Abstract
As medical technology advances in the area of cancer therapeutics, dental practitioners will encounter patients with active or history of cancer. Typically, these patients may have or are currently undergoing therapies such as surgery, radiation, chemotherapy, or combinations of the above. These patients may present with multiple side effects that can be preventable and manageable by the practitioner. Here, we will discuss about some of these lesions and provide management strategies for the dental practitioners.
Keywords: Oral cancers, xerostomia, oral mucositis, osteoradionecrosis, trismus, secondary infection, MRONJ, management
INTRODUCTION
Recent study showed that close to 14.5 million Americans have experiences with cancers as of 2014, and about 1.7 million new cancer cases are expected to be diagnosed in the year of 20151. Among them, 2% is attributed to oral and oropharyngeal cancers, ranking them as the sixth most commonly occurring cancer with 63% and 51% of overall 5- and 10-year survival rates, respectively, in the United States. The similar trend can also be seen worldwide2, suggesting that oral and oropharyngeal cancers in the oral cavity impose significant health issues not only in the United States but also in the world.
About 45,780 new diagnoses of oral and oropharyngeal cancers alone are expected in 2015 in the United States1. While these patients may seek a large cancer center in which their dental needs can be addressed at the hospital-based settings before, during, or after cancer therapy, significant numbers of these cancer patients are often being referred to local general dental practitioners for their dental care3. With progressive increase in life expectancy due to the advancement in medical technology, these cancer patients seeking general dentists to address their dental needs would only escalate. Therefore as a general dental practitioner, it is important to know about, and to be better prepared for, any disease or pathology that may specifically develop in the oral cavity in patients who are undergoing or have undergone therapy for their cancer.
Depending on the treatment modality, the side effects commonly seen in the oral cavity are diverse, ranging from xerostomia, oral mucositis, osteoradionecrosis, trismus, and opportunistic infection. These side effects may be overlooked due to their asymptomatic nature in limited cases but can be severe such that normal functioning in the daily life may be significantly compromised. Detailed description of screening and examining oral cancers in patients can be found in the website for Foundation for Oral-facial Rehabilitation (http://www.ffofr.org) and in other reviews4. Here, we will primarily focus on etiology, clinical presentation, and management of these oral lesions in patients undergoing or underwent therapy for their cancers. The use of adjuvant cancer therapeutic agents such as bisphosphonates and denosumab are increasingly common to treat metastatic cancers, especially in the advanced stages where cancer has metastasis to bone. These patients are at the risk of developing oral-specific lesions called medication-related osteonecrosis of the jaw (MRONJ). We will also discuss about managing MRONJ lesions at the end of this review article.
CANCER THERAPY OPTIONS
To better manage oral-specific side effects that are induced during or after the cancer therapy, it is helpful to understand the nature of each therapeutic modality. The principal methods of cancer treatment are largely determined by surgical, radiation, chemo or combinatorial therapy.
Surgical therapy
Surgical therapy for cancer is a method of choice for cancer treatment as it allows for physical removal the entire tumor mass. Following the surgical removal, patients may undergo adjuvant radiation or chemotherapy for complete eradication of cancers. However, this approach is often limited due to compromised functions and esthetics5. Surgical removal of cancer mass in the oral cavity often creates large structural defects, and the outcomes may be disfiguring. In addition, intraoral surgical removal may result in significantly altered oral functions in speech and mastication. Therefore, patients may opt out from this therapeutic modality because of these functional and esthetic concerns.
Radiation therapy
Radiation therapy takes an advantage of inducing DNA damage in highly proliferating cancer cells by ionizing radiation via generating reactive oxygen species (ROS). Because cancer cells constantly replicate DNA for their continual proliferation, DNA damage by ionizing radiation through radiation therapy leads to cell death. Radiation is delivered to the tumor sites by fractionating the doses with different radiation path in multiple visits. Typically, an average of 2 Gy per fraction gets delivered over a course of 6–7 weeks, resulting in a total dose of 60–72 Gy.
Chemotherapy
Chemotherapy is usually treated in outpatient basis, but hospitalization may be required if serious sequalae develops. The modality of chemotherapy is largely dependent on cytotoxicity of the drug and the patient’s body defense condition. Combinations of different drugs (e.g., alkylating agents, antimetabolites, antitumor antibiotics, antineoplastics, and monoclonal antibody such as cetuximab) are preferred to avoid development of single agent resistance in cancer cells. In addition, combination chemotherapy can lower the doses of drug, and results in better remission and cure rate.
ORAL COMPLICATIONS ASSOCIATD WITH CANCER THERAPY
Although these treatment modalities are specifically formulated to reduce cancer burden by inducing cancer cell death, normal cells that are responsible for maintaining body homeostasis by continually proliferating, differentiating, and replenishing tissue structures and functions are also affected. As such, there are multiple complications associated with cancer therapy such as nausea, vomiting, hair loss, myelosuppression, or stomatotoxicity. Among them, several side effects are observed in the oral-specific manner and compromise the quality of patients’ life. These complications include oral mucositis, xerostomia, ORN, trismus, and secondary infection.
Oral Mucositis
General description
Oral mucositis is one of the most common side effects in patients undergoing radio- and/or chemotherapy. The degree of mucositis severity varies depending on fields, doses, and fractionation of radiation, and the ulcerative mucositis lesions are more severe in patients receiving adjunctive or concurrent chemotherapy. The etiology of oral mucositis is primarily due to generation of reactive oxygen species (ROS) by radiation and/or chemotherapy which cause direct DNA damage on actively proliferating cells that are responsible for replenishing the tissues, leading to oral mucosal damages6, 7. Ironically, this is also the basic principle behind the use of radiation and/or chemotherapy to target cancer cells. The severity of mucosal reaction is more evident in the less keratinized oral mucosa such as under the tongue. The ulceration escalates in patients with chronic alcoholism, liver cirrhosis, and insulin-dependent diabetes.
Clinical manifestation
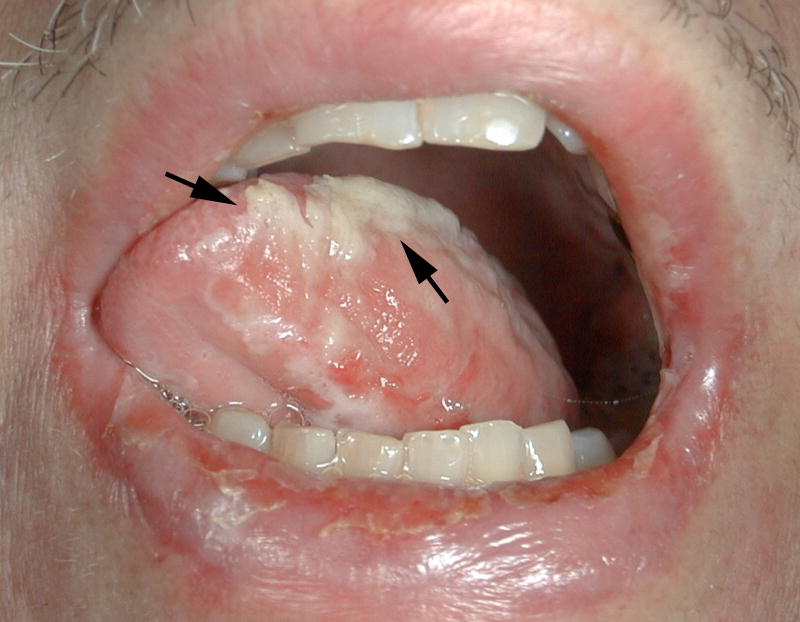
Mucositis initially presents as an erythematous lesion as early as 7 to 10 days after the initial treatment dose. These initial erythematous mucositis will soon develop into ulcerative mucositis that is typically covered by pseudomembranes (Figure 1). These lesions are usually confined to the tissues associated with the initial tumor site. Ulcerative mucositis lasts throughout the treatment period, but the lesion are usually self-limiting after 2–4 weeks following the completion of therapy during which they are re-epithelialized and covered by normal appearing oral mucosa.
Figure 1.
Mucositis covered by pseudomembranous layer with areas of erythema and ulceration
A caution should be noted when managing the irradiated oral mucosal tissues as they can be easily perforated by trauma, resulting in secondary ulceration that may take months to heal. The practitioner should carefully examine the localized ulcerative mucositis in oral mucosal tissues particularly around the metallic crown that is in the path of the radiation beam due to backscatter effects of radiation.
Management
As these lesions are often self-limiting, the primary goal of managing patients with oral mucositis should be focused on alleviating pain. Topical anesthetics in forms of sprays, ointments, gels or rinses can be used such as lidocaine, benzocaine, dyclonine, and capsaicin. The practitioners should examine loss of oral function, weight loss, and secondary infection8. It should be noted that patients with severe mucositis may require hospitalization. Patients should be informed of avoiding hot, spicy, and acidic foods or beverages. Any sharp or hard food intakes should be refrained, as they can be traumatic to the oral mucosal tissues. If the oral mucositis is generalized throughout the oral cavity, analgesics can be administered systemically, which may require hospitalization. Emphasizing good oral hygiene practices to patients is important to reduce the chances of getting infection secondary to mucositis. Fungal and bacterial infections are common to these lesions, and antifungal and/or antibacterial medications may be prescribed as needed.
Some of these patients have already undergone preventive therapeutic treatment such as cryotherapy, Palifermin or Amifostine, so practitioners should be aware of these methods. Palifermin, a truncated human keratinocyte growth factor (KGF) recombinant protein, is FDA-approved and currently available to use in the clinic; however, recent clinical trials demonstrated modest effects of Palifermin9, 10. A radioprotectant, Amifostine, can be administered intravenously or subcutaneously before therapy to reduce severity of oral mucositis, but it may induce several side effects such as headaches, nausea, or hypotension.
Xerostomia
General description
Xerostomia, or dry mouth, is another commonly occurring side effect in cancer patients undergoing radiation therapy or concomitant chemotherapy. Xerostomia occurs as a result of partial or complete damages, which may either be recoverable or irreversible, to the salivary glands (e.g., parotid, submandibular, and sublingual glands) especially when these glands reside in the path of radiation. Histologically, early changes at the tissue level include interstitial fibrosis, progressive loss of the fine vasculature, and vacuolization of serous acinar cells. Of notes, serous acinar cells seem to be more readily affected by radiation when compared to the mucous cells, presumably due to relatively rapid turnover rate and profuse vasculatures of serous cells. As such, saliva is more acidic and viscous with less buffering capacity. During the late stages of radiation therapy, glands become progressively fibrotic, leading to almost complete loss of acinar elements and the striated duct system. Ultimately, no saliva may be present. Because such environmental alterations make the oral cavity more susceptible to rampant caries, acute and chronic fungal infections, and compromised tolerance to prosthesis such as dentures, early detection and management of xerostomia in these patients are critical to alleviate discomforts and possible permanent structural damages in the oral cavity.
Clinical manifestation
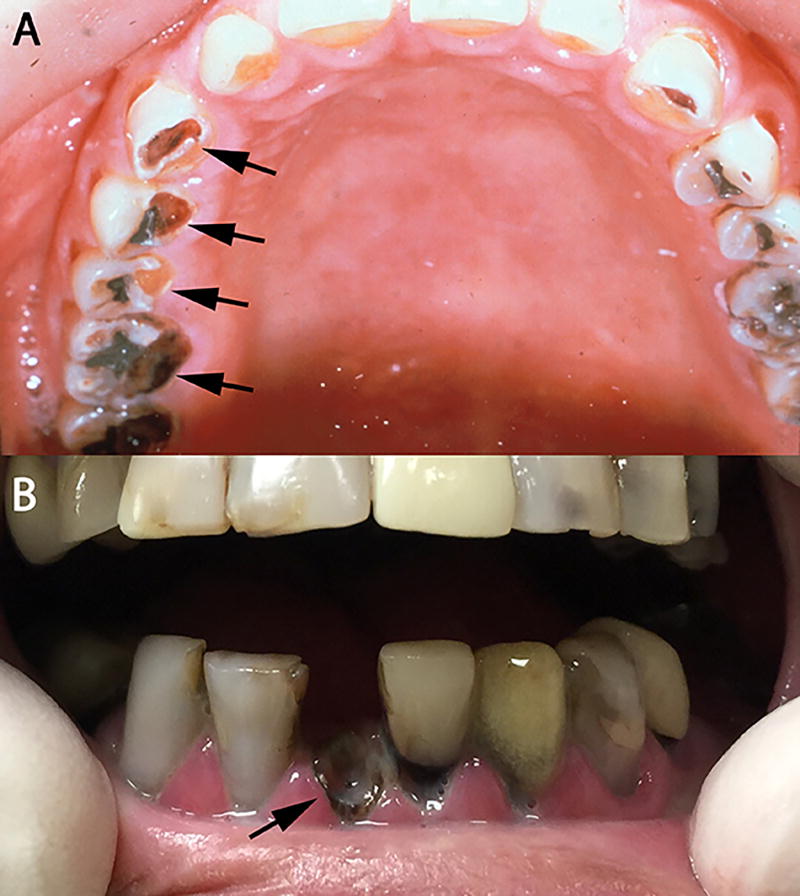
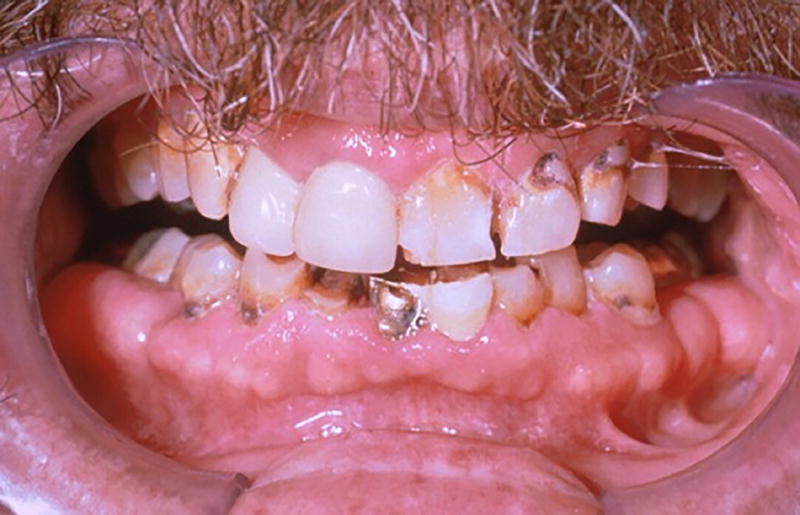
Practitioners should actively look for signs and symptoms related to salivary hypofunction including fissures at the lip commissures, difficulties in swallowing or chewing food as well as in speech. Salivary reduction up to 80% of its original flow11 and xerostomia can be specifically noted in cancer patients 2 weeks after initial radiation therapy, or at a cumulative dose of 20 Gy. The diminished salivary flows bring changes in the oral flora, increasing the chances of bacterial and/or fungal infection. These changes predispose the patient to radiation caries, which can be typically located at the incisal edge and cervical third of the teeth. Rampant caries progress rapidly and extensively such that teeth become non-restorable or fractured at the gingival margin (Figure 2a and 2b); therefore, early detection and immediate restorations are highly recommended.
Figure 2.
Rampant caries secondary to xerostomia (A) Xerostomia causing gross decay (arrows) (B) Gross decay leading to fracture at the gumline (arrow)
Management
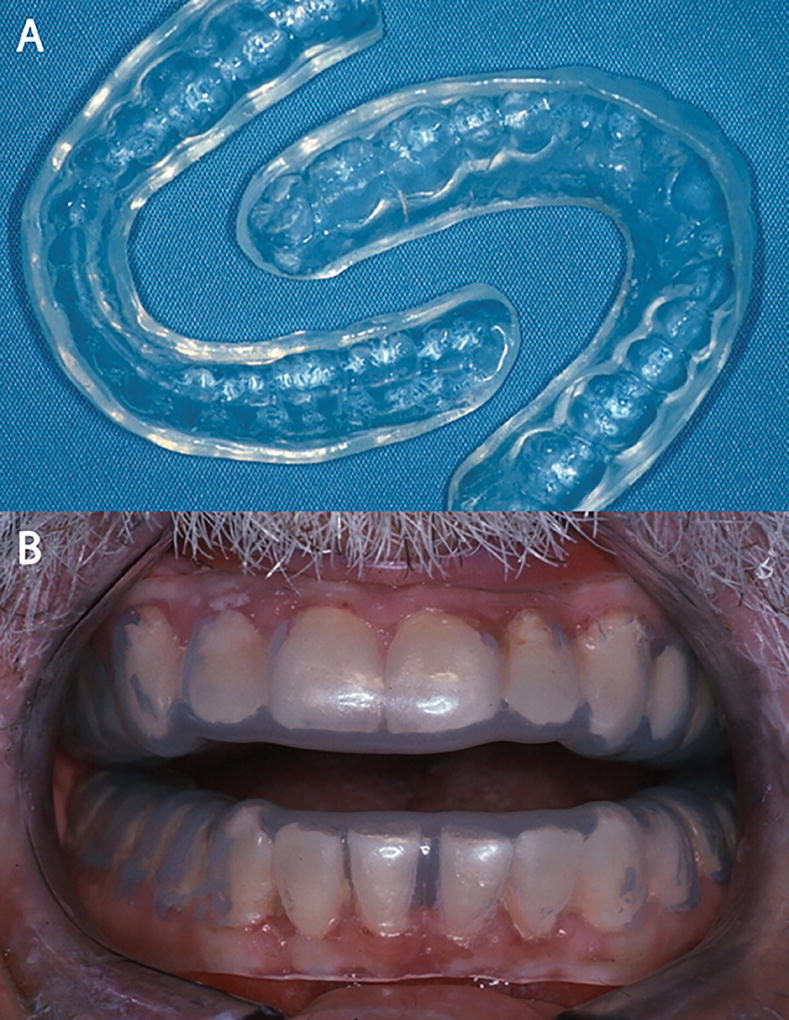
Early symptoms of xerostomia includes thick or ropey saliva in the oral cavity. Carboxymethylcellulose-, mucin-, water-, or glycerin-based saliva substitutes may be used, although effectiveness of these agents is somewhat questionable. If salivary glands are spared from complete eradication by radiation therapy, salivary stimulants such as pilocarpine or cevimeline may be used. The early use of stannous fluoride gel applied with custom carriers and five-minute daily applications is highly recommended to reduce the caries risk (Figure 3). If significant numbers of rampant caries are noted, the dental treatment should be performed without any delay. Due to the high caries risk, fluoride-releasing glass ionomers and/or amalgam restorations are more predictable when compared to composite restorations. Patients should be informed of eliminating sucrose diet and reducing the frequency of meals.
Figure 3.
Stannous fluoride gel application with custom tray. (A) Custom trays for maxillary and mandibular dentitions. (B) Custom trays filling onto the maxillary and mandibular dentition.
Osteoradionecrosis (ORN)
General description
The incidence of ORN ranges from 8% to 35%, largely depending on observational periods that range from months to years12, 13, and most of ORN (about 75%) occurs within the first 3 years of radiation therapy treatment14. ORN is more prevalent in the mandible than the maxilla, owning to the poor vascularization and increased density that allows for absorbing more radiation in the mandible. The cause of ORN is still unclear although there are several hypotheses such as bacterial infection, hypoxia, and fibroatrophy15–17. Risk factors include location of primary tumor, cancer staging, dose of radiation (> 60 Gy), poor oral hygiene, alcohol and tobacco uses, and invasive dental procedures such as tooth extraction18. It is noteworthy that, once received radiation therapy, the cancer patients have the risk of developing ORN that is lifelong and does not decrease over time. Therefore, thorough examination at each visit for periodic examination is essential.
Clinical manifestation
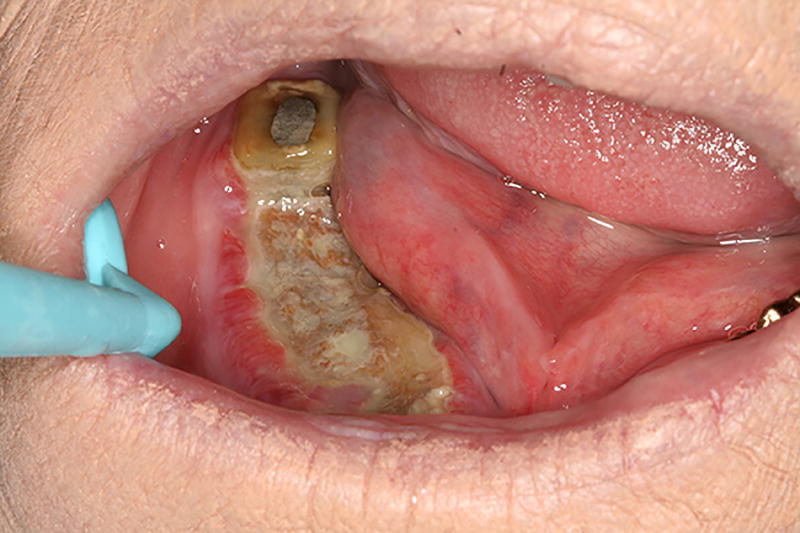
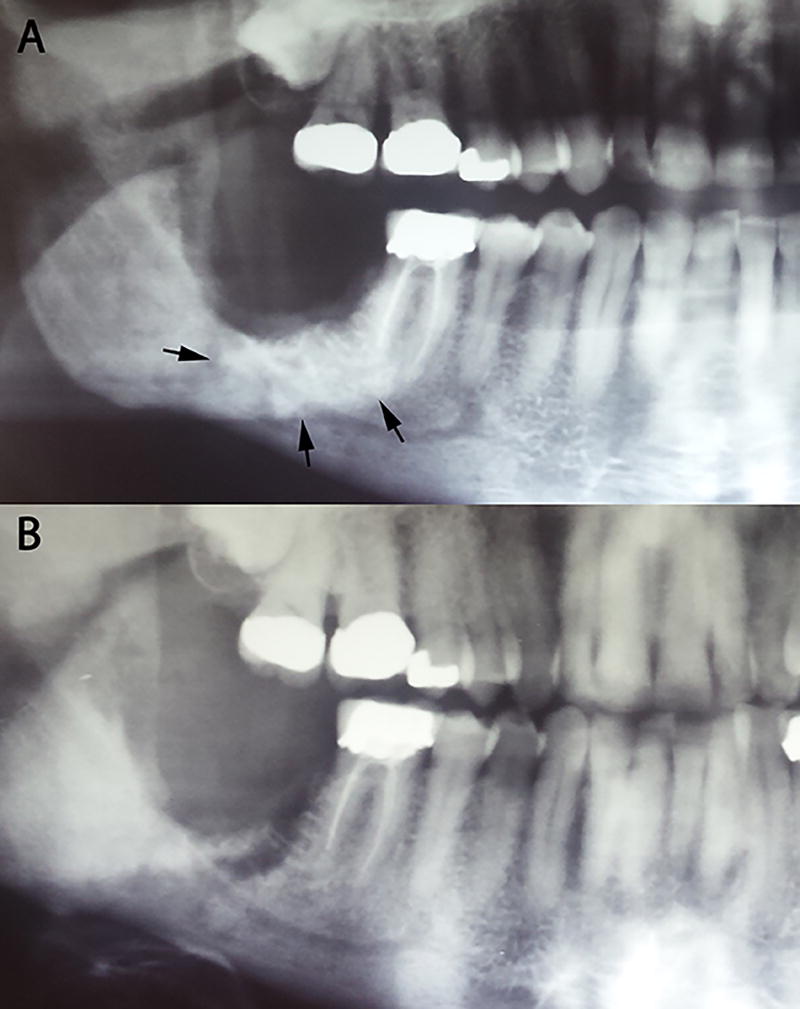
ORN is clinically defined as an area of exposed bone that persists for more than 3 months (Figure 4); however, radiographic findings of irregular radio-opacity that is indicative of sequestrum formation without breached overlaying mucosal closure are also common (Figure 5). Ulcerative or necrotic soft tissues can also be seen frequently around the exposed area. Long-term exposure without proper oral care may lead to accumulation of plaques that cover the exposed bone.
Figure 4.
Osteoradionecrosis lesion with exposed bone in the lower right mandibular arch. Note plaque accumulation associated with the exposed bone.
Figure 5.
Radiographic findings of irregular radio-opacity (A) Sclerotic changes around #31 area (arrows) after radiation therapy. (B) After two years, sequestrum was pushed out spontaneously.
Management
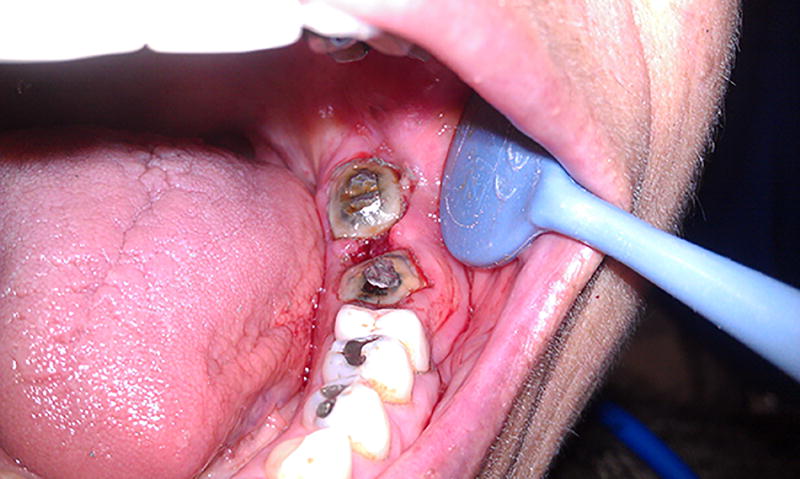
Tooth extraction accounts for ORN as high as 50%12; therefore, invasive dental procedures should be reserved. Periodontal procedures such as deep scaling and flap surgery are also contraindicated particularly in heavily irradiated patients. Instead, more conservative treatment approaches such as endodontic therapy with or without coronal restorations are preferable (Figure 6). When bone exposure is evident, Patients’ typical chief complaint is pain associated with bacterial infection secondary to exposed bone. Prescribing antibiotics may help resolve the pain. Regular checkups and dental prophylaxis for every four months are highly recommended to maintain optimal conditions in the oral cavity along with giving the clinician ample opportunity to catch dental disease at the early stages. Hyperbaric oxygen (HBO) therapy that provide high contents of oxygen has been used to manage the ORN conditions but without drastic improvement19. If possible, it is highly encouraged to remove any sources of dental diseases including advanced caries, periapical infection, and pathologic periodontal bone loss before undergoing radiation therapy. New alternatives to HBO treatments are introduced such as the use of pentoxyfylline and/or tocopherol20, 21, and the use of these medications may hold promising results in reducing the risk and managing patients with ORN.
Figure 6.
Endodontically treated #18 and 19 that are domed with amalgam restoration.
Trismus
General description
Trismus refers to limited mouth opening due to any etiological reasons related to sustained contraction of one or more mastication muscles22. The most common etiological factors involve radiation-induced fibrosis and post-surgical scar formation23. Trismus occurs most commonly when radiation is combined with a surgical procedure (e.g., radical maxillectomy) that affects the temporal mandibular joint (TMJ) and the muscles of mastication.
Clinical manifestation
clinically, mouth opening less than 35 mm is considered to be having trismus, although the degree of limited mouth opening may be subjective24. In severe case, the maximum opening may be reduced to less than 10 mm (Figure 7). The severity of trismus depends on age and concomitant chemotherapy.
Figure 7.
Trismus. Note the limited mouth opening.
Management
The treatment consist of exercise and the use of dynamic bite openers. Because fibrosis and scar formation becomes progressively worse and they are often irreversible, early identification of trismus and immediate initiation of an exercise program using devices such as Therabite® are critical to improve the condition significantly25, 26.
Oral candidiasis
General description
During the administration of radiation therapy, acute candidiasis is likely to occur due to altered immunity and xerostomia secondary to hyposalivary functions in the oral cavity27.
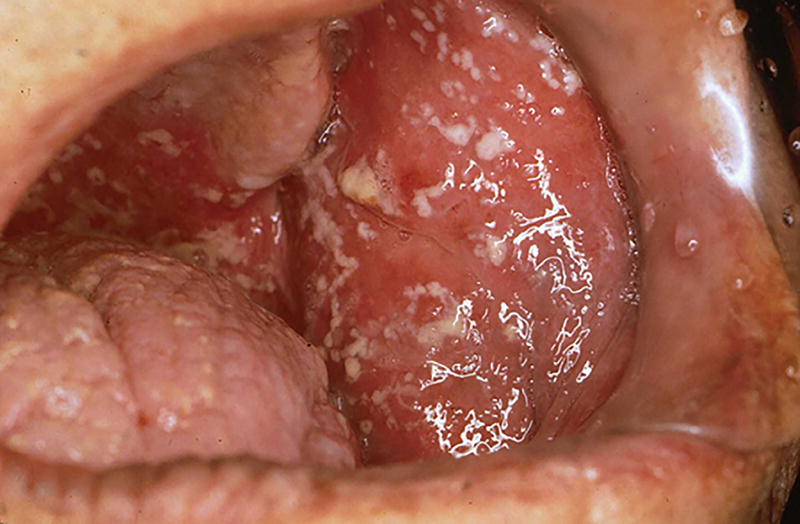
Clinical manifestation
Clinically, candidiasis is known to occur up to 27% of patients28 undergoing cancer therapy in forms of pseudomembranous (thrush), erythematous, and angular cheilitis (Figure 8).
Figure 8.
Candida albicans.
Management
Nystatin is a drug of choice, which can be dispensed in a number of different configurations such as lozenges, powder, creams or oral suspension.
Altered taste buds
Alterations in taste acuity are first noticed as early as the second week of radiation therapy (approximately 30 Gy of radiation). Perception of bitter and acid flavors are more susceptible to impairment than salt and sweet. Architecture of the taste buds is almost completely eliminated at 50 Gy. However, taste generally returns to normal 2–4 weeks after the completion of therapy as long as salivary flow is reasonable. In case of severe xerostomia following radiation therapy, the number of buds is significantly decreased and their morphology is altered. The perception of taste may be altered lifelong.
ORAL COMPLICATIONS ASSOCIATD WITH ADJUVANT CHEMOTHERAPY
A typical nature of cancers in the advanced stages is metastasis to the other parts of the body distant from the primary site. In particular, some cancers including breast, prostate, lung, thyroid and kidney cancers are more prone to metastasize to bone. These lesions may also lead to high calcium levels in the blood stream called hypercalcemia. Medications commonly prescribed for management of bone metastasis includes bisphosphonates and denosumab. The use of these medications increase the risk of delayed healing of surgical wounds and developing bone necrosis. SUCH intraoral side lesion called Medication-related osteonecrosis of the jaw (MRONJ) is highly specific to the oral cavity. Therefore, the general practitioners should be aware of these MRONJ lesions when managing patients who are receiving such adjuvant chemotherapy.
Medication-related osteonecrosis of the jaw (MRONJ)
General description
The first formal report on ONJ by bisphosphonates was published in 200329, but the etiology is still unknown. Multiple hypotheses have been suggested including suppression of bone remodeling, inflammation, inhibition of angiogenesis, and soft tissue toxicity30. The terminology of bisphosphonate-related osteonecrosis of the jaw, or simply BRONJ, was recently updated to medication-related osteonecrosis of the jaw, MRONJ, in order to be more inclusive for medications other than bisphosphonates such as denosumab or bevacizumab31. MRONJ is clinically defined as patients with history of receiving treatment with anti-resportive or anti-angiogenic agents, exposed bone for more than 8 weeks, and no history of radiation therapy to the head and neck regions31. The detail classification of MRONJ can be found in elsewhere31. Individuals with cancers in advanced stages that invade bone may take these medications, usually with doses given intravenously or subcutaneously to prevent cancer spreading to bone. As such, the practitioners should keep in mind that patients with osteoporosis also take these drugs as pills by mouth, and that, although the incidence of MRONJ taking by this route is relatively less, these patients may still develop MRONJ lesions when they have been on these medications for more than 4 years32. The practitioners should be also aware of risk factors for MRONJ such as high (e.g., iv or subcutaneous administration) and long duration (e.g., > 4 years) of doses, pre-existing inflammatory dental diseases (e.g, periodontal disease or periapical lesions), dentoalveolar surgery (e.g., tooth extraction), age, and corticosteroids32–38, all of which may exacerbate ONJ lesions.
Clinical manifestation
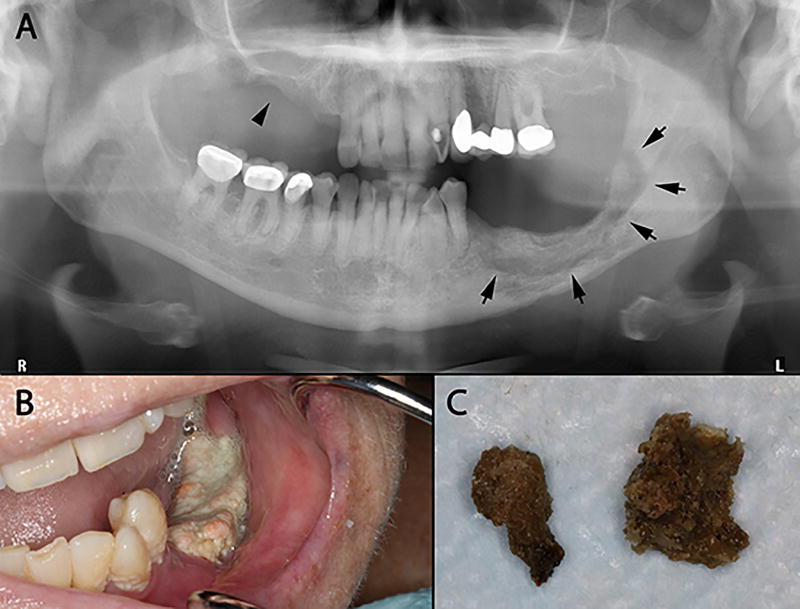
A typical clinical presentation is very similar to that of ORN. Long-term exposure of bone is almost inevitably accompanied by plague accumulation (Figure 9b). The practitioners should note for patients’ pain with unidentifiable origin as this may indicate MRONJ at the staging “0.” Abnormal findings (e.g., sclerosis) from radiography and computed tomography (CT) should also be noted, but interpreted with caution, as it may be a suggestive of MRONJ39, 40. The bone exposure are likely to be seen at the previously extracted areas but can also occur spontaneously in thin oral mucosal areas such as tori. Spontaneous bone exposure may be associated with chronic inflammation (e.g., periodontal or periapical diseases) and previously traumatized areas. Radiographically, non-viable bone can be predicted based on radiolucent periphery around the affected area (Figure 9a). A periodontal probe instrument can be used to detect bony surface through mucosal fistulas, which is an indicative of MRONJ at the stage 1, 2, or 3.
Figure 9.
MRONJ lesions induced by the long-term bisphosphonate use. (A) Note radiolucency around the affected area (arrows), which is an indicative of non-viable bone. (B) A typical MRONJ lesions with plaque formation induced by the long-term use of bisphosphonates on the lower left mandibular arch. (C) Bony sequelae that fell out spontaneously from the upper right maxillary arch (arrow head in the Fig. 9A) that the patient brought to the clinic.
Management
Similar to ORN, invasive dental procedures should be refrained, but conservative approaches are recommended. It is important to know whether cancer patients are taking aforementioned medications as dental treatment options are significantly limited due to increased risk of having MRONJ after invasive dental treatment. Once identified, patients with MRONJ should be managed according to the MRONJ staging. As a general practitioner, the primary goals of managing these patients are: 1) to maintain good oral hygiene in a non-surgical manner in patient with staging 2 or less; and 2) to monitor progression the lesions such that, when the lesions meet the stage 3 criteria, the patients can be referred to oral surgeon for possible surgical interventions. For patients who are taking these mediations without signs/symptoms of MRONJ, routine oral hygiene including scaling and root planning should be continued. For the Stage 0 patients with chief complaint of pain with unidentifiable origins, the use of medication to control pain is helpful. In patients with stage 1 or 2 with exposed bone, the use of oral antimicrobial rinses (e.g., 0.12% chlorhexidine) is recommended. Although infection as a primary etiological factor in causing MRONJ is still controversial, the use of antibiotics is also recommended in order to reduce bacterial colonization particularly at the area with exposed bone. It is not uncommon to observe a tooth with class 3 mobility. In such case, extraction should be avoided; instead, the occlusal plane can be reduced as needed to eliminate occlusal contacts and contact-associated pain until the tooth falls out spontaneously. In certain instance, patients may present with bony sequelae that naturally sequester out (Figure 9c), and such sign is usually accompanied by reepithelialization and healing site. Such sites should be continuously monitored.
CONCLUSION
Once established, the relationship between dentists and patients can last for many years. As increased life expectancy and advancement of medical technology continues to grow, these relations may potentially life-long. During that time, dentists are likely to encounter patients undergoing or have a history of cancer therapy. Many side effects including oral mucositis, xerostomia, ORN, trismus, and secondary infection from cancer therapy are inevitable but can also be managed (Table 1). This holds true for MRONJ lesions in patients with cancers other than oral/oropharyngeal cancers undergoing adjuvant therapy with bisphosphonates and denosumab. Therefore, it is of paramount importance to know what cancer therapy entails in the oral health and to manage these patients accordingly in order to provide full spectrum of dental services at the local offices. Because it is a team effort in managing cancer patients, it is also equally important for general practitioners to communicate with medical practitioners and the patients and determine optimal managing plans for these patients in an individual-basis.
Table 1.
Summary of oral-specific side effects and management in cancer patients
| Types of side effects |
Types of cancer Therapy |
Common sites (in the oral cavity) |
Patient's chief complaints |
Clinical manifestation |
Goals of management | Management |
|---|---|---|---|---|---|---|
| Oral mucositis | Radio/Chemo | All |
|
|
|
|
| Xerostomia | Radio/Chemo | All |
|
|
|
|
| Osteoradionecrosis | Radio | Mandible |
|
|
|
|
| Trismus | Surgical/Radio/Chemo | Mastication muscles |
|
|
|
|
| Oral candidiasis | Radio/Chemo | All |
|
|
|
|
| Altered taste | Radio/Chemo | All |
|
|
|
|
| MRONJ | Chemo (for advanced cancer) | Maxilla/mandible |
|
|
|
|
Acknowledgments
This study was supported by the grants (R01DE023874 and R01DE023348) from NIDCR/NIH.
References
- 1.Society AC. Cancer Facts and Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Barker GJ, Epstein JB, Williams KB, Gorsky M, Raber-Durlacher JE. Current practice and knowledge of oral care for cancer patients: a survey of supportive health care providers. Support Care Cancer. 2005;13(1):32–41. doi: 10.1007/s00520-004-0691-5. [DOI] [PubMed] [Google Scholar]
- 4.Beumer J, Marunick MT, Esposito SJ. Maxillofacial Rehabilitation: Prosthodontic and Surgical Management of Cancer-related, Acquired, and Congenital Defects of the Head and Neck: Quintessence Pub. 2011 [Google Scholar]
- 5.Biglioli F. Surgical therapy of oral cancer. Minerva Stomatol. 2009;58(4):157–80. [PubMed] [Google Scholar]
- 6.Prasanna PG, Stone HB, Wong RS, et al. Normal tissue protection for improving radiotherapy: Where are the Gaps? Transl Cancer Res. 2012;1(1):35–48. [PMC free article] [PubMed] [Google Scholar]
- 7.Villa A, Sonis ST. Mucositis: pathobiology and management. Curr Opin Oncol. 2015;27(3):159–64. doi: 10.1097/CCO.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 8.Eilers J, Epstein JB. Assessment and measurement of oral mucositis. Semin Oncol Nurs. 2004;20(1):22–9. doi: 10.1053/j.soncn.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Henke M, Alfonsi M, Foa P, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2011;29(20):2815–20. doi: 10.1200/JCO.2010.32.4103. [DOI] [PubMed] [Google Scholar]
- 10.Le QT, Kim HE, Schneider CJ, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol. 2011;29(20):2808–14. doi: 10.1200/JCO.2010.32.4095. [DOI] [PubMed] [Google Scholar]
- 11.Burlage FR, Coppes RP, Meertens H, Stokman MA, Vissink A. Parotid and submandibular/sublingual salivary flow during high dose radiotherapy. Radiother Oncol. 2001;61(3):271–4. doi: 10.1016/s0167-8140(01)00427-3. [DOI] [PubMed] [Google Scholar]
- 12.Reuther T, Schuster T, Mende U, Kubler A. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients--a report of a thirty year retrospective review. Int J Oral Maxillofac Surg. 2003;32(3):289–95. doi: 10.1054/ijom.2002.0332. [DOI] [PubMed] [Google Scholar]
- 13.Rice N, Polyzois I, Ekanayake K, Omer O, Stassen LF. The management of osteoradionecrosis of the jaws--a review. Surgeon. 2015;13(2):101–9. doi: 10.1016/j.surge.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Thorn JJ, Hansen HS, Specht L, Bastholt L. Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J Oral Maxillofac Surg. 2000;58(10):1088–93. doi: 10.1053/joms.2000.9562. discussion 93–5. [DOI] [PubMed] [Google Scholar]
- 15.Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73(2):119–31. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Marx RE. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg. 1983;41(6):351–7. doi: 10.1016/s0278-2391(83)80005-6. [DOI] [PubMed] [Google Scholar]
- 17.Meyer I. Infectious diseases of the jaws. J Oral Surg. 1970;28(1):17–26. [PubMed] [Google Scholar]
- 18.O'Dell K, Sinha U. Osteoradionecrosis. Oral Maxillofac Surg Clin North Am. 2011;23(3):455–64. doi: 10.1016/j.coms.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Bennett MH, Feldmeier J, Hampson N, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2012;5:CD005005. doi: 10.1002/14651858.CD005005.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Delanian S, Chatel C, Porcher R, Depondt J, Lefaix JL. Complete restoration of refractory mandibular osteoradionecrosis by prolonged treatment with a pentoxifylline-tocopherol-clodronate combination (PENTOCLO): a phase II trial. Int J Radiat Oncol Biol Phys. 2011;80(3):832–9. doi: 10.1016/j.ijrobp.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi M, Pellecer M, Chung E, Sung E. The efficacy of pentoxifylline/tocopherol combination in the treatment of osteoradionecrosis. Spec Care Dentist. 2015 doi: 10.1111/scd.12124. [DOI] [PubMed] [Google Scholar]
- 22.Rapidis AD, Dijkstra PU, Roodenburg JL, et al. Trismus in patients with head and neck cancer. Etiopathogenesis, diagnosis and management. Clin Otolaryngol. 2015 doi: 10.1111/coa.12488. [DOI] [PubMed] [Google Scholar]
- 23.Ichimura K, Tanaka T. Trismus in patients with malignant tumours in the head and neck. J Laryngol Otol. 1993;107(11):1017–20. doi: 10.1017/s0022215100125149. [DOI] [PubMed] [Google Scholar]
- 24.Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg. 2006;35(4):337–42. doi: 10.1016/j.ijom.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Lee LY, Chen SC, Chen WC, Huang BS, Lin CY. Postradiation trismus and its impact on quality of life in patients with head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(2):187–95. doi: 10.1016/j.oooo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Pauli N, Fagerberg-Mohlin B, Andrell P, Finizia C. Exercise intervention for the treatment of trismus in head and neck cancer. Acta Oncol. 2014;53(4):502–9. doi: 10.3109/0284186X.2013.837583. [DOI] [PubMed] [Google Scholar]
- 27.Bensadoun RJ, Patton LL, Lalla RV, Epstein JB. Oropharyngeal candidiasis in head and neck cancer patients treated with radiation: update 2011. Support Care Cancer. 2011;19(6):737–44. doi: 10.1007/s00520-011-1154-4. [DOI] [PubMed] [Google Scholar]
- 28.Groll AH, Piscitelli SC, Walsh TJ. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol. 1998;44:343–500. doi: 10.1016/s1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 29.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115–7. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 30.Reid IR, Cornish J. Epidemiology and pathogenesis of osteonecrosis of the jaw. Nat Rev Rheumatol. 2012;8(2):90–6. doi: 10.1038/nrrheum.2011.181. [DOI] [PubMed] [Google Scholar]
- 31.Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–56. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 32.Lo JC, O'Ryan FS, Gordon NP, et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68(2):243–53. doi: 10.1016/j.joms.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thumbigere-Math V, Michalowicz BS, Hodges JS, et al. Periodontal disease as a risk factor for bisphosphonate-related osteonecrosis of the jaw. J Periodontol. 2014;85(2):226–33. doi: 10.1902/jop.2013.130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malden N, Lopes V. An epidemiological study of alendronate-related osteonecrosis of the jaws. A case series from the south-east of Scotland with attention given to case definition and prevalence. J Bone Miner Metab. 2012;30(2):171–82. doi: 10.1007/s00774-011-0299-z. [DOI] [PubMed] [Google Scholar]
- 35.Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679–87. doi: 10.1007/s00520-013-2022-1. [DOI] [PubMed] [Google Scholar]
- 36.Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341–7. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 37.Vahtsevanos K, Kyrgidis A, Verrou E, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol. 2009;27(32):5356–62. doi: 10.1200/JCO.2009.21.9584. [DOI] [PubMed] [Google Scholar]
- 38.Fehm T, Beck V, Banys M, et al. Bisphosphonate-induced osteonecrosis of the jaw (ONJ): Incidence and risk factors in patients with breast cancer and gynecological malignancies. Gynecol Oncol. 2009;112(3):605–9. doi: 10.1016/j.ygyno.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 39.Khan AA, Morrison A, Hanley DA, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30(1):3–23. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- 40.Bedogni A, Fedele S, Bedogni G, et al. Staging of osteonecrosis of the jaw requires computed tomography for accurate definition of the extent of bony disease. Br J Oral Maxillofac Surg. 2014;52(7):603–8. doi: 10.1016/j.bjoms.2014.04.009. [DOI] [PubMed] [Google Scholar]