Abstract
Primary gaze fixation in healthy individuals is frequently interrupted by microsaccades and saccadic intrusions (SI). The neural systems responsible for the control of attention and eye movements are believed to overlap and in line with this, the behaviour of microsaccades appears to be affected by exogenous and endogenous attention shifts. In the current work we wished to establish whether SI would also be influenced by attention. Twelve participants performed a cue-target task where they were cued exogenously or endogenously and had to respond to the appearance of a peripheral target with either a button press or saccade. Our results indicate that SI are influenced by exogenous and endogenous attention and in a very similar manner to microsaccades. In all conditions, SI frequency initially decreased following the cue, then rose to a maximum before falling to below baseline levels. Following the exogenous cue, SI were more frequently directed away from the cue as predicted by inhibition of return. Additionally, SI direction following the endogenous cue was biased towards the cue for the saccadic response mode only, suggesting that the degree to which the eye movement and attention systems overlap depends on whether an eye movement is required. In summary, our findings indicate that SI characteristics are modulated by exogenous and endogenous attention and suggest that SI and microsaccades may lie on a continuum of fixational instabilities and furthermore, SI are likely to provide greater insights into the relationship between attention and the oculomotor systems.
Introduction
Primary gaze fixation characteristics
Primary gaze fixation is never perfectly stable but consists of small involuntary physiological eye movements (Carpenter, 1988; Ditchburn, 1973). These are comprised of disconjugate slow drifts (1-3′ arc), small conjugate microsaccades (5-10′ arc, 1-2 per second) and disconjugate tremors (15″ arc, 30-80Hz) (Ditchburn, 1973; Kowler, 1991; Ratliff and Riggs, 1950; Steinman, Cushman and Martins, 1982). A further class of involuntary eye movement has also been described; saccadic intrusions (SI). SI consist of conjugate, horizontal saccadic eye movements that take the form of an initial fast eye movement away from the desired eye position, followed after a variable duration by a return saccade or drift (Abadi and Gowen, 2004). Recent work suggests that SI characteristics such as amplitude and frequency are affected by the attentional requirements of the task (Gowen, Abadi and Poliakoff, 2005).
Attention and eye movements
Attention orienting is often divided into two forms: Exogenous orienting occurs when attention is automatically drawn to a stimulus in a transduction initiated or bottom-up manner and endogenous orienting occurs when attention is guided in a voluntary manner, by cognitive top-down mechanisms (Klein and Shore, 2000; Posner, 1980). Exogenous orienting develops within the first 100ms after stimulus presentation, whereas the effects of endogenous attention are observed after 200-300ms (Muller and Rabbitt, 1989). Findings from behavioural, lesion and imaging studies indicate that these forms of attention are mediated by separate neural substrates with exogenous orienting under greater temporo-parietal and ventral frontal cortex control while the posterior parietal and frontal cortex play a stronger role in endogenous orienting (Berger, Henik and Rafal, 2005; Deubel, 1995; Corbetta and Shulman, 2002; Muller and Rabbit, 1989; Grosbras, Laird and Paus, 2005; Mayer et al., 2004; Mort et al., 2003; Rafal et al., 1988; Rafal and Henik, 1994; although see Peelen, Heslenfeld and Theeuwes, 2004). Either of these two attentional mechanisms can occur with (overt orienting) or without (covert orienting) an accompanying eye movement. It remains unclear as to whether covert shifts of attention can occur independently of oculomotor planning (independence hypothesis) or whether they actually represent oculomotor programming (identity hypothesis). These two views are encapsulated in the spotlight metaphor of attention (Posner, Snyder and Davidson, 1980) and the oculomotor readiness hypothesis (Klein, 1980) or the premotor theory (Rizzolatti et al., 1987) which both emphasise that covert orienting of attention is the same as preparing to make an overt eye movement to look at that location.
A vast effort has been devoted to disentangling this issue and it would appear that an intermediate stance whereby the two systems share resources at some stage (interdependence hypothesis) appears most likely (for a review see Awh, Armstrong and Moore, 2006). Evidence indicates that it is not possible to make an eye movement without a prior shift in attention to the desired location (Shepherd, Findlay and Hockey, 1986) and that attention directed towards a target both facilitates saccades and enhances perceptual identification at the saccade goal (Kowler et al., 1995). Nevertheless, once saccade programming is complete some attention can be diverted from the saccade goal with little cost to the saccade latency or accuracy (Kowler et al., 1995). More recently, imaging studies have strengthened evidence for overlapping neural areas involved in covert and overt attention orienting (Corbetta, 1998; Grosbras, Laird and Paus, 2005; Nobre et al., 2000) although evidence at the single cell level argues that any dissociation may be apparent on a finer scale (Ignashchenkova et al., 2005; Sato and Schall, 2003; Thompson et al., 2005) and that task differences may recruit the two systems to differing degrees (Abrams and Pratt, 2000; Hunt and Kingstone, 2003; Tse, Sheinberg and Logthetis, 2002; Sumner et al., 2004; Theeuwes et al., 1998). Any relationship found between SI characteristics and covert exogenous or endogenous attention shifts would support the view that eye movement programming occurs during covert attention shifts.
A common paradigm used to investigate the effects of exogenous and endogenous attention is the cue-target task (Posner, 1980; Posner and Cohen, 1984). During the exogenous form of the task, a non informative peripheral cue is briefly presented (usually at one of two locations), whereas during the endogenous condition, a central informative cue is presented indicating which location is the most likely location for the target. Following the cue, a target is presented at the same or the opposite location to the cue. Manual and saccadic responses to the target exhibit distinct patterns of facilitation or inhibition depending on the cue type. In the case of exogenous cueing, latencies are faster (facilitation) if the target is presented at the cued location less than 200ms following the cue, but are longer if the target is presented over 300ms later (Klein, 2000). This inhibitory effect is termed inhibition of return (IOR) and can last for up to 3 seconds (Posner et al., 1985; Posner and Cohen, 1984). With endogenous cueing, facilitation towards the cue occurs later (from approximately 150ms) and IOR is absent during manual response conditions unless the cue triggers a saccade to be prepared or executed (Rafal et al., 1989; Fecteau, Bell and Morris, 2003; Muller and Rabbitt, 1989).
Attention and microsaccades
Recent work employing such a cue-target task has demonstrated that microsaccade direction and frequency are affected by exogenous and endogenous attention shifts (Engbert and Kliegl, 2003; Hafed and Clark, 2002; Laubrock, Engbert and Kliegl, 2004; Rolfs, Engbert and Kliegl, 2004). During both exogenous and endogenous cuing, microsaccade frequency first decreases then increases to a maximum approximately 400ms following the cue. In contrast, microsaccade direction is affected differently between the two attention conditions: Approximately 200ms following the exogenous cue, microsaccades tend to be directed away from the cue, whereas during endogenous cuing this effect is absent or reversed. Hafed and Clark (2002) and Laubrock, Engbert and Kliegl (2004) have speculated that microsaccades may be a consequence of exogenous and endogenous influence on the superior colliculus (SC) build up cells. The SC controls saccadic initiation, amplitude and direction through mutually inhibitory connections between rostral and caudal build up cells (Krauzlis, Basso and Wurtz, 2000; Munoz and Istvan, 1998; Munoz and Wurtz, 1992;1993a-b;1995a-b). The rostral build up cells (also known as fixation cells) represent the foveal region of the visual field and are active during fixation when they suppress larger saccades through inhibition of the caudal build up cells. The level and location of activity within these build up cells may dictate microsaccade frequency and direction.
At present, the question of whether SI and microsaccades represent distinct or related forms of fixational eye movements remains unanswered. Findings that attention and task instructions (Barlow, 1952; Gowen, Abadi and Poliakoff, 2005; Steinman et al., 1967; Steinman et al., 1973; Winterson and Collewijn, 1976; Kowler and Steinman, 1980) exert similar effects on both support the latter conclusion. However, in contrast to SI, microsaccades are of smaller amplitude, exhibit a higher frequency, are multiplanar and are uncoupled (do not possess a return saccade) (Abadi and Gowen, 2004; Carpenter, 1988a; Ditchburn, 1980; Kowler, 1991; Steinman et al., 1967;1973; Steinman, Cushman and Martins, 1982). If modulation of SI frequency and direction by exogenous and endogenous attention was found to resemble that for microsaccades, this would support the argument for a close association.
Consequently, in the current study we wished to explore whether SI characteristics such as amplitude, frequency and direction would also be influenced by exogenous or endogenous changes in attention. Therefore, as a more rigorous test of the preliminary study by Gowen, Abadi and Poliakoff (2005), we investigated the effect of both exogenous and endogenous cuing on SI characteristics in 12 subjects. Our aims were threefold. Firstly, we wished to examine whether exogenous and endogenous attention have differential effects on SI. Specifically, are SI directed away from the cue during the exogenous condition, but towards the cue during endogenous conditions. Secondly, earlier findings hinted that SI characteristics were influenced differently depending on the response mode; SI amplitude was larger and SI frequency lower during saccadic as opposed to manual trials (Gowen, Abadi and Poliakoff, 2005). Differences between saccadic and manual IOR have been observed previously (Abrams and Pratt, 2000; Hunt and Kingstone, 2003; Sumner et al., 2004) and any differential affect on SI would support a decoupling between covert attention orienting and eye movement planning. Thirdly, we wished to discover whether exogenous and endogenous attention shifts would affect SI in a similar manner to microsaccades, which would provide evidence as to whether the two forms of eye movement are linked.
2. Materials and Methods
2.1. Subjects
We tested 12 healthy volunteers whose average age was 28.67 years (range, 19-42). The subjects had no previous or current history of ocular disease, strabismus, general health problems or medication that had been linked to any ocular complications. Subjects demonstrated a corrected visual acuity of 0.2 LogMAR or better in either eye. Each gave written informed consent to participate and the study was approved by a local ethical committee.
2.2. Eye movement recording and instrumentation
Horizontal eye movements were recorded binocularly using an IRIS 6500 infrared limbal tracker (Skalar Medical, Delft, The Netherlands). The analogue output was filtered through a 100Hz low pass filter, digitised to 12-bit resolution and then sampled at intervals at 5ms (200Hz). The system was linear to ± 20º and had a resolution of >5'arc. Each subject’s head was restrained using a chin rest and cheek pads.
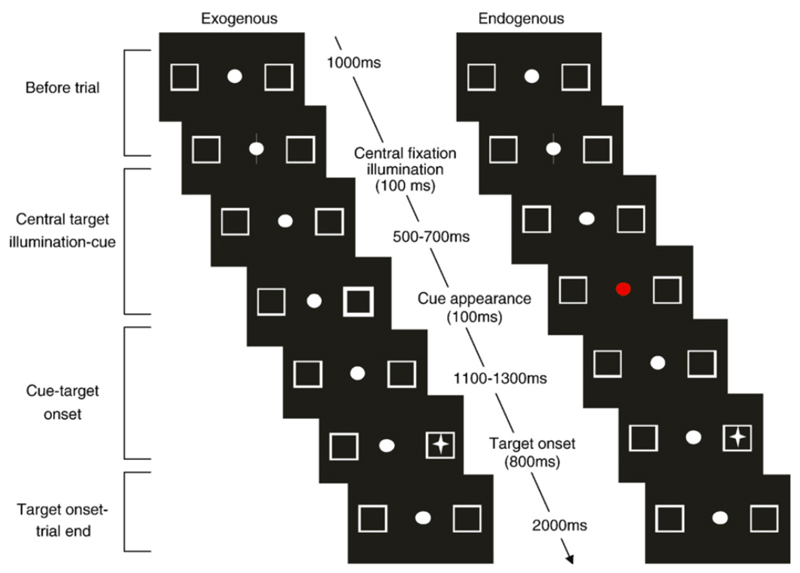
Visual stimuli were displayed on a cathode ray tube monitor and viewed from a distance of 55cms giving a field of 27.1°×38.5°. They consisted of a central fixation circle (1.04°) that was flanked by two peripheral boxes (3.85°×3.85°). The boxes were positioned 13.02° from the fixation circle. An asterisk (0.42°) served as the target and appeared in the centre of each box (Figure 1). The distance between the central fixation target and the peripheral asterisk subtended 14.58°. Testing was carried out in a quiet, near dark room. Subject’s eye movements were calibrated by moving a circular calibration target (0.42°) sinusoidally at 0.19Hz over a horizontal range of ±17.7º.
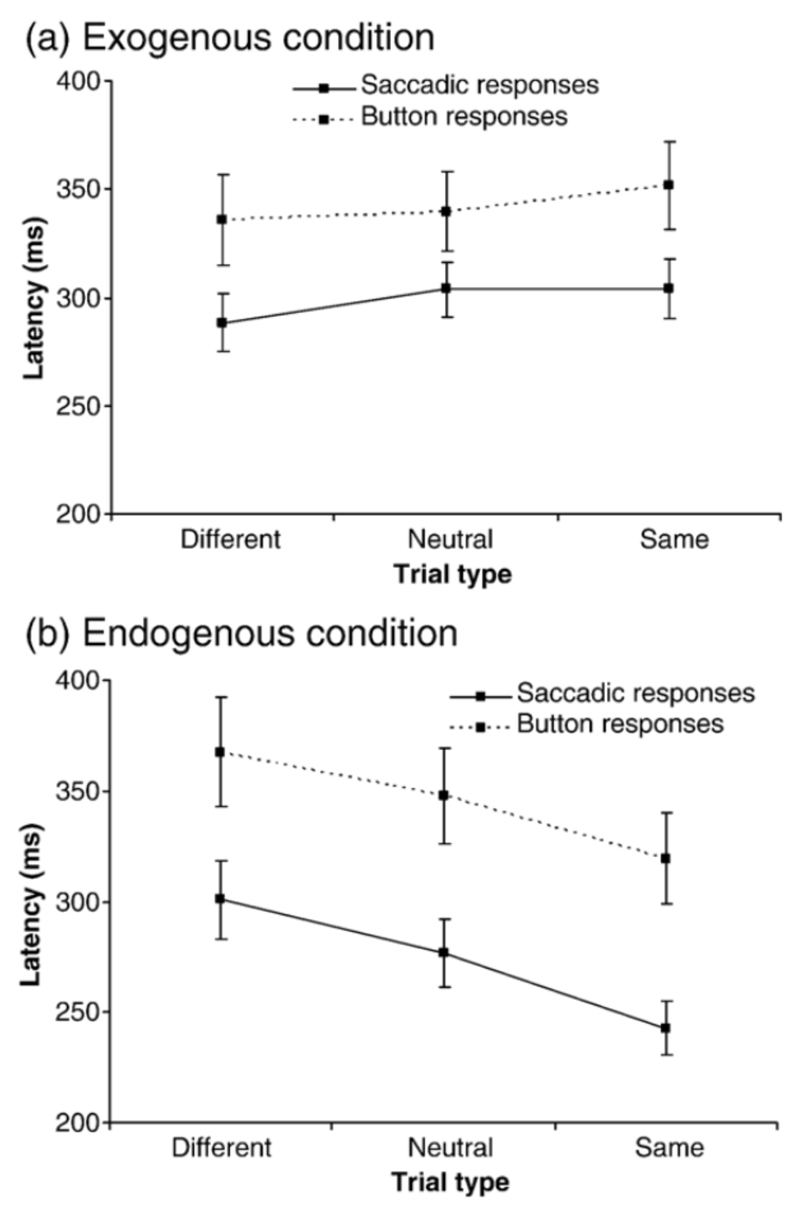
Fig. 1.
Saccade latencies (continuous line) and manual latencies (dashed line) over the different trial types for (a) exogenous cuing condition and (b) endogenous cuing condition. Standard error bars are shown.
The experimental procedure
In order to gain baseline measurements of SI frequency, amplitude and directional bias (the % of SI directed rightward) subjects were instructed to fixate a central point (0.42°) for 2 minutes. Following this they were required to perform two separate attention paradigms where the target was either cued exogenously or endogenously. Response mode to the target could either be a manual button press or saccade giving a total of four separate tasks: (1) Exogenous manual (2) Exogenous saccadic (3) Endogenous manual and (4) Endogenous saccadic. These were performed separately and counterbalanced over two 75 minute sessions.
Exogenous task
Subjects were required to fixate the central circular point which was continually present throughout the trials. A trial began (Fig 1a) with brightening (100ms) of the central point and following a pseudo-randomised delay of between 500 and 700ms, the outline of one of the peripheral boxes would brighten for 100ms. The subject was instructed to ignore this cue and continue fixating the central target. There was then a further delay of 1100 or 1300ms and the target (an asterisk) appeared randomly in one of the peripheral boxes for 800ms. Non-predictive 50% valid cues were used to summon attention to the cue by pure exogenous mechanisms. Depending on response mode, the subject was required to either press a button (exogenous manual task) or make a saccade to the target, then back to the centre once it was extinguished (exogenous saccade task). A new trial commenced 3 seconds later with brightening of the central target. Stimulus onset asynchrony (SOA), the time between the onset of the cue and the onset of the target was either 1200 or 1400ms.
Eight conditions were tested: Cue location (left, right) x SOA (1200, 1400) x Target location (left, right). Each condition was repeated 12 times giving a total of 96 trials. Neutral trials, where the non-informative cue consisted of both peripheral boxes flashing, followed by the appearance of the target in one of the boxes occurred in 24 trials (20%) giving a grand total of 120 trials. The 120 trials were repeated under the two different response modes (manual and saccadic). The trials were separated into blocks of 40 and at the beginning of each, eye movements were calibrated.
Endogenous condition
The timing of the trial was identical to the exogenous task (Fig 1b). The difference was that the colour of a centrally presented cue informed the subject of the likely target location: Yellow = left, Blue = right, Red = non-informative neutral cue. The subject was instructed to use the information present in these cues but to continue fixating at the central target. Depending on the response mode, the subject was required to either press a button (endogenous manual task) or make a saccade to the target, then back to the centre once it was extinguished (endogenous saccade task). Central informative 80% valid cues (yellow/blue) were used to summon attention by pure endogenous mechanisms, in contrast to the non-informative (50% valid) red cue. Circular colour cues (as opposed to arrow cues) were chosen so that any influence of cue form and shape on SI behaviour would be minimal
Eight conditions were tested: Cue location (left, right) x SOA (1200, 1400) x Target location (left, right). The four conditions where the cue correctly predicted the target location were repeated 48 times and the four conditions where the cue incorrectly predicted the target were repeated 12 times giving a total of 240 trials. Neutral trials, occurred in 60 trials (20%) giving a grand total of 300 trials. The 300 trials were conducted under the two different response modes. The trials were separated into blocks of 50 and at the beginning of each eye movements were calibrated.
Data analysis
Saccadic and manual responses
Eye position was recorded binocularly to allow selection of conjugate saccades, but only data from the right eye was used in the analysis. Saccade start and end points were determined by a 20º/second cut-off criterion, using bespoke Matlab scripts and the calculated amplitude and latency stored to disc. Artefacts such as blinks and drift were discarded together with data from trials where saccades were made in the direction opposite the target or where saccadic responses were <80ms and >2 standard deviations from the average. Removal of such trials amounted to <12%. Trials with button responses <100ms and >1000ms were also removed (<1% of trials).
SI analysis
SI start and end points were determined by a 20º/second cut-off criterion. SI with amplitudes > 4° and durations > 800ms were not included in the analysis as it has been shown that these are the upper limits of SI (Abadi and Gowen, 2004). Once the SI was detected its direction (right or left) and amplitude were stored and it was manually assigned to a category: Monophasic square wave intrusion, Biphasic square wave intrusion, Double saccadic pulse or Saccadic pulse (Abadi and Gowen, 2004). These all commence with an initial saccade but differ according to duration and whether the return movement consists of a saccade or drift. In the remainder of the study these are all analysed together and collectively termed SI. A total of 24260 SI were collected.
Three SI parameters were calculated: SI amplitude, SI frequency (per minute) and the % of SI directed towards the cue. For neutral trials the % of rightward directed SI was calculated instead of % towards the cue. In order to record the effect of the different trial events on the SI, they were sorted according to:
-
(1)
Attention condition – whether they occurred during an exogenous or endogenous trial
-
(2)
Response mode – whether they occurred during a manual or saccadic trial
-
(3)
Trial period – what time they occurred. Trials were separated into four time periods: (a) Before trial (1000ms before the central cue illuminated) (b) Central target illumination to cue onset (c) Cue to target onset (SOA) and (d) Target onset to trial end (Figure 1).
-
(4)
Cue type – whether they occurred in a trial where the cue appeared in the same/different direction as the target or where the cue was neutral.
As the period between the cue and target was of particular interest we performed a separate analysis on this period by segregating it into bins of 12 ×100ms bins and allocating the SI characteristic accordingly. For each subject and each time window, an average was computed and used in the statistical analysis.
Statistical analysis
Normality of the data was verified through histogram and Shapiro Wilk analysis. Data for SI amplitude (Shapiro Wilk test, p = 0.99; mean = 0.62, median = 0.63) and % of SI directed towards the cue (Shapiro Wilk test, p = 0.81; mean = 0.46, median = 0.46) were normally distributed whereas SI frequency was not; therefore non-parametric statistics were conducted on the latter. In cases where the data violated the assumption of sphericity, the greenhouse Geisser correction was employed.
Results
SI characteristics
Results from the control primary position fixation task revealed that mean (± standard deviation) SI amplitude and frequency were 0.54±0.26° and 18.66±12.86 per minute respectively. Mean SI directional bias (% of SI directed to the right) was 63.9%±23.51. These characteristics are consistent with those previously reported in a healthy population (Abadi and Gowen, 2004).
Response Latencies
Mean reaction times for the exogenous and endogenous conditions can be seen in Figures 2a-b respectively. A 2×3×2 within factor ANOVA with factors of attention condition (exogenous/endogenous), cue type (same/neutral/different) and response mode (button press/saccade) revealed a significant effect of cue type (F(2,22) = 5.09, p =0.015) and response mode (F(1,11) = 16.1, p =0.002) but no significant main effect of attention condition (F(1,11) = 2.19, p =0.17). The interaction between attention condition and cue type was significant (F(2,22) = 34.28, p <0.000); all other interactions were not significant. Although there was a trend for subjects to respond faster to the different cue types during the exogenous condition, follow up paired t tests with a corrected α of 0.00 revealed that there was no significant difference between same and different cues for the exogenous saccadic (t=-1.74, p=0.11) or exogenous manual (t=-2.19, p=0.05) task. There was however, a significant difference between same and different cues types for the endogenous saccadic (t=4.82. p=0.001) and endogenous manual (t=3.58. p=0.004) tasks. These results indicate that our experimental conditions produced different effects on response times that were dependent on attention condition. That is during exogenous conditions subjects were non-significantly slower to respond when the target direction was the same as the preceding cue direction (IOR) whereas during endogenous conditions, subjects were significantly faster to respond when the target direction was the same as the proceeding cue. Finally, manual responses were slower than saccadic responses during both attention conditions.
Fig. 2.
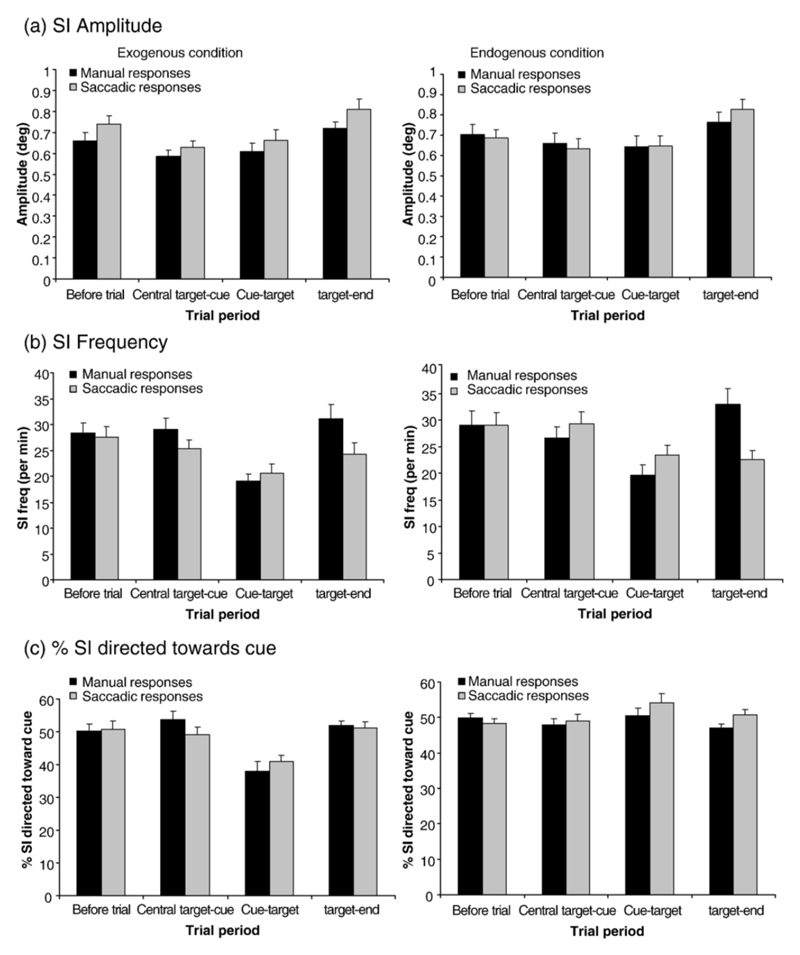
SI amplitude (a), frequency (b) and percent of SI directed towards the cue (c) for manual (black bars) and saccadic (grey bars) responses over the four different trial periods. Exogenous and endogenous conditions are shown on the left and right respectively. Cue types (same/different/neutral) are combined. Standard error bars are shown.
The effect of trial time on SI characteristics
SI amplitude, frequency and % to cue were analysed separately across attention condition (exogenous/endogenous), response mode (manual button press/saccade), trial period (Before trial/Central to cue/Cue to target/Target to end) and cue type (same/different/neutral). Planned comparisons of the SI features during the critical cue-target interval were also undertaken. In the following analysis, parametric statistics have been employed for the analysis of SI amplitude and SI % to cue and non-parametric statistics (Friedman’s, Wilcoxon signed ranks test) for SI frequency (see methods section) A Bonferroni adjustment to a corrected level of α = 0.01 was employed for all non-parametric statistics.
SI characteristics across different trial periods
SI Amplitude
A 2×2×3×4 within factor ANOVA with factors of attention condition (exogenous/endogenous), response mode (button press/saccade), cue type (same/different/neutral) and trial period revealed no significant effects of attention condition (F(1,10) = 0.006, p =0.939), response mode (F(1,10) = 3.34, p =0.1) or trial type (F(2,20) = 0.174, p =0.842) but that SI amplitude significantly varied across the trial periods (F(3,30) = 16, p =<0.001). This can be observed in Figures 3a where SI amplitudes are larger at the beginning and end of a trial compared with the centre portion. Paired T tests revealed significant differences between all trial periods (t≥3.73, p≤0.00), except between the centre cue to target and the cue-target periods (t=1.29, p=0.28). No significant interactions were observed (F≤2.08, p =≥0.12). Therefore, SI amplitude was similar under all task conditions and only varied during the different trial periods.
Fig. 3.
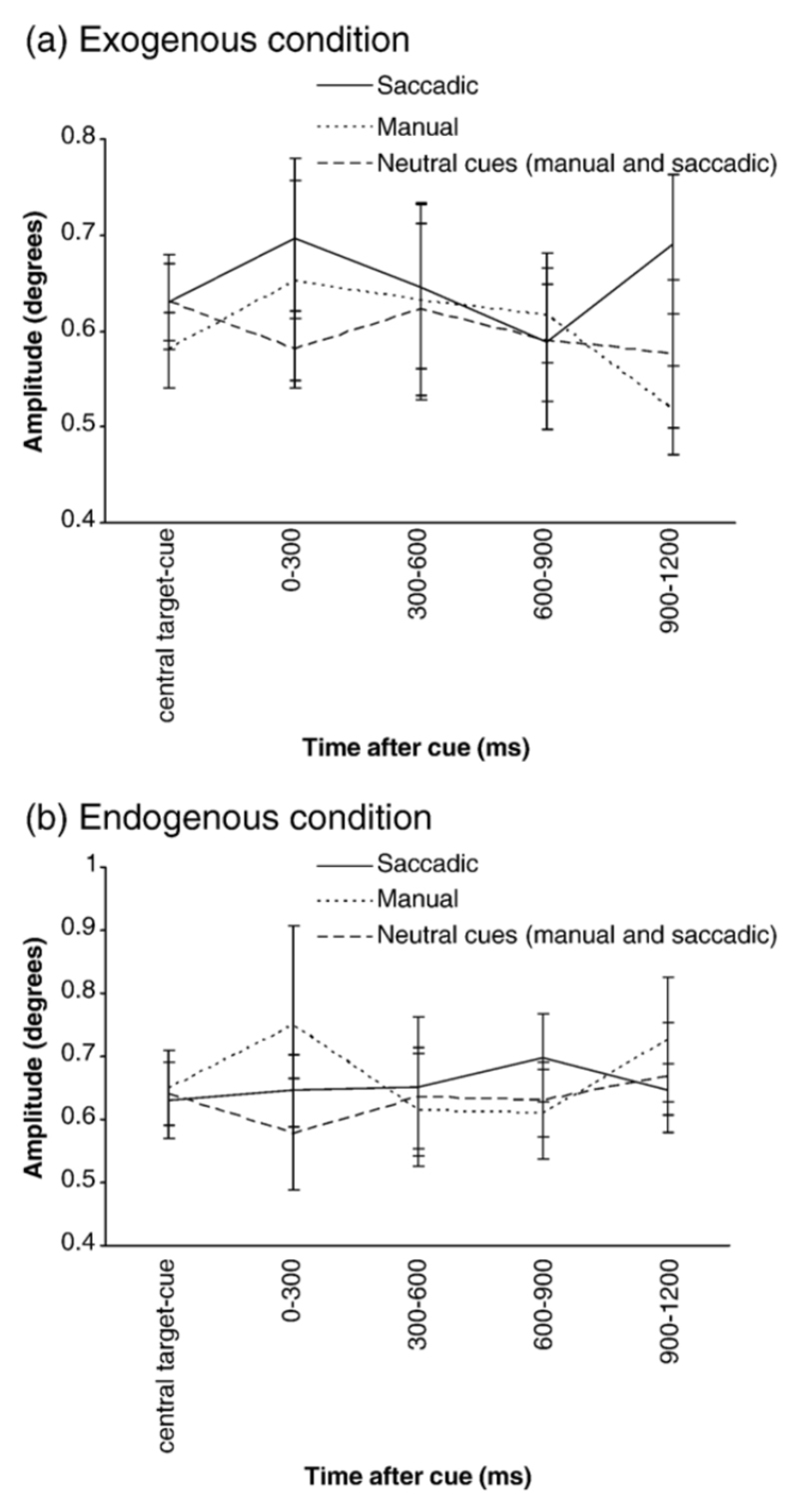
SI amplitude over the different time windows during the cue-target interval for the exogenous (a) and endogenous (b) conditions. Central target-cue = average SI amplitude over the preceding trial period (central target illumination-cue). Manual trials are denoted by the continuous line, saccadic trials by the dotted line and combined manual and saccadic neutral trials by the dashed line. Standard error bars are shown.
SI frequency
Figures 3b display SI frequency characteristics for exogenous (a) and endogenous (b) conditions. A Friedman’s test revealed that SI frequency did not significantly differ between exogenous and endogenous trial conditions (χ2=0.5, p=0.48) or between response modes (χ2=1.13, p=0.29). No significant interactions were observed between attention condition and response mode (exogenous v endogenous manual trials, z=-0.16, p=0.87; exogenous v endogenous saccadic trials, z=-2.1, p=0.04).
Comparison of SI frequency across trial periods revealed significant differences (χ2=59.48, p<0.001). This was the case for all trial conditions (χ2≥13.23, p≤0.004) and for each response mode (χ2≥26.42, p≤0.001). A Wilcoxon test across all trial conditions indicated that SI frequency was significantly lower during the cue - target period then during the before trial (z=-6.7, p< 0.00) and central illumination-cue (z=-6.78, p< 0.00) and target-end (z=-5.96, p<0.00) periods. Saccadic response mode was compared to manual response mode for each trial period. For the exogenous condition, SI was significantly higher for manual responses (33.75 per sec) as opposed to saccadic responses (23.6 per sec) during the target appearance-end period only (z=-3.11, p<0.0021) but for the endogenous conditions there were no differences between the response modes for any trial period (z≤2.23, p≥0.03).
SI frequency was significantly affected by cue type (χ2=10.8, p=0.006; same = 26.49 per min; different = 26.71 per min; neutral = 25.1 per min; Table 1). This was not significant over the different attention conditions, (χ2≤6.3, p≥0.04) or the different response modes (χ2≤5.44, p≥0.07). However, when cue type was analysed against trial period, SI frequency was significantly lower for neutral trials during the target-end period (χ2=17.37, p<0.001; same = 28.98 per min; different = 28.54 per min; neutral = 25.6 per min).
Table 1.
SI frequency during each trial period for cues that were in the same or different direction as the target or were neutral in respect to the target direction. Both attention conditions and response modes have been combined.
| Trial period | SI frequency (per min) for each cue type | ||
|---|---|---|---|
| Same | Different | Neutral | |
| Before trial | 28.35 | 29.05 | 28.04 |
| CC-Cue | 28.04 | 27.72 | 26.9 |
| Cue-target | 20.04 | 21.51 | 19.85 |
| Target-end | 28.98 | 28.54 | 25.6 |
In summary, SI frequency did not differ across the attention conditions, response mode or cue type but was significantly lower during the cue-target interval (i.e. SOA of 1200ms) for all these conditions. Furthermore, SI frequency was higher for manual responses during the target appearance-end period, particularly during the exogenous condition and appeared to be lower for neutral trials during this period.
SI direction
Neutral trials were analysed separately as the outcome parameter (% rightward) differs from directional cue trials (% to cue). Figures 3c displays the % of SI directed towards the cue across the different trial periods for exogenous and endogenous conditions respectively.
A 2x2x4 repeated measures ANOVA with factors of attention condition (exogenous/endogenous), response mode (manual/saccadic) and trial period revealed no significant effects of attention condition, response mode or trial interval (F≤1.89, p≥0.16). However, there was a significant interaction between attention condition and trial period (F(3,69) = 8.18, p =0.001). A paired sampled t test indicated that there was a higher percentage of SI directed towards the cue in the endogenous condition compared to the exogenous condition for the cue-target period (exogenous = 39.42%, endogenous = 52.35%, t=-4.11, p = <0.000). This can be observed in Figures 3c.
A second repeated measures ANOVA with the same factors as above examined the pattern of rightward directed SI in the neutral trials over the different conditions. There was no effect of attention condition (F(1,11) = 1.1, p =0.32), response mode (F(1,11) = 0.63, p =0.45) or trial period (F≤1.1, p ≥0.32) and crucially no interaction between attention condition and trial period (F(3,33) = 1.4, p =0.26).
In summary, the % of SI directed towards the cue tended to be lower during the cue-target period in the exogenous condition compared to the endogenous condition. However, there was no effect of experimental conditions on the right-ward bias in the neutral trials. In order to examine this effect in more detail we next analysed SI characteristics during the cue-target period only.
SI characteristics during cue-target period
SI characteristics were investigated across the cue-target interval separated in 12 ×100ms bins (see method section (SI analysis) for details).
SI amplitude
These group results can be seen in Figs 4a-b. As data was missing from some time windows (where SI had not occurred for certain subjects), amplitude averages were collapsed across three bins (300ms time windows) giving a total of four time windows. This was performed for each subject and each task condition and response mode. These were submitted to a 4×2×2 repeated measures ANOVA with factors of time window, attention condition (exogenous/endogenous) and response mode (manual/saccadic). There was a significant main effect of response mode (F(1,10) = 6.02, p =0.02) indicating that SI amplitude was higher for saccade trials and a significant 3 way interaction (F(3,30) = 4.65, p =0.01) which was further analysed using two separate repeated measures ANOVA’s for each response type with factors of attention condition (exogenous/endogenous) and time window. For the saccade response mode there was only a main effect of time window (F(3,30) = 3.44, p =0.03), whereas for the manual response condition there was an interaction between attention condition and time (F(3,33) = 3.35, p =0.03). Paired t tests comparing SI amplitude across the different time windows between the exogenous and endogenous manual conditions (corrected α 0.01) revealed that SI amplitude was marginally higher during the last time window for the manual endogenous condition (t=2.8, p=0.02). Therefore, SI amplitude was higher in the endogenous manual condition compared to the exogenous manual condition, but only during the last time window following the cue, whereas SI amplitude did not vary between the exogenous and endogenous saccadic conditions.
Fig. 4.
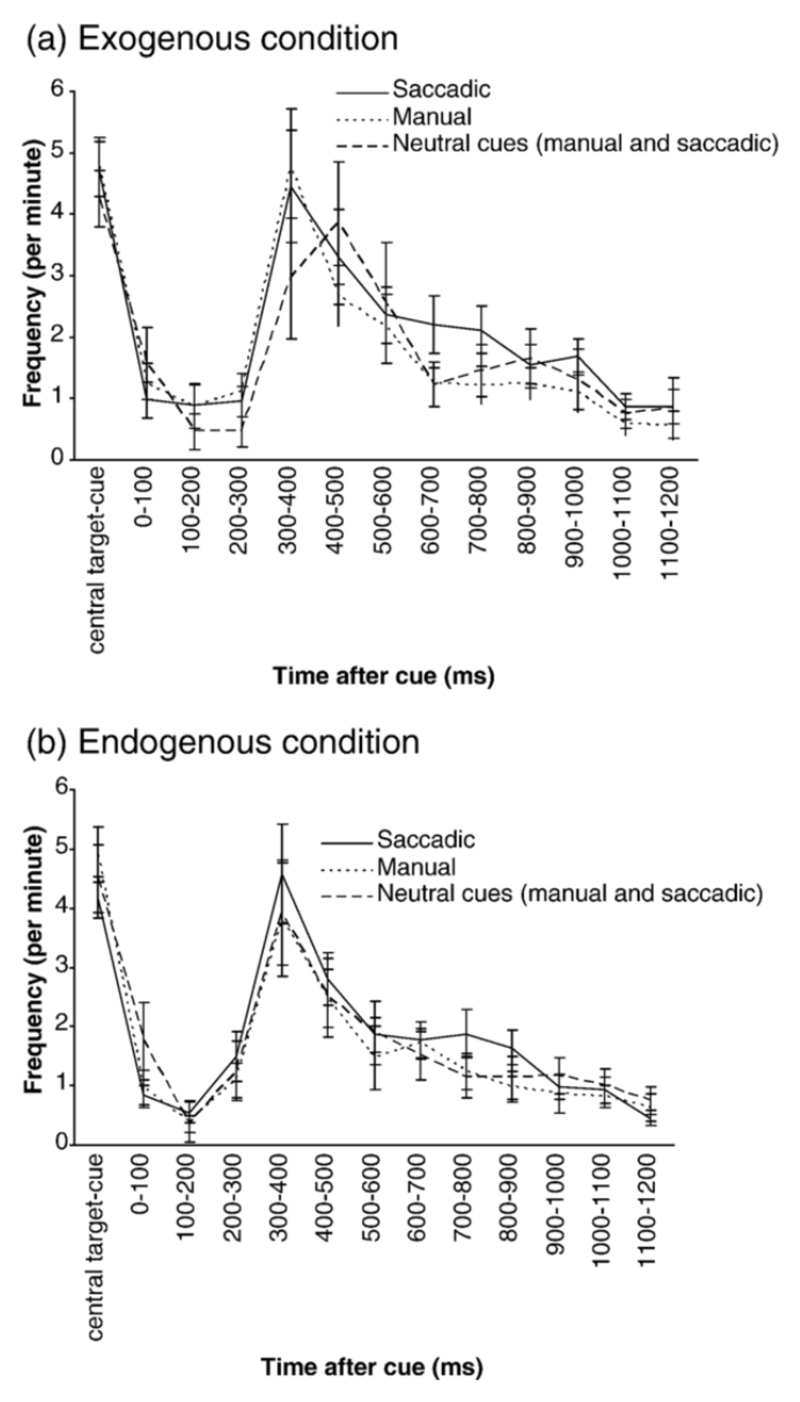
SI frequency over the different time windows during the cue-target interval for the exogenous (a) and endogenous (b) conditions. Central target-cue = average SI frequency over the preceding trial period (central target illumination-cue). Manual trials are denoted by the continuous line, saccadic trials by the dotted line and combined manual and saccadic neutral trials by the dashed line. The CC-cue interval represents the average frequency during any 100-ms window during the central cue-cue interval. Standard error bars are shown.
Presentation of fewer neutral trials led to insufficient numbers of SI to perform the above analysis so the averages across each time window during the cue-target period were collapsed for all subjects and submitted to a 2×2×2 repeated measures ANOVA with factors of cue type (neutral or informative cue) attention condition (exogenous or endogenous) and response type (manual or saccadic). There was a significant effect of cue type (F(1, 11) = 12.76, p =0.004) but no significant effect of attention (F(1, 11) = 2.09, p =0.18) or response mode (F(1, 11) = 3.66, p =0.08) and no interactions between cue type and attention (F(1, 11) = 0.49, p =0.5), cue type and response type (F(1, 11) = 0.02, p =0.89) or attention and response type (F(1, 11) = 0.02, p =0.9). It can be observed in Fig 6a-b that although SI amplitude modulation across the different time periods appears similar in both neutral and informative cue trials, SI amplitude in neutral trials was significantly smaller.
Fig. 6.
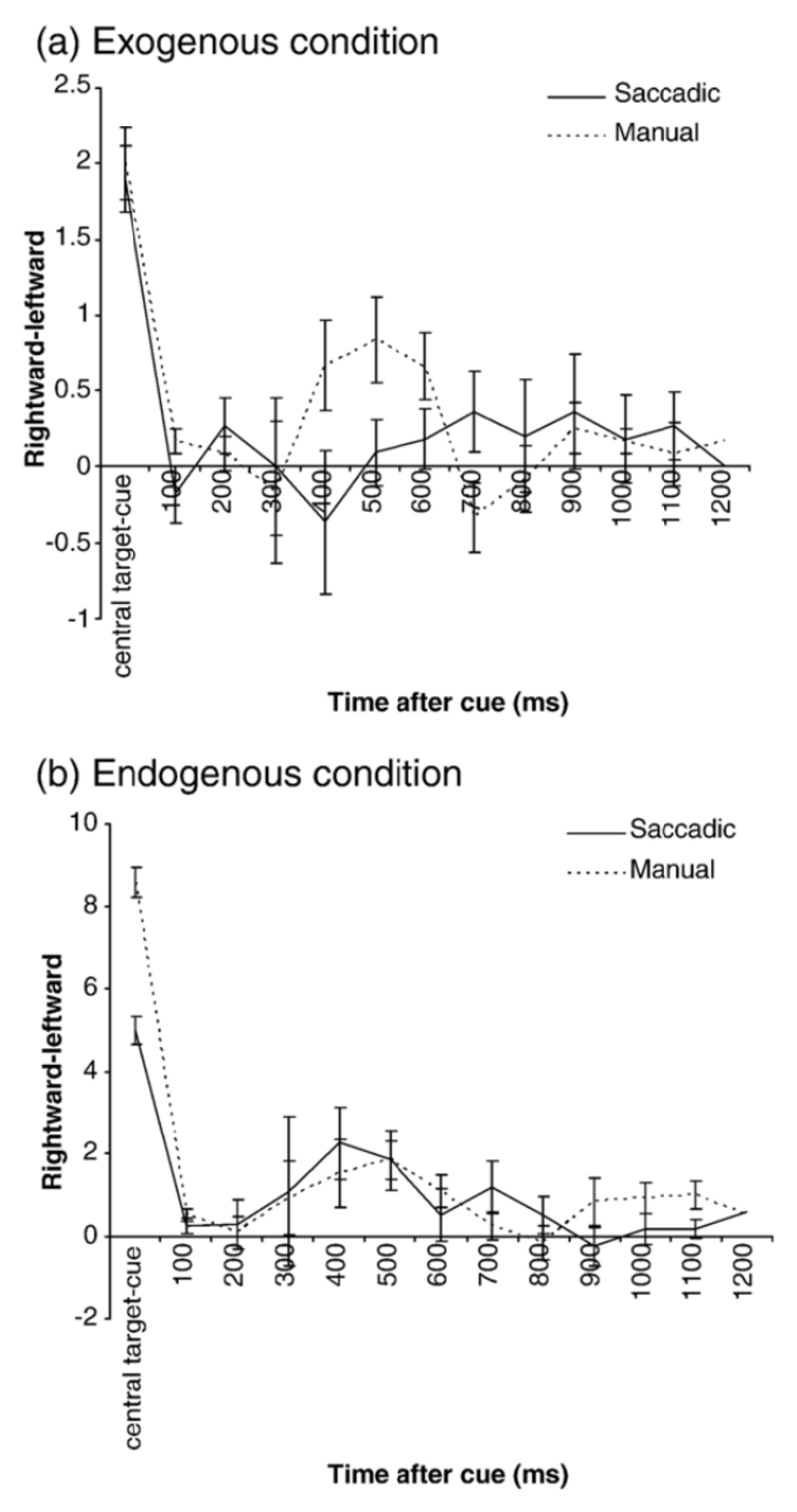
The number of rightward SI subtracted from the number of leftward SI over the different time windows following a neutral cue for the exogenous (a) and endogenous (b) conditions. Positive values indicate a larger number of rightward SI, negative values indicate a larger number of leftward SI. Central target-cue = average SI direction over the preceding trial period (central target illumination-cue). Manual trials are denoted by the continuous line, and saccadic trials by the dotted line. Standard error bars are shown.
SI frequency
SI frequency changes over the different time windows can be seen in Figures 5a-b. Friedman testing revealed that SI frequency differences were highly significant across the twelve time windows, for all task conditions (χ2≥44.07, p≤0.000). There was no significant difference between exogenous or endogenous conditions (z=-1.39, p=0.17), but the frequency was significantly higher for saccadic (1.75 per min) compared to manual (1.47 per min) responses (z=-3.49, p<0.000). In the neutral cue conditions, there was a significant effect of time for all conditions (χ2≥23.77, p≤0.01) but there was no difference between exogenous or endogenous conditions (z=-0.35, p=0.73) or between saccadic and manual response mode (z=-0.86, p=0.39).
Fig. 5.
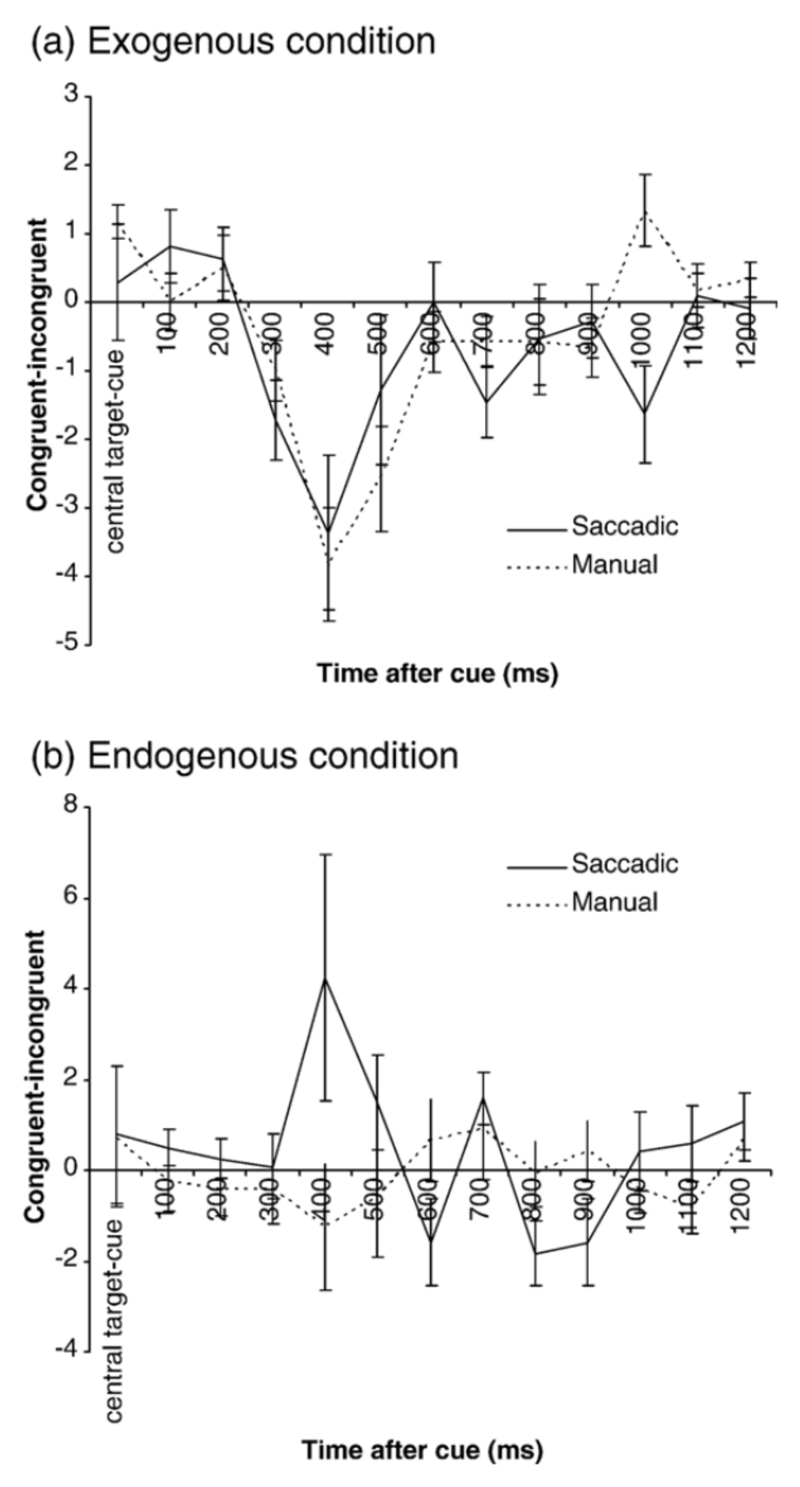
The number of congruent SI subtracted from the number of incongruent SI over the different time windows during the cue-target interval for the exogenous (a) and endogenous (b) conditions. Positive values indicate a larger number of congruent SI, negative values indicate a larger number of incongruent SI. Central target-cue = average SI direction over the preceding trial period (central target illumination-cue). Manual trials are denoted by the continuous line, and saccadic trials by the dotted line. Standard error bars are shown.
In summary for both attention conditions, response modes and all cue types, SI frequency tended to decrease to a minimum following the cue onset, then rise to reach a peak at approximately 300-400ms post cue before slowly returning to minimum levels by 1200ms after the cue. SI frequency during saccadic response trials was higher than manual trials. During neutral cue trials, SI frequency followed a similar pattern for all attention conditions and response modes, although there was a non-significant tendency for frequency to be lower than directional cues and a trends for the pattern to occur later during the exogenous condition.
SI direction
In order to statistically examine how trial conditions affected SI direction, the number of SI directed towards the cue (Same) were subtracted from the number of SI in the opposite direction to the cue (Different) (Laubrock, Engbert and Kliegl, 2004). Zero indicates that an equal number of SI were directed towards and away from the cue, positive values indicate a higher frequency of SI directed towards the cue and negative values indicate a higher frequency of SI directed away from the cue (Figures 6a-b). These bias values were submitted to a repeated measures ANOVA with factors of time, response mode (manual/saccadic) and attention condition (exogenous/endogenous). This revealed a main effect of attention condition (F(1,11) =5.45, p =0.04), but no main effect of time (F(11,121) = 1.41, p =0.25) or response mode (F(1,11) = 0.61, p =0.45). There was a significant interaction between attention condition and time (F(11,121) = 3.35, p =0.03) and response mode and time (F(11,121) =2.93, p =0.02) and a significant three-way interaction between attention condition, response mode and time (F(11,121) =2.62, p =0.05). No significant interaction was observed between response mode and attention condition (F(1,11) = 2.0, p =0.19).
Follow up paired t tests (corrected α=0.01) indicated that the attention×time interaction was due to a greater number of congruent SI in the endogenous compared to the exogenous condition 400ms (t=-2.66, p=0.01) and 700 ms (t=-4.21, p=0.00) following the cue. The response×time interaction was due to more incongruent SI in the manual response condition 400ms following the cue which was not significant using the adjusted α (t=-2.27, p=0.03). The three way interaction was analysed for exogenous and endogenous conditions using 2 separate ANOVA’s with factors of response mode and time interval. Both conditions showed an interaction between response mode and time interval (F(11,121) ≥2.14, p≤0.02). For the exogenous condition there were more incongruent SI during the saccadic response mode 1000ms following the cue (t=-3.48, p=0.005). However, for the endogenous condition there were more congruent SI during the saccadic condition 400 and 800ms following the cue. These results are shown in Figures 6a-b. In short, during exogenous conditions, approximately 400ms following the cue, SI tended to be directed away from the cue whereas during endogenous conditions SI direction remained stable (manual condition) or were directed towards the cue (saccadic condition).
A second repeated measures ANOVA with factors of time, response mode (manual/saccadic) and attention condition (exogenous/endogenous) compared the number of SI directed rightward with those directed leftward for neutral cues. This revealed no significant effect of attention condition (F(1,9) =3.27, p =0.1), or an interaction between attention condition and time (F(11,99) =0.47, p =0.92), indicating that SI direction was only biased differently in the exogenous and endogenous conditions when a directional cue was presented (Figures 7a-b). Figures 7a-b also show that following the neutral cue the usual rightward SI bias is reduced
Fig. 7.
Time course of exogenous (left figure) and endogenous (right figure) tasks. The different trial periods in which the SI were analysed are described to the left.
Discussion
We have examined the effect of exogenous and endogenous attention orienting on SI through the use of a cue-target paradigm and our work provides three main findings: (1) SI characteristics are influenced by both exogenous and endogenous attention orienting (2) SI characteristics are dependent on the response mode and (3) SI responses to exogenous and endogenous cues resemble previously published microsaccade behaviour.
The influence of exogenous and endogenous attention on SI characteristics
A similar pattern in the modulation of SI amplitude and frequency across a ~5 second trial occurred regardless of attention condition or response mode. SI amplitude was largest during the before trial and trial-end periods and SI frequency lowest during the cue-target interval. These results can be explained by the requirement to maintain the eyes at a central location while having to respond to the peripheral cue and are in agreement with Gowen, Abadi and Poliokoff (2005) who found that increased endogenous attention led to a central fixation target leads to decreased SI frequency and amplitude. The observation that SI frequency was lower for neutral cues than directional cues (for both attention conditions and response modes), particularly during the target-end interval, suggests that the prior modulation by a directional cue affects post response SI. Perhaps the presence of two peripheral cues during the exogenous neutral cue condition and the absence of expectation during the endogenous neutral cue task provoke greater suppression of SI. The effect of two peripheral cues on SI frequency is explored in more detail later.
The time course of the modulations in SI frequency and direction during the cue-target interval suggests that SI are influenced by both exogenous and endogenous attention. Early exogenous orienting to the cue onset (in both attentional tasks) may have caused the initial frequency decrease which is most likely an automatic response to stimulus change, rather than attention shifts as this was observed with both peripheral and central cues and directional and neutral cues. Indeed, irrelevant visual stimuli produce similar decreases in microsaccade frequency (Engbert and Kliegl, 2003) and in larger voluntary saccades (Reingold and Stampe, 2002, 2004). Unlike voluntary saccades where exogenous facilitation occurs during the first 100ms post cue, SI did not appear to be preferentially directed towards the cue. This has also been observed with microsaccades (Rolfs et al., 2004; Galfano, Betta and Turatto, 2004) and may be due to the small amount of data collected during this low frequency period or the requirement to ignore the cue and maintain fixation. The time when SI were at their maximum frequency corresponded with a change in SI direction where SI were predominantly directed away from the cue during the exogenous task or towards the cue during the endogenous saccadic task, resembling IOR (Klein, 2000) and endogenous facilitation (Muller and Rabbitt, 1989) respectively. This increase in SI frequency during the change in SI direction suggests that shifting attention (toward the cued location in the endogenous task and away from the cued location in the exogenous task) leads to a release of SI and that the processes controlling spatial attention and fixation are similar. However, the time (approximately 600ms) at which SI frequency and direction for all conditions reach equilibrium, is much earlier than IOR or facilitation usually disappears (Muller and Rabbitt, 1989; Posner and Cohen, 1984; Tassinari et al., 1987). This suggests that at this point, voluntary attentional mechanisms that are separate to IOR or endogenous facilitation and perhaps due the fixation demands of the task prevent further influence of IOR or facilitation on SI. On the other hand, processing demands related to IOR and endogenous attention may diminish, enabling greater resources to be available for fixation control. Alternatively, SI may be more sensitive to shifts in attention rather than maintaining attention at one location.
The relationship between attention and eye movements: What can SI contribute?
Our findings indicate that the degree to which attention and oculomotor control overlap during a particular task may also be apparent in changes SI behaviour. Across exogenous and endogenous conditions SI amplitude and frequency was higher for the saccadic response mode during the cue-target interval, suggesting that saccade planning may influence SI frequency. SI frequency was higher for manual responses during the target appearance-end period, indicating that either attention may have been diverted from maintaining steady fixation to responding in a different modality or that making a saccade allowed less opportunity for SI to occur. A further difference between the response modes occurred during endogenous conditions, when SI direction was biased towards the cue in the saccadic but not manual trials. Reaction times were shorter in the valid cue conditions compared to the invalid type for both manual and saccadic response trials indicating that subjects were using the cues appropriately. We cannot discount that spatial preparation of the saccadic response may have had greater influence on SI direction than non-spatially dependent manual responses (Simon, 1990). However, even if this were to be the case, our findings still suggest a dissociation between attention and saccade processing.
This dissociation between the response modes did not occur for the exogenous condition when SI were affected equally by IOR. Interestingly, generation of IOR requires activation of oculomotor systems regardless of response mode (Ro, Farnè and Chang, 2003) which is substantiated by the fact that IOR during manual endogenous conditions only occurs if a saccade to the cue has been prepared or executed (Rafal et al., 1989) and that IOR is influenced by a saccadic response being made to the cue or target (Taylor and Klein, 2000). This suggests that SI direction was not affected during the endogenous manual condition because oculomotor planning did not occur. Therefore our findings indicate that under conditions that demand an endogenous shift of attention without a saccade (i.e. a covert attention task), minimal activation of the oculomotor system us required and that this may manifest as a decoupling of SI direction with attention orienting. This is in keeping with studies highlighting that task conditions may determine how closely attention and saccade planning are associated (Abrams and Pratt, 2000; Briand, Larrison and Sereno, 2000; Fischer, 1999; Hunt and Kingstone, 2003; Ignashchenkova et al., 2005; Sato and Schall, 2003; Sumner et al., 2004; Taylor and Klein, 2000; Thompson et al., 2005).
Neural substrate of SI
Our results support the contention that SI may arise from the interaction between bottom up and top down influences at the level of SC. SI are affected by the presence of IOR, a response which is considered to be in part generated at the level of the SC (Abrams and Dobkins, 1994; Posner et al., 1985; Sapir et al., 1999). Reaction times of voluntary saccades correlate strongly with target-related activity within the SC, suggesting that the activity of these neurons influences the threshold of saccade initiation (Bell, Fecteau and Munoz, 2004; Dorris and Munoz, 1998; Fecteau, Bell and Munoz, 2004). Our data also show that SI dynamics during the cue-target task resemble those of larger voluntary saccades indicating that they may also be influenced by SC cell activity. Both voluntary saccades and, SI are affected by IOR and exhibit similar post cue inhibition effects that have been attributed to increased SC rostral build up cell activity (Levy-Schoen, 1969; Reingold and Stampe, 2002, 2004; Walker et al., 1997). Moreover, SI displayed greater attenuation for exogenous neutral cues (illumination of both peripheral boxes) than exogenous directional cues (illumination of one peripheral box), a trend that was not observed for central endogenous cues. This is reminiscent of the remote distracter effect where two targets that are presented simultaneously in different hemi fields delay reaction times (Levy-Schoen, 1969; Walker et al., 1997) and is thought to be due to increased SC rostral build up cell activity produced by the two targets Finally, as SI characteristics are influenced by both exogenous and endogenous inputs, this supports the view that they arise from an area that receives input from both these systems. Moreover the SC receives projections from frontal and parietal areas (Sparks and Hartwich-Young, 1989) that process both exogenous and endogenous signals (Chambers and Mattingley, 2005; Corbetta and Shulman, 2002; Grosbras, Laird and Paus, 2005; Mayer et al., 2004) and activity relating to attention has been observed within the SC (Cavanaugh and Wurtz, 2004; Ignashchenkova et al., 2005).
We therefore propose that exogenous bottom up and endogenous top down projections may have a direct influence on the coupling between the rostral and caudal build-up cells, thus producing SI whose characteristics are dependent on the locus and level of activity within these circuits. If the rostral build up cells are more active and the focus of activity tightly constrained around the area representing the foveal region, SI will be of a smaller amplitude and lower frequency (as during the cue-target interval when steady fixation was important). Following an exogenous cue, increased activity may occur in build up cells opposite to the original cue-elicited activity, resulting in a greater number of SI being directed away from the cue. In response to an endogenous cue, activity may favour the rostral build up cells which encode the direction of the endogenous cue, causing SI to also be predominantly directed towards the cue. Importantly, our tasks involved fixation, so any fluctuation in rostral build up cell activity would be expected to remain tightly focussed around the foveal zone, producing SI in the amplitude range observed.
The relationship between Microsaccades and SI
The observed changes in SI frequency and direction in response to exogenous and endogenous cues resemble those reported for microsaccades (Engbert and Kliegl, 2003; Galfano, Betta and Turatto, 2004; Hafed and Clarke, 2002 Laubrock, Engbert and Kliegl, 2004; Rolfs, Engbert and Kliegl, 2004). There is also suggestion that a similar dissociation in SI direction between saccadic and manual endogenous orienting is apparent in microsaccade direction (Engbert and Kliegl, 2003; Laubrock, Engbert and Kliegl, 2004), although a direct comparison between the two tasks has not been performed. These similarities together with previous findings that they are both of an involuntary nature, are conjugate and display a decreased frequency when attention levels are raised (Abadi and Gowen, 2004; Barlow, 1952; Kowler and Steinman, 1980; Møller et al., 2002; Steinman, 1965; Steinman et al., 1967; see Martinez-Conde, Macknik and Hubel, 2004 for a review) support the conjecture that the two saccadic behaviours are related and perhaps share common final pathways. However, there remain some differences between SI and microsaccades.
Firstly, the microsaccade amplitude (~0.15º) (Ditchburn, 1980; Steinman, Cushman and Martins, 1982; Carpenter, 1988a; Kowler, 1991) is smaller than SI amplitude (~0.6 º) (Abadi and Gowen, 2004). Some studies have included microsaccades of up to 2º (Engbert and Kliegl, 2003; Hafed and Clarke, 2002; Laubrock, Engbert and Kliegl, 2004) which we consider to be in the range of SI. However, Hafed and Clarke (2002) noted that division of microsaccades into those above and below 0.5° did not affect the relationship between attention and microsaccades. This indicates that microsaccades and SI are closely related and that their amplitudes may differ according to the location of SC rostral build cell activity. Secondly, microsaccades are reported as having a mean frequency of 1-2 per second (Ditchburn, 1980; Engbert and Kliegl, 2003; Steinman, Cushman and Martins, 1982; Carpenter, 1988a; Kowler, 1991) which is well above the mean SI frequency of 18 per minute (Abadi and Gowen, 2004). However, as the task is to fixate centrally, one would expect larger SI to be less frequent compared to smaller microsaccades. Thirdly, microsaccades are multiplanar whereas SI are predominantly recorded in the horizontal plane (Gowen and Abadi, 2004). This could be attributed to the coordinates of the SC saccadic motor map where contralateral upward saccades are represented medially and downward saccades laterally (Robinson, 1972). The separation between cells encoding horizontal and those encoding vertical saccades may be smaller for more rostral sites within the build up cells. This would result in stimulation of a number of cells that may encode different directions and result in a spatially averaged microsaccade (Findlay, 1982). Indeed, SC buildup cell activity for a 1º saccade exhibits a certain degree of spread around the focus (Munoz and Wurtz, 1995b). Therefore, as fixational eye movements increase in size there is more likelihood of a uniplanar movement due to less spatial averaging. Fourthly, the waveform characteristics of microsaccades and SI also differ in that microsaccades consist of a single saccade, whereas SI tend to feature a return saccade or drift (Abadi and Gowen, 2004). As visual resolution decreases when the retinal image leaves the central 0.3° of the fovea, (Steinman et al., 1973; Steinman, Cushman and Martins, 1982; Westheimer and McKee, 1975) and microsaccade amplitude does not frequently exceed this area, error detection and correction may occur more frequently for SI than microsaccades. Indeed, Hafed and Clarke observed “coupled” microsaccades in the amplitude range of SI and coupling of microsaccades has been reported when their amplitude is larger (Ditchburn and Ginsborg, 1953).
Therefore, we propose that microsaccades and SI lie on continuum of fixational instabilities that owe their differences to the properties of the SC fixation cells and the nature of top down and bottom up inputs. In order to further strengthen this argument it remains to be seen whether microsaccade and SI frequency co vary in a dependent manner and whether there is a correlation between size and coupling. In support of the latter, we note from our work that saccadic instabilities of the single sided variety are rarely seen in the amplitude range of SI.
Conclusions
In conclusion, SI characteristics are affected by changes in the level and orientation of exogenous and endogenous attention. Although these observations are in keeping with a close link between eye movements and attention we also found that attention had less influence on SI under conditions where eye movements were not required, suggesting a degree of flexibility between the two systems. The similarity with previously reported microsaccade characteristics suggests that SI and microsaccades are tightly related and perhaps lie on a continuum. Finally, we believe that exploration of SI may provide a useful tool in examining the relationship between eye movements and attention and that the use of attentional paradigms may assist in distinguishing those SI that are of a physiological origin from the those that may represent pathology.
Acknowledgments
We would like to thank Andrew Jerrison for his programming advice. This work was supported by James McDonnell Foundation and the Wellcome Trust.
References
- Abadi RV, Gowen E. Characteristics of saccadic intrusions. Vision Res. 2004;44:2675–2690. doi: 10.1016/j.visres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Abrams R, Dobkin RS. The gap effect and inhibition of return: interactive effects on eye movement latencies. Exp Brain Res. 1994;98:483–487. doi: 10.1007/BF00233985. [DOI] [PubMed] [Google Scholar]
- Abrams RA, Pratt J. Oculocentric coding of inhibited eye movements to recently attended locations. J Exp Psychol Hum Percept Perform. 2000;26:776–788. doi: 10.1037//0096-1523.26.2.776. [DOI] [PubMed] [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 2006;10:124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Eye movements during fixation. J Phsyiol. 1952;116:290–306. doi: 10.1113/jphysiol.1952.sp004706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AH, Fecteau JH, Munoz DP. Using auditory and visual stimuli to investigate the behavioral and neuronal consequences of reflexive covert orienting. J Neurophysiol. 2004;91:2172–2184. doi: 10.1152/jn.01080.2003. [DOI] [PubMed] [Google Scholar]
- Berger A, Henik A, Rafal R. Competition between endogenous and exogenous orienting of visual attention. J Exp Psychol Gen. 2005;134:207–221. doi: 10.1037/0096-3445.134.2.207. [DOI] [PubMed] [Google Scholar]
- Briand KA, Larrison AL, Sereno AB. Inhibition of return in manual and saccadic response systems. Percept and Psychophys. 2000;62:1512–1524. doi: 10.3758/bf03212152. [DOI] [PubMed] [Google Scholar]
- Carpenter RHS. Chapter 6 Miniature eye movements. In: Carpenter RHS, editor. Movements of the eyes. Pion Press; London: 1988. pp. 124–138. [Google Scholar]
- Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Mattingley JB. Neurodisruption of selective attention: insights and implications. Trends Cogn Sci. 2005;9:542–550. doi: 10.1016/j.tics.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci U S A. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Danziger S, Fendrich R, Rafal RD. Inhibitory tagging of locations in the blind field of hemianopic patients. Conscious Cogn. 1997;6:291–307. [PubMed] [Google Scholar]
- Deubel H. Separate adaptive mechanisms for the control of reactive and volitional saccadic eye movements. Vision Res. 1995;35:3529–3540. doi: 10.1016/0042-6989(95)00058-m. [DOI] [PubMed] [Google Scholar]
- Ditchburn W. Eye movements and visual perception. Clarendon Press; Oxford: 1973. [Google Scholar]
- Ditchburn W, Ginsborg BL. Involuntary eye movements during fixation. J Physiol. 1953;119:1–17. doi: 10.1113/jphysiol.1953.sp004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci. 1998;18:7015–7026. doi: 10.1523/JNEUROSCI.18-17-07015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Klein RM, Everling S, Munoz DP. Contribution of the primate superior colliculus to inhibition of return. J Cogn Neurosci. 2002;14:1256–1263. doi: 10.1162/089892902760807249. [DOI] [PubMed] [Google Scholar]
- Engbert R, Kliegl R. Microsaccades uncover the orientation of covert attention. Vision Res. 2003;43:1035–1045. doi: 10.1016/s0042-6989(03)00084-1. [DOI] [PubMed] [Google Scholar]
- Engbert R, Kliegl R. Microsaccades keep the eyes' balance during fixation. Psychol Sci. 2004;15:431–436. doi: 10.1111/j.0956-7976.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Bell AH, Munoz DP. Neural correlates of the automatic and goal-driven biases in orienting spatial attention. J Neurophysiol. 2004;92:1728–1737. doi: 10.1152/jn.00184.2004. [DOI] [PubMed] [Google Scholar]
- Findlay JM. Global visual processing for saccadic eye movements. Vision Res. 1982;22:1033–1045. doi: 10.1016/0042-6989(82)90040-2. [DOI] [PubMed] [Google Scholar]
- Fischer MH. An investigation of attention allocation during sequential eye movement tasks. Q J Exp Psychol A. 1999;52:649–677. doi: 10.1080/713755838. [DOI] [PubMed] [Google Scholar]
- Galfano G, Betta E, Turatto M. Inhibition of return in microsaccades. Exp Brain Res. 2004 doi: 10.1007/s00221-004-2111-y. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Keller EL. Comparison of saccades perturbed by stimulation of the rostral superior colliculus, the caudal superior colliculus and the omnipause neuron region. J Neurophysiol. 1999;82:3236–3253. doi: 10.1152/jn.1999.82.6.3236. [DOI] [PubMed] [Google Scholar]
- Gowen E, Abadi RV. Saccadic instabilites and voluntary saccadic behaviour. Exp Brain Res. 2005;164:29–40. doi: 10.1007/s00221-004-2209-2. [DOI] [PubMed] [Google Scholar]
- Gowen E, Abadi RV, Poliakoff E. Paying attention to saccadic intrusions. Cognitive Brain Res. 2005;25(3):810–825. doi: 10.1016/j.cogbrainres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Laird AR, Paus T. Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum Brain Mapp. 2005;25:140–154. doi: 10.1002/hbm.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Clark JJ. Microsaccades as an overt measure of covert attention shifts. Vision Res. 2002;42:2533–2545. doi: 10.1016/s0042-6989(02)00263-8. [DOI] [PubMed] [Google Scholar]
- Hunt AR, Kingstone A. Inhibition of return: Dissociating attentional and oculomotor components. J Exp Psychol Hum Percept Perform. 2003;29:1068–1074. doi: 10.1037/0096-1523.29.5.1068. [DOI] [PubMed] [Google Scholar]
- Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci. 2004;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM. Does oculomtor readiness mediate cognitive control of visual attention? In: Nickerson R, editor. Attention and Performance VIII. Hillsdale, N.J: Erlbaum; 1980. [Google Scholar]
- Klein RM. Inhibition of return. Trends Cogn Sci. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Klein RM, Shore DI. Chapter 8 Relations among modes of visual orienting. In: Monsell S, Driver J, editors. Control of Cognitive Processes. Attention and Performance XV111. The MIT Press; Cambridge, Massachusetts, London, England: 2000. pp. 195–208. [Google Scholar]
- Kowler E. The stability of gaze and its implications for vision. In: Carpenter RHS, editor. Vision and Visual Dysfunction. Eye movements. Vol. 8. 1991. pp. 71–92. [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Kowler E, Steinman RM. Small Saccades serve no useful purpose: Reply to a letter by R.W Ditchburn. Vision Res. 1980;20:273–276. doi: 10.1016/0042-6989(80)90113-3. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH. Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol. 2000;84:876–891. doi: 10.1152/jn.2000.84.2.876. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- Laubrock J, Engbert R, Kliegl R. Microsaccade dynamics during covert attention. Vision Res. 2005;45:721–730. doi: 10.1016/j.visres.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Levy-Schoen A. Determination et latence de la response oculomotrice a deux stimulus. L’Anne Psychology. 1969;74:43–66. [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. The role of fixational eye movements in visual perception. Nat Rev Neurosci. 2004;5:229–240. doi: 10.1038/nrn1348. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Dorflinger JM, Rao SM, Seidenberg M. Neural networks underlying endogenous and exogenous visual-spatial orienting. Neuroimage. 2004;23:534–541. doi: 10.1016/j.neuroimage.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Moller F, Laursen ML, Tygesen J, Sjolie AK. Binocular quantification and characterization of microsaccades. Graefes Arch Clin Exp Ophthalmol. 2002;240:765–770. doi: 10.1007/s00417-002-0519-2. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci U S A. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Perry RJ, Mannan SK, Hodgson TL, Anderson E, Quest R, McRobbie D, McBride A, Husain M, Kennard C. Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage. 2003;18:231–246. doi: 10.1016/s1053-8119(02)00028-9. [DOI] [PubMed] [Google Scholar]
- Muller HJ, Rabbitt PM. Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. J Exp Psychol Hum Percept Perform. 1989;15:315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol. 1998;79:1193–1209. doi: 10.1152/jn.1998.79.3.1193. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Role of the rostral superior colliculus in active visual fixation and execution of express saccades. J of Neurophysiol. 1992;67:1000–1002. doi: 10.1152/jn.1992.67.4.1000. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus 1. characteristics of cell discharge. Journal of Neurophysiol. 1993a;70:559–575. doi: 10.1152/jn.1993.70.2.559. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus II. Reversible activation and deactivation. Journal of Neurophysiol. 1993b;70:576–589. doi: 10.1152/jn.1993.70.2.576. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus I. Characteristics of burst and buildup cells. Journal of Neurophysiol. 1995a;73:2313–2333. doi: 10.1152/jn.1995.73.6.2313. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus II. Spread of activity during saccades. Journal of Neurophysiol. 1995b;73:2334–2348. doi: 10.1152/jn.1995.73.6.2334. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Heslenfeld DJ, Theeuwes J. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. Neuroimage. 2004;22:822–830. doi: 10.1016/j.neuroimage.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J of Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis D, editors. Attention and Performance. London; 1984. pp. 531–556. [Google Scholar]
- Posner MI, Rafal R, Choate LS, Vaughan J. Inhibition of return: Neural basis and function. Cogn Neuropsychol. 1985;2:211–228. [Google Scholar]
- Posner MI, Snyder CRR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Rafal R, Calabresi PA, Brennan CW, Sciolto TK. Saccade preparation inhibits reorienting to recently attended locations. J Exp Psychol Hum Percep Perform. 1989;15(4):673–685. doi: 10.1037//0096-1523.15.4.673. [DOI] [PubMed] [Google Scholar]
- Rafal R, Henik A. The neurology of inhibition integrating controlled and automatic processes. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory and language. Academic Press; San Diego: 1994. pp. 1–51. [Google Scholar]
- Ratliff F, Riggs LA. Involuntary motions of the eye during monocular fixation. J Exp psychol. 1950;40:687–701. doi: 10.1037/h0057754. [DOI] [PubMed] [Google Scholar]
- Reingold EM, Stampe DM. Saccadic inhibition in voluntary and reflexive saccades. J Cogn Neurosci. 2002;14:371–388. doi: 10.1162/089892902317361903. [DOI] [PubMed] [Google Scholar]
- Reingold EM, Stampe DM. Saccadic inhibition in reading. J Exp Psychol Hum Percept Perform. 2004;30:194–211. doi: 10.1037/0096-1523.30.1.194. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Drascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians:Evidence in favor or a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Ro T, Farne A, Chang E. Inhibition of return and the human frontal eye fields. Exp Brain Res. 2003;150:290–296. doi: 10.1007/s00221-003-1470-0. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Engbert R, Kliegl R. Microsaccade orientation supports attentional enhancement opposite a peripheral cue: commentary on Tse, Sheinberg, and Logothetis 2003. Psychol Sci. 2004;15:705–707. doi: 10.1111/j.0956-7976.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- Sapir A, Soroker N, Berger A, Henik A. Inhibition of return in spatial attention: direct evidence for collicular generation. Nat Neurosci. 1999;2:1053–1054. doi: 10.1038/15977. [DOI] [PubMed] [Google Scholar]
- Sato TR, Schall JD. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron. 2003;38:637–648. doi: 10.1016/s0896-6273(03)00237-x. [DOI] [PubMed] [Google Scholar]
- Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Res. 2004;44:1453–1467. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Shepherd M, Findlay JM, Hockey R. The relationship between eye movements and spatial attention. Q J Exp Psychol A. 1986;38:475–491. doi: 10.1080/14640748608401609. [DOI] [PubMed] [Google Scholar]
- Simon JR. The effects of an irrelevant directional cue on human information processing. In: Proctor RW, Reeve TG, editors. Stimulus Response Compatibility: An integrated perspective Adv Psychol. Vol. 65. North Holland, Amsterdam: 1990. pp. 31–86. [Google Scholar]
- Smith DT, Rorden C, Jackson SR. Exogenous Orienting of Attention Depends upon the Ability to Execute Eye Movements. Curr Biol. 2004;14:792–795. doi: 10.1016/j.cub.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hartwich – Young R. The deep layers of the superior colliculus. In: Wurtz RH, Goldberg ME, editors. Research in Eye movements. The Neurobiology of Saccadic Eye Movements. Elsevier Science Publishers BV; 1989. pp. 213–255. [Google Scholar]
- Steinman RM, Haddad GM, Skavenski AA, Wyman D. Minature eye movements. Science. 1973;181:810–819. doi: 10.1126/science.181.4102.810. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Cunitz RJ, Timberlake GT, Herman M. Voluntary control of microsaccades during maintained monocular fixation. Science. 1967;155:1577–1579. doi: 10.1126/science.155.3769.1577. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Cushman WB, Martins AJ. The precision of gaze. Hum Neurobiol. 1982;1:97–109. [PubMed] [Google Scholar]
- Sumner P, Nachev P, Vora N, Husain M, Kennard C. Distinct cortical and collicular mechanisms of inhibition of return revealed with s cone stimuli. Curr Biol. 2004;14:2259–2263. doi: 10.1016/j.cub.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Tassinari G, Aglioti L, Chelazzi L, Marzi CA, Belucchi G. Distribution in the visual field of the costs of voluntarily allocated attention and of the inhibitory after-effects of covert orienting. Neuropsychologia. 1987;25:55–71. doi: 10.1016/0028-3932(87)90043-1. [DOI] [PubMed] [Google Scholar]
- Taylor TL, Klein RM. Visual and motor effects in inhibition of return. J Exp Psychol Hum Percept Perform. 2000;26:1639–1656. doi: 10.1037//0096-1523.26.5.1639. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Kramer AF, Hahn S, Irwin DE. Our eyes do not always go where we want them to go: capture of the eyes by new objects. Psychol Science. 1998;9:379–385. [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper C, Kingstone A. Is inhibition of return a reflexive effect? Cognition. 2005;97:B55–B62. doi: 10.1016/j.cognition.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Tse PU, Sheinberg DL, Logothetis NK. Fixational eye movements are not affected by abrupt onsets that capture attention. Vision Res. 2002;42:1663–1669. doi: 10.1016/s0042-6989(02)00076-7. [DOI] [PubMed] [Google Scholar]
- Van der Lubbe RH, Neggers SF, Verleger R, Kenemans JL. Spatiotemporal overlap between brain activation related to saccade preparation and attentional orienting. Brain Res. 2006;1072:133–152. doi: 10.1016/j.brainres.2005.11.087. [DOI] [PubMed] [Google Scholar]
- Walker R, Deubel H, Schneider WX, Findlay JM. Effect of remote distractors on saccade programming: evidence for an extended fixation zone. J Neurophysiol. 1997;78:1108–1119. doi: 10.1152/jn.1997.78.2.1108. [DOI] [PubMed] [Google Scholar]
- Westheimer G, McKee SP. Visual acuity in the presence of retinal image motion. J Opt Soc Am. 1975;65:847–850. doi: 10.1364/josa.65.000847. [DOI] [PubMed] [Google Scholar]
- Winterson BJ, Collewijn H. Microsaccades during finely guided visuomotor tasks. Vision Res. 1976;16:1387–1390. doi: 10.1016/0042-6989(76)90156-5. [DOI] [PubMed] [Google Scholar]