immunotherapy. We noticed inconsistences in the DCs and investigated its nature and cause.
Methods
adHER2/neu DCs for 21 patients were manufactured from autologous peripheral blood monocytes which were treated with GM-CSF and IL-4 for 3 days, transduced with Ad5f35HER2ECTM and then treated with lipopolysaccharide and IFN-γ for one day. The cells were cultured in RPMI-1640 supplemented with either 10% heat inactivated autologous or AB plasma.
Results
Twenty-eight adHER2/neu DCs were manufactured for 21 patients using autologous plasma and 68 were manufactured for 20 of those patients using AB plasma. The expression of HER2/neu was less for DCs manufactured with autologous plasma (70.3±33.3% vs 86.1±22.8%, p<0.01). Manufacturing adHER2/neu DCs using monocytes from 3 healthy subjects and plasma from one patient with low HER2/neu expression (18%) resulted in low HER2/neu expression by all three DCs (13%, 16% and 23%). Analysis of the levels of 1322 proteins in 8 plasma samples associated with low HER2/neu expression and in 12 associated with high HER2/neu expression revealed that the levels of 14 predicted HER2/neu transduction efficiency.
Conclusion
The manufacture of adHER2/neu DC using autologous plasma as a media supplement resulted in inconsistent HER2/neu expression. It is likely that variability in the levels of multiple proteins in autologous plasma contributed to low HER2/neu expression.
Keywords: adHER2/neu, cancer immunotherapy, dendritic cells (DCs)
Introduction
The manufacturing of many clinical cell therapies involves ex vivo culture in specialized medium that is supplemented with growth factors and other proteins. Fetal Bovine Serum (FBS) is often used as a culture media protein supplement, but exposure of cellular therapy recipients to FBS can result in allergic reactions or the transmission of xenogeneic infections (1–3). Consequently, most clinical cell therapy manufacturing protocols avoid using FBS containing media for cell culture and expansion. Defined media that is free from animal-derived proteins and human serum has been developed for the culture of some, but not all, cell types. Another alternative to FBS is AB serum prepared from pools of serum or plasma collected from many healthy subjects. Donors of blood products, including AB serum, undergo health history screening and testing for the exposure to or presence of transfusion transmitted pathogens. Despite the screening and testing of AB serum donors, their serum can still transmit infectious diseases. Exposure to transfusion transmitted diseases can also be avoided by using autologous serum or plasma as a culture media protein supplement rather than FBS or pooled third party donor AB serum.
Many cancer immunotherapies are made from autologous leukocytes. Most Chimeric Antigen Receptor (CAR) T cell products are made from autologous lymphocytes and many Dendritic Cell (DC) cancer vaccines are made from autologous monocytes (4–7). These autologous lymphocytes and monocytes are collected by apheresis as a peripheral blood mononuclear cell (PBMC) concentrate using a blood cell separator. After the PBMC concentrate is collected, an additional 200 to 300 mL of autologous plasma can be collected which can be used as a culture media protein supplement for manufacturing the autologous cell therapy.
We developed a protocol to manufacture an autologous DC vaccine expressing Human Epidermal Growth Factor Receptor 2 (HER2/neu) to treat patients with HER2/neu expressing cancers. The HER2/neu (ErbB2) oncogene is a member of the epidermal growth factor receptor tyrosine kinase family that encodes a 185-kd transmembrane receptor that functions to regulate cell proliferation, metabolism, and invasion. Overexpression of HER2/neu is associated with tumorigenesis and human cancer pathogenesis. The oncogene is overexpressed in 25 to 30% of all human breast and ovarian cancers, and is associated with higher recurrence and lower survival rates (8).
DCs for this clinical trial were manufactured from autologous monocytes that were isolated from peripheral blood mononuclear cell (PBMC) concentrates by counter-flow elutriation. The monocytes were cultured with GM-CSF and IL-4 to produce immature DCs and then with interferon-gamma (IFN-γ) and lipopolysaccharide (LPS) to produce mature DCs. The immature DCs were transduced with a chimeric adenoviral vector, Ad5 serotype with the knob and fiber of the Ad35 serotype (Ad5f35), that expressed the extracellular and transmembrane (ECTM) domains of human HER2 (Ad5f35HER2ECTM). This chimeric adenovirus is less susceptible to anti-Ad5 neutralizing antibodies and is effective at transducing human DCs. We initially manufactured the adHER2/neu DCs in media supplemented with autologous plasma; however, we found that the expression of HER2/neu was highly variable. We then changed the manufacturing protocol and used third party AB plasma collected from a single donor rather than autologous plasma as a media supplement. This study compared the variability in expression of HER2/neu among DCs manufactured with autologous serum and DCs manufactured with AB plasma and investigated the source of this variability.
Material and Methods
Dendritic Cell Manufacturing Process
Dendritic cells were manufactured according to a standard procedure established in the Cell Processing Section (CPS), Department of Transfusion Medicine (DTM), Clinical Center (CC), NIH, Bethesda, MD. Briefly, peripheral blood mononuclear (PBMC) cell concentrates were collected by apheresis. For the 21 patients treated with adHER2/neu that were studied, PBMC concentrates were collected with the Cobe Spectra (Terumo BCT) blood cell separator and an additional 100 to 200 mL of autologous plasma was collected. For some mechanistic studies PBMC concentrates were collected by apheresis from healthy subjects using the Amicus Separator (Baxter Healthcare, Fenwal Division, Deerfield, IL). All donors signed an informed consent approved by a NIH institutional review board. Monocytes were enriched directly from the PBMC concentrates by elutriation using the Elutra (Gambro BCT Lakewood, CO) automatic mode according to the manufacturer's recommendations and the monocytes were cryopreserved in aliquots of 100×106 cells each.
Donor monocytes were thawed and manufactured in RPMI-1640 supplemented with either 10% autologous plasma or 10% healthy donor AB plasma that had been heat inactivated at 56°C for 120 minutes. The media was also supplemented with GM-CSF (Leukine Sargramostin, 2000 IU/ml, Genzyme, Cambridge, MA, USA), 10 mcg/ml gentamicin, IL-4 (USP grade recombinant human IL-4, 2000 IU/ml, CellGenix, Gmbh, Freiburg, Germany) at a final concentration of 1.5×106 cells/mL in T75 flasks (Corning Incorporated Life Sciences, Lowell, MA). The flask was incubated at 37°C in 5% CO2. On day 2, fresh cytokines IL-4 (2000 IU/ml) and GM-CSF (2000 IU/ml) were added in addition to Keyhole limpet hemocyanin (KLH, 10mcg/ml, Stellar Biotechnology Port Hueneme, CA, USA). Day 3, immature DCs were transduced with Vector Ad5f35HER2ECTM (Center for Cell and Gene Therapy, CACT, Baylor Medical College, Houston, TX) at a 1 monocyte to 3000 physical viral particles (measured by optical density) ratio. After transduction, the cells were incubated at 37°C in 5% CO2 for 4 hours then maturation cocktail containing lipopolysaccharide (30ng/ml, CTEP, NIH Frederick MD) and Interferon gamma 1b (Actimmune interferon gamma-1b, 1000 IU/ml, Intermune, Brisbane, CA, USA) were added to the culture. On day 4, 24 hours after the addition of the adenoviral vector, the cells were washed, harvested and re-suspended in cold HBSS.
Gene Expression Profiling
Total RNA was extracted from the DCs, using a miRNeasy kit (Qiagen, Valencia, CA). Universal Human Reference RNA (Stratagene, Santa Clara, CA) was used as reference. Test samples and reference RNA were amplified and labeled using Agilent kit according to the manufacturer's instructions and hybridized on Agilent Chip (Whole Human genome, 4×44k; Agilent Technologies, Santa Clara, CA). Raw images were obtained by scanning the slides with an Agilent Scan G2505B and Agilent Scan Control software (version 9.5). The images were extracted using the Feature Extraction Software (Agilent Technologies). Partek Genomic Suite 6.4 (Partek Inc., St. Louis, MO, USA) was used for data visualization, identification of differentially expressed transcripts and hierarchical cluster analysis. We transformed the fluorescence intensity data to log2 ratios of each sample versus the universal human RNA reference (Stratagene, Santa Clara, CA, USA). Then t-tests were used to identify the differentially expressed genes (p value less than 0.05). The Ingenuity Pathway Analysis (IPA) tool (http://www.ingenuity.com, Ingenuity System Inc., Redwood City, CA, USA) was used for analysis of functional pathways.
Supernatant Cytokine and Growth Factor Analysis Using Multiplex SOMAscan
Supernatants from the DC samples were evaluated using a SOMAmer-based capture array called SOMAscan (SomaLogic Inc, Boulder, CO) performed at CHI (Center for Human Immunology, NIH). In brief, each of the 1322 proteins measured has its own binding reagent made of chemically modified DNA, referred to as modified aptamer7. Each sample of plasma was incubated with the mixture of modified aptamers to generate modified aptamer-protein complexes. Unbound modified aptamers and unbound or nonspecifically bound proteins were eliminated by 2 bead-based immobilization steps. After eluting the modified aptamers from the target protein, the fluorescently labeled modified aptamers were directly quantified on an Agilent hybridization array (Agilent Technologies). After the data normalization, Calibrators (Partek Genomic Suite 6.4 (Partek Inc., St. Louis, MO, USA) and BRB-ArrayTools (9) were used for data analysis and visualization. Univariate two sample t-test was performed to identify significantly expressed proteins between plasma samples associated with high DC HER2/neu expression and those associated with low DC HER2/neu expression. Global permutation p-value for significant proteins was computed based on 10000 random permutations. Efron-Tibshirani’s maxmean test was used in the functional enrichment pathway analysis on 151 Biocarta pathways. A class prediction model was built using the PAM method (10) to predict the plasma samples with either high or low DC HER2/neu expression. PAM is based on an enhancement of the simple nearest prototype (centroid) classifier. Performance of PAM classifier was estimated using 10 fold cross-validation.
Adenovirus Antibodies
Antibodies to adenovirus were measured using a complement fixation assay (Quest Diagnostics)
Flow Cytometry Analysis
Qualitative determinations of specific sub-populations were performed using fluorescent-labeled antibodies and flow cytometry. The purity of the elutriated monocytes was evaluated by flow cytometry using CD33-PE, CD15-FITC, CD3/CD19/CD56-APC and CD45-APC-Cy7 (Becton Dickinson, Mountain View, CA) and isotype controls (Becton Dickinson). The analysis of mDCs was undertaken after harvest on day 4. This included the standard “DC panel” adopted in our institution as lot release for mDCs products and other investigational markers. The panel consisted of CD86-FITC, CD83-PE, CD14-APC, CD209-FITC, CCR7-PE, CD40-APC, HLA-DRFITC, CD123-PE, CD11c-APC, CD80-FITC, CD154-PE, CD54-APC, CD16-FITC, CCR7-PE and CD1a-APC. The expression of HER2/neu was assessed by flow cytometry with anti-CD340 antibodies (BioLegend, San Diego, CA). Flow cytometry acquisition and analysis were performed with FACScanto flow cytometer (Becton Dickinson and Company, Franklin Lakes, NJ) according to CPS procedures. Spectral overlap was electronically compensated using single color controls. Quality controls were run before each session according to internal quality control policy.
Results
Patient Characteristics
Cells and plasma from 21 patients were collected and studied. The patients’ age ranged from 36 to 72 years; 12 were female. Among the 21 patients; 8 had colon cancer, 5 ovarian cancer, 4 bladder cancer and 1 each had gastric, cervical, prostate and non-small cell lung cancer (Table 1).
Table 1.
Characteristics of the patients treated with adHER2/neu DCs
| Patient # | Age (years) | Gender | Cancer Type | Tumor HER2 Expression* |
|---|---|---|---|---|
| 1 | 48 | Male | Colon | 2+ |
| 2 | 60 | Female | Ovarian | 2+ |
| 3 | 67 | Female | Cervix | 2+ |
| 4 | 36 | Female | NSCLC | 2+ |
| 5 | 72 | Male | Bladder | 3+ |
| 6 | 43 | Male | Bladder | 3+ |
| 7 | 46 | Female | Colon | 1 + |
| 8 | 60 | Female | Colon | 3+ |
| 9 | 63 | Female | Gastric | 3+ |
| 10 | 55 | Male | Colon | 2+ |
| 11 | 63 | Male | Colon | 2+ |
| 12 | 51 | Female | Colon | 1 + |
| 13 | 52 | Female | Ovarian | 2+ |
| 14 | 43 | Female | Colon | 2+–3+ |
| 15 | 64 | Female | Ovarian | 2+ |
| 16 | 57 | Male | Colon | 1 + |
| 17 | 63 | Male | Bladder | 3+ |
| 18 | 56 | Female | Ovarian | 3+ |
| 19 | 67 | Male | Prostate | 3+ |
| 20 | 72 | Female | Bladder | 1 + |
| 21 | 69 | Female | Ovarian | 1 + |
Measured by immunohistochemistry
Manufacturing adHER2/neu DCs with Autologous Plasma
Each patient enrolled could receive up to 5 doses of the adHER2/neu DC vaccine. Each dose was manufactured from an aliquot of cryopreserved monocytes. Initially, all DCs were manufactured using autologous plasma and after three adHER2/neu DCs were manufactured for patients #1 and #2, two DCs for patient #3 and three DCs for patient #4, it was noted that that the transduction efficiency was variable. Transduction efficiency was <28% for the three DCs for patient #1 and the two DCs for patient #3 (Table 2). In contrast, the transduction efficiency for the two DCs for patients #2 and the three DCs for patient #4 were all greater than 90% (Table 2).
Table 2.
The expression of HER2/neu by DCs manufactured with autologous and healthy donor AB plasm for 21 patients
| Patient # | DCs Manufactured with AB Plasma (% of Cells Expressing CD340) |
DCs Manufactured with Autologous Plasma (% of Cells Expressing CD340) |
||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | A | B | C | |
| 1 | 98.5 | 98.6 | 13.9 | 12.8 | 27.3 | |||
| 2 | 96 | 94.9 | 91.3 | |||||
| 3 | 95.7 | 95.3 | 96.6 | 10.1 | 15.1 | |||
| 4 | 95.2 | 97.8 | 98.3 | 97.6 | ||||
| 5 | 98.7 | 95.1 | ||||||
| 6 | 90.2 | 88.8 | 79.8 | 89.8 | 83.7 | 72.6 | ||
| 7 | 72.3 | 78.3 | 62.2 | 50.3 | ||||
| 8 | 95.9 | 94.5 | 95.7 | 40.8 | ||||
| 9 | 93 | 90.8 | 93.1 | 92.9 | 67 | 83.3 | ||
| 10 | 76.1 | 85.8 | ||||||
| 11 | 97.8 | 97.8 | 96.3 | 96.1 | 96.5 | |||
| 12 | 95.1 | 94 | 14.5 | 96.6 | 97.4 | 98.2 | ||
| 13 | 90 | 93.2 | 94.3 | 18.2 | ||||
| 14 | 98.2 | 6.3 | 97.9 | 94.2 | 97.7 | 98.5 | ||
| 15 | 90.2 | 4.5 | 89.2 | 84.1 | 86.4 | |||
| 16 | 94.9 | 96.6 | 95.3 | 96.7 | ||||
| 17 | 97.6 | 98.5 | 98.8 | 98.9 | 98.3 | 33.7 | ||
| 18 | 82.5 | 97.3 | 97.2 | 98.2 | 95.5 | 60.6 | ||
| 19 | 97 | 13.3 | 85 | 86.9 | 97.3 | 97.9 | ||
| 20 | 98.7 | 86.3 | 11 | 99 | ||||
| 21 | 86.6 | 97.1 | 98.5 | |||||
DC #C from patient #12, DC #B from patient #14 and DC #B from patient #15 were manufactured with AB plasma 635395
Samples highlighted in yellow had less than 70% of DCs expressing CD340.
Because of the poor transduction efficiency for patient #1’s and patient #3’s adHER2/neu DCs, the remaining DCs for these patients were manufactured with single donor AB plasma. Two more DCs were manufactured for patient #1 and three more DCs for patient #3 and the transduction efficiency for all five DCs produced with AB plasma was greater than 95% (Table 2). For all further patients enrolled in this protocol, adHER2/neu DCs were manufacturing with single donor AB plasma; however, in order to investigate the cause of the poor transduction efficiency, we continued to manufacture for laboratory evaluation only one DC preparation from each patient’s monocytes using autologous plasma.
Comparison of adHER2/neu DCs Manufactured with Autologous and Healthy Donor AB Plasma
At least one preparation of DCs was manufactured with AB plasma for 20 patients and autologous plasma for 21 patients; no adHER2/neu DCs were manufactured with AB plasma for patient #2. The average transduction efficiency for the 68 adHER2/neu DCs manufactured with AB plasma for 20 patients was 86.1±22.8% while the average transduction efficiency of the 28 DCs manufactured with autologous plasma for 21 patients was 70.3±33.3%. (p =0.0073). HER2/neu expression was low, <70%, for 6 or 8.8% of the 68 DC preparations manufactured with AB plasma and 10 or 35.7% of the 28 DC preparations manufactured with autologous plasma. Interestingly, among the 6 DC preparations manufactured with AB plasma that had low HER2/neu expression, 3 were manufactured with AB plasma from the same healthy donor, 635395. The expression of HER2/neu by the 3 DCs manufactured with AB plasma 635395 was 14.5%, 6.3% and 4.5%.
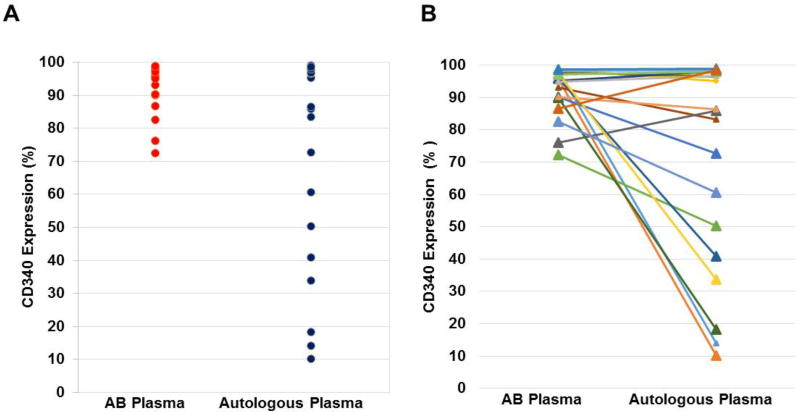
For the 20 patients for whom at least one HER2/neu DC preparation was manufactured with both autologous and AB plasma, the expression of HER2/neu was compared among the first preparation from each patient manufactured with autologous plasma and the first preparation from each patient manufactured with AB plasma. The HER2/neu expression for the 20 DCs manufactured with AB plasma was significantly greater than that of the 20 DCs manufactured with autologous plasma (92.2±7.6% versus 71.7±32.1%; p=0.01, paired t test) (Figure 1).
Figure 1. Comparison of HER2/neu expression by adHER2/neu DCs manufactured with autologous and AB plasma.
At least one HER2/neu DC was manufactured with autologous plasma and healthy donor AB plasma from 20 patients. The expression of HER2/neu for the first manufactured DC of each type, autologous plasma and AB plasma, is shown. Panels A and B show the same data with Panel B showing the relationship of expression by HER2/neu DCs for each patient. HER2/neu expression was assessed by flow cytometry using a CD340 antibody.
Among the 21 patients, diagnosis, gender and age did not affect the frequency of association of autologous plasma with DC HER2/neu expression of <70%. Plasma from 5 of the 13 female and 2 of the 8 male patients was associated with low HER2/neu expression. The mean age of patients whose plasma was associated with DC HER2/neu expression of <70% was 56 years and for those with high HER2/neu expression of >70% was 58 years.
Manufacture of adHER2/neu DCs from Healthy Donor Monocytes and Patient Plasma Associated with Poor DC adHER2/neu Expression
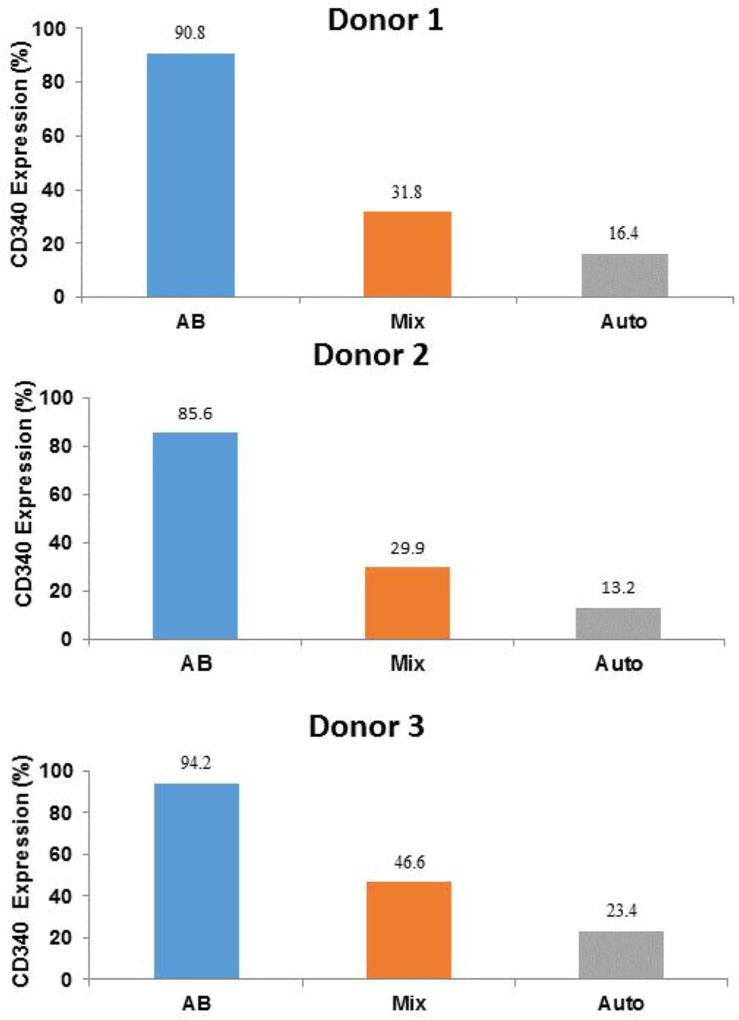
To investigate the role of plasma factors in the low HER2/neu expression, autologous plasma from one patient, #13, which was associated with poor HER2/neu expression when used to manufacture autologous DCs, was used to manufacture adHER2/neu DCs using monocytes from healthy donors. adHER2/neu DCs were manufactured with monocytes from 3 healthy donors using patient #13 plasma, healthy donor AB plasma and an equal mix of patient #13 and AB plasma. The expression of HER2/neu was high (>90%) for all three DC preparations manufactured with AB plasma (Figure 2). In contrast, the expression of HER2/neu was low (<25%) for all 3 DCs manufactured with plasma from patient #13. HER2/neu expression by DCs manufactured with the mix of patient #13 and AB plasma was also low (<50%) (Figure 2). These results confirm that autologous plasma from patient #13 contains a factor or factors that prevent full HER2/neu expression by DCs.
Figure 2. Expression of HER2/neu by DCs manufactured with monocytes from healthy subjects and patient plasma associated with poor HER2/neu expression.
Monocytes from 3 different healthy subjects were used to manufacture adHER2/neu DCs using plasma from patient #13 which was associated with poor HER2/neu expression when used to manufacture autologous DCs. AdHER2/neu DCs were also manufactured with the same healthy donor monocytes and heathy donor AB plasma and an equal mix of Patient #13 plasma and health donor AB plasma. In all cases the final concentration of plasma was 10%. HER2/neu expression was measured by flow cytometry with a CD340 antibody.
Effects of Patient Plasma on adHER2/neu DCs
Evaluation of DCs manufactured with autologous monocytes and plasma
In order to investigate the effects patient plasma on adHER2/neu DCs, DC surface marker expression by the first adHER2/neu DCs manufactured with autologous plasma for each patient was compared among those with HER2/neu expression ≥70% and those with HER2/neu expression <70%. Among the 14 DC preparations with high HER2/neu expression and the 7 with low HER2/neu expression, differences were seen in the expression of CD197, CD14 and CD83. There was no difference in the expression of CD86, CD38 and CD54 (Table 3).
Table 3.
Comparison of surface marker expression by adHER2/neu DCs manufactured with autologous plasma among 14 patients whose first manufactured DCs had high HER2/neu, CD340, expression, >70% of cells, and 7 patients whose first manufactured DC had low HER2/neu, CD340, expression, <70%.
| CD340 (%) |
CD197 (%) |
CD14 (%) |
CD86 (%) |
CD83 (%) |
CD38 (%) |
CD54 (%) |
CD340 (MFI) |
HLA DR (MFI) |
|
|---|---|---|---|---|---|---|---|---|---|
| High Transduction Efficiency | 96 | 43.6 | 13.7 | 98.8 | 81.3 | 98.3 | 98.8 | 17062 | 9258 |
| 97.8 | 25.4 | 11.3 | 98.8 | 81.8 | 98.1 | 98.6 | 19481 | 10653 | |
| 95.1 | 12.1 | 13.5 | 99.6 | 44.7 | 83.6 | 99.7 | 18620 | 2542 | |
| 83.2 | 34.1 | 6.9 | 99.2 | 91.2 | 98.4 | 99.8 | 10564 | 12020 | |
| 85.8 | 6.6 | 2.6 | 97.8 | 85.4 | 94.7 | 99.1 | 14898 | 393 | |
| 96.5 | 13.6 | 1.9 | 99.7 | 93.9 | 99.5 | 99.4 | 13706 | 6648 | |
| 98.2 | 24.6 | 8.9 | 99.5 | 92.2 | 98.3 | 99.7 | 20186 | 10780 | |
| 98.5 | 11.5 | 8.6 | 99.4 | 80.3 | 96.2 | 99.8 | 21792 | 6335 | |
| 86.4 | 63.5 | 34.2 | 96.7 | 83.9 | 95.5 | 97.5 | 14490 | 23845 | |
| 96.7 | 11.1 | 12.9 | 98.3 | 66.3 | 95.1 | 99.3 | 20162 | 2416 | |
| 97.9 | 20 | 4.9 | 99.7 | 75.7 | 93.9 | 99.7 | 10,313 | 1,949 | |
| 99 | 13.4 | 9.6 | 99.5 | 91.9 | 99.6 | 99.1 | 11,082 | 2,210 | |
| 98.5 | 9.7 | 3.9 | 99.9 | 77.2 | 97.8 | 99.3 | 11,203 | 2,958 | |
| 72.6 | 3.8 | 9.5 | 82.8 | 51.9 | 78.5 | 87.8 | 160 | 16784 | |
| Average ± SD | 93.0±7.93 | 20.9±16.5 | 10.2±7.9 | 97.8±4.4 | 78.4±14.9 | 94.8±6.2 | 98.4±3.1 | 14551±5737 | 7770± 6673 |
| Low Transduction Efficiency | 13.9 | 52.8 | 43 | 99.8 | 98.9 | 99.8 | 99.8 | 1432 | 28925 |
| 10.1 | 79.5 | 26.6 | 99.8 | 99.4 | 99.7 | 99.7 | 989 | 18487 | |
| 50.3 | 63.9 | 46 | 99 | 86.2 | 97.3 | 98.7 | 6054 | 46261 | |
| 40.8 | 57.6 | 38.5 | 99.4 | 92.3 | 99.3 | 99.8 | 3348 | 34608 | |
| 18.2 | 75 | 32 | 97.5 | 94.4 | 98 | 98 | 1122 | 24594 | |
| 33.7 | 74.9 | 27.9 | 99.4 | 98.7 | 99.4 | 99.2 | 1517 | 4446 | |
| 60.6 | 77.6 | 35.6 | 99.6 | 91.4 | 99 | 99.9 | 3213 | 13472 | |
| Average ± SD | 32.5±19.3 | 68.8±10.6 | 35.7±7.4 | 99.2±0.8 | 94.5±4.9 | 98.9±0.9 | 99.3±0.7 | 2525±1832 | 24399±13875 |
| P-value | 9.06×10−05 | 2.82×10−07 | 6.14×10−06 | N.S. | 1.85×10−03 | 2.89×10−02 | N.S. | 1.48×10−06 | 1.86×10−02 |
NS = not significant
Evaluation of DCs manufactured with healthy donor monocytes and patient plasma associated with poor DC HER2/neu expression
The expression of surface markers assessed by flow cytometry was compared among adHER2/neu DCs manufactured with the monocytes from 3 healthy subjects using patient #13 plasma and AB plasma. We found that CD197 expression was lower on adHER2/neu DCs manufactured using AB plasma than on those manufactured using patient #13 plasma (8.5±1.5% vs 32.0±6.4%), but the expression of CD14, CD86, CD83, CD38, and CD54 expression were similar between adHER2/neu DCs manufactured with AB and patient plasma was similar (Table 4).
Table 4.
Surface marker expression by adHER2/neu DCs manufactured from healthy donor monocytes using AB and patient #13 plasma.
| Monocyte Donor |
CD340 (%) |
CD197 (%) |
CD14 (%) |
CD86 (%) |
CD83 (%) |
CD38 (%) |
CD54 (%) |
CD340 (MFI) |
HLA DR (MFI) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 90.8 | 11.9 | 21.0 | 99.0 | 82.2 | 96.3 | 99.2 | 7571 | 3035 | |
| AB | 2 | 85.6 | 11.0 | 41.2 | 99.1 | 94.4 | 99.0 | 99.5 | 5238 | 4732 |
| 3 | 94.2 | 2.6 | 27.2 | 98.9 | 73.6 | 94.3 | 98.7 | 6261 | 10402 | |
| Aver ± SD | 90.2±4.3 | 8.5±5.1 | 29.8±10.3 | 99.0±0.1 | 83.4±10.5 | 96.5±2.4 | 99.1±0.4 | 6356±1169 | 6056±3857 | |
| 1 | 16.4 | 26.0 | 23.5 | 99.0 | 93.0 | 99.4 | 98.9 | 686 | 7074 | |
| Pt #13 | 2 | 13.2 | 38.7 | 34.6 | 99.4 | 98.7 | 99.9 | 99.5 | 404 | 5534 |
| 3 | 23.4 | 31.3 | 26.8 | 97 | 93.4 | 99.1 | 96.1 | 978 | 6811 | |
| Aver ± SD | 17.7±5.2 | 32.0±6.4 | 28.3±5.7 | 98.5±1.3 | 95.0±3.2 | 99.5±0.4 | 98.2±1.8 | 689±287 | 6473±823 | |
| 1 | 31.8 | 19.1 | 28.3 | 99.3 | 93.5 | 99.8 | 99.5 | 1105 | 9092 | |
| Mix | 2 | 29.9 | 30.4 | 39.6 | 99.7 | 96.7 | 99.9 | 99.8 | 856 | 8334 |
| 3 | 46.6 | 10.9 | 34.8 | 98 | 88.6 | 99.4 | 96.8 | 1923 | 5794 | |
| Ave±SD | 36.1±9.1 | 20.1±9.8 | 34.2±5.7 | 99.0±0.9 | 92.9±4.1 | 99.7±0.3 | 98.7±1.7 | 1294±558 | 7740±1727 | |
| AB vs Auto | 2.06×10−04 | 3.79×10−02 | N.S. | N.S. | N.S. | N.S. | N.S. | 1.18×10−02 | N.S. | |
| AB vs Mix | 3.90×10−03 | 9.63×10−02 | N.S. | N.S. | N.S. | N.S. | N.S. | 1.87×10−02 | N.S. | |
| Auto vs Mix | 1.67×10−02 | 1.09×10−01 | 2.91×10−02 | N.S. | N.S. | N.S. | 4.72×10−02 | N.S. | N.S. |
AB = AB plasma
Auto = autologous patient #13 plasma
Mix = equal mixture of AB and patient #13 plasma
Surface marker expression was measured by flow cytometry
The adHER2/neu DCs manufactured with healthy donor monocytes and patient #13 plasma, AB plasma or a mixture containing 50% AB plasma and 50% patient #13 plasma were also analyzed by global gene expression analysis (Figure 3). A total of 2085 genes were differentially expressed among the DCs manufactured with patient #13 plasma and AB plasma (t-tests, p≤0.05). Unsupervised hierarchical clustering analysis using the 2085 differentially expressed genes separated the DCs manufactured with patients #13 plasma, the DCs manufactured with AB plasma and those manufactured with a mixture of patient and AB plasma. Ingenuity analysis of the 2085 genes whose expression was greater in DCs manufactured with patient #13 plasma showed that these genes were over represented in a variety of pathways (Table 5).
Figure 3. Global gene expression analysis of HER2/neu DCs manufactured with healthy donor monocytes using patient and healthy donor AB plasma.
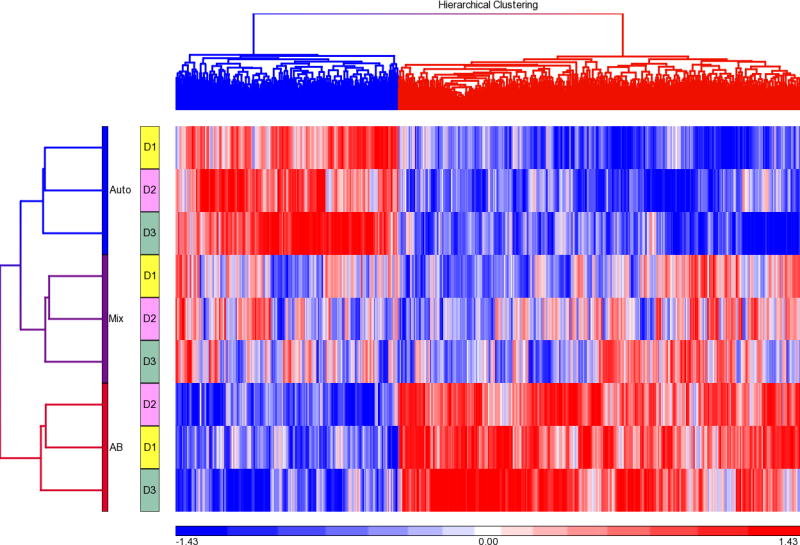
Monocytes from 3 different healthy subjects were used to manufacture HER2/neu DCs using plasma from patient #13 which was associated with poor HER2/neu expression when used to manufacture autologous DCs (Auto). AdHER2/neu DCs were also manufactured with the same healthy donor monocytes and heathy donor AB plasma (AB) and an equal mixture of patient #13 and AB plasma (Mix). The 9 DCs were analyzed by global gene expression analysis. A total of 2085 genes were differentially expressed among the DCs manufactured with patient #13 and AB plasma. The 9 DCs were subjected to hierarchical clustering analysis using the 2085 differentially expressed genes.
Table 5.
Ingenuity pathway analysis of 2085 genes differentially expressed between DCs manufactured with healthy donor monocytes, inhibitory patient plasma and AB plasma*
| Protein Ubiquitination Pathway | Cell Cycle Control of Chromosomal Replication |
| PPAR Signaling | IL-6 Signaling |
| fMLP Signaling in Neutrophils | Cardiac Hypertrophy Signaling |
| IL-10 Signaling | Agrin Interactions at Neuromuscular Junction |
| Cell Cycle: G2/M DNA Damage Checkpoint Regulation | SAPK/JNK Signaling |
| LPS/IL-1 Mediated Inhibition of RXR Function | P2Y Purigenic Receptor Signaling Pathway |
| Card iomyocyte Differentiation via BMP Receptors | IGF-1 Signaling |
| Actin Nucleation by ARP-WASP Complex | LPS-stimulated MAPK Signaling |
| Estrogen Receptor Signaling | Fatty Acid Activation |
| ATM Signaling | Pyrimidine Ribonucleotides De Novo Biosynthesis |
| GNRH Signaling | PPARα/RXRα Activation |
| Thio-molybdenum Cofactor Biosynthesis | CD27 Signaling in Lymphocytes |
| Sorbitol Degradation I | CXCR4 Signaling |
| Dolichyl-diphosphooligosaccharide Biosynthesis | NRF2-mediated Oxidative Stress Response |
| Hepatic Cholestasis | IL-2 Signaling |
All pathways were up regulated in DCs manufactured with inhibitory plasma
Analysis of Patient Plasma
Adenovirus antibodies
Since antibodies to adenovirus could prevent DC transduction with adHER2/neu (11–13), anti-adenovirus levels were tested for patients #8 through #21 at the time of enrollment into the clinical trial. The antibody titers by a complement fixation assay were less than 1 to 8 for all 14 patients including for 4 whose plasma was associated with poor DC HER2/neu expression. These results suggest that poor HER2/neu expression was not due to antibodies directed to adenovirus.
Plasma Protein levels
We evaluated the levels of 1322 factors in 11 patient plasma samples that supported a HER2/neu transduction of >70% and 7 patient plasma samples associated with a transduction efficiency of <70%. We also analyzed the expression of the same 1322 factors in AB plasma from a healthy subject that was associated with high DC HER2/neu expression, 90.0%, and AB plasma from one subject that was associated with poor expression of HER2/neu, 14.5%. Principal component analysis of the 20 samples and 1322 proteins separated the samples into 2 groups; one group associated with low HER2/neu expression and one associated with high HER2/neu expression, except for two patient samples associated with 85.8% and 96.7% HER2/neu expression that clustered with the low expression group (Figure 4).
Figure 4. PCA analysis of soluble factors in patient plasma.
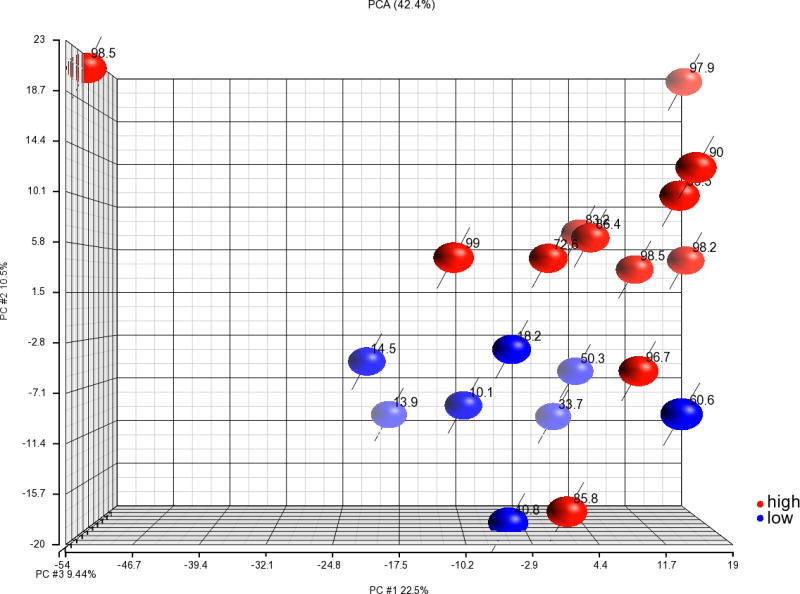
The levels of 1322 soluble factors were assessed in plasma from the 18 patients and 2 AB blood group healthy donors using a multiplex SOMAmer-based capture array. Among the 20 plasma samples 7 patient and 1 AB plasma donor samples were associated with low HER2/neu expression, <70%, and 11 patient and 1 AB donor plasmas were associated with high HER2/neu expression, >70%. The results of PCA of the 20 samples using all 1322 proteins are shown. Plasma samples associated with high HER2/neu expression are shown in red and those with low expression are shown in blue. The number by each circle indicates the HER2/neu expression of the first adHER2/neu DC manufactured with each plasma sample.
Functional enrichment pathway analysis of the 1322 proteins and 20 samples using Efon-Tibshirani’s maxmean test found 8 significant Biocarta pathway sets among 151 Biocarta pathways (p<0.005); the levels of proteins in 4 pathways were increased in plasma samples associated with low HER2/neu expression and the protein levels in 4 pathways were increased among plasma samples associated with high HER2/neu expression. Among the 4 pathways with protein levels that were increased in the samples associated with high HER2/neu expression 3 were immune pathways (Table 6).
Table 6.
Functional Enrichment Pathway Analysis of the levels of 1322 proteins using Efron-Tibshirani's maxmean test reveals 8 significant Biocarta pathways (under 200 permutations)
| Biocarta Pathway | Pathway description | Number of Proteins |
Efron-Tibshirani's GSA test p-value |
|---|---|---|---|
| h_trkaPathway | Trka Receptor Signaling Pathway | 9 | < 0.005 (−) |
| h_srcRPTPPathway | Activation of Src by Protein-Tyrosine Phosphatase Alpha | 5 | 0.005 (−) |
| h_eif4 Pathway | Regulation of eIF4e and p70 S6 Kinase | 11 | 0.005 (−) |
| h_plcPathway | Phospholipase C Signaling Pathway | 7 | 0.005 (−) |
| h_longevityPathway | The IGF-1 Receptor and Longevity | 12 | < 0.005 (+) |
| h_il6Pathway | IL-6 Signaling Pathway | 14 | < 0.005 (+) |
| h_stat3Pathway | Stat3 Signaling Pathway | 5 | < 0.005 (+) |
| h_IL12Pathway | IL12 and Stat4 Dependent Signaling Pathway in Th1 Development | 9 | < 0.005 (+) |
(−) = pathways with proteins whose levels were increased in plasma samples associated with low HER2/neu expression
(+) = pathways with proteins whose levels were increased in plasma samples associated with high HER2/neu expression
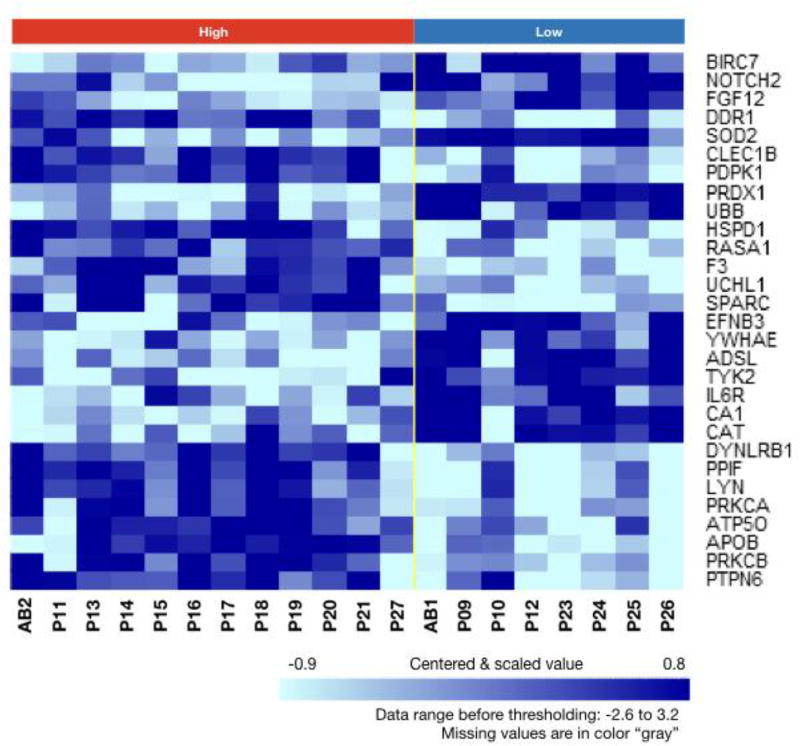
Among the 12 plasma samples associated with high DC HER2/neu expression and 8 associated with low DC HER2/neu expression, 29 proteins were differentially expressed (t-tests p<0.005 and global permutation test p<0.018) (Supplemental Table 1). A clustered heatmap of the 20 plasma samples using the 29 differentially expressed proteins shows a separation of the samples into two groups; one with samples associated with high HER2/neu expression and another with samples associated with low HER2/neu expression (Figure 5). The 29 proteins included immune system factors Notch2, FGF12 and IL-6R. Prediction Analysis of Microarrays (PAM) analysis identified that the levels of 14 proteins were able to predict which plasma samples were associated with high or low DC HER2/neu expression (Table 7). Performance indicators of the PAM classifier during cross validation are good with 91.7% sensitivity, 75% specificity, 84.6% PPV (Positive Prediction Value), 85.7 NPV (Negative Prediction Value), and 85% overall accuracy.
Figure 5. Clustered heat map of 29 significantly expressed proteins with plasma samples grouped by class.
The levels of 29 proteins were significantly different among 8 plasma sample associated with low HER2/neu expression, <70%, and 12 plasma samples associated with high expression (t test p<0.005 and Global permutation test p<0.018). The clustered heat map separated plasma samples associated with higher HER2/neu expression from those associated with lower HER2/neu expression. Higher protein levels are indicated by darker color. AB indicates AB plasma samples and P indicates patient plasma samples.
Table 7.
PAM analysis of the 1322 proteins identifies 14 proteins that can best characterize HER2/neu transduction efficiency with 85 percent prediction accuracy from 10 fold cross validation.
| Symbol | Fold-change (high vs low) |
Description | |
|---|---|---|---|
| 1 | CA1 | −2.6316 | Carbonic Anhydrase I |
| 2 | ADSL | −1.9231 | Adenylosuccinate Lyase |
| 3 | PRDX1 | −1.5385 | Peroxiredoxin 1 |
| 4 | LYN | 2.04 | LYN Proto-Oncogene, Src Family Tyrosine Kinase |
| 5 | APOB | 2.05 | Apolipoprotein B |
| 6 | PRKCA | 2.12 | Protein Kinase C, Alpha |
| 7 | PTPN6 | 2.22 | Protein Tyrosine Phosphatase, Non-Receptor Type 6 |
| 8 | PPIF | 2.4 | Peptidylprolyl Isomerase F |
| 9 | EIF4G2 | 2.5 | Eukaryotic Translation Initiation Factor 4 Gamma, 2 |
| 10 | PDPK1 | 2.53 | 3-Phosphoinositide Dependent Protein Kinase 1 |
| 11 | TPM4 | 2.58 | Tropomyosin 4 |
| 12 | GRB2 | 2.73 | Growth Factor Receptor-Bound Protein 2 |
| 13 | DYNLRB1 | 3.27 | Dynein, Light Chain, Roadblock-type 1 |
| 14 | C3 | 3.56 | Complement Component 3 |
Discussion
When we first manufactured adHER2/neu DCs for a cancer immunotherapy clinical trial we used autologous monocytes and media supplemented with 10% heat inactivated autologous plasma. However, after noting that 5 of the first 11 DC preparations manufactured had much lower HER2/neu expression than expected, AB plasma was used in place of autologous plasma. Low DC HER2/neu expression was less likely when AB plasma was used as a media supplement for manufacturing the adHER2/neu DCs. Among the 21 patients in this study, the manufacture of DCs with autologous plasma from 7 was associated with low HER2/neu expression. When adHER2/neu DCs were manufactured with monocytes from 6 of these 7 patients using AB plasma from healthy subjects, HER2/neu expression increased for all 6 patients. Manufacturing adHER2/neu DCs using healthy donor monocytes and patient plasma associated with low HER2/neu expression, found that the patient plasma also inhibited HER2/neu expression by healthy donor DCs. These results suggested that factors present in plasma from some patients with cancer inhibited HER2/neu expression.
To identify the factor or factors that contributed to the reduced expression of HER2/neu by DCs, patient plasma was evaluated. Comparison of 1322 protein levels among plasma samples associated with high and low DC HER2/neu expression found that 8 pathways and the levels of 29 proteins differed among the groups and the level of 14 proteins could predict high or low DC HER2/neu transduction efficiency. The 8 Biocarta pathways were associated with HER2/neu transduction included 3 immune related pathways: IL-6 signaling, Stat 3 signaling and IL-12 and Stat 4 Dependent Signaling Pathway in Th1 Development. While some of these proteins may be markers of difference among the patients rather than contributing to differences in HER2/neu transduction, our results show that multiple factors are likely responsible for the differences in HER/neu transduction. We speculate that changes in plasma factor levels that resulted in reduced transfusion of DCs by HER2/neu may contribute to systemic changes in immune function in these patients. The nature of the immune modulation and its clinical implications are not certain, but they merit further study.
Evaluation of the adHER2/neu DCs of the 21 patients produced with autologous plasma found changes in addition to low HER2/neu expression. Comparison of surface marker expression and transcriptomes among adHER2/neu DCs manufactured with plasma associated with high and low HER2/neu expression found many differences and suggested that plasma induced changes in DCs that were responsible for the poor HER2/neu expression. Zhang and colleagues have found that the transduction of peripheral blood mononuclear cells with Ad5f35 chimeric adenovirus is increased following stimulation with anti-CD3 and cytokines (15). Using Jurkat T cells as a model they found that changing the cells’ physiological state by starvation reduced Ad5f35 expression (16). Starvation did not affect viral binding but it decreased cell membrane fluidity and adf35 endocytosis and Ad5f35 endosomal escape which resulted in a reduction in the quantity of virus entering the cells and reaching the cytoplasma. It is possible that in the current study plasma that was associated with low HER2/neu expression by DCs caused changes in DC membrane fluidity or Ad5f35 endosomal escape.
While the use of autologous plasma may provide some level of patient safety by preventing exposure to allogeneic blood products, we found that it can cause some undesirable consequences. The use of autologous plasma produced inconsistent adHER2/neuDCs as assessed by HER2/neu expression. In fact, when autologous plasma was used to manufacture adHER2/neu DCs, HER2/neu expression was so highly variable that it is likely that product potency was, in some cases, affected. For this reason we have stopped using autologous plasma to manufacture clinical adHER2/neu DCs.
In conclusion, we found that the use of autologous plasma as media supplement for the manufacture of adHER2/neu DCs results in a final product of highly variable HER2/neu expression. Multiple factors in the plasma were responsible for the reduced expression of HER2/neu and may be contributing to immune suppression in some patient with cancer.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, Clinical Center and National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selvaggi TA, Walker RE, Fleisher TA. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood. 1997;89(3):776–9. [PubMed] [Google Scholar]
- 2.Macy E, Bulpitt K, Champlin RE, Saxon A. Anaphylaxis to infusion of autologous bone marrow: an apparent reaction to self, mediated by IgE antibody to bovine serum albumin. J Allergy Clin Immunol. 1989;83(5):871–5. doi: 10.1016/0091-6749(89)90099-7. [DOI] [PubMed] [Google Scholar]
- 3.Mackensen A, Drager R, Schlesier M, Mertelsmann R, Lindemann A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2000;49(3):152–6. doi: 10.1007/s002620050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688–700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood LV, Fojo A, Roberson BD, Hughes MS, Dahut W, Gulley JL, et al. TARP vaccination is associated with slowing in PSA velocity and decreasing tumor growth rates in patients with Stage D0 prostate cancer. Oncoimmunology. 2016;5(8):e1197459. doi: 10.1080/2162402X.2016.1197459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 9.Simon R, Lam A, Li MC, Ngan M, Menenzes S, Zhao Y. Analysis of gene expression data using BRB-ArrayTools. Cancer Inform. 2007;3:11–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A. 2002;99(10):6567–72. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Gordo E, Podgorski II, Downes N, Alemany R. Circumventing antivector immunity: potential use of nonhuman adenoviral vectors. Hum Gene Ther. 2014;25(4):285–300. doi: 10.1089/hum.2013.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17(3):295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahi YS, Bangari DS, Mittal SK. Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther. 2011;11(4):307–20. doi: 10.2174/156652311796150372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riol-Blanco L, Sanchez-Sanchez N, Torres A, Tejedor A, Narumiya S, Corbi AL, et al. The chemokine receptor CCR7 activates in dendritic cells two signaling modules that independently regulate chemotaxis and migratory speed. J Immunol. 2005;174(7):4070–80. doi: 10.4049/jimmunol.174.7.4070. [DOI] [PubMed] [Google Scholar]
- 15.Zhang WF, Wu FL, Shao HW, Wang T, Huang XT, Li WL, et al. Chimeric adenoviral vector Ad5F35L containing the Ad5 natural long-shaft exhibits efficient gene transfer into human T lymphocytes. J Virol Methods. 2013;194(1–2):52–9. doi: 10.1016/j.jviromet.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 16.Zhang WF, Shao HW, Wu FL, Xie X, Li ZM, Bo HB, et al. Influence of cell physiological state on gene delivery to T lymphocytes by chimeric adenovirus Ad5F35. Sci Rep. 2016;6:22688. doi: 10.1038/srep22688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.