Abstract
BACKGROUND
Babesia microti is the foremost infectious risk to the US blood supply for which an FDA licensed test is unavailable for donation screening. Characterization of the antibody response to B. microti and correlation with parasitemia is necessary to guide screening and donor management policies.
STUDY DESIGN AND METHODS
During an FDA-licensure trial, blood donors were prospectively screened (July-November 2013) using a B. microti-specific antibody enzyme immunoassay (EIA-Immunetics) in highly endemic (New York-NY; n=13,688), moderately endemic (Minnesota-MN; n=4,583) and non-endemic (New Mexico-NM; n=8,451) regions. Blood donors with repeat reactive (RR) results participated in a 12-month prospective cohort study using B. microti EIA, IFA, PCR, blood smear and clinical questionnaire.
RESULTS
Thirty-seven (61.67%) (24 NY, 7 MN, 6 NM) of 60 eligible RR donors enrolled in the study; 20/37 (54%) completed the 12-month follow-up visit of which 15 (75%) were still seroreactive. Nine PCR positive donors were identified during index screening; 5 participated in the follow-up study, 3 were PCR positive at 6 months and 2 remained positive at final follow-up (378 and 404 days). Most RR donors displayed low-level seroreactivity that was either stable or waning during follow-up. The level and pattern of reactivity correlated poorly with PCR positivity.
CONCLUSION
The findings indicate prolonged seropositivity in blood donors. Although rare, asymptomatic, persistent PCR positivity supports the current policy of indefinite deferral for donors with a history of babesiosis or positive test results. Repeat testing by PCR and serology will be necessary if reinstatement is to be considered.
Keywords: Babesia microti, blood donors, ELISA
INTRODUCTION
Babesia microti is a tick-borne, intraerythrocytic protozoan parasite that has gained recent attention for its transfusion transmissibility. Indeed, B. microti is widely regarded as the foremost infectious risk to the US blood supply for which an effective mitigation strategy has yet to be implemented. To date there have been over 200 cases of transfusion transmitted babesiosis (TTB) due to B. microti, three quarters of which have been reported since 2000, including at least 31 transfusion-associated fatalities since initial reporting in 19791–3. For immunocompetent adults, babesiosis characteristically manifests as a subclinical or mild febrile illness. Nonetheless, B. microti has the ability to establish asymptomatic, infection that can persist for months to years in some infected individuals, escaping notice at time of blood donation. For certain patient subsets such as neonates, patients aged >50 years, the asplenic and the immunocompromised (HIV, cancer and immunosuppressant therapy), babesiosis may be severe or even fatal. Those same patient populations at risk are notably over-represented among transfusion recipients, which is the likely basis for the high mortality (~19%) associated with TTB4.
Currently, the only required mitigation strategy in use for TTB is indefinite deferral of those donors who report a history of babesiosis. This approach has proven to be suboptimal as evidenced by the increase in cases of TTB, thus providing the impetus for the development of laboratory-based screening assays for detection of B. microti5,6. However, babesia poses novel challenges for transfusion screening in the US. First, B. microti is regionally endemic (i.e., primarily in the Northeast and upper Midwestern US) and screening outside of endemic areas is projected to incur high costs with little gain7. Conversely, TTB – unlike tick borne infection- is not geographically restricted given routine shipment of blood that is collected in endemic states to those where Babesia is non-endemic4. Donors also travel outside of their state of residence further detracting from the efficacy and rationale of regional screening approaches. Second, unlike the major transfusion transmissible viral infections (e.g. HIV, HBV and HCV) that are detectable in plasma, Babesia is an intraerythrocytic parasite, rendering recovery of target nucleic acids comparatively difficult if molecular testing were to be considered. In contrast, serology-based screening is well established and comparatively inexpensive, yet has the potential for high rates of donor deferral given a large, ill-defined area of endemicity where many donors are likely to be seroreactive, including those with resolved infections that do not pose a risk for TTB.
An understanding of the kinetics of Babesia infection (i.e., durations and levels of parasitemia and antibodies from initial acquisition of parasitemia through seroconversion, resolution of persistent infection and eventual seroreversion) is critical to inform strategies to interdict infectious donations. Furthermore, the relationship between test reactivity (by EIA and/or PCR) and infectivity (parasitemia) is important for donor counseling and management and facilitates development of guidelines for reinstating test positive individuals into the donor pool. For example, high rates of seroreactivity in the absence of parasitemia could support reinstatement of seroreactive donors; conversely, the demonstration of high rates of acute or chronic parasitemia could motivate for inclusion of PCR in screening algorithms. These issues are particularly important in endemic areas where incidence and seroprevalence are high,8–13 yet many seropositive donors will have resolved their infection and are likely not infectious (absent parasitemia).
To address these questions, we enrolled and followed a cohort of B. microti seroreactive blood donors. By employing sensitive and semiquantitative serological and PCR testing, we strived to determine what proportion of B. microti seropositive donors develops persistent seroreactivity with or without evidence of persistent parasitemia. This infectious-risk categorization (transient vs. enduring evidence of exposure and infection) is important to understanding the natural evolution of B. microti infection and guide blood screening strategies.
MATERIALS AND METHODS
Blood Systems Research Institute (BSRI), Immunetics, Creative Testing Solution (CTS), New York Blood Center (NYBC), United Blood Services-New Mexico (UBS-NM) and Memorial Blood Center division of Innovative Blood Resources (MBC) conducted an FDA-licensure trial for an EIA developed for high throughput blood donor screening5. Prospective (real-time) screening was conducted July 29 – November 15 2013 using an EIA (Immunetics, MA) against B. microti antibodies on a sample of donations that originated in B. microti highly endemic (New York; n=13,688), moderately endemic (Minnesota; n=4,583) and non-endemic areas (New Mexico; n=8,451).
Repeat reactive (S/C ≥1.0) and gray zone (S/C ≥0.934-<1.0) donations were subjected to immunofluorescent assay (IFA), PCR and peripheral blood smear (PBS) examination. The methods and results for each of the test modalities (EIA, IFA and PBS) from the index screening study have previously been described (reference Levin et al. IND paper). Further analysis of the index donation data from both the validation and clinical trial studies supported an increase in the original S/C for defining reactivity from 1.0 to 1.6 which resulted in improved specificity without compromising sensitivity for detection of PCR positive donors (i.e., none of the PCR positive donors or clinical babesiosis cases had EIA S/C ratios below 1.6)(reference Levin et al. IND paper). However, the revised criterion for seroreactivity reduced the number of eligible donors as noted in Table 1. The follow-up study analysis reported here is confined to those donors who had an index S/C >1.6 x the original cut-off.
Table 1.
Babesia Study Demographics by EIA
| All | EIA 1.0 – 1.59 | EIA ≥ 1.6 | χ2 | ||||
|---|---|---|---|---|---|---|---|
| n=87 | n=50 | n=37 | p | ||||
| Sex | |||||||
| Male | 41 | 47.1% | 23 | 46.0% | 18 | 48.6% | 0.8 |
| Female | 46 | 52.9% | 27 | 54.0% | 19 | 51.4% | |
| Race | |||||||
| White | 75 | 86.2% | 43 | 86.0% | 32 | 86.5% | 0.3 |
| Black | 2 | 2.3% | 2 | 4.0% | 0 | 0.0% | |
| Asian | 1 | 1.1% | 0 | 0.0% | 1 | 2.7% | |
| American Indian/Native Alaskan | 2 | 2.3% | 2 | 4.0% | 0 | 0.0% | |
| Other | 6 | 6.9% | 2 | 4.0% | 4 | 10.8% | |
| Refused | 1 | 1.1% | 1 | 2.0% | 0 | 0.0% | |
| Ethnicity | |||||||
| Non-Hispanic | 70 | 80.5% | 39 | 78.0% | 31 | 83.8% | 0.8 |
| Hispanic | 11 | 12.6% | 7 | 14.0% | 4 | 10.8% | |
| Missing | 6 | 6.9% | 4 | 8.0% | 2 | 5.4% | |
| Age | |||||||
| Median (Range) | 50 (18–89) | 49 (18–84) | 51 (19–89) | ||||
| 18–29 | 12 | 13.8% | 7 | 14.0% | 5 | 13.5% | 0.5 |
| 30–39 | 11 | 12.6% | 8 | 16.0% | 3 | 8.1% | |
| 40–49 | 18 | 20.7% | 11 | 22.0% | 7 | 18.9% | |
| 50–59 | 21 | 24.1% | 13 | 26.0% | 8 | 21.6% | |
| 60–89 | 25 | 28.7% | 11 | 22.0% | 14 | 37.8% | |
Eligible donors were contacted via mail and/or telephonically regarding enrollment into a prospective cohort study. Eligibility for participation in the follow-up study was based on a grey zone or RR EIA test result during clinical trial/index screening (see above). This was later revised during the analysis phase to focus exclusively on those donors who had index S/C values ≥1.6 x the original cutoff. Participation in the follow-up study was conducted under full informed consent and ethical approval was obtained from the ethics committees that represent each of the participating institutions (Blood Systems Research Institute, New York Blood Center, Memorial Blood Center/Innovative Blood Resources, UBS-New Mexico, Creative Testing Solutions and Immunetics) prior to initiation of the research activities. Donors who agreed to participate in the follow-up study completed a questionnaire (see below) and underwent phlebotomy for repeat testing using the full complement of tests employed on index donation samples (EIA, IFA, PCR and PBS). An abbreviated follow-up clinical questionnaire was administered and phlebotomy undertaken at each of 5 follow-up visits (1–2 weeks, 3-, 6-, 9- and 12- months post-index donation). Subject follow-up concluded December 24, 2014. The follow-up test results were communicated to subjects by mail along with an interpretation and recommendation to share the results with the subject’s health care provider. Multiple serum, plasma and residual red cell aliquots were prepared from the index donation and follow-up blood samples and saved frozen in a dedicated specimen repository at CTS for use both in this study as well as in future research.
Variables captured in the questionnaires included donor demographics, travel outside state of residence, comorbid medical or surgical conditions (e.g. splenectomy), infections that may be associated, confused or co-infect with B. microti (e.g. Lyme borreliosis, anaplasmosis, or malaria), time spent in grassy or wooded areas, tick bites, exposure and/or clinical symptoms of babesiosis. The questionnaire was administered either telephonically or in person; this accounts for discrepancies between the blood draws and questionnaires i.e. completed questionnaires in the absence of a blood draw where the subject failed to return after being contacted telephonically.
Donors implicated in cases of TTB
In addition donors who had been implicated in cases of TTB were contacted and enrolled in a sub-study. Those donors who responded and provided consent, returned for a single blood draw; the associated specimen was evaluated using EIA, IFA, PCR and PBS.
Statistical Analysis
Demographic data were summarized and descriptive statistics were reported for all variables. For the purposes of analysis, designation as highly- and moderately endemic was collapsed into a single endemic group (for comparison with data from the non-endemic group). Sample median, minimum and maximum were provided for continuous variables. Categorical values were summarized with frequencies and percentages. Point estimates and 95% confidence intervals are reported. Two-tailed tests were conducted with p=0.05 as the criterion for statistical significance. Statistical analyses were performed with StataMP version 12 (StataCorp, College Station, TX).
RESULTS
A total of 26,702 blood donors were screened in the clinical trial. At a provisional S/C of 1.0, 134 (0.5%) donors screened seropositive (126 repeat reactive and 8 gray zone) of whom 87/134 (64.93%) [48 NY, 15 MN and 24 NM] consented to participate in the follow-up study. Given revision of the S/C ratio from 1.0 to 1.6, the results of this analysis are confined to that subset. A total of 37 (24 NY, 7 MN, 6 NM) out of 60 eligible donors with an index S/C ≥1.6 enrolled; the median age of the enrolled donors was 51 [19–89] years; 48.6% were male; and 86.5% were non-Hispanic Caucasian) (Table 1). The number of follow-up visits attended did not vary by donor demographics (data not shown.
Laboratory Testing at Provisional cut-off of 1.6
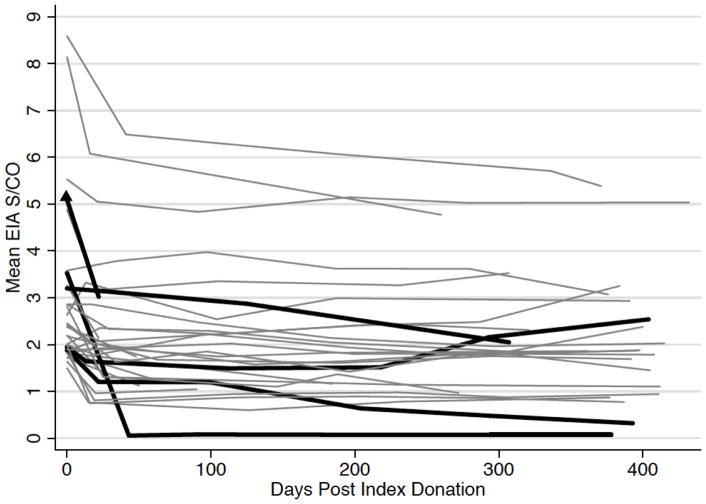
Of the 37 donors who enrolled in the follow-up study 20 (54.05%) completed the 12-month follow-up blood draws. Fifteen (75%) of these donors were still EIA seroreactive at their final visits, a median of 398 (362–432) days following their index donations. A total of 9 PCR positive donors were identified during screening (8 from NY and 1 from MN) 5 of whom participated in the follow-up study. Three of the five PCR positive donors remained PCR positive at 6 months, 2 were still positive at 9 months and remained PCR positive at final follow-up (378 and 404 days) (Figure 1).
Figure 1. Longitudinal follow-up EIA results on index seroreactive blood donors (S/C>1.6 at index donation).
Note: Each line corresponds to a single subject. Bold lines indicates those donors who were PCR positive on index donation
Donor Risk Factors (Table 2)
Table 2.
Risk Factors by Endemic Area
| EIA 1.6 | ||||||
|---|---|---|---|---|---|---|
| Endemic | Non-endemic | All | ||||
| n=31 | n=6 | n=37 | ||||
| Travel to Endemic State in Prior Month | 7 | 22.6% | 0 | 0.0% | 7 | 18.9% |
| Travel to Endemic State in Prior Year | 14 | 45.2% | 0 | 0.0% | 14 | 37.8% |
| Outdoor lifestyle | 24 | 77.4% | 4 | 66.7% | 28 | 75.7% |
| Hiking | 10 | 32.3% | 2 | 33.3% | 12 | 32.4% |
| Camping | 8 | 25.8% | 2 | 33.3% | 10 | 27.0% |
| Hunting | 0 | 0.0% | 1 | 16.7% | 1 | 2.7% |
| Tick bite | 8 | 25.8% | 0 | 0.0% | 8 | 21.6% |
| Tick exposure | 14 | 45.2% | 1 | 16.7% | 15 | 40.5% |
| Post-outdoor rash | 3 | 9.7% | 0 | 0.0% | 3 | 8.1% |
| Post-outdoor symptoms | 6 | 19.4% | 2 | 33.3% | 8 | 21.6% |
| Contact with person with post-outdoor symptoms* | 8 | 25.8% | 1 | 16.7% | 9 | 24.3% |
| History of Lyme Disease | 5 | 16.1% | 0 | 0.0% | 5 | 13.5% |
The question relates to reported symptoms and signs of tick borne illness in a friend or family member; while not specific, an affirmative answer may be used as a crude proxy for exposure risk in the donor given potential for shared activities (e.g. hiking, hunting)
None of the seroreactive subjects in non-endemic areas reported travel to endemic states in the year prior to donation. Donors in both endemic and non-endemic states reported similar rates of outdoor activities (77.4% and 66.7% respectively) as determined by the self-reported time spent outdoors. Likewise, the rates of hiking (32.3 vs. 33.3%) and camping (25.8% vs. 33.3%) were similar. The rates of tick bite, tick exposure and history of Lyme disease were 25.8%, 45.2% and 16.1% in the endemic subjects vs. 0%, 16.7% and 0% in the non-endemic group. None of the donors reported either history of splenectomy or organ transplantation.
Donor Symptoms (Table 3)
Table 3.
Symptoms Reported in Seroreactive donors in the three months prior to donation (Index EIA 1.6)
| Symptoms | N=37 | |
|---|---|---|
| Body aches | 17 | 46.0% |
| Headache | 15 | 40.5% |
| Fatigue | 14 | 37.8% |
| Joint pain | 11 | 29.7% |
| Chills | 7 | 18.9% |
| Sore throat | 7 | 18.9% |
| Sweats | 6 | 16.2% |
| Fever | 5 | 13.5% |
| Diarrhea | 5 | 13.5% |
| Joint swelling | 5 | 13.5% |
| Nausea | 3 | 8.1% |
| Swollen lymph nodes | 2 | 5.4% |
| Poor appetite | 2 | 5.4% |
| Shortness of breath | 2 | 5.4% |
| Conjunctivitis | 2 | 5.4% |
| Skin rash | 1 | 2.7% |
| Dark red/brown urine | 1 | 2.7% |
| Vomiting | 0 | 0.0% |
| Jaundice | 0 | 0.0% |
The most commonly reported symptoms in seroreactive donors were body aches (n=17 [46%]), headache (n=15 [40.5%]), fatigue (n=14 [37.8%]) and joint pain (n=11 [29.7%]). One (2.7%) donor each reported rash and red/brown urine.
Donors implicated in cases of TTB
A total of 5 donors were implicated in TTB during the study enrollment period. Two of the five consented to participate in the study. The implicated units had been collected in September 2013 and February 2014 with a pre-transfusion interval of 22 and 14 days respectively. At time of evaluation, the post-donation interval was 152 and 99 days respectively; both donors were repeat reactive by EIA (mean S/C 3/96 and 4.24), IFA IgG positive, IFA IgM negative and both PCR and PBS negative.
DISCUSSION
Characterization of the immunopathogenesis of B. microti infection (i.e. kinetics of the antibody response and its correlation with parasitemia) is critical to the optimal selection of a mitigation strategy. Of those donors who were index repeat reactive and completed a 12-month follow-up blood draw, the overwhelming majority were still seroreactive, a median of 398 (362–432) days after index donation. While the number of enrolled PCR positive donors was low, at least one donor was still PCR positive at final follow-up (404 days). Furthermore, most donors displayed borderline or low level seroreactivity that either waned or remained stable over time. In contrast, only a few donors demonstrated high-level seroreactivity as reflected by their S/C ratios (Table 5 and Figure 1). Importantly, both the pattern and the level of seroreactivity (i.e. the S/C) correlated poorly with parasitemia rendering it impossible to predict active infection from the S/C alone.
Table 5.
S/CO by Study Visit
| EIA ≥ 1.6 n=37 |
Index PCR+ n=5 |
|||
|---|---|---|---|---|
| Median (Range) | Observations | Median (Range) | Observations | |
| Index donation | 2.3 (1.5–8.6) | 37 | 3.2 (1.9–5.1) | 5 |
| Visit 1 | 1.9 (0.1–6.5) | 31 | 2.3 (1.2–3.1) | 4 |
| Visit 2 | 1.9 (0.1–6.3) | 24 | 1.5 (1.2–2.9) | 3 |
| Visit 3 | 1.8 (0.1–6.1) | 25 | 1.5 (0.6–2.0) | 3 |
| Visit 4 | 1.8 (0.1–5.7) | 19 | 1.3 (0.5–2.2) | 2 |
| Visit 5 | 1.9 (0.1–5.4) | 20 | 1.4 (0.3–2.5) | 2 |
Collectively, the demonstration of persistent seroreactivity and PCR positivity lends support to current babesia donor deferral policy. This is not new, having already been reported previously in both asymptomatic blood donors as well as patients following a diagnosis of acute babesiosis 10,14,15 Moritz et al. recently reported on longitudinal follow-up of 262 seroreactive and/or PCR positive blood donors (from 2010 to 2015) using an arrayed fluorescent immunoassay (AFIA) and PCR. The median time to seroreversion was 410 days (range 24–1156) and among 129 donors followed to 1 year, 104 (81%) remained seroreactive. Of those donors who were PCR positive 69/73(93%) resolved PCR reactivity by 1 year16.
As was evident in our findings, the risk factors for B. microti infection lack sufficient specificity to be used practically. Behaviors that place individuals at risk of tick exposure such as hiking, camping, spending time outdoors etc. are common and independent of Babesia’s geographic distribution. One might consider recall of tick bite to be convincingly associated but this is not the case. One study observed no significant difference in the Babesia seroprevalence rates between those donors who self-reported tick exposure and those who did not recall tick exposure 17. The authors postulated that those who report tick exposure are the same vigilant subset who take routine precautions against tick bites, removing them following frequent self-examination. Another reason why a history of tick exposure has no bearing on Babesia risk is the size of the infecting ticks. It is frequently assumed, erroneously, that infections are ascribed to the adult ticks, which are clearly visible. Instead, the majority of infections are transmitted by nymphal ticks, each of which is approximately the same size as a poppy seed thus readily going unnoticed 18.
Donor travel is an important –albeit complicated- risk factor for babesiosis, particularly as regional strategies have been proposed i.e. screening in those states that are regarded as endemic 4. As stated, TTB, unlike naturally acquired transmission, is not geographically restricted. Residents from non-endemic areas may become infected when traveling to Babesia endemic areas and blood from endemic areas may be transfused in non-endemic areas (resource sharing). Indeed, 13% of cases of TTB have occurred in non-endemic states4. Even the notion of endemic vs. non-endemic is ill defined and will continue to be so with increasing populations of deer (which carry the tick vectors), human encroachment on deer habitats and climate change that affects both deer and tick populations. Therefore, implementation of a regional laboratory screening strategy will be challenging. Surprisingly, in our study six seroreactive donors were residents of New Mexico, (a non-endemic state), none of whom reported travel to endemic states in the year prior to donation. Given convincingly positive results (corroborated by western blot and IFA data), the donors were re-contacted and additional history obtained. This confirmed the absence of travel to endemic states in the two years prior to donation. There are several possible explanations as to why this might occur, including the possibility that B. microti or a Babesia variant species is present in New Mexico. Babesia is a ubiquitous genus and is well described in domestic19,20 and wild animal populations across the United States. While assay specificity was considered, several of the non-endemic donors were convincingly positive by several test modalities.
Selected medical (e.g. cancer, immunosuppressive) or surgical (e.g. splenectomy) conditions place individuals at risk for babesiosis. None of our cohort reported any of these risks. This is unsurprising given that blood donation selects for a healthy subset of the population. Similarly, clinical symptoms and signs were also evaluated and were shown to be unrevealing. The prerequisite for donation eligibility is the absence of symptoms or signs of disease, which is unlikely to be the case for patients with acute babesiosis. In the three months prior to donation, the most commonly reported symptoms and signs were fatigue (38%), joint pain (30%) and sore throat (19%). These overlap with a diverse array of infectious and even non-infectious conditions. Only three percent reported “dark red/brown urine” that might suggest hemaglobinuria secondary to hemolysis. The absence of clinical risk factors to discriminate between those who have and those who have not been exposed to Babesia, underlies the very need for laboratory screening or alternative mitigation strategy (e.g. pathogen reduction technology).
As the industry draws closer toward licensed assays for screening, the recent surge in activity has helped to gain insight into the scope of Babesia infection, enlisting support for informed development of screening and donor management strategies. In May 2015, The Blood Product Advisory Committee meeting voted in favor (11/14 yes votes) of nation-wide, year round serological testing of blood donations for Babesia-risk21. There was also unanimous support (14/14) for NAT-based testing in certain high-risk states, with most (8 votes) supporting a 9 high-endemic state screening strategy. Alternative strategies include pathogen reduction technology (PRT); with both whole blood and red cell PRT in advanced development (i.e. trials already complete or underway)22, which may well be added to the armamentarium in the future.
Our study has limitations. Foremost, while it was advantageous to leverage the larger screening study (IND licensure trial), this introduced unexpected challenges. Specifically, the provisional screening cut-off was selected based on prior validation studies. Analysis of the screening data, with attention to PCR positivity, showed the cut-off to be too conservative, thus compromising specificity (see methods). This motivated for a proposed revision of the EIA cut-off to 1.6. However, given that the enrollment of subjects into the follow-up study had been contingent on the provisional cut-off (1.0), this reduced the original cohort of 134 enrolled subjects to 60. Second, while PCR positivity correlates better with parasitemia and infectivity, there are caveats to its use. For example, PCR positivity does not discriminate between viable and non-viable parasites and may persist following treatment with or without resolution of infection15,23. Repeated PCR positivity in the same donor can reflect reinfection i.e. the test in use does not discriminate between persistent and reinfection. Third, to ascertain whether a given risk factor is over or under-represented in the subject group, one ideally should compare the prevalence in a control group (i.e. denominator data). This is problematic as only risk factor data were captured on those who were enrolled. Fourth, there were unanticipated challenges surrounding subject follow-up; therefore attrition limited the complete longitudinal characterization of donors to a subset of the original cohort. Nonetheless, there are data on index screening and 12-month follow-up for the majority of donors; those two time points were critical to determine the proportion of donors that remained seropositive and/or PCR positive at follow-up. Fifth, we failed to include questions on the follow-up questionnaire regarding whether there had been any treatment for babesiosis following notification of the results. Sixth, while not a deficiency of the follow-up study as such, we acknowledge that primary serological rather than PCR screening will not identify incident (pre-seroconversion or “window phase”) infections. Furthermore, the assay was designed for capture of antibodies against B. microti. While this is overwhelming responsible for TTB in the US, other species of Babesia may have been missed or may have marred the findings24. Finally, only a small number of blood donors were enrolled following their having been implicated in cases of TTB. This limits how much one can glean from the TTB donor results. Nonetheless, the high S/C values for those two cases is contrary to what has been stated about those enrolled in the prospective study i.e. the TTB findings might suggest that high S/C values in donors are more predictive of transmissibility. However, this merits further investigation given the small sample size.
The study served to validate and optimize the EIA for transfusion screening and has contributed data to support laboratory screening. It also suggests lower rates of seropositivity than have been reported in prior studies. In part this may be ascribed to the different methods used in primary screening (e.g. IFA vs. EIA) as well as seasonal variation in rates of infection. The study has also yielded a large sample repository that will be used for future babesia research. Future directions include investigation of antigens that might discriminate active vs. resolved infection as well as development of an antigen/antibody assay, analogous to fourth generation assays in use for the detection of other TTIs e.g. HIV.
In conclusion, the findings refine our understanding of Babesia infection and are consistent with prior reports of persistent seroreactivity in blood donors. PCR positivity, although rare, may be protracted and can result in competent infection. Assays in use for transfusion screening are ideally optimized to capture these high-risk donors. Germane to donor management, the collective findings support current deferral policy for those with a history of babesiosis or test reactivity, pending availability of a biomarker of active infection. Should donor re-entry be considered in the future, repeat screening should be undertaken after a minimum of one to two years.
Table 4.
Index EIA 1.6 & PCR Positive
| N=5 | ||
|---|---|---|
| Sex | ||
| Male | 3 | 60.0% |
| Female | 2 | 40.0% |
| Race | ||
| White | 5 | 100.0% |
| Ethnicity | ||
| Non-Hispanic | 4 | 80.0% |
| Hispanic | 1 | 20.0% |
| Age | ||
| Median (Range) | 49 (24–71) | |
| 18–29 | 2 | 40.0% |
| 30–39 | 0 | 0.0% |
| 40–49 | 1 | 20.0% |
| 50–59 | 0 | 0.0% |
| 60–89 | 2 | 40.0% |
| Risk Factors | ||
| Travel to Endemic State in Prior Month | 0 | 0.0% |
| Travel to Endemic State in Prior Year | 4 | 80.0% |
| Outdoor lifestyle | 5 | 100.0% |
| Hiking | 1 | 20.0% |
| Camping | 2 | 40.0% |
| Hunting | 0 | 0.0% |
| Tick bite | 2 | 40.0% |
| Tick exposure | 3 | 60.0% |
| Post-outdoor rash | 0 | 0.0% |
| Post-outdoor symptoms | 0 | 0.0% |
| Contact with person with post-outdoor symptoms | 2 | 40.0% |
| History of Lyme Disease | 1 | 20.0% |
Acknowledgments
Funding: Funding was provided by the National Heart Lung and Blood Institute through R21HL117140
This authors wish to thank, the staff at NYBC, MBC, UBS-NM and BSI for their invaluable assistance in donor enrollment and follow-up as well as Joan Clifford (Immunetics) and Valerie Winkelman (CTS) for their contribution to database and inventory management respectively.
References
- 1.Gubernot DM, Lucey CT, Lee KC, Conley GB, Holness LG, Wise RP. Babesia infection through blood transfusions: reports received by the US Food and Drug Administration, 1997–2007. Clin Infect Dis. 2009;48:25–30. doi: 10.1086/595010. [DOI] [PubMed] [Google Scholar]
- 2.FDA. Fatalities Reported to FDA Following Blood Collection and Transfusion: Annual Summary for Fiscal Year 2012. 2014. [Google Scholar]
- 3.FDA. Fatalities Reported to FDA Following Blood Collection and Transfusion: Annual Summary for Fiscal Year 2013. 2013. [Google Scholar]
- 4.Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med. 2011;155:509–19. doi: 10.7326/0003-4819-155-8-201110180-00362. [DOI] [PubMed] [Google Scholar]
- 5.Levin AE, Williamson PC, Erwin JL, Cyrus S, Bloch EM, Shaz BH, Kessler D, Telford SR, 3rd, Krause PJ, Wormser GP, Ni X, Wang H, Krueger NX, Caglioti S, Busch MP. Determination of Babesia microti seroprevalence in blood donor populations using an investigational enzyme immunoassay. Transfusion. 2014;54:2237–44. doi: 10.1111/trf.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moritz ED, Winton CS, Johnson ST, Krysztof DE, Townsend RL, Foster GA, Devine P, Molloy P, Brissette E, Berardi VP, Stramer SL. Investigational screening for Babesia microti in a large repository of blood donor samples from nonendemic and endemic areas of the United States. Transfusion. 2014;54:2226–36. doi: 10.1111/trf.12693. [DOI] [PubMed] [Google Scholar]
- 7.Goodell AJ, Bloch EM, Krause PJ, Custer B. Costs, consequences, and cost-effectiveness of strategies for Babesia microti donor screening of the US blood supply. Transfusion. 2014;54:2245–57. doi: 10.1111/trf.12805. [DOI] [PubMed] [Google Scholar]
- 8.Leiby DA, Chung AP, Gill JE, Houghton RL, Persing DH, Badon S, Cable RG. Demonstrable parasitemia among Connecticut blood donors with antibodies to Babesia microti. Transfusion. 2005;45:1804–10. doi: 10.1111/j.1537-2995.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 9.Linden JV, Wong SJ, Chu FK, Schmidt GB, Bianco C. Transfusion-associated transmission of babesiosis in New York State. Transfusion. 2000;40:285–9. doi: 10.1046/j.1537-2995.2000.40030285.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson ST, Cable RG, Leiby DA. Lookback investigations of Babesia microti-seropositive blood donors: seven-year experience in a Babesia-endemic area. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03345.x. [DOI] [PubMed] [Google Scholar]
- 11.Ruebush TK, 2nd, Juranek DD, Chisholm ES, Snow PC, Healy GR, Sulzer AJ. Human babesiosis on Nantucket Island. Evidence for self-limited and subclinical infections. N Engl J Med. 1977;297:825–7. doi: 10.1056/NEJM197710132971511. [DOI] [PubMed] [Google Scholar]
- 12.Filstein MR, Benach JL, White DJ, Brody BA, Goldman WD, Bakal CW, Schwartz RS. Serosurvey for human babesiosis in New York. J Infect Dis. 1980;141:518–21. doi: 10.1093/infdis/141.4.518. [DOI] [PubMed] [Google Scholar]
- 13.Krause PJ, Telford SR, 3rd, Pollack RJ, Ryan R, Brassard P, Zemel L, Spielman A. Babesiosis: an underdiagnosed disease of children. Pediatrics. 1992;89:1045–8. [PubMed] [Google Scholar]
- 14.Krause PJ, Spielman A, Telford SR, 3rd, Sikand VK, McKay K, Christianson D, Pollack RJ, Brassard P, Magera J, Ryan R, Persing DH. Persistent parasitemia after acute babesiosis. N Engl J Med. 1998;339:160–5. doi: 10.1056/NEJM199807163390304. [DOI] [PubMed] [Google Scholar]
- 15.Leiby DA, Johnson ST, Won KY, Nace EK, Slemenda SB, Pieniazek NJ, Cable RG, Herwaldt BL. A longitudinal study of Babesia microti infection in seropositive blood donors. Transfusion. 2014;54:2217–25. doi: 10.1111/trf.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moritz EDSS. Antibody and DNA Duration in Asymptomatic Blood Donors Who Screen Positive in Investigational Testing for Babesia microti. Transfusion. 2015;55:192A. [Google Scholar]
- 17.Leiby DA, Chung AP, Cable RG, Trouern-Trend J, McCullough J, Homer MJ, Reynolds LD, Houghton RL, Lodes MJ, Persing DH. Relationship between tick bites and the seroprevalence of Babesia microti and Anaplasma phagocytophila (previously Ehrlichia sp) in blood donors. Transfusion. 2002;42:1585–91. doi: 10.1046/j.1537-2995.2002.00251.x. [DOI] [PubMed] [Google Scholar]
- 18.CDC. Parasites- Babesiosis- Epidemiology & Risk Factors. 2015. [Google Scholar]
- 19.Birkenheuer AJ, Correa MT, Levy MG, Breitschwerdt EB. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000–2003) J Am Vet Med Assoc. 2005;227:942–7. doi: 10.2460/javma.2005.227.942. [DOI] [PubMed] [Google Scholar]
- 20.Yancey CB, Hegarty BC, Qurollo BA, Levy MG, Birkenheuer AJ, Weber DJ, Diniz PP, Breitschwerdt EB. Regional seroreactivity and vector-borne disease co-exposures in dogs in the United States from 2004–2010: utility of canine surveillance. Vector Borne Zoonotic Dis. 2014;14:724–32. doi: 10.1089/vbz.2014.1592. [DOI] [PubMed] [Google Scholar]
- 21.FDA. Blood Products Advisory Committee Meeting Summary Minutes. 2015 May 13;2015 [Google Scholar]
- 22.Snyder EL, Stramer SL, Benjamin RJ. The safety of the blood supply--time to raise the bar. N Engl J Med. 2015;372:1882–5. doi: 10.1056/NEJMp1500154. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Villafuerte P, Zhuge J, Visintainer P, Wormser GP. Comparison of a quantitative PCR assay with peripheral blood smear examination for detection and quantitation of Babesia microti infection in humans. Diagn Microbiol Infect Dis. 2015 doi: 10.1016/j.diagmicrobio.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Bloch EM, Herwaldt BL, Leiby DA, Shaieb A, Herron RM, Chervenak M, Reed W, Hunter R, Ryals R, Hagar W, Xayavong MV, Slemenda SB, Pieniazek NJ, Wilkins PP, Kjemtrup AM. The third described case of transfusion-transmitted Babesia duncani. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03467.x. [DOI] [PubMed] [Google Scholar]