Introduction
The bacterium Staphylococcus aureus is a major cause of persistent inflammation of the mammary gland, or mastitis, in bovines. Mastitis is one of the most important diseases affecting dairy cattle worldwide, with an estimated economic impact of $1.7–2 billion annually in the U.S [1]. This disease may be caused by a number of microorganisms, however S. aureus is significant for its ability to establish contagious, chronic disease that is difficult or impossible to treat. Improved control practices have reduced incidence, but S. aureus remains a leading cause of mastitis in dairy herds worldwide. U.S. studies have estimated that 10% of all dairy cows are infected, and 84% of herds are positive for S. aureus [2, 3]. While reports indicate that bovine and human isolates are host-adapted, some evidence exists for transmission of antibiotic resistant S. aureus between humans and cattle, increasing the urgency to find methods of prevention [4–6]. S. aureus can survive on the skin and mucous membranes and is transmitted between cows, most commonly, during the milking process [7]. Importantly, survival of S. aureus within bovine mammary cells, including alveolar cells and macrophages, is believed to contribute to chronic disease [8].
Vaccines targeting S. aureus have been in development for many years and approaches have included attenuated or inactivated bacteria, toxoids, purified capsule polysaccharide and purified protein subunit. Current vaccines, that include a lysed whole cell vaccine of three prevalent capsular S. aureus serotypes (Lysigin®, Boehringer Ingelheim Vetmedica, Inc.) and a polyvalent inactivated vaccine (STARTVAC®, Hippa, Spain) have shown reduction in clinical severity, but limited ability to prevent colonization [9–12]. Reduced efficacy may be due to inadequate humoral responses against heterologous isolates [13]. These vaccines also require multiple intramuscular doses and subsequent follow up. It is recognized t hat future strategies against S. aureus must focus on multiple conserved virulence factors to promote strain cross-protection. Inducing mucosal responses to block bacterial attachment, and cellular responses to reduce intracellular infection, are also key for preventing colonization and chronic disease. In this report, we present the immunogenicity and initial safety profile of a mucosal vaccine to prevent S. aureus mastitis as determined by two field trials in dairy cows. The vaccine is based upon the S. aureus iron-regulated surface protein A (IsdA) and clumping factor A (ClfA) antigens fused into a Vibrio cholerae cholera toxin (CT) A2/B chimera (IsdA-CTA2/B + ClfA-CTA2/B). CTA2/B chimeras are purified holotoxin-like molecules that have the toxic domain of CT genetically removed and replaced with an antigen of interest [14, 15]. These molecules have been reported to induce bactericidal mucosal and systemic responses after intranasal delivery [16]. Intranasal delivery has also recently been shown to induce antigen-specific responses in bovine milk [17].We hypothesized that a CTA2/B-based S. aureus mucosal vaccine would be effective to reduce or eliminate S. aureus colonization of the udder. Our results indicate that intranasal delivery of IsdA-CTA2/B + ClfA-CTA2/B during the dry period is safe in dairy cows and induces antigen-specific humoral responses in blood and milk that are active in vitro to trigger uptake and killing of S. aureus.
Materials and Methods
Bacterial strains, growth conditions and plasmids
S. aureus Newbould 305 was used for cloning of isdA and clfA and opsonophagocytosis assay (OPA) [18, 19]. S. aureus was grown in Luria Broth (LB) at 37°C for DNA isolation and in low iron media (LIM) without shaking at 37°C for OPA [20]. E. coli TE1 was used for cloning and protein expression of HIS-tagged antigens from pCK001 and pMaH001. These strains were grown in LB for cloning, or terrific broth (TB) for protein expression, + 100 µg/mL ampicillin at 37°C. E. coli Clear Coli BL21(DE3) (Lucigen, Madison, WI) was used for expression of CTA2/B chimeras from pLR001 and pLR003 and grown in TB + 35 ug/mL chloramphenicol at 37°C. pLR001 was constructed by amplification of Newbould 305 DNA using isdA primers for cloning into the vector pARLDR19 (SphI-ClaI) [21]. pLR003 was similarly constructed into pARLDR19 (SphI-XhoI) using clfA primers. pCK001 was constructed by insertion of isdA (Newbould 305) into pTRCHIS (BamHI-HindIII) to express HIS-IsdA. pMaH001 was constructed by insertion of clfA (Newbould 305) into pBAD18 (NheI-HindIII) to express ClfA-HIS. Strains, plasmid details and primer sequences (with restriction sites underlined) are shown in Supplementary Table 1. All plasmids were sequenced through cloning junctions.
Protein expression and purification
Chimeras were purified as described [22]. Briefly, to express IsdA-CTA2/B and ClfA-CTA2/B, ClearColi® (Lucingen, Madison, WI) transformed with pLR001 or pLR003 was grown to an optical density (O.D.600) of 0.9 and induced for 24 h with 0.2 % l-arabinose. Proteins were isolated from the periplasmic extract with 1 mg/mL polymyxin B and purified by affinity chromatography on immobilized d-galactose (Pierce™ D-Galactose Agarose, Thermo Fisher, Waltham, MA). Vaccine proteins were dialyzed into sterile 20% glycerol + 1×PBS and concentrations determined by BCA (Pierce™ BCA, Thermo Fisher). Vaccines were tested to ensure endotoxin levels below 0.05 EU/mL (LAL Endpoint Chromogenic, Lonza, Allendale, NJ), plated for sterility and stored at −80°C until use. For ELISA, IsdA and ClfA were isolated from the cytosol or periplasm of E.coli Top10 (Thermo Fisher) + pCK001/pMAH001 after overnight induction with 1M IPTG. Proteins were purified on cobalt (HisPur™, Thermo Fisher) and dialyzed into sterile 1×PBS. F or use in flow cytometry and OPA, endotoxin was removed (Pierce™ Endotoxin Removal Columns, Thermo Fisher).
Animals, trial design and sample collection
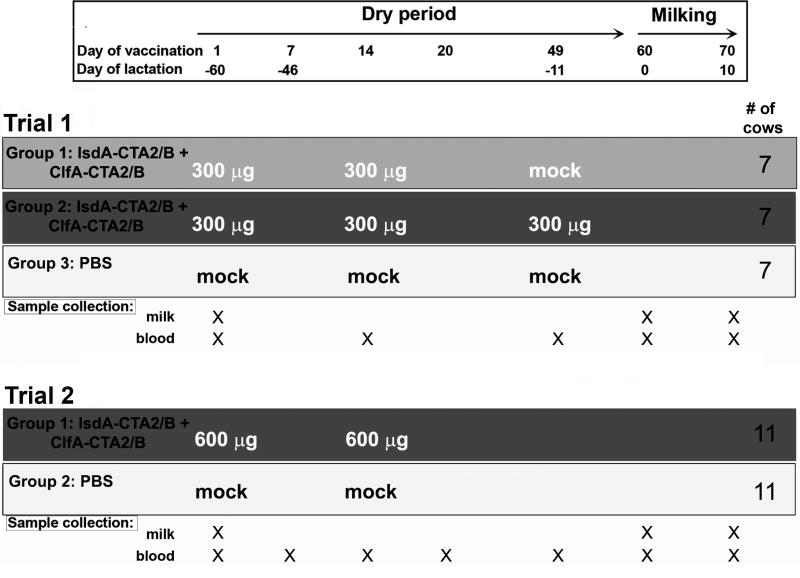
All animal protocols were pre-approved by the Boise State University Institutional Animal Care and Use Committee. Sample size was determined prior to Trial 1 by power analysis based upon predicted immune responses in milk and serum using data from a small pilot study (n=3) (Supplementary Figure 1). The A sample size of 7 cows per group was predicted to provide, at a 95% level of confidence, 92% power to detect a minimum difference between the control and any other group of 15%. This is based on a univariate two-group repeated measures ANOVA, assuming that the between groups error term is 0.13 and the within-groups error term is 0.08 [23]. Healthy Holstein cows in the third or fourth lactation were enrolled in the study (21 total Trial 1, n =7; 22 total Trial 2, n=11). Animals were screened as those with at least one of two previous somatic cell counts (SCC) below 200×103 cells/mL and no clinical evidence of mastitis. Further enrollment criteria included: 1) no growth of S. aureus from milk, 2) low baseline milk anti-IsdA responses, and 3) no evidence of bovine leukemia virus (BLV) infection. Cows were randomized into two or three groups (Figure 2). One cow in Trial 1 (group 2) and four cows in Trial 2 (groups 1 and 2) left the study prior to day 60 due to unrelated complications. Milk was not able to be obtained by day 70 from two additional cows in Trial 1 (groups 1 and 3). The vaccine was delivered in 2.5 mL volumes into each nare using a nasal cannula (Merck & Co., Kenilworth, NJ). Blood was isolated by tail vein and coagulated at room temperature (RT) for 1hr prior to removal of serum and dilution into 1:10 Inhibitor Solution (IS =1× HALT™ protease inhibitor, and 5% glycerol in 1× PBS). Whole blood was collected on day 60 in vacutainer tubes for peripheral blood mononuclear cell (PBMC) isolation (Becton Dickinson, Franklin Lakes, NJ). Composite milk samples were obtained aseptically after washing teat ends with 70% ethanol. M ilk was centrifuged at 700 ×g for 20 min at 4°C and skim milk stored in 1:10 IS at −20°C prior to use in ELISA.
Figure 2.
Trial 1 and Trial 2 summary showing vaccination schedule, dosage, sample collection and number of animals. Groups are: 1) Trial 1, Group 1, vaccinated 2× on days 1 and 14 (light grey); 2) Trial 1, Group 2, vaccinated 3× on days 1, 14 and 49 (dark grey); 3) Trial 1, Group 3, not vaccinated (white); 4) Trial 2, Group 1, vaccinated 2× on days 1 and 14 (dark grey); and 5) Trial 2, Group 2, not vaccinated (white).
Milk culture, PCR and clinical assessment
100 µL and 10 µL of milk and nasal culture was plated on MP2 (Udder Health Systems, Inc., Meridian, ID). The presence of small, white, esculin-negative colonies was considered presumptive Staphylococcus. Genomic DNA from these colonies was analyzed by PCR for staphylococcal species and for nuc, isdA and clfA (primers Supplemental Table 3). All animals were scored for clinical mastitis by temperature and observation of udder on days of vaccination and milk sampling [24, 25]. SCC analysis was performed on milk using the California Mastitis Test (CMT, Udder Health Systems, Inc.).
IgG and IgA ELISA
IsdA and ClfA specific responses on serum, milk and nasal wash were detected using ELISA, as described [22]. Briefly, 96-well plates (Nunc, Thermo Fisher) were coated with 10 µg of IsdA or ClfA (from pCK001 and pMAH001, as described above) in 50µL 1×PBS, blocked for 2 hr at 37°C in 1% goat milk + 1× PBS, and incubated with two-fold dilutions of bovine serum, purified milk IgG or nasal wash from Trials 1 and 2. Purified milk IgG was obtained using protein G affinity (Nab™ Protein G and Zeba™ Spin, Thermo Fischer) and dilutions for ELISA initiated at 25 µg. Plates with antibody dilutions were incubated at 4°C for 12 hr prior to washing and addition of HRP-anti-bovine IgG (1:10,000 Bethyl Laboratories, Montgomery, TX) or HRP-anti-bovine IgA (1:10,000 Bethyl Laboratories, Montgomery, TX). Samples were developed with ™B One ™ (Promega, Thermo Fisher) and read at 370 nm. For Trial 1, serum and milk ELISAs were performed on individual cows (n=6–7), or pools of 6–7 cows by vaccine group for nasal wash. For Trial 2, ELISA results are reported using pools of 1–2 cows (n=5) for consistency with cellular analysis. Data are presented as the ratio of Day Fresh (or Day X for serum)/Day1 of the optical density (O.D. 370 nm) from a representative antibody dilution in the linear part of the curve. Results are the average of three independent assays.
PBMC isolation, flow cytometry and cytokine qPCR
PBMCs were isolated from whole bovine blood from Trial 2 on day 60 using a density gradient established by layering blood diluted 1:2 with PBS on Histopaque®1077 (Sigma-Aldrich, St. Louis, MO). Samples were centrifuged at 800 ×g for 30 min at RT. The buffy coat was removed, washed 3× with Hank’s buffered salt solution (HBSS) and counted with 0.2% Trypan blue. PBMCs were pooled to contain cells from 2 cows (n=4 or 5), plated (1 × 105 cells/well) in tissue-culture 96-well plates (Corning, Sigma-Aldrich) and grown in RPMI-1640 at 37 °C, 5% CO2. Cells were stimulated with 10 µg of endotoxin-free IsdA + ClfA (from pCK001 and pMAH001, as described above) after 24 and 72 hr, and harvested after 6 days. Cells were stained with 10 µL/106 anti-CD4 FITC (CC8, BioRad, Hercules, CA) and anti-CD8 PE (CC63, BioRad), or equivalent amounts of isotype control (IgG2a FITC, Becton Dickenson), for 30 min at 4 °C. Samples were washed with 1×PBS, centrifuged at 400×g for 5 min, and resuspended in 3% FBS/0.02% NaN3/PBS. CountBright™ beads (10 µL) (Thermo Scientific) were added to determine absolute numbers. Samples were analyzed for fluorescence intensity on a FACS Calibur flow cytometer (Becton Dickinson) using FlowJo software (Tree Star, Inc.). A minimum of 10,000 total events were collected for each reaction. For cytokine assays, total RNA from stimulated pooled PBMCs (n=4–5) was extracted (RNeasy, Qiagen, Germantown, MD; DNase I, Promega, Madison, WI). cDNA was produced (High-Capacity RNA-to-cDNA™ Kit, Thermo Fisher) and qRT PCR was conducted using SYBR fast (Kapa Biosystems, Thermo Fisher) and IFN-γ, IL-4 and IL-17 primers, with bovine GAPDH as reference (Supplementary Table 1). The data are presented as fold changes determined by stimulated vs non-stimulated PBMC. All qRT-PCR experiments were performed twice using biological triplicates.
Opsonophagocytosis assay (OPA)
OPA was performed as described [26, 27]. Briefly, equal concentrations of endotoxin free IsdA (50 ug/well), and pooled (n=5) heat inactivated serum (50ug/well), or purified milk IgG (50ug/well), from days 20 and 60 or Fresh of Trial 2 were mixed and incubated at 37°C, 5% CO2 for 1 hr. Equal volumes of S. aureus Newbould 305 (2×105 CFU) and bovine PBMCs (2×105 cells) were added, and the reaction was incubated for 90 min. PBMCs were isolated as described above from whole citrated bovine blood (Hardy Diagnostics, Santa Maria, CA). After 90 min, 10 µl of reaction mixture was plated on MP2 in triplicate at 1: 100,000 to determine CFU/mL. Results are reported as percent killing, or reduction in CFU/ml in the presence of serum/milk IgG compared to PBMC’s and S. aureus alone.
Statistical methods and analysis
Statistical analyses were conducted using JMP (Cary, NC) and SAS software (Cary, NC). Logged values were used where necessary to stabilize variance in residual plots, and nonparametric exact methods were used with the smallest sample sizes and where logging did not correct the skew of the data. P-values are reported as p ≤ 0.05(*), p ≤ 0.01(**), p ≤ 0.001 (***) or p ≤ 0.0001(****) and all reflect two-sided tests.
Results
Expression and purification of IsdA-CTA2/B and ClfA-CTA2/B
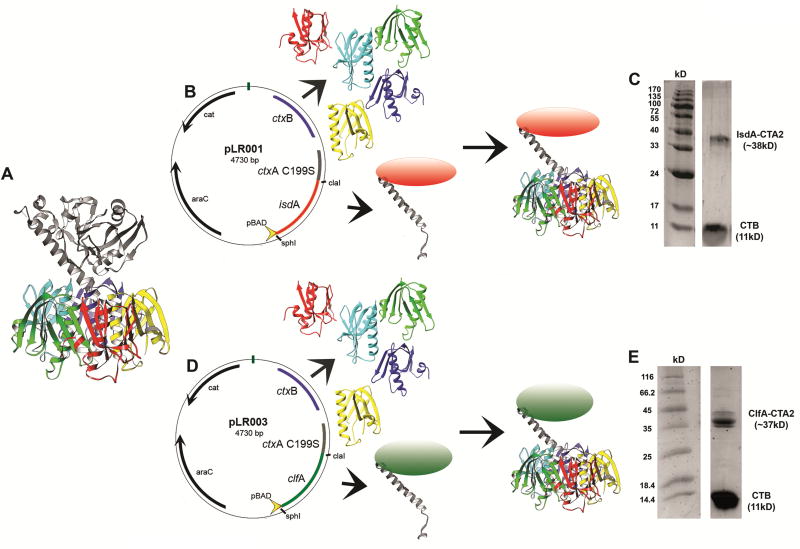
The crystal structure of CT reveals an AB5 structure with a toxic A subunit and non-toxic pentameric B subunit (Figure 1A)[28]. To construct the vaccine, the toxic CTA1 domain (ctxA) was genetically replaced by isdA or clfA to form chimera expression plasmids (pLR001 and pLR003, Figure 1B and D and Supplementary Table 1). IsdA-CTA2/B and ClfA-CTA2/B were expressed in E.coli for purification from the periplasm [21]. Folded chimeras were confirmed by SDS-PAGE (Figure 1C and 1E).
Figure 1.
Construction and purification of S. aureus CTA2/B chimeric mucosal vaccines. (A) ribbon diagram of Vibrio cholerae cholera toxin [28], (B) expression of the IsdA-CTA2/B chimera from E.coli + pLR001, (C) SDS-PAGE of d-galactose affinity-purified IsdA-CTA2/B (IsdA-CTA2 = 38kD, CTB = 11kD) vaccine preparation, (D) expression of the ClfA-CTA2/B chimera from E.coli + pLR003, and (E) SDS-PAGE of d-galactose affinity-purified ClfA-CTA2/B (ClfA-CTA2 = 37kD, CTB =11kD) vaccine preparation.
Trial design, safety assessment and culture analysis
Field trials were conducted in healthy Holstein heifers during the dry-off period (Figure 2). Trial 1 group 1 (light grey) received 300 µg intranasal doses of IsdA-CTA2/B + ClfA-CTA2/B on days 1 and 14, group 2 (dark grey) on days 1, 14 and 49, and group 3 (white) received mock (PBS) vaccination. Trial 2 group 1 (dark grey) received 600 µg intranasal doses on days 1 and 14 and group 2 (white) received mock vaccination. Animals were assessed during both trials for changes in temperature, SCC and clinical outcome (Supplementary Table 2), and milk/nasal wash samples were cultured for the presence of Staphylococcus and genotyping of isolates (Supplementary Table 3). No animals showed evidence of inflammation or clinical mastitis during either trial, and temperatures, as well as SCC, were not affected by vaccination. No animal, in either trial, was S. aureus positive in milk or nasal wash at any time. The presence of all Staphylococcus, and those specifically harboring isdA and clfA, was monitored throughout by culture and PCR. Results revealed that vaccination did not affect the presence and number of Staphylococcus. A small number of animals in Trial 2 were found to harbor isdA and clfA positive Staphylococcus prior to vaccination (4 total, determined to be S. hemolyticus and S. chromogenes), but no isdA/clfA positive staphylococci was present after vaccination in either trial.
IsdA and ClfA-specific humoral responses in Trials 1 and 2
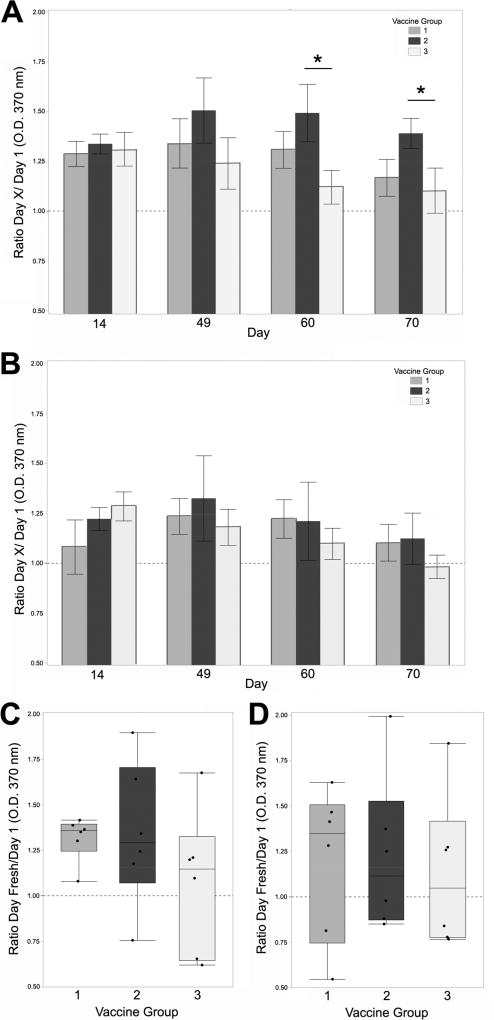
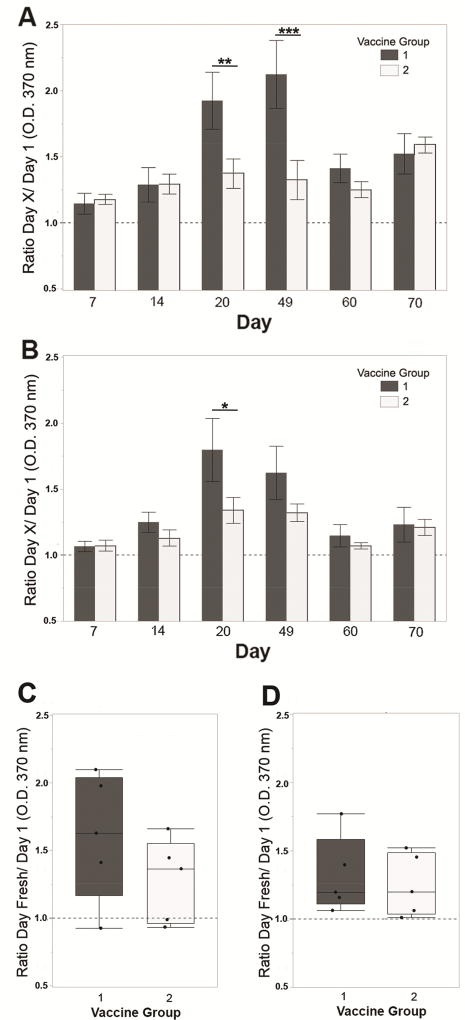
Antigen-specific humoral responses were quantified by ELISA from blood, milk and nasal wash collected during Trial 1, and from blood and milk of Trial 2. In Trial 1, group 2 (dark grey bars, Figure 3A), showed significantly higher anti-IsdA IgG in serum on days 60 and 70 with a consistent increase in vaccine group 1 (light grey bars), over the unvaccinated group 3 (white bars). ClfA serum ELISA was supportive of an increase in anti-ClfA IgG from groups 1 and 2 on days 60 and 70, but not significant (Figure 3B). Similarly, milk anti-IsdA at freshening was the highest in vaccine group 2 followed by vaccine group 1, relative to unvaccinated, but not significant (Figure 3C). Milk anti-ClfA was lower, but with a consistent pattern (Figure 3D). In Trial 2, group 1 (dark grey bars, Figure 4A) showed a significant increase in anti-IsdA serum IgG over group 2 (white bars) on days 20 and 49, and a supportive increase on day 60. Serum anti-ClfA was consistent with anti-IsdA, but significant on day 20 only (Figure 4B). Trial 2 milk anti-IsdA IgG was higher in group1 (Figure 4C), but without significance. Milk anti-ClfA IgG did not show differences over unvaccinated in Trial 2 (Figure 4D). Similar anti-IsdA responses were observed from a small pilot trial performed prior to Trials 1 and 2 (Supplementary Figure 1). In this trial, three cows were vaccinated during milking via the intranasal route with only the IsdA-CTA2/B chimera and three cows vaccinated with purified IsdA-HIS (pCK001) in the absence of adjuvant. Responses from this pilot provide evidence that the IsdA antigen alone is not immunogenic. Together, results demonstrate that antigen-specific humoral responses can be stimulated after intranasal vaccination with IsdA-CTA2/B + ClfA-CTA2/B.
Figure 3.
Trial 1 humoral responses in serum and milk. Anti-IsdA (A) and anti-ClfA (B) IgG responses as determined by ELISA using serum from days 14, 49, 60 and 70. Results are reported as ratios of Day X/Day (O.D. 370 nm) at serum dilution of 1:320. Anti-IsdA (C) and anti-ClfA (D) IgG responses as determined by ELISA using IgG purified from milk on day 60 or 70 (Day Fresh). Results are reported as ratios of Day Fresh/Day 1 (O.D. 370 nm) at IgG dilution of 1:80 (3.125 µg). Groups are: 1) vaccinated 2× on days 1 and 14 (light grey), 2) vaccinated 3× on days 1, 14 and 49 (dark grey), and 3) not vaccinated (white). Serum ELISA results were analyzed using repeated measures ANOVA on logged values in a mixed-model framework with compound symmetric within-cow covariance. Vaccine groups were compared on each day with hypotheses not adjusted due to pre-planned comparisons. Data are presented as the mean ± standard error for serum (n=6–7), or quartile box plots with medians for milk (n=6). Significance on Day 60 and Day 70 (p = 0.027 * and p = 0.047 *) is shown.
Figure 4.
Trial 2 humoral responses in serum and milk. Anti-IsdA (A) and anti-ClfA (B) IgG responses as determined by ELISA using serum from days 7, 14, 20, 49, 60 and 70. Results are reported as ratios of Day X/Day 1 (O.D. 370 nm) at serum dilutions of 1:1280 (400 ng total protein). Anti-IsdA (C) and anti-ClfA (D) IgG responses as determined by ELISA using IgG purified from milk on day 60 or 70 (Day Fresh). Results are reported as ratios Day Fresh/Day 1 (O.D. 370 nm) at milk dilution of 1:80 (3.125 µg). Groups are: 1) vaccinated 2× on days 1 and 14 (dark grey), 2) not vaccinated (white). As with Trial 1, serum ELISA results were analyzed using repeated measures ANOVA on logged values in a mixed-model framework with compound symmetric within-cow covariance and groups were compared on each day with hypotheses not adjusted due to pre-planned comparisons. Data are presented as the mean ± standard error for serum or quartile box plots with medians for milk (n=5). Significance on Days 20 and 49 for anti-IsdA responses (p = 0.009 ** and p = 0.0003 ***) and on Day 20 for anti-ClfA response (p = 0.013 *) is shown.
Induction of cellular immune responses from Trial 2
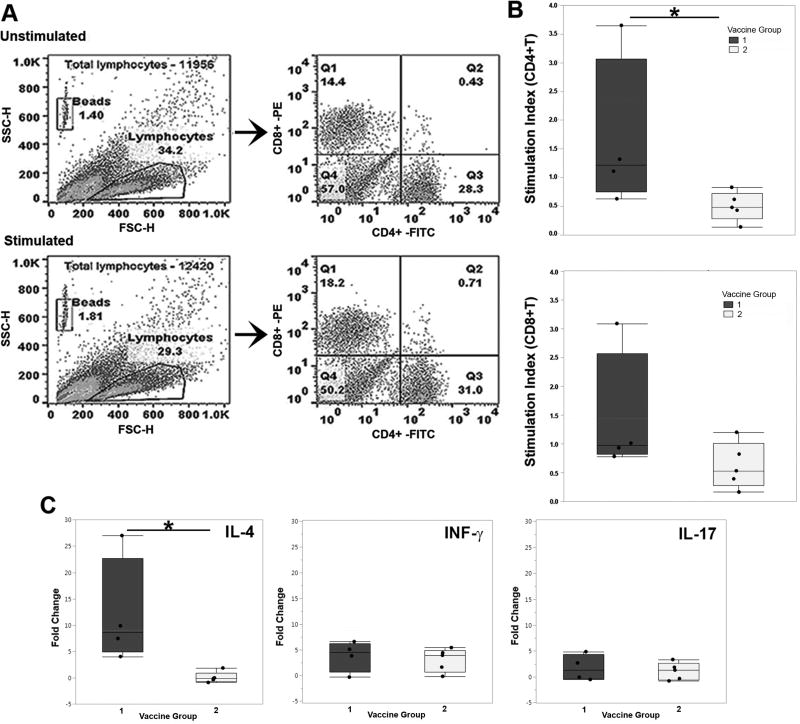
Antigen-specific T cell proliferation was determined using PBMCs from cows on day 60 of Trial 2. Concentrations of CD8+ and CD4+ cells in antigen-stimulated (IsdA + ClfA) and unstimulated PBMCs was determined by flow cytometry (Figure 5A). Group 1 (vaccinated) showed a significant increase in CD4+ T cells compared to non-vaccinated group 2 (Figure 5B). Group 1 also revealed a modest increase in the proliferation of CD8+ T cells (Figure 5B). The levels of IFN-γ, IL4 and IL17 cytokine expression was further assessed by qRT-PCR from antigen stimulated cells. Group 1 showed significantly higher expression levels of IL-4 upon stimulation, but no detectable increase in INF-γ or IL-17 (Figure 5C). These results indicate that mucosal immunization with IsdA-CTA2/B + ClfA-CTA2/B stimulated a Th2-type cellular response.
Figure 5.
Trial 2 cellular and cytokine responses. Flow cytometry analysis to determine proliferation of CD4+ and CD8+ T-lymphocytes from Day 60 after stimulation with IsdA + ClfA: (A) representative light scatter dot plots showing gated bead and lymphocyte populations for unstimulated and stimulated PBMCs, as well as the lymphocyte gated population of CD8-PE vs CD4-FITC, where the CD8 population falls in quadrant 3 (Q3) and CD4-FITC population in Q4, and (B) quantification of flow cytometry showing the CD4+ T cell and CD8+ T cell stimulation index in vaccinated (dark grey) and unvaccinated (white) groups. (C) Quantitative RT-PCR to determine cytokine expression from day 60 antigen-stimulated PBMCs isolated from vaccinated (dark grey) and unvaccinated (white) cows, showing: IL-4, INF-γ and IL-17 expression. Data is reported as normalized Ct values fold change over GAPDH. A Wilcoxon test in an exact testing framework was used to compare vaccine treatments for Trial 2 flow cytometry and qPCR data. Significance for CD4+ T cell (p = 0.032 *) and IL-4 expression (p=0.016 *) is shown.
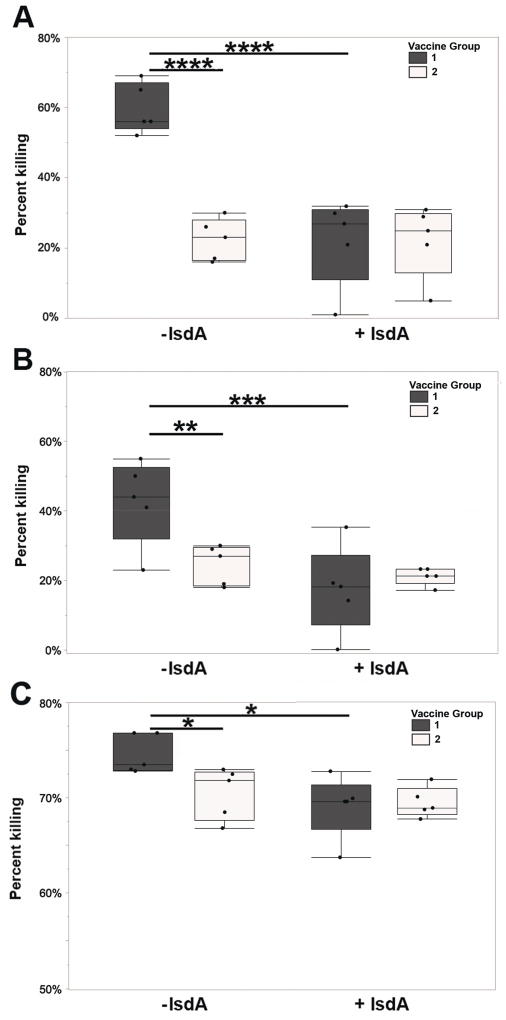
Opsonophagocytic activity of IsdA specific IgG antibodies from Trial 2
The in vitro activity of anti-IsdA antibodies was assessed by OPA using serum and milk IgG from Trial 2. Serum from group 1 on days 20 and 60 was superior to serum from group 2 in killing activity in the absence of purified IsdA (Figure 6A and B). When purified IsdA was added to the assay, percent killing was significantly reduced in the vaccinated group on both days respectively (Figure 6A and B). Purified milk IgG from group 1 at freshening (day 60 or 70) also showed significant activity relative to IgG from group 2, and consistent blocking of killing in the presence of IsdA (Figure 6C). Overall results indicate that antibodies stimulated against IsdA are active against isogenic S. aureus in vitro and induce opsonophagocytic responses.
Figure 6.
In vitro activity of anti-IsdA responses from Trial 2 serum and milk. Opsonophagocytic assay (OPA) to determine the ability of anti-IsdA humoral responses from days 20 (A) and 60 (B), or pooled milk from day Fresh (C), of vaccinated (dark grey) and unvaccinated (white) cows, to promote phagocytic killing. Samples were also pre-mixed with (+IsdA) or without (−IsdA) purified IsdA to assess antigen-specific inhibition of serum activity. Results are reported as percent killing (CFU/mL) of S. aureus Newbould 305 by bovine PBMCs after mixture with pooled serum compared to a control of PBMCs plus S. aureus in the absence of serum (n=5). Serum percent kill from OPA was analyzed with a 3-way ANOVA and vaccine group was compared within day and IsdA status, and IsdA status was compared within vaccine status. Sets of tests were adjusted for multiple comparisons using the false discovery rate method [60]. Milk percent kill from OPA was analyzed with a 2-way ANOVA with only day Fresh. Significance between serum in vaccine groups without IsdA on days 20 and 60 is shown (p = < 0.0001 **** and p = 0.009 ** respectively); as is significance between IsdA presence and absence with serum from vaccinated cows on days 20 and 60 (p < 0.0001 **** and p = 0.001*** respectively). In milk, vaccine groups differ (p = 0.041 *) and within the vaccinated group, percent killing differs by IsdA status (p = 0.01 *) as shown.
Discussion
This report describes the outcome of two bovine trials conducted to determine the immunogenicity of a CT-based mucosal S. aureus mastitis vaccine (IsdA-CTA2/B + ClfA-CTA2/B). CT, and the homologous E. coli LTI enterotoxin, are gold-standard mucosal vaccine adjuvants that have been used extensively in animal studies. The AB5 holotoxin of CT and LTI have known potent immunostimulatory activity, however the non-toxic subunits (CTB and LTIB) retain adjuvanticity [29, 30]. While the exact mechanism remains unknown, binding of the B pentamer to ganglioside GM1, found on many host cells including dendritic cells and macrophages, likely triggers antigen delivery. This interaction enhances antigen presentation, upregulation of surface molecules, and B-cell isotype switching [31, 32]. While many reports reveal that CT induces a Th2 response, the polarity of immune induction depends upon the co-delivered antigen and route of immunization [33–35]. CTB has also been shown to stimulate Th17 responses, and may also have anti-inflammatory properties [36–39]. CTA2/B chimeras have advantages over CT and CTB that include: the absence of the toxic A1 domain, the non-covalent association of antigen, and a native receptor-binding subunit. A2/B chimeras have shown efficacy in animal models, mostly notably after intranasal delivery [22, 40–45]. We predicted that the capacity of CTA2/B to stimulate humoral and cellular responses from the mucosa, induce IL-17, and reduce inflammation, would support its efficacy as a bovine S. aureus vaccine. Further, CTA2/B chimeras are flexible and safe, and needle-free vaccination of cattle would reduce transmission of disease. Our results indicate that while we did not see induction of IL-17, significant antigen-specific responses were detected in the serum of vaccinated animals, and the vaccine induced Th2-type cellular responses. IsdA-CTA2/B + ClfA-CTA2/B was also well tolerated and safe.
IsdA and ClfA are well described S. aureus cell wall associated extracellular matrix (ECM) binding proteins. IsdA is a fibrinogen and fibronectin adhesin that contributes to iron sequestration and S. aureus dissemination [46]. Reports have described IsdA as a central human vaccine target [47, 48]. The presence of isdA is strongly conserved between human strains of S. aureus, and its presence and expression have been described in bovine isolates [49–51]. ClfA is also a conserved S. aureus fibrinogen adhesin found in bovine clinical isolates [52, 53]. clfA mutants show reduced ability to colonize human nares, and ClfA is a promising antigen for passive or active immunity against mastitis [54–56]. Binding to the ECM is a key component of S. aureus colonization of the udder, and facilitates bacterial intracellular uptake into bovine mammary cells [57, 58]. We targeted this bacterial-ECM interaction by incorporating both of these adhesins into IsdA-CTA2/B + ClfA-CTA2/B, however, results indicated that higher anti-IsdA responses were stimulated in both trials. This may be due to a more stable IsdA-CTA2/B holotoxin structure, or improper folding of ClfA in the chimera. The potential instability or loss of antigen tertiary structure, and resulting antibody specificity, are recognized limitations of CTA2/B chimeras. Future optimization of antigen composition, as well as chimera stability, will promote vaccine efficacy.
The development of an effective vaccine to prevent bovine mastitis caused by S. aureus would have benefits to both animal and human health. Opsonophagocytosis, or functional antibody activity, is a known critical feature of a successful S. aureus vaccine [59]. While in vivo efficacy of IsdA-CTA2/B + ClfA-CTA2/B is still to be determined, the ability of both serum and milk from vaccinated animals during freshening to trigger S. aureus killing in vitro was highly significant, antigen-specific, and supportive of the protective capacity of this vaccine. New vaccine approaches for S. aureus, that are adaptable and inexpensive, but can induce complex responses to block colonization and transmission are needed. The IsdA-CTA2/B + ClfA-CTA2/B vaccine is a novel approach that may address these needs.
Supplementary Material
Supplementary Figure 1. Pilot immunogenicity trial vaccination schedule, dosage and sample collection (A). Anti-IsdA IgA responses in serum on days 14, 28, 49 and 70 (B), and anti-IsdA response in milk on days 14, 28, 49 and 70 (C). Results are reported as ratios of Day X/Day1 (O.D. 370) at a serum dilution of 1:320 or milk dilution of 1:80. Groups are:1) vaccinated with IsdA-CTA2/B 2× on days 1 and 14 (light grey), and 2) vaccinated with IsdA-HIS (no adjuvant) 2× on days 1 and 14 (dark grey). Logged humoral responses were analyzed with a 2-way ANOVA, with vaccine groups compared within each day. Data are presented mean ± standard error (n=3).
Acknowledgments
Acknowledgements and support
We would like to thank Laura Rogers, Macey Horch, Hannah Weaver, Connor Richmond, Eric Swiecki, Emily Price, Kimberly Brown, Montana Marks, Kristina Chapman, Orion Thomson-Vogel, Danielle Holt and Travis Williams for field work and technical support. This work was supported by a 2009 USDA AFRI seed grant (#2009-01778, PI-Tinker), a 2013 USDA AFRI standard grant (#2013-01189, PI-Tinker, Co-PI McGuire), and a faculty seed grant to J.K.T. from an Institutional Development Awards (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (#P20GM103408 and P20GM109095). We also acknowledge support from The Biomolecular Research Center at Boise State with funding from the National Science Foundation, Grants # 0619793 and #0923535; the MJ Murdock Charitable Trust; and the Idaho State Board of Education.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
All authors were blinded throughout trials with the exception of J.K.T. N.M. was the lead for all project experimentation and manuscript preparation; T.F.W. purified vaccines and was directly involved in ELISA, culture analysis and field work; C.L.K. performed culture analysis, PCR and field work; R.H. was instrumental in flow cytometry and analysis; L.B. contributed to study design through power analysis and data analysis; B.M. performed vaccinations and conducted all field work; M.A.M. contributed to study development and funding acquisition; J.K.T. was lead investigator for the study, including: design, implementation, data analysis, manuscript preparation and funding acquisition.
Conflicts of interest
J.K.T. holds an unlicensed patent for the use of cholera toxin chimera as a staphylococcal vaccine (Tinker, US 13/328,686). B.M. holds a contract for services with Boise State University Office of Technology Transfer, however this author was blinded throughout both trials and not involved in laboratory experimentation or data analysis.
References
- 1.Current Concepts in Bovine Mastitis. 4 National Mastitis Council; Madison WI: 1996. [Google Scholar]
- 2.Haran KP, Godden SM, Boxrud D, Jawahir S, Bender JB, Sreevatsan S. Prevalence and characterization of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, isolated from bulk tank milk from Minnesota dairy farms. J Clin Microbiol. 2012;50:688–95. doi: 10.1128/JCM.05214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones GM, Pearson RE, Clabaugh GA, Heald CW. Relationships between somatic cell counts and milk production. J Dairy Sci. 1984;67:1823–31. doi: 10.3168/jds.S0022-0302(84)81510-6. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt T, Kock MM, Ehlers MM. Diversity and antimicrobial susceptibility profiling of staphylococci isolated from bovine mastitis cases and close human contacts. J Dairy Sci. 2015;98:6256–69. doi: 10.3168/jds.2015-9715. [DOI] [PubMed] [Google Scholar]
- 5.Juhasz-Kaszanyitzky E, Janosi S, Somogyi P, Dan A, van der Graaf-van Bloois L, van Duijkeren E, et al. MRSA transmission between cows and humans. Emerg Infect Dis. 2007;13:630–2. doi: 10.3201/eid1304.060833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado S, García P, Fernández L, Jiménez E, Rodríguez-Baños M, del Campo R, et al. Characterization of Staphylococcus aureus strains involved in human and bovine mastitis. FEMS Immunol Med Microbiol. 2011;62:225–35. doi: 10.1111/j.1574-695X.2011.00806.x. [DOI] [PubMed] [Google Scholar]
- 7.Roberson JR, Fox LK, Hancock DD, Gay JM, Besser TE. Sources of intramammary infections from Staphylococcus aureus in dairy heifers at first parturition. J Dairy Sci. 1998;81:687–93. doi: 10.3168/jds.S0022-0302(98)75624-3. [DOI] [PubMed] [Google Scholar]
- 8.Hebert A, Sayasith K, Senechal S, Dubreuil P, Lagace J. Demonstration of intracellular Staphylococcus aureus in bovine mastitis alveolar cells and macrophages isolated from naturally infected cow milk. FEMS Microbiol Lett. 2000;193:57–62. doi: 10.1111/j.1574-6968.2000.tb09402.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith GW, Lyman RL, Anderson KL. Efficacy of vaccination and antimicrobial treatment to eliminate chronic intramammary Staphylococcus aureus infections in dairy cattle. J Am Vet Med Assoc. 2006;228:422–5. doi: 10.2460/javma.228.3.422. [DOI] [PubMed] [Google Scholar]
- 10.Middleton JR, Ma J, Rinehart CL, Taylor VN, Luby CD, Steevens BJ. Efficacy of different Lysigin formulations in the prevention of Staphylococcus aureus intramammary infection in dairy heifers. J Dairy Res. 2006;73:10–9. doi: 10.1017/S0022029905001354. [DOI] [PubMed] [Google Scholar]
- 11.Schukken YH, Bronzo V, Locatelli C, Pollera C, Rota N, Casula A, et al. Efficacy of vaccination on Staphylococcus aureus and coagulase-negative staphylococci intramammary infection dynamics in 2 dairy herds. J Dairy Sci. 2014;97:5250–64. doi: 10.3168/jds.2014-8008. [DOI] [PubMed] [Google Scholar]
- 12.Bradley AJ, Breen JE, Payne B, White V, Green MJ. An investigation of the efficacy of a polyvalent mastitis vaccine using different vaccination regimens under field conditions in the United Kingdom. J Dairy Sci. 2015;98:1706–20. doi: 10.3168/jds.2014-8332. [DOI] [PubMed] [Google Scholar]
- 13.Luby CD, Middleton JR, Ma J, Rinehart CL, Bucklin S, Kohler C, et al. Characterization of the antibody isotype response in serum and milk of heifers vaccinated with a Staphylococcus aureus bacterin (Lysigin) J Dairy Res. 2007;74:239–46. doi: 10.1017/S0022029907002476. [DOI] [PubMed] [Google Scholar]
- 14.Jobling MG, Holmes RK. Fusion proteins containing the A2 domain of cholera toxin assemble with B polypeptides of cholera toxin to form immunoreactive and functional holotoxin-like chimeras. Infect Immun. 1992;60:4915–24. doi: 10.1128/iai.60.11.4915-4924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajishengallis G, Hollingshead SK, Koga T, Russell MW. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154:4322–32. [PubMed] [Google Scholar]
- 16.Price GA, Russell MW, Cornelissen CN. Intranasal administration of recombinant Neisseria gonorrhoeae transferrin binding proteins A and B conjugated to the cholera toxin B subunit induces systemic and vaginal antibodies in mice. Infect Immun. 2005;73:3945–53. doi: 10.1128/IAI.73.7.3945-3953.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boerhout E, Vrieling M, Benedictus L, Daemen I, Ravesloot L, Rutten V, et al. Immunization routes in cattle impact the levels and neutralizing capacity of antibodies induced against S. aureus immune evasion proteins. Vet Res. 2015;46:115. doi: 10.1186/s13567-015-0243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouchard D, Peton V, Almeida S, Le Maréchal C, Miyoshi A, Azevedo V, et al. Genome sequence of Staphylococcus aureus Newbould 305, a strain associated with mild bovine mastitis. J Bacteriol. 2012;194:6292–3. doi: 10.1128/JB.01188-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad LBNF. Inoculation of the bovine teat duct with Staph aureus:the relationship of teat duct lenght, milk yield and milking rate to development of intramammary infection. Can Vet Journal. 1968;9:107–15. [PMC free article] [PubMed] [Google Scholar]
- 20.Baratela KC, Saridakis HO, Gaziri LC, Pelayo JS. Effects of medium composition, calcium, iron and oxygen on haemolysin production by Plesiomonas shigelloides isolated from water. J Appl Microbiol. 2001;90:482–7. doi: 10.1046/j.1365-2672.2001.01270.x. [DOI] [PubMed] [Google Scholar]
- 21.Tinker JK, Erbe JL, Holmes RK. Characterization of fluorescent chimeras of cholera toxin and Escherichia coli heat-labile enterotoxins produced by use of the twin arginine translocation system. Infect Immun. 2005;73:3627–35. doi: 10.1128/IAI.73.6.3627-3635.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arlian BM, Tinker JK. Mucosal immunization with a Staphylococcus aureus IsdA-cholera toxin A2/B chimera induces antigen-specific Th2-type responses in mice. Clin Vaccine Immunol. 2011;18:1543–51. doi: 10.1128/CVI.05146-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elashoff JD. In: nQuery Advisor® Version 7.0 User’s Guide. Lawrence V, editor. Los Angeles, CA: 2007. [Google Scholar]
- 24.Wenz JR, Garry FB, Barrington GM. Comparison of disease severity scoring systems for dairy cattle with acute coliform mastitis. J Am Vet Med Assoc. 2006;229:259–62. doi: 10.2460/javma.229.2.259. [DOI] [PubMed] [Google Scholar]
- 25.Atalla H, Gyles C, Wilkie B, Leslie K, Mallard B. Somatic cell scores and clinical signs following experimental intramammary infection of dairy cows with a Staphylococcus aureus small colony variant (S. aureus SCV) in comparison to other bovine strains. Vet Microbiol. 2009;137:326–34. doi: 10.1016/j.vetmic.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Theilacker C, Kropec A, Hammer F, Sava I, Wobser D, Sakinc T, et al. Protection against Staphylococcus aureus by antibody to the polyglycerolphosphate backbone of heterologous lipoteichoic acid. J Infect Dis. 2012;205:1076–85. doi: 10.1093/infdis/jis022. [DOI] [PubMed] [Google Scholar]
- 27.Maira-Litran T, Bentancor LV, Bozkurt-Guzel C, O'Malley JM, Cywes-Bentley C, Pier GB. Synthesis and Evaluation of a Conjugate Vaccine Composed of Staphylococcus aureus Poly- N-Acetyl-Glucosamine and Clumping Factor A. PLoS ONE. 2012;7:e43813. doi: 10.1371/journal.pone.0043813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang RG, Scott DL, Westbrook ML, Nance S, Spangler BD, Shipley GG, et al. The three-dimensional crystal structure of cholera toxin. J Mol Biol. 1995;251:563–73. doi: 10.1006/jmbi.1995.0456. [DOI] [PubMed] [Google Scholar]
- 29.Holmgren J, Czerkinsky C, Lycke N, Svennerholm AM. Strategies for the induction of immune responses at mucosal surfaces making use of cholera toxin B subunit as immunogen, carrier, and adjuvant. Am J Trop Med Hyg. 1994;50:42–54. [PubMed] [Google Scholar]
- 30.Russell MW, Wu HY, Hajishengallis G, Hollingshead SK, Michalek SM. Cholera toxin B subunit as an immunomodulator for mucosal vaccine delivery. Adv Vet Med. 1999;41:105–14. doi: 10.1016/s0065-3519(99)80011-1. [DOI] [PubMed] [Google Scholar]
- 31.George-Chandy A, Eriksson K, Lebens M, Nordstrom I, Schon E, Holmgren J. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect Immun. 2001;69:5716–25. doi: 10.1128/IAI.69.9.5716-5725.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnitzler AC, Burke JM, Wetzler LM. Induction of cell signaling events by the cholera toxin B subunit in antigen-presenting cells. Infect Immun. 2007;75:3150–9. doi: 10.1128/IAI.00581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eriksson K, Fredriksson M, Nordstrom I, Holmgren J. Cholera toxin and its B subunit promote dendritic cell vaccination with different influences on Th1 and Th2 development. Infect Immun. 2003;71:1740–7. doi: 10.1128/IAI.71.4.1740-1747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu-Amano J, Jackson RJ, Fujihashi K, Kiyono H, Staats HF, McGhee JR. Helper Th1 and Th2 cell responses following mucosal or systemic immunization with cholera toxin. Vaccine. 1994;12:903–11. doi: 10.1016/0264-410x(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 35.Hajishengallis G, Arce S, Gockel CM, Connell TD, Russell MW. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J Dent Res. 2005;84:1104–16. doi: 10.1177/154405910508401205. [DOI] [PubMed] [Google Scholar]
- 36.Lee JB, Jang JE, Song MK, Chang J. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PLoS ONE. 2009;4:e5190. doi: 10.1371/journal.pone.0005190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meza-Sanchez D, Perez-Montesinos G, Sanchez-Garcia J, Moreno J, Bonifaz LC. Intradermal immunization in the ear with cholera toxin and its non-toxic beta subunit promotes efficient Th1 and Th17 differentiation dependent on migrating DCs. Eur J Immunol. 2011;41:2894–904. doi: 10.1002/eji.201040997. [DOI] [PubMed] [Google Scholar]
- 38.Burkart V, Kim YE, Hartmann B, Ghiea I, Syldath U, Kauer M, et al. Cholera toxin B pretreatment of macrophages and monocytes diminishes their proinflammatory responsiveness to lipopolysaccharide. J Immunol. 2002;168:1730–7. doi: 10.4049/jimmunol.168.4.1730. [DOI] [PubMed] [Google Scholar]
- 39.Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169:6408–16. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- 40.Boyaka PN, Ohmura M, Fujihashi K, Koga T, Yamamoto M, Kweon MN, et al. Chimeras of labile toxin one and cholera toxin retain mucosal adjuvanticity and direct Th cell subsets via their B subunit. J Immunol. 2003;170:454–62. doi: 10.4049/jimmunol.170.1.454. [DOI] [PubMed] [Google Scholar]
- 41.Kweon MN, Yamamoto M, Watanabe F, Tamura S, Van Ginkel FW, Miyauchi A, et al. A nontoxic chimeric enterotoxin adjuvant induces protective immunity in both mucosal and systemic compartments with reduced IgE antibodies. J Infect Dis. 2002;186:1261–9. doi: 10.1086/344526. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Erbe JL, Lockatell CV, Johnson DE, Jobling MG, Holmes RK, et al. Use of translational fusion of the MrpH fimbrial adhesin-binding domain with the cholera toxin A2 domain, coexpressed with the cholera toxin B subunit, as an intranasal vaccine to prevent experimental urinary tract infection by Proteus mirabilis. Infect Immun. 2004;72:7306–10. doi: 10.1128/IAI.72.12.7306-7310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price GA, Holmes RK. Evaluation of TcpF-A2-CTB chimera and evidence of additive protective efficacy of immunizing with TcpF and CTB in the suckling mouse model of cholera. PLoS ONE. 2012;7:e42434. doi: 10.1371/journal.pone.0042434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sultan F, Jin LL, Jobling MG, Holmes RK, Stanley SL., Jr Mucosal immunogenicity of a holotoxin-like molecule containing the serine-rich Entamoeba histolytica protein (SREHP) fused to the A2 domain of cholera toxin. Infect Immun. 1998;66:462–8. doi: 10.1128/iai.66.2.462-468.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tinker JK, Yan J, Knippel RJ, Panayiotou P, Cornell KA. Immunogenicity of a West Nile virus DIII-cholera toxin A2/B chimera after intranasal delivery. Toxins (Basel) 2014;6:1397–418. doi: 10.3390/toxins6041397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarke SR, Wiltshire MD, Foster SJ. IsdA of Staphylococcus aureus is a broad spectrum, iron-regulated adhesin. Mol Microbiol. 2004;51:1509–19. doi: 10.1111/j.1365-2958.2003.03938.x. [DOI] [PubMed] [Google Scholar]
- 47.Kim HK, DeDent A, Cheng AG, McAdow M, Bagnoli F, Missiakas DM, et al. IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine. 2010;28:6382–92. doi: 10.1016/j.vaccine.2010.02.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clarke SR, Andre G, Walsh EJ, Dufrene YF, Foster TJ, Foster SJ. Iron-regulated surface determinant protein A mediates adhesion of Staphylococcus aureus to human corneocyte envelope proteins. Infect Immun. 2009;77:2408–16. doi: 10.1128/IAI.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf C, Kusch H, Monecke S, Albrecht D, Holtfreter S, von Eiff C, et al. Genomic and proteomic characterization of Staphylococcus aureus mastitis isolates of bovine origin. Proteomics. 2011;11:2491–502. doi: 10.1002/pmic.201000698. [DOI] [PubMed] [Google Scholar]
- 50.Herron-Olson L, Fitzgerald JR, Musser JM, Kapur V. Molecular Correlates of Host Specialization in Staphylococcus aureus. PLoS ONE. 2007;2:e1120. doi: 10.1371/journal.pone.0001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Misra N, Wines TF, Knopp CL, McGuire MA, Tinker JK. Expression, immunogenicity and variation of iron-regulated surface protein A from bovine isolates of Staphylococcus aureus. FEMS Microbiol Lett. 2017;364 doi: 10.1093/femsle/fnx082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikawaty R, Brouwer EC, Van Duijkeren E, Mevius D, Verhoef J, Fluit AC. Virulence factors of genotyped bovine mastitis Staphylococcus aureus isolated in the Netherlands. International Journal of Dairy Science. 2010;5:60–70. [Google Scholar]
- 53.Fluit AC. Livestock-associated Staphylococcus aureus. Clin Microbiol Infect. 2012;18:735–44. doi: 10.1111/j.1469-0691.2012.03846.x. [DOI] [PubMed] [Google Scholar]
- 54.Castagliuolo I, Piccinini R, Beggiao E, Palu G, Mengoli C, Ditadi F, et al. Mucosal genetic immunization against four adhesins protects against Staphylococcus aureus-induced mastitis in mice. Vaccine. 2006;24:4393–402. doi: 10.1016/j.vaccine.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 55.Gong R, Hu C, Xu H, Guo A, Chen H, Zhang G, et al. Evaluation of clumping factor A binding region A in a subunit vaccine against Staphylococcus aureus-induced mastitis in mice. Clin Vaccine Immunol. 2010;17:1746–52. doi: 10.1128/CVI.00162-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wertheim HF, Walsh E, Choudhurry R, Melles DC, Boelens HA, Miajlovic H, et al. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med. 2008;5:e17. doi: 10.1371/journal.pmed.0050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lammers A, Nuijten PJ, Smith HE. The fibronectin binding proteins of Staphylococcus aureus are required for adhesion to and invasion of bovine mammary gland cells. FEMS Microbiol Lett. 1999;180:103–9. doi: 10.1111/j.1574-6968.1999.tb08783.x. [DOI] [PubMed] [Google Scholar]
- 58.Brouillette E, Grondin G, Shkreta L, Lacasse P, Talbot BG. In vivo and in vitro demonstration that Staphylococcus aureus is an intracellular pathogen in the presence or absence of fibronectin-binding proteins. Microb Pathog. 2003;35:159–68. doi: 10.1016/s0882-4010(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 59.Anderson AS, Miller AA, Donald RG, Scully IL, Nanra JS, Cooper D, et al. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum Vaccin Immunother. 2012;8:1585–94. doi: 10.4161/hv.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benjamani Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- 61.Tinker JK, Erbe JL, Hol WG, Holmes RK. Cholera holotoxin assembly requires a hydrophobic domain at the A-B5 interface: mutational analysis and development of an in vitro assembly system. Infect Immun. 2003;71:4093–101. doi: 10.1128/IAI.71.7.4093-4101.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzales VK, de Mulder EL, de Boer T, Hannink G, van Tienen TG, van Heerde WL, et al. Platelet-rich plasma can replace fetal bovine serum in human meniscus cell cultures. Tissue Eng Part C Methods. 2013;19:892–9. doi: 10.1089/ten.tec.2013.0009. [DOI] [PubMed] [Google Scholar]
- 63.Puech C, Dedieu L, Chantal I, Rodrigues V. Design and evaluation of a unique SYBR Green real-time RT-PCR assay for quantification of five major cytokines in cattle, sheep and goats. BMC Vet Res. 2015;11:65. doi: 10.1186/s12917-015-0382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rainard P, Cunha P, Bougarn S, Fromageau A, Rossignol C, Gilbert FB, et al. T helper 17-associated cytokines are produced during antigen-specific inflammation in the mammary gland. PLoS One. 2013;8:e63471. doi: 10.1371/journal.pone.0063471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morot-Bizot SC, Talon R, Leroy S. Development of a multiplex PCR for the identification of Staphylococcus genus and four staphylococcal species isolated from food. J Appl Microbiol. 2004;97:1087–94. doi: 10.1111/j.1365-2672.2004.02399.x. [DOI] [PubMed] [Google Scholar]
- 66.Brakstad OG, Aasbakk K, Maeland JA. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol. 1992;30:1654–60. doi: 10.1128/jcm.30.7.1654-1660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Pilot immunogenicity trial vaccination schedule, dosage and sample collection (A). Anti-IsdA IgA responses in serum on days 14, 28, 49 and 70 (B), and anti-IsdA response in milk on days 14, 28, 49 and 70 (C). Results are reported as ratios of Day X/Day1 (O.D. 370) at a serum dilution of 1:320 or milk dilution of 1:80. Groups are:1) vaccinated with IsdA-CTA2/B 2× on days 1 and 14 (light grey), and 2) vaccinated with IsdA-HIS (no adjuvant) 2× on days 1 and 14 (dark grey). Logged humoral responses were analyzed with a 2-way ANOVA, with vaccine groups compared within each day. Data are presented mean ± standard error (n=3).