Summary
Poor bone quality contributes to bone fragility in diabetes, aging, and osteogenesis imperfecta. However, the mechanisms controlling bone quality are not well understood, contributing to the current lack of strategies to diagnose or treat bone quality deficits. TGFβ signaling is a crucial mechanism known to regulate the material quality of bone, but its cellular target in this regulation is unknown. Studies showing that osteocytes directly remodel their perilacunar/canalicular matrix led us to hypothesize that TGFβ controls bone quality through perilacunar/canalicular remodeling (PLR). Using inhibitors and mice with an osteocyte-intrinsic defect in TGFβ signaling (TβRIIocy−/−), we show that TGFβ regulates PLR in a cell-intrinsic manner to control bone quality. Altogether, this study emphasizes that osteocytes are key in executing the biological control of bone quality through PLR, thereby highlighting the fundamental role of osteocyte mediated perilacunar/canalicular remodeling in bone homeostasis and fragility.
Keywords: Osteocyte, TGFβ, bone quality, perilacunar/canalicular remodeling, bone fragility
Introduction
Bone fragility is determined by bone mass and quality. Bone quality encompasses parameters including bone geometry, porosity, trabecular microarchitecture, and bone extracellular matrix (ECM) material properties (Hernandez and Keaveny, 2006; Seeman, 2008). Historically, the prognosis of fragility fractures has focused on bone mass, but it is now known that compromised ECM properties play a causal role in bone fragility in diabetes, aging, and osteogenesis imperfecta (OI) (Delmas and Seeman, 2004; Fleischli et al., 2006; Grafe et al., 2014; Lane et al., 2006; Nalla et al., 2004; Ott, 1993; Van Staa et al., 2003). Despite the clinical importance of this and other aspects of bone quality, the management of fragility currently focuses on improving bone mass. Overcoming this clinical gap in diagnosing and treating bone quality requires elucidation of mechanisms that orchestrate the biological control of bone quality in skeletal health and disease.
Currently, the transforming growth factor beta (TGFβ) pathway is one of the few signaling pathways known to regulate bone mass and quality (Alliston, 2014; Balooch et al., 2005; Mohammad et al., 2009; Chang et al., 2010; Edwards et al., 2010). In bone, TGFβ produced by bone forming osteoblasts is sequestered in the ECM in an inactive, latent form (Sinha et al., 1998). When released upon osteoclastic resorption of the ECM, TGFβ exerts pleiotropic effects on osteoblasts, osteoclasts, and their progenitors to coordinate bone remodeling (Dallas, 2008; Tang and Alliston, 2013). Aberration in TGFβ signaling leads to altered bone mass and poor bone quality in multiple skeletal diseases including Camurati Engelman Disease (CED) and OI (Grafe et al., 2014; Kinoshita et al., 2000). While the pathogenesis of the poor bone quality associated with these diseases has been attributed to imbalanced osteoclast and osteoblast activity, not much is known about the causal role of osteocytes and osteocyte intrinsic TGFβ signaling in bone fragility.
In addition to regulating the activity of osteoclasts and osteoblasts, osteocytes also engage in perilacunar/canalicular remodeling (PLR), during which they directly resorb and deposit bone matrix surrounding their intricate lacuno-canalicular network (Qing and Bonewald, 2009). This process was originally called ‘osteocyte osteolysis’ when it was observed in pathologic conditions, or ‘perilacunar/canalicular remodeling’ in metabolically demanding situations such as lactation or hibernation (Haller and Zimny, 1977; Qing et al., 2012; Qing and Bonewald, 2009; Teti and Zallone, 2009; Wysolmerski, 2013). It is now clear that PLR is a homeostatic mechanism that helps to maintain mineral homeostasis and the lacuno-canalicular network. Several studies demonstrate the essential role in PLR of matrix metalloproteinases (Mmps, namely Mmp2, Mmp13 and Mmp14), cathepsin K (Ctsk), carbonic anhydrase 2, and tartrate resistant acid phosphatase (Acp5/TRAP) (Kogawa et al., 2013; Qing et al., 2012; Qing and Bonewald, 2009; Wysolmerski, 2013). Through loss-of-function studies in mice, these genes were found to be essential for an intact lacuno-canalicular network, organization of collagen, and bone matrix mineralization (Holmbeck et al., 2005; Inoue et al., 2006; Kogawa et al., 2013; Kulkarni et al., 2012; Qing and Bonewald, 2009; Tang et al., 2012; Tang and Alliston, 2013; Wysolmerski, 2013), all of which contribute to bone quality. Macro-mechanical testing of MMP13-deficient bone revealed a correlation between the loss of PLR and bone fragility (Tang et al., 2012). Nonetheless, many questions remain about the relationship between osteocyte-mediated PLR and bone quality.
In an effort to elucidate the cellular and molecular mechanisms that control bone quality, we tested the hypothesis that TGFβ acts directly on osteocytes to control PLR, and that this mechanism accounts for the TGFβ-dependent control of bone quality. Several lines of evidence support this model, including the ability of TGFβ to directly regulate the expression of several Mmps implicated in PLR (Krstic and Santibanez, 2014; Selvamurugan et al., 2004). To investigate this possible mechanism, we employed unique in vivo and in vitro models and pharmacologic TGFβ antagonists similar to those in human clinical trials for the treatment of bone fragility in OI. Using this approach, we uncovered the essential role of osteocyte-intrinsic TGFβ signaling in the control of PLR and fracture resistance and demonstrate the importance of PLR in bone fragility.
Results
Pharmacologic inhibition of TGFβ signaling dysregulates perilacunar/canalicular remodeling
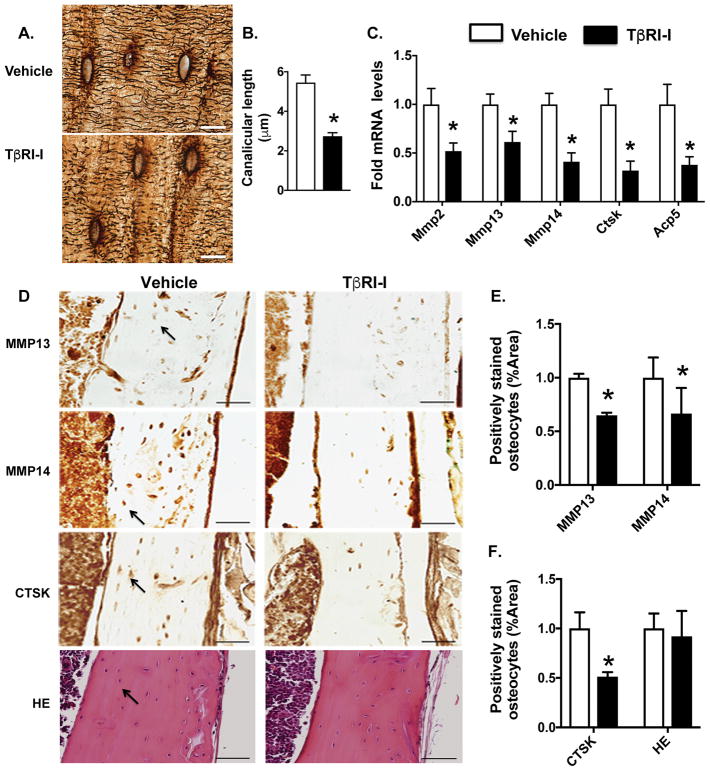
We previously showed that pharmacologic inhibition of the TGFβ receptor type I (TβRI- inhibitor, SD-208) increases trabecular bone mass through stimulating osteoblastic bone formation and repressing osteoclastic resorption (Mohammad et al., 2009). However, the effect of SD-208 or other TβRI-inhibitory agents on osteocytes (OCY) is unknown. To investigate the role of TGFβ signaling in osteocytes, the most abundant bone cells in cortical bone (Franz-Odendaal et al., 2006), we examined histologic and molecular outcomes of osteocyte-mediated perilacunar/canalicular remodeling (PLR) in mice treated with SD-208. As expected, 6 weeks of TβRI-inhibitor treatment significantly increased trabecular bone mass (Fig. S1). Histologic analysis shows a dense and organized network of osteocyte canaliculi in cortical bone of vehicle-treated mice. However, TβRI-inhibitor treatment caused severe deterioration of the osteocyte canalicular network with a 50% reduction in canalicular length (Fig. 1A–B).
Figure 1. Pharmacologic TβRI inhibition impairs perilacunar remodeling (PLR).
(A, B) Silver nitrate stained images of femoral cortical bone from vehicle and TGFβ receptor I kinase inhibitor (TβRI-I, SD-208) treated mice show osteocyte lacuno-canalicular network (A) and canalicular length (B). Scale bar, 20 μm (n=6 mice/group). (C) qPCR analysis of PLR genes Mmp2, Mmp13, Mmp14, Ctsk, and Acp5 in bones from vehicle and TβRI-I treated animals (n=8 mice/group). (D–F) Immunohistochemistry (IHC) of MMP13, MMP14 CTSK and H&E staining of femoral cortical bone of vehicle and TβRI-I treated animals. Arrows in the image indicate positively stained osteocytes that were quantified and normalized to total bone area. Scale bar, 50 μm (n=4 mice/group). Error bars indicate mean ± SEM, *p<0.05 compared to vehicle from Student’s t test.
The dysregulated canalicular network in TβRI-I-treated bone resembles that seen in bones deficient in enzymes essential for PLR (Holmbeck et al., 2005; Inoue et al., 2006; Kulkarni et al., 2012; Tang et al., 2012). Therefore, we evaluated the effect of TβRI- inhibition on the expression of genes encoding PLR enzymes, including matrix metalloproteinases Mmp2, Mmp13 and Mmp14, Cathepsin K (Ctsk), and tartrate resistant acid phosphatase (Acp5) in cortical bone. TβRI-I treatment coordinately reduced the level of mRNA encoding all five enzymes, relative to vehicle-treated controls (Fig. 1C). Expression of the ATPase Atp6v0d, is increased in TβRI-treated bone (Fig. S2). Moreover, TβRI-I treatment also causes a decline in osteocytic protein expression of MMP13, MMP14 and CTSK without impacting their viability (Fig. 1D–F). This strong, concerted repression of several genes required for osteocyte-mediated PLR upon TGFβ inhibition indicates the critical role of TGFβ in controlling osteocyte function.
TGFβ regulates perilacunar/canalicular remodeling in a cell intrinsic manner
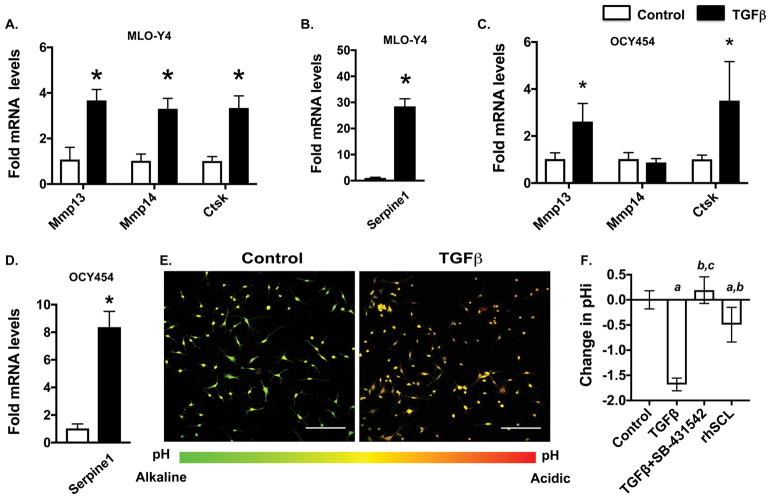
While systemic inhibitors of TGFβ clearly impact lacuno-canalicular networks and the expression of genes associated with PLR, it was unclear if TGFβ exerts its effects on osteocytes directly or indirectly. Therefore, we examined the cell-intrinsic effects of TGFβ on MLO-Y4 osteocyte-like cells and OCY454 osteocytes, which more faithfully mimic osteocytic gene expression. Within 6 hours of treatment, TGFβ induced expression of Mmp13, Mmp14 and Ctsk mRNA, as well as Serpine1, a well-known TGFβ-inducible gene in MLO-Y4 cells (Fig. 2A–B) (Graycar et al., 1989). TGFβ also induced expression of Mmp13 and Ctsk, but not Mmp14, in OCY454 cells (Fig. 2C–D). Further supporting the osteocyte intrinsic role of TGFβ, TGFβ induced the expression of the osteocyte marker genes Sclerostin (Sost) and dentin matrix protein-1 (Dmp1), without affecting Phosphate Regulating Endopeptidase Homolog, X-Linked (Phex) (Fig. S3).
Figure 2. TGFβ promotes cell intrinsic osteocytic perilacunar remodeling.
(A–D) qPCR analysis of PLR genes Mmp13, Mmp14 and Ctsk and Serpine1 upon TGFβ (5ng/mL) treatment in MLO-Y4 (A, B) and OCY454 (C, D) cells. (n=3 replicates/group). (E, F) Intracellular pH (pHi) of MLO-Y4 cells after 3 days of TGFβ (5ng/ml), TβRI inhibitor SB-431542 (10 μM), or recombinant sclerostin (rhSCL, 10 ng/ml). The representative image (E) shows the shift in the emission peak from 580 nm to 640 nm after TGFβ treatment of MLO-Y4 cells. Scale bar, 100 μm). TGFβ-induced acidification is blocked by SB-431542 (F) (n=4 replicates/group). Error bars indicate mean ± SD of 3 independent experiments, *p<0.05 different from control mRNA, a-p<0.05 different from control pHi, b-p<0.05 different from TGFβ pHi, and c-p<0.05 different from rhSCL pHi. Statistics calculated from Student’s t test.
In addition to expressing PLR enzymes, osteocytes engaged in PLR acidify their microenvironment. Using the pH-sensitive dye 5-(and-6)-carboxy SNARF-1, AM, we examined the effect of TGFβ on MLO-Y4 cell acidification. As shown by others (Kogawa et al., 2013), recombinant sclerostin (rhSCL) induces PLR and lowers the intracellular pH (pHi) of MLO-Y4 cells. TGFβ treatment resulted in a larger acidification than sclerostin treatment. In contrast, blocking TGFβ signaling with an in vitro inhibitor of TβRI (SB-431542) relieved this acidification, such that pHi was equivalent to untreated cells (Fig. 2E–F). Altogether, our findings support the possibility that TGFβ induces PLR in an osteocyte-intrinsic manner.
Osteocyte-specific inhibition of TGFβ signaling impairs perilacunar/canalicular remodeling
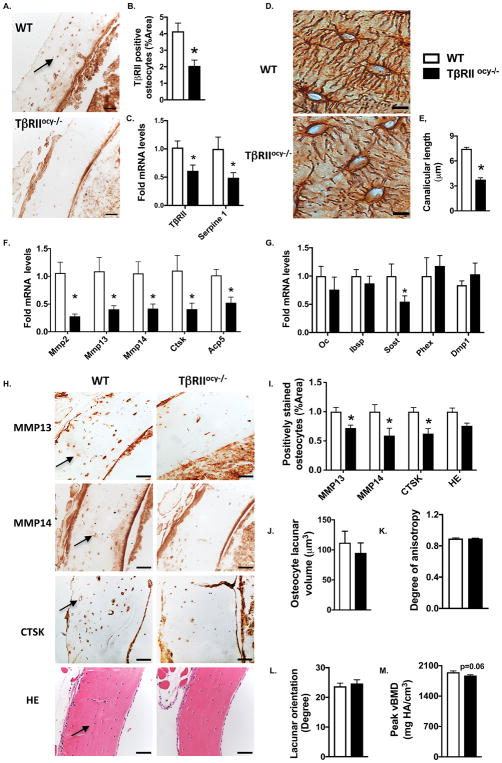
To evaluate the osteocyte-intrinsic role of TGFβ signaling in vivo, TGFβ receptor II (TβRII) was deleted in osteocytes using DMP1-Cre mice, resulting in TβRIIocy−/− mice. We validated the specific reduction of TβRII expression in osteocytes (but not in other cell types) of TβRIIocy−/− bone relative to DMP1Cre−/−; TβRIIfl/fl (WT) littermate controls (Fig. 3A–B). Abrogation of TGFβ signaling in TβRIIocy−/− bone was validated by reduced TβRII and Serpine1 gene expression (Fig. 3C). Furthermore, using primary bone marrow cultures from WT and TβRIIocy−/− mice, we verified the osteocyte-specific defect in TGFβ signaling by confirming that osteogenic gene expression is normal until after these cells differentiate into osteocytes (Fig S5A–D).
Figure 3. Osteocytic deletion of TβRII dysregulates perilacunar remodeling.
(A, B) TβRII-stained osteocytes (A) (arrow, scale bar, 50 μm) in the femoral cortical bone from WT and TβRIIocy−/− mice (8-week old males) were quantified as percentage of positively stained osteocytes normalized to total bone area (B) (n=5 mice/group) (C) qPCR analysis of TβRII and Serpine1 in WT and TβRIIocy−/− femoral bones. (n=8–10 mice/group). (D, E) Silver nitrate stained images of WT and TβRIIocy−/− femoral cortical bone shows the osteocyte lacuno-canalicular network (D) and canalicular length (E) (scale bar, 20 μm, n=5 mice/group). (F, G) qPCR analysis of PLR genes, Mmp2, Mmp13, Mmp14, Ctsk, and Acp5 (F) and OCY-specific genes, Sost, Dmp1 and Phex (G) in the WT and TβRIIocy−/− bones (n=8–10 mice/group) (H, I) IHC of MMP13, MMP14, CTSK and H&E staining of WT and TβRIIocy−/− femoral cortical bone. Arrows in the image indicate positively stained osteocytes (H) that were quantified and normalized to total bone area (I), (n=4 mice/group).(J–M) SRμT shows volume (J), degree of anisotropy (K), orientation (L) and mineralization (N) of osteocyte lacunae of WT and TβRIIocy−/− bone (n=3–4 mice/group). Error bars indicate mean ± SEM with *p<0.05 compared to WT from Student’s t test.
Since systemic inhibition of TGFβ signaling causes severe deterioration of the osteocyte canalicular network, and since TGFβ regulates osteocytic expression of PLR enzymes, we evaluated the lacuno-canalicular network in TβRIIocy−/− cortical bone. Upon osteocytic deletion of TβRII, the canalicular network was abrogated and visibly blunted. Relative to WT, the length of canalicular projections in TβRIIocy−/− bone were reduced by 50% and the total lacuno-canalicular area was reduced by 32% (Fig. 3D–E, Fig. S4A).
Among the panel of PLR genes, expression of Mmp2, Mmp13, Mmp14, Ctsk, and Acp5 was downregulated in TβRIIocy−/− mice (Fig. 3F, Fig. S4B). In fact, the effect of osteocyte-intrinsic TβRII ablation on PLR gene expression was even more profound than that produced by TβRI-inhibitor treatment. Expression of osteocalcin (Oc) and bone sialoprotein (Ibsp), Dmp1 and Phex genes that control systemic mineral homeostasis was unaffected by the absence of osteocytic TGFβ signaling. Expression of Sost, which is known to be induced by TGFβ (Loots et al., 2012; Nguyen et al., 2013), was downregulated in TβRIIocy−/− bones (Fig. 3G). Protein expression of MMP13, MMP14 and CTSK in osteocytes of TβRIIocy−/− mice was also significantly reduced 27–40% compared to WT mice, without apparent changes in osteocyte number and viability as determined by H&E and TUNEL staining (Fig. 3H–I, Fig S4B–C). These findings corroborated the observations in the TβRI-I mouse model and revealed the direct role of osteocytic TGFβ signaling in the regulation of PLR.
To rigorously evaluate the effect of TβRII deletion on lacunar size, orientation and shape, we utilized synchrotron radiation micro-tomography (SRμT), which visualizes and quantifies the osteocyte lacunae in a 3-D space. In spite of the dramatic differences in the canalicular network seen histologically, osteocyte lacunar volume, shape, and orientation relative to the long axis of the bone did not differ significantly between TβRIIocy−/− and WT cortical bone (Fig. 3J–M, Fig. S4E–H). Also using SRμT, we detected a 3% reduction (P=0.06) in peak bone mineral concentration in diaphyseal cortical bone of TβRIIocy−/− mice compared to WT (Fig. 3M, Fig. S4H). Therefore, osteocyte-intrinsic TGFβ signaling regulates the expression of enzymes required for PLR, and is essential for the integrity of the canalicular network and bone matrix mineralization. Not only does this reveal a previously unidentified role of TGFβ in osteocytes, but it also adds TGFβ to the short list of factors shown to regulate PLR.
Osteocyte-specific deletion of TβRII increases trabecular bone mass by inhibiting bone resorption
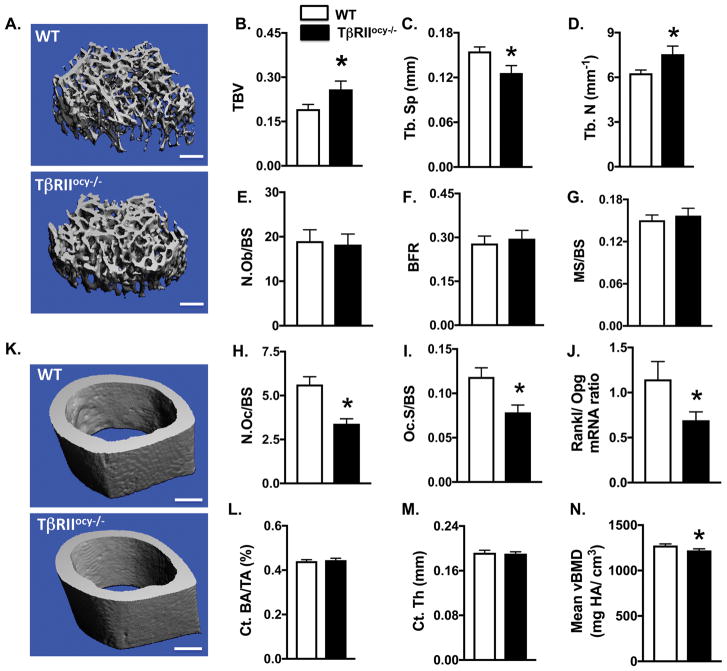
Because alterations in TGFβ signaling often impact bone mass (Balooch et al., 2005; Mohammad et al., 2009), we analyzed the impact of ablated osteocyte-specific TGFβ signaling on trabecular and cortical bone mass and geometry. Bones of TβRIIocy−/− mice showed no gross abnormalities relative to WT mice. Micro-computed tomography (μCT) analysis revealed a 35% increase in trabecular bone mass in 8-week old TβRIIocy−/− mice relative to WT littermates. This gain in mass was attributed to the corresponding increase in trabecular number (26%) and complementary decrease in trabecular spacing (25%) (Fig. 4A–D, Table 1). However, osteocytic deletion of TβRII did not affect cortical bone thickness or geometry (Fig. 4K–M). Cortical bone mineralization (Fig. 4N) of TβRIIocy−/− mice was reduced by 4.8%, consistent with the 3% decrease in peak bone mineral concentration detected by SRμT (Fig. 3M). Therefore, osteocyte-intrinsic TGFβ regulates the mass and geometry of trabecular, but not cortical bone.
Figure 4. Osteocytic deletion of TβRII increases trabecular bone mass but does not affect cortical bone mass.
(A–D) μCT analysis of femur from WT and TβRIIocy−/− mice (8-week old males), Representative μCT reconstructions of trabecular bone (A) from mice and trabecular bone parameters: trabecular bone volume fraction (BV/TV) (B), trabecular number (Tb.N.) (C), and separation (Tb.Sp.) (D). Scale bar, 100 μm (n=10–11 mice/group)(E–I) Histomorphometric analysis of femurs from WT and TβRIIocy−/− mice (8-week old males) measures osteoblast number normalized to bone surface (N.Ob/BS), bone formation rate (BFR) and percent mineralizing bone surface per bone surface (MS/BS), osteoclast number normalized to bone surface (N.Oc/BS), osteoclast suface normalized to bone surface (Oc.S/BS) (n=6–7 mice/group). (J) qPCR analysis of mRNA harvested from WT and TβRIIocy−/− bones shows the Rankl/Opg ratio (n=8–10 mice/group).(K–N) Representative μCT reconstructions of femoral cortical bone femur from WT and TβRIIocy−/− mice (8-week old males) (K) and cortical bone parameters: cortical area fraction (Ct. BA/TA) (L), cortical thickness (Ct. Th) (M), and cortical mineralization (Ct. Min) (N). Scale bar,100 μm (n=10–11 mice/group). Data are presented as mean ± SEM and *p<0.05 compared to WT from Student’s t test.
Table 1. Skeletal phenotyping of 8-week old WT and TβRIIocy−/− mice.
μCT and histomorphometry analysis revealed significant differences in trabecular bone phenotype, cortical mineralization, and osteoclast behavior between wildtype and TβRIIocy−/− mice, but no differences were observed in cortical bone volume or osteoblast behavior. Bone parameters measured by microCT include trabecular bone volume fraction (TBV); connectivity density (Conn D); trabecular number (Tb. N); trabecular thickness (Tb. Th); trabecular separation (Tb. Sp); structural model index (SMI); trabecular mineralization (Tb. Min); cortical bone volume fraction (Ct. BV/TV); cortical thickness (Ct. Th); cortical SMI, and cortical mineralization (Ct. Min), with n=10–11 mice per group. Histomorphometry parameters measured include osteoid volume (OV/BV); osteoid surface (OS); osteoid width (O. Wi); osteoblast number (N. Ob); osteoblast surface (N. Ob/BS); osteoclast surface (Oc.S); eroded bone surface (Oc.S/BS); osteoclast number (N. Oc); osteoclast number (N.Oc/BS); mineralization surface (MS/BS); bone formation rate (BFR); and mineral apposition rate (MAR), with n=6–7 mice per group. Data are presented as mean ± SEM with *p < 0.05, # p = 0.06, and † p = 0.07 different from WT group from Student’s t-test.
| Parameters | WT | TβRIIocy−/− |
|---|---|---|
| MicroCT | ||
| Distal Femur | ||
| TBV (BV/TV) (%) | 0.192 ± 0.016 | 0.259 ± 0.028 * |
| Conn D (1/mm3) | 377.74 ± 29.29 | 493.37 ± 71.58 |
| Tb. N (1/mm) | 6.275 ± 0.215 | 7.557 ± 0.542 * |
| Tb. Th (μm) | 0.043 ± 0.002 | 0.043 ± 0.001 |
| Tb. Sp (mm) | 0.155 ± 0.006 | 0.126 ± 0.009 * |
| SMI | 1.720 ± 0.184 | 1.063 ± 0.287 # |
| Tb. Min (mg HA/cm3) | 1020.98 ± 9.31 | 1014.23 ± 13.36 |
| Midshaft Femur | ||
| Ct. BA/TA (%) | 0.437 ± 0.006 | 0.445± 0.008 |
| Ct. Th (μm) | 0.188 ± 0.004 | 0.189 ± 0.003 |
| Ct. SMI | −0.442 ± 0.239 | −1.066 ± 0.339 |
| Ct. Min (mg HA/cm3) | 1279.69 ± 15.30 | 1217.59 ± 16.136 * |
| Histomorphometry | ||
| Static parameters | ||
| OV/BV | 0.007 ± 0.001 | 0.004 ± 0.001 |
| OS | 0.14 ± 0.02 | 0.12 ± 0.04 |
| OS/BS (%) | 0.04 ± 0.01 | 0.03 ± 0.01 |
| O. Wi (μm) | 3.28 ± 0.21 | 2.79 ± 0.36 |
| N.Ob | 61.00 ± 5.61 | 60.94 ± 8.81 |
| N.Ob/BS (/mm) | 19.01 ± 2.57 | 18.24 ± 2.38 |
| Oc.S | 0.83 ± 0.07 | 0.68 ± 0.01 † |
| Oc.S/BS (%) | 0.12 ± 0.01 | 0.08 ± 0.01 * |
| N.Oc | 39.57 ± 3.10 | 30.17 ± 1.66 * |
| N.Oc/BS (/mm) | 5.63 ± 0.44 | 3.40 ± 0.28 * |
| Dynamic parameters | ||
| MS/BS (%) | 0.15 ± 0.01 | 0.16 ± 0.01 |
| MAR (μm/d) | 1.84 ± 0.13 | 1.89 ± 0.17 |
| BFR/BS (μm2. μm3. d) | 0.28 ± 0.03 | 0.30 ± 0.03 |
To understand the cellular mechanism underlying the elevated trabecular bone phenotype of TβRIIocy−/− mice, histomorphometry was performed. Neither static nor dynamic histomorphometric analyses revealed significant differences in osteoblast or bone formation parameters in TβRIIocy−/− bone (Fig. 4E–G, Table 1). On the other hand, measures of bone resorption implicate osteocyte-intrinsic TGFβ in the control of osteoclast function. Specifically, TRAP-positive osteoclasts were reduced by 40%, along with a 34% reduction in osteoclast surface in TβRIIocy−/− mice (Fig. 4H–I, Table 1). Furthermore, TβRIIocy−/− mice showed a substantial reduction in the ratio of RANKL/OPG mRNA expression due to low levels of the osteoclastogenic factor RANKL (Rankl), but unaffected levels of OPG (Opg), a RANKL antagonist (Fig. 4J, Fig. S5E–F). Together these results attribute the high trabecular bone mass phenotype of TβRIIocy−/− mice to decreased osteoclast function, which essentially results from decreased production of RANKL by TβRII-deficient osteocytes.
Osteocyte deletion of TβRII reduces fracture resistance of bone
Given that TβRIIocy−/− cortical bone mass and thickness are normal, evidence of bone fragility in these mice would be consistent with defects in bone quality. Despite our prior implication of TGFβ in bone quality regulation (Balooch et al., 2005; Chang et al., 2010; Mohammad et al., 2009), the cellular target responsible for the control of bone quality has since been elusive. The disruption of PLR and cortical bone mineralization in TβRIIocy−/− bone led us to hypothesize that TGFβ controls bone quality through regulation of osteocytic PLR. To test this hypothesis, we performed a series of tests to evaluate the macromechanical and material behavior of TβRIIocy−/− bone.
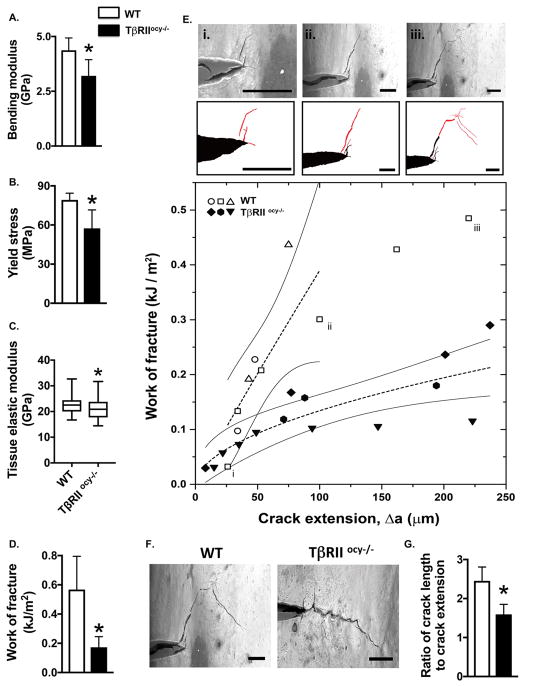
Macromechanical testing showed reduced fracture resistance of TβRIIocy−/− cortical bone. Using flexural testing, we found that TβRIIocy−/− femora exhibited a 26% decline in the bending modulus relative to WT bone, indicating a reduced capacity to resist elastic deformation (Fig. 5A). Similarly, the yield stress was reduced by 27% in TβRIIocy−/− bones (Fig. 5B). Using nanoindentation to examine the material properties of the TβRIIocy−/− bone revealed that the Young’s modulus of TβRIIocy−/− bone matrix was significantly lower than that of WT bone (Fig. 5C), a finding that is consistent with the reduced TβRIIocy−/− cortical bone mineralization (Fig. 4N). The most dramatic effects were observed in fracture toughness testing, in which notched TβRIIocy−/− cortical bone exhibited a 65% decrease in total work of fracture compared to WT bone (Fig. 5D). These findings are particularly remarkable, given that the severe fragility of TβRIIocy−/− bone could not be attributed to differences in cortical bone mass or geometry.
Figure 5. Osteocytic deletion of TβRII reduces bone material properties at multiple length scales.
Mechanical testing on femurs from WT and TβRIIocy−/− mice (8-week-old males). (A–B) Flexural tests of intact femurs shows bending modulus (A) and yield stress (B) (n=5 mice/group). (C) Nanoindentation of mid-diaphyseal femoral bone shows that tissue elastic modulus (n=3 mice/group). (D–E) In situ fracture toughness testing of notched femurs subjected to 3-point bending in a variable pressure SEM shows total work of fracture (WoF) (D) (n=5 mice/group), WoF R-curves produced by calculating WoF at each instance of crack propagation (E) (n=3 mice/group). Three stages of crack growth are shown for a WT sample (i–iii), with pre-existing notch or crack in black and new crack extension in red, and the corresponding points are indicated in the R-curve. (F–G) The decrease in TβRIIocy−/− bone of the extrinsic toughening mechanism, crack deflection, is readily seen in two representative samples (F) (scale bar = 100 μm) and in quantification of the ratio of total crack length to crack extension (G) (n=5 mice/group). Data are presented as mean ± SD. 95% confidence intervals in (E) are calculated based on a power fit. *p<0.05 different from WT group from Student’s t-test.
Accordingly, we sought to learn more about the material mechanisms responsible for TβRIIocy−/− bone fragility. For example, resistance to crack initiation is primarily imparted through intrinsic toughening mechanisms, representing a material’s inherent resistance to microstructural damage. On the other hand, crack growth toughness stems from extrinsic toughening mechanisms, which act to shield the crack from the applied driving force to limit crack propagation (Launey and Ritchie, 2009).
To distinguish between the effects of osteocyte TβRII deficiency on crack initiation and crack growth, we conducted fracture toughness testing in a variable pressure scanning electron microscope to simultaneously visualize and quantify crack behavior. While crack initiation toughness could not be conclusively differentiated between genotypes, the shallow slope of the R-curve for TβRIIocy−/− bones is indicative of reduced crack growth toughness and a loss of extrinsic toughening mechanisms (Fig. 5E). In situ images of crack growth show evidence of extrinsic toughening by crack deflection and uncracked ligament bridging in WT bone (Fig. 5Ei–iii). Conversely, the path of cracks in TβRIIocy−/− bone tended to be more linear and shorter relative to their profile extension (Fig. 5F–G). Therefore we conclude that TGFβ regulates bone quality in an osteocyte-intrinsic manner, specifically through extrinsic toughening mechanisms that limit crack growth. Identification of osteocytes as crucial cellular targets in the biological control of bone quality raises new questions about the role of osteocytes and PLR in human bone fragility.
Discussion
This study advances our understanding of bone homeostasis and fragility by revealing an osteocyte-intrinsic role for TGFβ signaling. Here we implicate TGFβ as a crucial regulator of perilacunar/canalicular remodeling and pinpoint osteocytes as the cell type principally responsible for the biological control of bone quality. Either using pharmacologic TGFβ receptor type I kinase inhibitors or a genetic model of osteocyte-specific TGFβ receptor ablation, we demonstrate that suppression of TGFβ signaling causes a severe deterioration of osteocyte canalicular network and dysregulates the expression of a host of PLR genes. Loss of osteocyte-intrinsic TGFβ signaling also reduces bone matrix mineralization. Since TβRIIocy−/− cortical bone mass and geometry are normal, the profound fragility of these bones reveals that TGFβ controls bone quality through an osteocyte-intrinsic mechanism that relies on PLR. These findings strongly support the idea that PLR plays a fundamental role in bone homeostasis, specifically as the cellular mechanism responsible for the maintenance of the lacuno-canalicular network and bone quality.
Our findings revealed TGFβ signaling to be a cell-intrinsic regulator of perilacunar/canalicular remodeling. Osteocyte-specific inhibition of TGFβ signaling decreases the expression of several genes that have been functionally implicated in PLR, including Mmp2, Mmp13, Mmp14, Ctsk and Acp5. The coordinated regulation of these PLR genes by TGFβ is consistent with the effects of other PLR-regulatory pathways. Most of these genes are induced by PLR agonists, such as sclerostin and PTH, but repressed by PLR antagonists such as glucocorticoids (Fowler et al., 2017; Kogawa et al., 2013; Qing et al., 2012). In each case, including in this study, these changes in gene expression correspond to alterations in the organization of the canalicular network. Interestingly, expression of vacuolar ATPases that function in osteocyte acidification are upregulated in TβRIIocy−/− bone, raising the possibility that a feedback loop compensates for the low level of proteases mediating PLR.
The effects of TGFβ and other PLR-regulatory pathways on the lacuno-canalicular network and on bone matrix differ in important ways. In addition to alterations in the canalicular network, lactation and glucocorticoid treatment cause changes in lacunar size (Fowler et al., 2017; Qing et al., 2012). Furthermore, collagen organization is disrupted in MMP13-deficient mice and in mice treated with glucocorticoids. In TβRIIocy−/− mice neither collagen organization nor lacunar volume, shape, and orientation was impacted. In this study, PLR mediated changes were observed at osteocyte canaliculi alone. Interestingly, emerging data from our lab and others (Fowler et al., 2017; Kaya et al., 2017; Tang et al., 2012) suggest that remodeling by osteocytes may be spatially defined, such that some circumstances favor remodeling at lacunae, whereas others will promote remodeling around canaliculi. Additional studies will be needed to determine the extent to which this is true.
The in vitro analysis of osteocyte acidification is a useful surrogate of PLR, but additional research is needed to better understand the cell biology of PLR. Nonetheless, TGFβ clearly acts directly on osteocytes to calibrate the extent of PLR and is required for the maintenance of the lacuno-canalicular network. Importantly, it is possible that the degenerated canalicular networks in our mouse models of impaired TGFβ signaling result from defective osteocyte integration into the bone matrix. A shorter time course or an inducible model would be needed to conclusively address this question. However, our previous studies have shown similar canalicular degeneration within 21 days of glucocorticoid treatment (Fowler et al., 2017) or a week of lactation (unpublished data)(Kaya et al., 2017; Qing et al., 2012; Qing and Bonewald, 2009; Wysolmerski, 2013), thereby indicating that changes in the canalicular network can occur rapidly in a manner that is independent of a maturation defect.
The critical role of TGFβ in osteocytes complements its actions in osteoblasts, osteoclasts, and their progenitors, where it couples bone formation to resorption (Dallas, 2008). Thus, it is not surprising that osteocyte-intrinsic ablation of TβRII would inhibit osteoclast function due to reduced levels of RANKL expression by osteocytes. Whether by systemic TβRI inhibition, expression of a dominant negative TGFβ type II receptor in osteoblasts, or in TβRIIocy−/− mice, trabecular bone mass is increased due to reduced RANKL expression and reduced osteoclastogenesis (Edwards et al., 2010; Filvaroff et al., 1999; Mohammad et al., 2009). Though we cannot completely exclude a causal role of TβRIIocy−/− osteocyte canalicular degeneration in the trabecular bone phenotype, our current and previous data suggest that TGFβ’s regulation of RANKL expression is cell intrinsic. On the other hand, the complexity of TGFβ crosstalk in bone underlies the unique, and at times apparently contradictory, bone phenotypes that result from manipulating TGFβ signaling in one cell type or another (Dallas, 2008; Tang and Alliston, 2013). Furthermore, in bone and in many other tissues, the effect of TGFβ is nonlinear; such that either increased and decreased TGFβ signaling can produce an osteoporotic phenotype (Balooch et al., 2005; Borton et al., 2001; Erlebacher and Derynck, 1996). Despite this known complexity, we were surprised by the low mineral concentration of TβRIIocy−/− bone, given that mineralization is increased by systemic post-natal TβRI-I treatment (Edwards et al., 2010; Mohammad et al., 2009). Given that osteocyte canaliculi are sites of secondary mineralization, it is possible that the reduced canalicular length in the TβRIIocy−/− bones reduces surface area available for mineralization. Moreover, the increased expression of vacuolar ATPase may create an acidic microenvironment that is unfavorable for mineralization. Additional studies will be needed to discern the mechanisms by which pharmacologic disruption of TGFβ signaling affects bone mineralization differently from genetic ablation of TβRII specifically in osteocytes.
Bone strength relies on bone mass and bone quality, both of which depend on the ability of TGFβ to coordinate the function of osteoblasts, osteoclasts and osteocytes. In spite of the fact that bone quality contributes to at least half of fractures in people with clinically normal bone mass (Schuit et al., 2004; Sornay-Rendu et al., 2007), the cellular mechanisms controlling bone quality have remained unclear. Understanding these mechanisms is a critical step in improving the diagnostics and therapeutics for fragility fractures (Hernandez and Keaveny, 2006; Seeman, 2008). This study represents the most definitive evidence so far implicating osteocyte-mediated PLR in the control of bone quality. Previous studies by our group and others show that bone quality is impaired following glucocorticoid treatment or systemic ablation of MMP13, and that PLR is impaired in each case (Fowler et al., 2017; Lane et al., 2006; Tang et al., 2012). Here we find that osteocyte-specific deletion of TGFβ signaling caused defects in PLR and in cortical bone mineralization, flexural strength, ECM material properties, and fracture toughness without impacting cortical bone mass. In diseases like Camurati Engelman syndrome and osteogenesis imperfecta, both of which are characterized by excessive TGFβ signaling (Grafe et al., 2014; Kinoshita et al., 2000; Tang et al., 2009), deregulation of osteocyte-mediated remodeling may contribute to bone fragility. Similarly the extent to which dysregulated PLR contributes to the fragility in other skeletal diseases, including renal osteodystrophy, secondary hyperparathyroidism, and glucocorticoid-induced osteoporosis, is an important area of further investigation. If so, PLR could be an attractive therapeutic target for improving fracture resistance in many conditions.
In conclusion, this study emphasizes the need to identify the cellular and molecular mechanisms regulating bone quality to develop new therapies to address the significant unmet clinical need for the treatment of bone fragility. Current therapeutics can improve 70% of trabecular fractures but only 20–40% of cortical bone fractures, which is precisely where PLR dysregulation is most profound (Ahmed et al., 2015; Chen and Sambrook, 2011; Rivadeneira and Mäkitie, 2016). A combination of systems analysis of GWAS data from clinical cohorts, along with functional in vivo and in vitro studies, can shed light on new molecular targets to control bone fragility and expand the pool of genetic markers needed for fracture risk assessment and prevention.
Experimental Procedures
Mice
To block TGFβ signaling systemically, 5-week old equally weighing C57BL/6 male mice were administered either vehicle (1% methylcellulose) or a specific inhibitor of the TGFβ type I receptor (TβRI-I), (SD-208, 60 mg/kg twice daily by oral gavage) for 6 weeks (Mohammad et al., 2009). We also generated mice with osteocyte-specific ablation of TGFβ type II receptor (TβRII), which effectively blocks osteocyte sensitivity to TGFβ ligand. Homozygous TβRII-floxed mice that possess loxP sites flanking exon 4 of the targeted gene were backcrossed for 3 generations into a C57BL/6 background and subsequently bred with hemizygous −10kb-DMP1-Cre+/− mice, which express Cre recombinase primarily in osteocytes (Leveen et al., 2002; Lu et al., 2007). Half of the mice from the resulting cross were DMP1-Cre+/−;TβRIIflfl (named TβRIIocy−/− mice) and half were DMP1-Cre−/−;TβRIIfl/fl littermate controls (named Wild-type (WT) mice), as confirmed by PCR genotyping. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of California San Francisco and the Indiana University School of Medicine.
Morphological analysis
For skeletal phenotyping, femurs harvested from 8-week old male mice (n=8 mice per group) were cleaned of soft tissue and fixed in 10% neutral buffered formalin for microcomputed tomography (microCT, μCT). For μCT, fixed femurs were stored in 70% ethanol. For histomorphometry, 7-week old male mice were administered with two intraperitoneal injections of calcein (20 mg/kg body weight, Sigma) 10 and 3 days prior to euthanasia and the harvested femurs were fixed in 10% neutral buffered formalin for 48 hours, processed and embedded in a MMA plastic resin. Additional details of μCT and histomorphometry procedures are described in the Supplemental Data.
Quantitative RT-PCR analysis
We purified RNA from cells in culture and from bones dissected from soft tissues using the miRNeasy mini kit (Qiagen, Valencia, CA), following the manufacturer’s protocol. In vitro results are representative of n=3 replicates/group and 3 independent experiments. For bones (humeri from n≥8 mice/group), proximal and distal regions were cut off and marrow was removed by centrifugation before RNA extraction. The majority of RNA obtained from bone using this method is osteocyte derived, with very little contribution from osteoblasts (Halleux et al., 2012). Additional details of quantitative RT-PCR analysis are described in the Supplemental Data).
Immunohistochemistry
For immunohistochemistry, paraffin embedded (7μm thick) sections were incubated with primary antibodies for anti-MMP13 (1:100; Abcam, ab39012); anti-MMP14 (1:100; Abcam, ab38971); anti-CTSK (1:75; Abcam, ab19027), or anti-TβRII (1:500; Abcam, ab186838). This was followed by incubation with corresponding biotinylated secondary antibody, avidin-conjugated peroxidase, and diaminobenzidine substrate chromogen system (Innovex Universal Animal IHC kit). Corresponding nonimmune IgGs were used as negative controls. Hematoxylin and eosin (H&E) and TUNEL-DAPI staining were performed to visualize osteocyte number and apoptosis. Ploton silver staining (Jauregui et al., 2016; Ploton et al., 1986) was performed for visualization of the osteocyte lacuno-canalicular network. Images were acquired using a Nikon Eclipse E800 bright-field microscope and analyzed with ImageJ. Sections were evaluated for one femur from each of n≥4 mice/group. Additional details of immunohistochemistry and image analysis are described in the Supplemental Data.
Cell culture
The MLO-Y4 osteocyte-like cell line (generously provided by L. Bonewald) was maintained in α-MEM supplemented with 2.5% fetal bovine serum, 2.5% bovine calf serum, and 1% penicillin-streptomycin. The OCY454 osteocyte cell line (generously provided by P. Divieti-Pajevic) was cultured in α-MEM supplemented with 10% fetal bovine serum and 1% antibiotic/antimycotic (Gibco). For treatment, cells were cultured in α-MEM containing 0.5–1% fetal bovine serum, supplemented with 5 ng/ml TGFβ1 (Humanzyme, HZ-1011), 10 μM SB431542 (Sigma, S4317) or 10 ng/ml recombinant human sclerostin (rhSCL, R&D Systems) for the indicated times.
Intracellular pH assay
Intracellular pH (pHi) was measured in transfected or untransfected MLO-Y4 cells treated with TGFβ1, SB431542 or rhSCL using the pH-sensitive fluorescent dye, 5-(and-6)-carboxy SNARF-1, AM (Molecular Probes, Inc.) as described (Kogawa et al., 2013). Briefly, after 3 days of culture in the indicated conditions, cells were washed with PBS and loaded with 5-(and-6)-carboxy-SNARF-1, AM at 37°C for 30 minutes, at a final concentration of 10 μM and visualized under a Leica TCS SPE confocal microscope. n=4 replicates/group and 3 independent experiments. Additional details of the procedure are provided in the Supplemental Data.
Synchrotron radiation Micro-Tomography (SRμT)
SRμT studies were used to assess the degree of mineralization of bone as well as the volume and degree of anisotropy of osteocyte lacunae. The mid-diaphysis of 8-week old male mouse femurs were scanned with 20 keV x-ray energy, with a 300 ms exposure time, using a 5X magnifying lens for a spatial resolution of 1.3 μm (N = 3–4 mice/group). Additional details of the SRμT procedure are described in the Supplemental Data.
Mechanical tests
To measure bone quality, we assessed the macromechanical properties and the bone matrix material properties using flexural strength tests, in situ fracture toughness tests, and nanoindentation. Briefly, from 8-week old TβRIIocy−/− and WT mice (n=3–5 mice/group) intact femurs were isolated, cleaned of soft tissue and stored in Hanks’ Balanced Salt Solution (HBSS). Details of the flexural strength tests, in situ fracture toughness tests, and nanoindentation procedures are described in the Supplemental Data.
Statistical Analysis
We expressed all values as mean ± S.E.M or mean ± S.D as appropriate for each assay. Group sizes were determined by power calculations providing 80% probability of detecting a significant difference (p ≤ 0.05). Group size “n” is denoted in figure legends. For in vivo data, n refers to the number of mice analyzed per group. For in vitro data, n refers to the number of independent experiments performed. Unpaired two-tailed Student’s t-test was used to compare the means of two groups using GraphPad Prism (GraphPad Software). Data points falling more than 2 standard deviations from the mean were excluded. Variances ranged from 12.5% to 20% and were similar between groups. No blinding was used during analysis. In all figures, p ≤ 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This research was supported by NIH-NIDCR R01 DE019284 (T.A.), DOD PRORP OR130191 (T.A.), NSF 1636331, NIH-NIAMS R21 AR067439, NIH-NIAMS P30 AR066262-01 (T.A.), Read Research Foundation (T.A.), OREF/ORS Postdoctoral Fellowship Grant 17-008 (N.S.D.), NIH T32 GM008155 (C.M.M., J.P.L., D.A.M.), NSF 1650113 (C.M.M.), Department of Defense (DoD) through the National Defense Science & Engineering Graduate Fellowship (NDSEG) Program (D.A.M.), and Swiss National Science Foundation grant P300P2_167583 (C.A.).The authors acknowledge the use of the x-ray synchrotron beamlines 8.3.2 at the Advanced Light Source (ALS) at LBNL. The ALS is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. The authors gratefully acknowledge J.J. Woo for expert technical assistance. Illustration kindly provided by Dr. M. Ouchida.
Footnotes
Author Contributions
Conceptualization, N.S.D, T.W.F., K.S.M., T.A.; Investigation, N.S.D., C.M.M., C.A., J.P.L., D.A.M., B.G., J.N.R., F.W., D.E., T.F.L., B.Z.; Data Curation, S.M.; Analysis, all authors; Writing – Original Draft, N.S.D.; Writing – Review & Editing, all authors; Visualization, N.S.D., C.M.M., T.A.; Supervision, R.O.R., K.S.M., T.A.; Project Leadership, N.S.D., T.A.; Funding Acquisition, T.A.
References
- Ahmed LA, Shigdel R, Joakimsen RM, Eldevik OP, Eriksen EF, Ghasem-Zadeh A, Bala Y, Zebaze R, Seeman E, Bjornerem A. Measurement of cortical porosity of the proximal femur improves identification of women with nonvertebral fragility fractures. Osteoporos Int. 2015;26:2137–2146. doi: 10.1007/s00198-015-3118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balooch G, Balooch M, Nalla RK, Schilling S, Filvaroff EH, Marshall GW, Marshall SJ, Ritchie RO, Derynck R, Alliston T. TGF-beta regulates the mechanical properties and composition of bone matrix. Proc Natl Acad Sci U S A. 2005;102:18813–18818. doi: 10.1073/pnas.0507417102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borton AJ, Frederick JP, Datto MB, Wang XF, Weinstein RS. The loss of Smad3 results in a lower rate of bone formation and osteopenia through dysregulation of osteoblast differentiation and apoptosis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2001;16:1754–1764. doi: 10.1359/jbmr.2001.16.10.1754. [DOI] [PubMed] [Google Scholar]
- Chang JL, Brauer DS, Johnson J, Chen CG, Akil O, Balooch G, Humphrey MB, Chin EN, Porter AE, Butcher K, et al. Tissue-specific calibration of extracellular matrix material properties by transforming growth factor-beta and Runx2 in bone is required for hearing. EMBO Rep. 2010;11:765–771. doi: 10.1038/embor.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JS, Sambrook PN. Antiresorptive therapies for osteoporosis: a clinical overview. Nat Rev Endocrinol. 2011;8:81–91. doi: 10.1038/nrendo.2011.146. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Alliston T, Bonewald LF. Transforming Growth Factor-beta. In: Bilezikian LGRJP, Rodan GA, editors. Principles of Bone Biology. San Diego, CA: Academic Press; 2008. pp. 1145–1166. [Google Scholar]
- Delmas PD, Seeman E. Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone. 2004;34:599–604. doi: 10.1016/j.bone.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Edwards JR, Nyman JS, Lwin ST, Moore MM, Esparza J, O’Quinn EC, Hart AJ, Biswas S, Patil CA, Lonning S, et al. Inhibition of TGF-beta signaling by 1D11 antibody treatment increases bone mass and quality in vivo. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25:2419–2426. doi: 10.1002/jbmr.139. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Derynck R. Increased expression of TGF-beta 2 in osteoblasts results in an osteoporosis-like phenotype. J Cell Biol. 1996;132:195–210. doi: 10.1083/jcb.132.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filvaroff E, Erlebacher A, Ye J, Gitelman SE, Lotz J, Heillman M, Derynck R. Inhibition of TGF-beta receptor signaling in osteoblasts leads to decreased bone remodeling and increased trabecular bone mass. Development. 1999;126:4267–4279. doi: 10.1242/dev.126.19.4267. [DOI] [PubMed] [Google Scholar]
- Fleischli JG, Laughlin TJ, Athanasiou K, Lanctot DR, Lavery L, Wang X, Agrawal CM. Effect of diabetes mellitus on the material properties of the distal tibia. J Am Podiatr Med Assoc. 2006;96:91–95. doi: 10.7547/0960091. [DOI] [PubMed] [Google Scholar]
- Fowler TW, Acevedo C, Mazur CM, Hall-Glenn F, Fields AJ, Bale HA, Ritchie RO, Lotz JC, Vail TP, Alliston T. Glucocorticoid suppression of osteocyte perilacunar remodeling is associated with subchondral bone degeneration in osteonecrosis. Sci Rep. 2017;7:44618. doi: 10.1038/srep44618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235:176–190. doi: 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- Grafe I, Yang T, Alexander S, Homan E, Lietman C, Jiang MM, Bertin T, Munivez E, Chen Y, Dawson B, et al. Excessive TGFβ signaling is a common mechanism in Osteogenesis Imperfecta. Nature medicine. 2014;20:670–675. doi: 10.1038/nm.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graycar JL, Miller DA, Arrick BA, Lyons RM, Moses HL, Derynck R. Human transforming growth factor-beta 3: recombinant expression, purification, and biological activities in comparison with transforming growth factors-beta 1 and -beta 2. Mol Endocrinol. 1989;3:1977–1986. doi: 10.1210/mend-3-12-1977. [DOI] [PubMed] [Google Scholar]
- Haller AC, Zimny ML. Effects of hibernation on interradicular alveolar bone. J Dent Res. 1977;56:1552–1557. doi: 10.1177/00220345770560122601. [DOI] [PubMed] [Google Scholar]
- Halleux C, Kramer I, Allard C, Kneissel M. Isolation of mouse osteocytes using cell fractionation for gene expression analysis. Methods in molecular biology (Clifton, NJ) 2012;816:55–66. doi: 10.1007/978-1-61779-415-5_5. [DOI] [PubMed] [Google Scholar]
- Hernandez CJ, Keaveny TM. A biomechanical perspective on bone quality. Bone. 2006;39:1173–1181. doi: 10.1016/j.bone.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Pidoux I, Inoue S, Billinghurst RC, Wu W, Chrysovergis K, Yamada S, Birkedal-Hansen H, Poole AR. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J Cell Sci. 2005;118:147–156. doi: 10.1242/jcs.01581. [DOI] [PubMed] [Google Scholar]
- Inoue K, Mikuni-Takagaki Y, Oikawa K, Itoh T, Inada M, Noguchi T, Park JS, Onodera T, Krane SM, Noda M, et al. A crucial role for matrix metalloproteinase 2 in osteocytic canalicular formation and bone metabolism. J Biol Chem. 2006;281:33814–33824. doi: 10.1074/jbc.M607290200. [DOI] [PubMed] [Google Scholar]
- Jauregui EJ, Akil O, Acevedo C, Hall-Glenn F, Tsai BS, Bale HA, Liebenberg E, Humphrey MB, Ritchie RO, Lustig LR, et al. Parallel mechanisms suppress cochlear bone remodeling to protect hearing. Bone. 2016;89:7–15. doi: 10.1016/j.bone.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya S, Basta-Pljakic J, Seref-Ferlengez Z, Majeska RJ, Cardoso L, Bromage TG, Zhang Q, Flach CR, Mendelsohn R, Yakar S, et al. Lactation-Induced Changes in the Volume of Osteocyte Lacunar-Canalicular Space Alter Mechanical Properties in Cortical Bone Tissue. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2017;32:688–697. doi: 10.1002/jbmr.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Saito T, Tomita H, Makita Y, Yoshida K, Ghadami M, Yamada K, Kondo S, Ikegawa S, Nishimura G, et al. Domain-specific mutations in TGFB1 result in Camurati-Engelmann disease. Nature genetics. 2000;26:19–20. doi: 10.1038/79128. [DOI] [PubMed] [Google Scholar]
- Kogawa M, Wijenayaka AR, Ormsby RT, Thomas GP, Anderson PH, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin regulates release of bone mineral by osteocytes by induction of carbonic anhydrase 2. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28:2436–2448. doi: 10.1002/jbmr.2003. [DOI] [PubMed] [Google Scholar]
- Krstic J, Santibanez JF. Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. Scientific World Journal. 2014;2014:521754. doi: 10.1155/2014/521754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RN, Bakker AD, Gruber EV, Chae TD, Veldkamp JB, Klein-Nulend J, Everts V. MT1-MMP modulates the mechanosensitivity of osteocytes. Biochem Biophys Res Commun. 2012;417:824–829. doi: 10.1016/j.bbrc.2011.12.045. [DOI] [PubMed] [Google Scholar]
- Lane NE, Yao W, Balooch M, Nalla RK, Balooch G, Habelitz S, Kinney JH, Bonewald LF. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2006;21:466–476. doi: 10.1359/JBMR.051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launey ME, Ritchie RO. On the Fracture Toughness of Advanced Materials. Advanced Materials. 2009;21:2103–2110. [Google Scholar]
- Leveen P, Larsson J, Ehinger M, Cilio CM, Sundler M, Sjostrand LJ, Holmdahl R, Karlsson S. Induced disruption of the transforming growth factor beta type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood. 2002;100:560–568. doi: 10.1182/blood.v100.2.560. [DOI] [PubMed] [Google Scholar]
- Loots GG, Keller H, Leupin O, Murugesh D, Collette NM, Genetos DC. TGF-beta regulates sclerostin expression via the ECR5 enhancer. Bone. 2012;50:663–669. doi: 10.1016/j.bone.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007;86:320–325. doi: 10.1177/154405910708600404. [DOI] [PubMed] [Google Scholar]
- Mohammad KS, Chen CG, Balooch G, Stebbins E, McKenna CR, Davis H, Niewolna M, Peng XH, Nguyen DH, Ionova-Martin SS, et al. Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS One. 2009;4:e5275. doi: 10.1371/journal.pone.0005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalla RK, Kruzic JJ, Kinney JH, Ritchie RO. Effect of aging on the toughness of human cortical bone: evaluation by R-curves. Bone. 2004;35:1240–1246. doi: 10.1016/j.bone.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Nguyen J, Tang SY, Nguyen D, Alliston T. Load Regulates Bone Formation and Sclerostin Expression through a TGFβ-Dependent Mechanism. PLOS ONE. 2013;8:e53813. doi: 10.1371/journal.pone.0053813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott SM. When bone mass fails to predict bone failure. Calcif Tissue Int. 1993;53(Suppl 1):S7–13. doi: 10.1007/BF01673395. [DOI] [PubMed] [Google Scholar]
- Ploton D, Menager M, Jeannesson P, Himber G, Pigeon F, Adnet JJ. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region at the optical level. Histochem J. 1986;18:5–14. doi: 10.1007/BF01676192. [DOI] [PubMed] [Google Scholar]
- Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, Wysolmerski J, Bonewald LF. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2012;27:1018–1029. doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing H, Bonewald LF. Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int J Oral Sci. 2009;1:59–65. doi: 10.4248/ijos.09019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivadeneira F, Mäkitie O. Osteoporosis and Bone Mass Disorders: From Gene Pathways to Treatments. Trends in Endocrinology & Metabolism. 2016;27:262–281. doi: 10.1016/j.tem.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Seeman E. Bone quality: the material and structural basis of bone strength. J Bone Miner Metab. 2008;26:1–8. doi: 10.1007/s00774-007-0793-5. [DOI] [PubMed] [Google Scholar]
- Selvamurugan N, Kwok S, Alliston T, Reiss M, Partridge NC. Transforming growth factor-beta 1 regulation of collagenase-3 expression in osteoblastic cells by cross-talk between the Smad and MAPK signaling pathways and their components, Smad2 and Runx2. J Biol Chem. 2004;279:19327–19334. doi: 10.1074/jbc.M314048200. [DOI] [PubMed] [Google Scholar]
- Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2007;22:425–433. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- Tang S, Herber RP, Ho S, Alliston T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2012 doi: 10.1002/jbmr.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SY, Alliston T. Regulation of postnatal bone homeostasis by TGFbeta. Bonekey Rep. 2013;2:255. doi: 10.1038/bonekey.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nature medicine. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti A, Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone. 2009;44:11–16. doi: 10.1016/j.bone.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–3229. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- Wysolmerski JJ. Osteocytes remove and replace perilacunar mineral during reproductive cycles. Bone. 2013;54:230–236. doi: 10.1016/j.bone.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.