Abstract
Duchenne muscular dystrophy (DMD) is the most severe childhood form of muscular dystrophy caused by mutations in the gene responsible for dystrophin production. There is no cure and treatment is limited to glucocorticoids that prolong ambulation and drugs to treat the cardiomyopathy. Multiple treatment strategies are under investigation and have shown promise for DMD. Molecular-based therapies that replace or correct the missing or non-functional dystrophin protein have gained momentum. These strategies include gene replacement with adeno-associated virus (AAV), exon skipping with antisense oligonucleotides, and mutation suppression with compounds that “read-through” stop codon mutations. Other strategies include cell therapy and surrogate gene products to compensate for the loss of dystrophin. All of these approaches are discussed in this review with particular emphasis on the most recent advances made in each therapeutic discipline. The advantages of each approach and challenges in translation are outlined in detail. Individually or in combination, all of these therapeutic strategies hold great promise for treatment of this devastating childhood disease.
Keywords: Duchenne Muscular Dystrophy (DMD), Gene Therapy, Exon Skipping, Stem Cell Therapy, Newborn screening
Introduction
Duchenne muscular dystrophy (DMD) is the most common, severe childhood form of muscular dystrophy resulting from mutations in the X-linked DMD gene. The most recent data compiled from worldwide newborn screening studies indicates that birth prevalence is about 1:5000 [1]**. Given the very large size of the DMD gene [2] spontaneous mutations lead to an unparalleled number of sporadic cases and new carriers. Thus, the disease will never be eradicated with genetic screening and mandates the need for an effective treatment. The disease is detected early in life due to early disease manifestations. Serum creatine kinase levels are elevated at birth, and motor and even speech milestones are often delayed. Reduced motor skills between ages 3 to 5 typically provoke diagnostic evaluation. Quality of life for DMD boys is affected early in life with inability to keep up with peers and loss of ambulation by age 12 [3]. Improved practices in protection of the respiratory system often unmask declines in cardiac function dictating a need for treatment of the dilated cardiomyopathy. DMD is relentlessly progressive and the medical need for treatment is undeniable. This review will summarize the growing body of strategies that are under investigation for treatment of DMD with particular emphasis on the most recent observations.
Gene Replacement
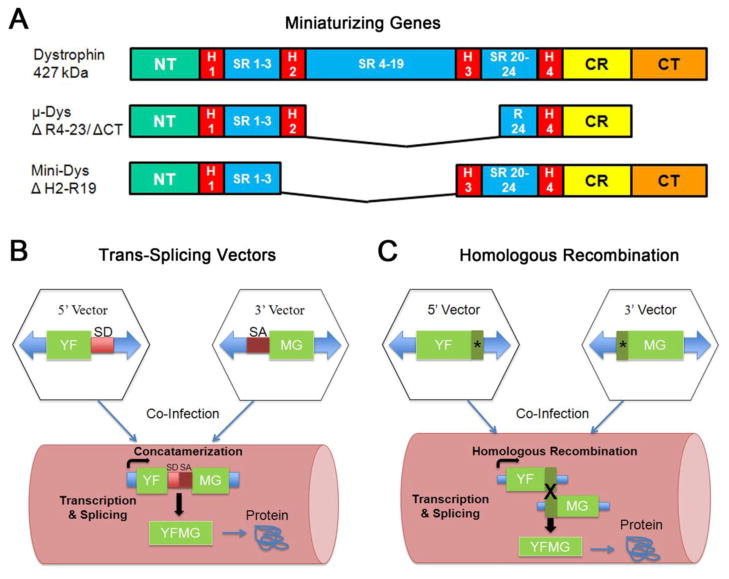
Multiple treatment strategies are under development for both the primary gene defect and secondary complications of DMD, however replacing the defective gene using adeno-associated virus (AAV) remains a promising approach for meaningful and long-term correction. AAV is non-pathogenic, remains stable in non-replicating cells (i.e. muscle) and multiple serotypes including AAV1,6,8,9 exhibit tropism for muscle. One caveat for AAV delivery as a treatment for DMD is the large size of the dystrophin gene which exceeds the packaging capacity of AAV (<5kb). Fortunately, there has been intense effort to develop an AAV based therapeutic which bypasses the packaging constraint. The first strategy was the development of mini and micro-dystrophins. The modular structure of dystrophin allows some functional flexibility; deletions of non-essential coding regions namely spectrin repeats and the C-terminus allow dystrophin to retain significant function if the reading frame is intact (Fig. 1A). This phenomena was initially based on a clinical observation in a BMD patient with a large in-frame deletion of exons 17–48 removing a significant portion of the rod domain [4]. The patient remained ambulatory until age 61 despite the absence of 46% of the dystrophin gene. This sparked investigation by numerous groups to establish the optimal dystrophin domains fulfilling two conditions: fitting the size limitations of AAV and demonstrating therapeutic efficacy [5, 6]. Based on these principles, mini-dystrophins were tested and showed reversal of the dystrophic phenotype and functional improvement in the mdx mouse model for DMD [7, 8]. These proof of principle studies provided the impetus for the first clinical trial with mini-dystrophin [9] **. This initial clinical trial for DMD defined potential obstacles regarding immune response to consider for future attempts to restore dystrophin using gene replacement. An important finding was the presentation of novel foreign epitopes resulting from delivery of a mini-dystrophin cDNA that expressed exogenous dystrophin in the region of the endogenous deletion. This experience has heightened awareness that not all mutations are necessarily amenable to gene replacement and patients must be carefully chosen as part of pre-screening for future gene therapy trials.
Figure 1. Current strategies to deliver larger dystrophin genes for DMD.
The full-length dystrophin cDNA (~14kb) precludes the use of AAV using standard methods. Proof of principle studies using three different methods have now demonstrated feasibility for dystrophin replacement. (A) Mini and micro (μ) versions of dystrophin which have deletions in spectrin repeat regions and/or the C-terminus of the protein have shown improvement in pre-clinical studies. (B) A trans-splicing approach is a dual vector strategy in which the transgene cassette is divided in two whereby a splice donor (SD) site is added to the 5′ vector and a splice acceptor (SA) site is added to the 3′ vector. Upon co-delivery to muscle the vectors concatamerize and undergo splicing to generate a full-length transcript. YFMG (your favorite muscle gene). (C) A second dual vector strategy employs an overlap sequence that is shared by both vectors (*). Following delivery to muscle, homologous recombination between the two vectors occurs to generate full-length transcript.
Additional strategies to deliver larger miniature dystrophin genes that exceed the packaging capacity of AAV are under investigation establishing proof of principle for translation to the clinic. This approach uses dual vectors delivered simultaneously allowing reconstitution of a larger functional dystrophin gene in vivo (Fig. 1). In one study, Odom and colleagues expressed a highly functional 6.2 kb mini-dystrophin gene containing the C-terminal signaling domain using this AAV-mediated homologous recombination strategy [10]* (Fig. 1B). The two transgene cassettes used in this experimental paradigm shared an overlap of 372 bp allowing the full-length minigene to be generated in vivo following homologous recombination. Functional studies demonstrated that the recombined mini-gene expressing in 41.7% of muscle fibers led to a greater improvement in peak force and protection from eccentric-contraction induced injury than a smaller micro-dystrophin gene fitting within AAV packaging limits and expressing in 72.6% of muscle fibers. These results emphasize the significance of including dystrophin functional domains such as the C-terminus to meaningfully improve muscle function. A second dual vector approach employs the use of synthetic intron splicing signals which lead to reconstitution of the gene following delivery to muscle (Fig. 1C). Using this trans-splicing strategy, the Duan laboratory developed a series of mini-dystrophin dual vectors and packaged them using AAV6 [11]. The mini-dystrophin vectors included the neuronal nitric oxide synthase (nNOS) binding site encoded by spectrin repeats 16 and 17. When activated by calcium, nNOS generates nitric oxide (NO), a critical regulator of muscle force [12]. Mislocalization of the muscle specific nNOS - nNOSμ - in DMD is thought to contribute to muscle weakening. Following intramuscular injection in mdx mice, the mini-dystrophin reconstituted through transplicing and restored a mini-dystrophin that included nNOSμ expression and its concomitant activity. Additional studies will be necessary to validate the functional significance of restoring nNOS activity in DMD muscle. Homologous recombination strategies have also been applied to gene delivery in limb girdle muscular dystrophy (LGMD) type 2B [13, 14] in pre-clinical studies. In addition, our group has demonstrated unexpected plasticity of the AAV5 serotype [15]. Without attempting to provide overlapped regions for homologous recombination, AAV5 spontaneously partially packaged two halves of a dysferlin transgene yielding virions with a region of homology that resulted in dual vectors expressing a full-length transgene. The future of gene replacement for muscular dystrophies resulting from mutations in large genes such as dystrophin and dysferlin will most likely incorporate the use of modified AAV strategies to maximize functional efficacy.
Exon Skipping
Exon skipping is a molecular treatment approach with considerable promise for a large portion of the DMD population, potentially applicable to 60–80% of DMD gene mutations, which cause frame-shifting. It is targeted at the pre-messenger RNA (mRNA) level allowing one or more exons to be omitted to restore the dystrophin reading frame. This is accomplished with antisense oligonucleotides (AONs) that are synthesized to hybridize in a complementary fashion to pre-mRNA resulting in splicing modification. Pre-clinical efficacy has been demonstrated in the mdx mouse, dystrophin/utrophin knock-out mouse, and CXMD dog [16–18]. Two phase I intramuscular safety trials were conducted in DMD patients targeting exon 51 using AONs with two different chemical backbones, 2-O -methyl RNA with a phosphorothioate backbone (2′ O-MePS, PRO051, Prosensa) and a phosphorodiamidate morpholino oligomer (PMO, eteplirsen, Sarepta Therapeutics) [19, 20]. Safety was demonstrated in both studies limited to intramuscular injections of the AON in single muscle groups. Phase I/II extension studies were performed with both AONs to assess efficacy and tolerability following systemic delivery. In the PRO051 trial, dose-related efficacy was achieved with evidence of new dystrophin expression in approximately 60–100% of muscle fibers in 10 of 12 patients and modest improvement in the 6-minute walk test [21]. In UK phase II open-label study with eteplirsen-treated patients confirmed the results of exon skipping with as many as 55% of muscle fibers expressing dystrophin post-treatment [22].
A USA phase IIb randomized, double-blind, placebo-controlled trials to assess multiple dose efficacy of eteplirsen has just been completed at Nationwide Children’s Hospital (Columbus, OH). After 6 months, dystrophin was expressed in 23 ± 6% of muscle fibers and the distance walked using the 6 minute walk test showed a change from baseline of 28 m compared to placebo. An open-label trial was extended for 1 year and 47% of muscle fibers expressed dystrophin compared to baseline and the distance on the 6MWT now improved 89m (p ≤ 0.016) compared to placebo [23]**. Two boys in the trial improved their walking distance by 65 and 63 m compared to baseline (personal communication JRM).
Both 2′O-MePS and PMO-based AONs have limited transduction efficiency in the heart as demonstrated in pre-clinical studies in mdx mice. Although safety and efficacy in skeletal muscle is currently being evaluated in clinical trials, development of peptide conjugated AONs is underway to transduce both heart and skeletal muscle more efficiently. These include PMOs conjugated to arginine-rich cell penetrating peptides [24] or others fused with a muscle-specific peptide (MSP) [25]. Studies in mdx mice using either mode of enhancement targeting exon 23 showed nearly 100% transduction efficiency in skeletal muscle and up to 40% in cardiac muscle [24, 25]. Cardiac expression of PMO-induced dystrophin expression is of considerable importance considering that unconjugated PMOs incapable of myocardial dystrophin restoration worsened myocardial pathology in long-term in mdx mouse studies [26]*. This implies that rescuing skeletal muscle activity through dystrophin expression in the absence of cardiac expression may have negative consequences for cardiomyocytes perhaps related to stress of increasing activity. Formulating AONs to improve cardiac transduction will be necessary to achieve satisfactory results with exon skipping alone, or require alternative cardiotherapy using anyone of multiple approaches [27–31].
Mutation Suppression
Stop codon mutations in the DMD gene comprise approximately 15% of all DMD cases [32]. The goal of mutation suppression is to read-through a premature stop codon (also called nonsense mutations) to generate full-length dystrophin protein. Two pharmacologic tactics have shown pre-clinical efficacy and have also been tested clinically. A proof of principle study in mdx mice demonstrated in vivo mutation suppression with functional benefit using the aminoglycoside antibiotic, gentamicin [33]. In a subsequent clinical trial, DMD patients (n = 16) with stop codons treated weekly or twice weekly for six months (7.5 mg kg IV), there was a modest increase in the number of muscle fibers expressing dystrophin, reaching 13 and 15% of normal. Functional correlates included stabilization of muscle strength and a modest increase in forced vital capacity. Although this study demonstrated proof of principle demonstrating gentamicin-induced dystrophin expression in a clinical setting, it is clear that higher doses would be necessary to improve functional outcomes for patients. Given the potential renal toxicity of aminoglycoside antibiotics and the need for regular intravenous infusions, attention shifted to safer pharmacologic products producing mutation suppression, especially those that could orally administered
A second drug, Ataluren formerly referred to as PTC124 (PTC therapeutics) demonstrated promise as an orally administered pharmacologic read-through agent for stop codon mutations [34]. Pre-clinical studies in the mdx mouse demonstrated dystrophin expression in skeletal, cardiac, and diaphragm muscle with protection against eccentric contraction-induced injury. A phase I study in healthy volunteers established safety and tolerability at doses exceeding those required for pre-clinical efficacy [35]. Dystrophin also increased post treatment in a phase IIa proof-of-concept 28 day study in DMD/BMD patients. Subsequently, a randomized, double-blind, placebo-controlled phase IIb trial was conducted evaluating safety and efficacy over a 48 week treatment period. In a press release, PTC, Inc. concluded that preliminary results indicated a convincing safety profile, but the primary endpoint of the 6 minute walk test did not reach statistical significance [36].
Recently, two new read-through compounds were identified in a high-throughput screen called RTC13 and RTC14. A pre-clinical study in mdx mice demonstrated modest results with RTC14, however RTC13 demonstrated higher levels of read-through accompanied by improved muscle function and increased dystrophin expression in skeletal muscle, diaphragm and heart following systemic delivery. The study compared RTC13 to gentamicin and Ataluren and showed it outperformed both compounds [38]. Further testing will be necessary to establish the safety and tolerability of RTC13, but this pre-clinical study offers hope for the treatment of DMD patients as well as other dystrophies caused by nonsense mutations.
Dystrophin Surrogates
There are a growing number of therapeutic strategies based on the concept of upregulating or overexpressing alternative genes to compensate for the loss of dystrophin [9]. In the face of potential immunity to exogenously expressed dystrophin in DMD patients these approaches are particularly attractive. Upregulation or replacement of utrophin, a developmental paralog of dystrophin was the first surrogate to show promise as a treatment DMD. Utrophin shares 80% sequence homology with dystrophin and has been shown to partially restore function in the absence of dystrophin in transgenic mice [39] or pre-clinical gene replacement studies [40]. Utrophin is expressed at the sarcolemma during development but is limited to the neuromuscular and myotendinous junctions in adult normal muscle. However, in both dystrophic mice and DMD patients it is overexpressed in the sarcolemma of all muscle fibers partially compensating for the lost mechanical function of dystrophin in the membrane.
Alternative strategies to further exploit this phenomenon have emerged to upregulate utrophin at the sarcolemma including several small molecules which demonstrate transcriptional upregulation through the utrophin-A promoter [41–43]. In addition to utrophin, there have been a number of studies that have shown improvement in mdx mice with other genes including biglyan (rhBGN), alpha 7 integrin, synaptic cytotoxic T-cell GalNAc transferase (Galgt2), SERCA, Hsp72 and L-arginine [44–49]. The overexpression of many of these genes results in the upregulation of dystrophin-associated proteins and also in the amount of utrophin. Although improvement has been demonstrated in the mdx mouse, results have been restricted to preservation rather than marked functional improvement. As a class of drugs, dystrophin surrogates may have the greatest potential in combination with dystrophin correction or replacement strategies.
Cell Therapy
Recurrent episodes of muscle fiber breakdown and regeneration characteristic of DMD, result in impaired satellite cell differentiation, loss of muscle fibers and replacement by fat and connective tissue. As a result, strategies to deliver normal or genetically corrected muscle cells or pluripotent stem cells have long been under investigation. Although myoblast transfer initially showed promise in the mdx mouse [50], subsequent clinical trials were disappointing due to immune rejection, failure to disperse from the site of injection, or poor cell fusion and survival [51, 52]. Various stem cell-myogenic precursor populations address some of these difficulties by offering the capacity to proliferate and ability to migrate via the vasculature. These populations include satellite cells, muscle derived stem cells, side population cells, bone marrow-derived stem cells, and mesangioblasts [53–57]. Of these approaches mesangioblasts demonstrated particular promise as multipotent progenitors: an exhibited potential for improving muscular dystrophy, ease of isolation from blood vessels, and their capacity for arterial transmigration [58]. The experimental paradigm is to use the mesangioblasts as delivery vehicles for the DMD gene. A series of studies in mdx mice and the golden retriever muscular dystrophy (GRMD) dog showed that mesangioblasts transduced with a lentiviral vector expressing micro-dystrophin resulted in expression of dystrophin positive fibers. Notably the studies in the GRMD dogs revealed improvements in muscle function and mobility together with increased levels of dystrophin [59]. There remains some concern regarding the disconnect between limited dystrophin replacement and the high degree of functional correction observed. In addition, the potential contribution of immunosuppressive drugs used in combination with these pre-clinical studies to the degree functional improvement in the treated GRMD dogs has not been fully addressed [60].
Impediments to translating cell therapies to patients with muscular dystrophy have been revealed over the past several years. The challenges parallel those for gene therapy strategies, including preference for autologous transplantation to avoid immune responses and identifying a viral vector to accommodate the large dystrophin gene [61]. The mesangioblast approach appeared to have addressed the issue of arterial dispersal, however it was found that muscular dystrophy patients had reduced numbers of mesangioblasts, which would be insufficient for autologous cell therapy [62]. To circumvent this issue, investigators found that they could reprogram fibroblasts and myoblasts from muscular dystrophy patients and generate induced pluripotent stem cells (iPSCs) from which mesangioblasts could be derived [62]*. iPSC-derived mesangioblasts have been used to genetically correct the dystrophic phenotype in alpha-sarcoglycan (α-SG) deficient mice using the human α-SG gene. These studies are not entirely relevant to DMD given the small size of the α-SG gene versus the inability to deliver the full-length dystrophin cDNA by viral vectors. Future studies may incorporate a dual vector strategy as discussed above to address this issue. In the meantime, Darabi and colleagues demonstrated proof-of-principle for transplantation of myogenic precursor cells derived by conditionally expressing Pax7 in human embryonic stem (ES)/iPS cells in mdx mice [63]. Dystrophin expression derived from the human iPS cells was modest but led to functional improvement in the mdx mouse. Additional studies will be necessary to determine the potential for translation for patients.
Conclusion
The challenge to find a treatment for DMD continues, although current therapeutic approaches under investigation do show promise. Corticosteroids represent the standard of care for this disease. This class of drugs can make a difference by prolonging ambulation and delaying or preventing the onset of scoliosis. Gene replacement strategies offer the potential for long-term correction. Improved gene therapy vectors that encompass critical dystrophin domains together with advances in understanding ways to circumvent immune responses provide a platform for meaningful translation to patients. In addition, the clinical benefit seen with the exon skipping drug represents a major advance for DMD treatment. Lastly, significant progress has been made for cell therapy based treatments and upregulation of genes that act as dystrophin surrogates. Increased expression of these alternative genes may be achieved with small molecules; therefore it is foreseeable that future therapeutic approaches would include both dystrophin restoration and compensatory molecules.
Footnotes
Disclosure
L. R. Rodino-Klapac: none; J. R. Mendell: PI on the eteplirsen trial for which he received no personal compensation; Z. Sahenk: none.
References
- 1**.Mendell JR, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71:304–13. doi: 10.1002/ana.23528. First report of two-teir system utilizing dried blood spots obtained at birth to identify cases of DMD. Creatine kinase levels are first determined and if they reach a level predictive of DMD, DNA sequencing follows. [DOI] [PubMed] [Google Scholar]
- 2.Koenig M, et al. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–17. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 3.Brooke MH, et al. Clinical investigation in Duchenne dystrophy: 2. Determination of the “power” of therapeutic trials based on the natural history. Muscle Nerve. 1983;6:91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- 4.England SB, et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–2. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- 5.Harper SQHM, DelloRusso C, et al. Modular flexibility of dystrophin: Implications for gene therapy of Duchenne muscular dystrophy. Nat Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Li J, Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci U S A. 2000;97:13714–9. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregorevic P, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12:787–9. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watchko J, et al. Adeno-associated virus vector-mediated minidystrophin gene therapy improves dystrophic muscle contractile function in mdx mice. Hum Gene Ther. 2002;13:1451–60. doi: 10.1089/10430340260185085. [DOI] [PubMed] [Google Scholar]
- 9**.Mendell JR, et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N Engl J Med. 2010;363:1429–37. doi: 10.1056/NEJMoa1000228. Demonstrated immune reponse to mini-dystrophin following AAV mediated gene therapy for DMD in a subset of patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Odom GL, et al. Gene therapy of mdx mice with large truncated dystrophins generated by recombination using rAAV6. Mol Ther. 2011;19:36–45. doi: 10.1038/mt.2010.205. Dual vector strategy delivering a mini-dystrophin cassette containing the dystrophin C-terminus demonstrated increased functional benefit in mdx mice over microdystrophin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Duan D. Novel mini-dystrophin gene dual adeno-associated virus vectors restore neuronal nitric oxide synthase expression at the sarcolemma. Hum Gene Ther. 2012;23:98–103. doi: 10.1089/hum.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D, et al. Nitrosative stress elicited by nNOSmicro delocalization inhibits muscle force in dystrophin-null mice. J Pathol. 2011;223:88–98. doi: 10.1002/path.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krahn M, et al. A naturally occurring human minidysferlin protein repairs sarcolemmal lesions in a mouse model of dysferlinopathy. Sci Transl Med. 2010;2:50ra69. doi: 10.1126/scitranslmed.3000951. [DOI] [PubMed] [Google Scholar]
- 14.Lostal W, et al. Efficient recovery of dysferlin deficiency by dual adeno-associated vector-mediated gene transfer. Hum Mol Genet. 19:1897–907. doi: 10.1093/hmg/ddq065. [DOI] [PubMed] [Google Scholar]
- 15.Grose WE, et al. Homologous Recombination Mediates Functional Recovery of Dysferlin Deficiency following AAV5 Gene Transfer. PLoS One. 2012;7:e39233. doi: 10.1371/journal.pone.0039233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann CJ, et al. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc Natl Acad Sci U S A. 2001;98:42–7. doi: 10.1073/pnas.011408598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyenvalle A, et al. Prevention of dystrophic pathology in severely affected dystrophin/utrophin-deficient mice by morpholino-oligomer-mediated exon-skipping. Mol Ther. 2010;18:198–205. doi: 10.1038/mt.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokota T, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–76. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Deutekom JC, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–86. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 20.Kinali M, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–28. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goemans NM, et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med. 2011;364:1513–22. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 22.Cirak S, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Neuromuscular Disorders; 17th International Congress of The World Muscle Society; 2012. pp. 771–922. Systemically delivered morpholino in DMD patients demonstrated clinical benefit. [Google Scholar]
- 24.Moulton HM, et al. Cellular uptake of antisense morpholino oligomers conjugated to arginine-rich peptides. Bioconjug Chem. 2004;15:290–9. doi: 10.1021/bc034221g. [DOI] [PubMed] [Google Scholar]
- 25.Yin H, et al. Functional rescue of dystrophin-deficient mdx mice by a chimeric peptide-PMO. Mol Ther. 2010;18:1822–9. doi: 10.1038/mt.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malerba A, Boldrin L, Dickson G. Long-term systemic administration of unconjugated morpholino oligomers for therapeutic expression of dystrophin by exon skipping in skeletal muscle: implications for cardiac muscle integrity. Nucleic Acid Ther. 2011;21:293–8. doi: 10.1089/nat.2011.0306. [DOI] [PubMed] [Google Scholar]
- 27.Casazza F, et al. Cardiac transplantation in Becker muscular dystrophy. J Neurol. 1988;235:496–8. doi: 10.1007/BF00314256. [DOI] [PubMed] [Google Scholar]
- 28.Stollberger C, Finsterer J. Left ventricular synchronization by biventricular pacing in Becker muscular dystrophy as assessed by tissue Doppler imaging. Heart Lung. 2005;34:317–20. doi: 10.1016/j.hrtlng.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Doing AH, Renlund DG, Smith RA. Becker muscular dystrophy-related cardiomyopathy: a favorable response to medical therapy. J Heart Lung Transplant. 2002;21:496–8. doi: 10.1016/s1053-2498(01)00316-3. [DOI] [PubMed] [Google Scholar]
- 30.Lai Y, Duan D. Progress in gene therapy of dystrophic heart disease. Gene Ther. 2012;19:678–85. doi: 10.1038/gt.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rafael-Fortney JA, et al. Early treatment with lisinopril and spironolactone preserves cardiac and skeletal muscle in Duchenne muscular dystrophy mice. Circulation. 2011;124:582–8. doi: 10.1161/CIRCULATIONAHA.111.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendell JR, et al. Diagnosis of Duchenne dystrophy by enhanced detection of small mutations. Neurology. 2001;57:645–50. doi: 10.1212/wnl.57.4.645. [DOI] [PubMed] [Google Scholar]
- 33.Barton-Davis ER, et al. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Invest. 1999;104:375–81. doi: 10.1172/JCI7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welch EM, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 35.Hirawat S, et al. Safety, tolerability, and pharmacokinetics of PTC124, a nonaminoglycoside nonsense mutation suppressor, following single- and multiple-dose administration to healthy male and female adult volunteers. J Clin Pharmacol. 2007;47:430–44. doi: 10.1177/0091270006297140. [DOI] [PubMed] [Google Scholar]
- 36.Gold RH. The evolution of mammography. Radiol Clin North Am. 1992;30:1–19. [PubMed] [Google Scholar]
- 37.Wilschanski M, et al. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur Respir J. 2011;38:59–69. doi: 10.1183/09031936.00120910. [DOI] [PubMed] [Google Scholar]
- 38.Kayali R, et al. Read-through compound 13 restores dystrophin expression and improves muscle function in the mdx mouse model for Duchenne muscular dystrophy. Hum Mol Genet. 2012;21:4007–20. doi: 10.1093/hmg/dds223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tinsley J, et al. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med. 1998;4:1441–4. doi: 10.1038/4033. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert R, et al. Adenovirus-mediated utrophin gene transfer mitigates the dystrophic phenotype of mdx mouse muscles. Hum Gene Ther. 1999;10:1299–310. doi: 10.1089/10430349950017987. [DOI] [PubMed] [Google Scholar]
- 41.Krag TO, et al. Heregulin ameliorates the dystrophic phenotype in mdx mice. Proc Natl Acad Sci U S A. 2004;101:13856–60. doi: 10.1073/pnas.0405972101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moorwood C, et al. Drug discovery for Duchenne muscular dystrophy via utrophin promoter activation screening. PLoS One. 2011;6:e26169. doi: 10.1371/journal.pone.0026169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tinsley JM, et al. Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS One. 2011;6:e19189. doi: 10.1371/journal.pone.0019189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hnia K, et al. L-arginine decreases inflammation and modulates the nuclear factor-kappaB/matrix metalloproteinase cascade in mdx muscle fibers. Am J Pathol. 2008;172:1509–19. doi: 10.2353/ajpath.2008.071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goonasekera SA, et al. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J Clin Invest. 2011;121:1044–52. doi: 10.1172/JCI43844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin PT, et al. Overexpression of Galgt2 in skeletal muscle prevents injury resulting from eccentric contractions in both mdx and wild-type mice. Am J Physiol Cell Physiol. 2009;296:C476–88. doi: 10.1152/ajpcell.00456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burkin DJ, et al. Enhanced expression of the alpha 7 beta 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice. J Cell Biol. 2001;152:1207–18. doi: 10.1083/jcb.152.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amenta AR, et al. Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc Natl Acad Sci U S A. 2011;108:762–7. doi: 10.1073/pnas.1013067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gehrig SM, et al. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature. 2012;484:394–8. doi: 10.1038/nature10980. [DOI] [PubMed] [Google Scholar]
- 50.Partridge TA, et al. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–9. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 51.Skuk D, et al. Dystrophin expression in myofibers of Duchenne muscular dystrophy patients following intramuscular injections of normal myogenic cells. Mol Ther. 2004;9:475–82. doi: 10.1016/j.ymthe.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 52.Mendell JR, et al. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med. 1995;333:832–8. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- 53.Asakura A, Rudnicki MA. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol. 2002;30:1339–45. doi: 10.1016/s0301-472x(02)00954-2. [DOI] [PubMed] [Google Scholar]
- 54.Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev. 2006;16:525–32. doi: 10.1016/j.gde.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Cossu G, Bianco P. Mesoangioblasts--vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537–42. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Dezawa M, et al. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–7. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 57.Qu-Petersen Z, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–64. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tagliafico E, et al. TGFbeta/BMP activate the smooth muscle/bone differentiation programs in mesoangioblasts. J Cell Sci. 2004;117:4377–88. doi: 10.1242/jcs.01291. [DOI] [PubMed] [Google Scholar]
- 59.Sampaolesi M, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–9. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 60.Davies KE, Grounds MD. Treating muscular dystrophy with stem cells? Cell. 2006;127:1304–6. doi: 10.1016/j.cell.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 61.Tedesco FS, Cossu G. Stem cell therapies for muscle disorders. Curr Opin Neurol. 2012;25:597–603. doi: 10.1097/WCO.0b013e328357f288. [DOI] [PubMed] [Google Scholar]
- 62.Tedesco FS, et al. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci Transl Med. 2012;4:140ra89. doi: 10.1126/scitranslmed.3003541. [DOI] [PubMed] [Google Scholar]
- 63.Darabi R, et al. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–9. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]