Abstract
Neuronal-glial relationships play a critical role in the maintenance of central nervous system architecture and neuronal specification. A deeper understanding of these relationships can elucidate cellular cross-talk capable of sustaining proper development of neural tissues. In the cerebellum, cerebellar granule neuron precursors (CGNPs) proliferate in response to Purkinje neuron-derived Sonic hedgehog (Shh) before ultimately exiting the cell cycle and migrating radially along Bergmann glial fibers. However, the function of Bergmann glia in CGNP proliferation remains not well defined. Interestingly, the Hh pathway is also activated in Bergmann glia, but the role of Shh signaling in these cells is unknown. In this study, we show that specific ablation of Shh signaling using the tamoxifen-inducible TNCYFP-CreER line to eliminate Shh pathway activator Smoothened in Bergmann glia is sufficient to cause severe cerebellar hypoplasia and a significant reduction in CGNP proliferation. TNCYFP-CreER; SmoF/− (SmoCKO) mice demonstrate an obvious reduction in cerebellar size within two days of ablation of Shh signaling. Mutant cerebella have severely reduced proliferation and increased differentiation of CGNPs due to a significant decrease in Shh activity and concomitant activation of Wnt signaling in SmoCKO CGNPs, suggesting that this pathway is involved in cross-talk with the Shh pathway in regulating CGNP proliferation. In addition, Purkinje cells are ectopically located, their dendrites stunted, and the Bergmann glial network disorganized. Collectively, these data demonstrate a previously unappreciated role for Bergmann glial Shh signaling activity in the proliferation of CGNPs and proper maintenance of cerebellar architecture.
Keywords: Bergmann glia, Sonic hedgehog, cerebellum, neuronal-glial relationships
INTRODUCTION
Cerebellar development proceeds in a tightly regulated manner, requiring the proper balance of neural progenitor cell expansion and differentiation to form a characteristically organized structure. However, our understanding of the cellular relationships and signaling pathways that contribute to this balance is incomplete. A cell type integral to development of the cerebellum is the cerebellar granular neuron precursor (CGNP), which occupies a transient layer on the outer surface of the cerebellum from embryonic day 14 to two weeks postnatally in mice. CGNPs proliferate in response to Sonic hedgehog (Shh) ligand, which is secreted by neighboring Purkinje cells (PC). The Shh signal is transduced in CGNPs by the Smoothened (Smo) transmembrane protein to initiate production of activator forms of the Gli transcription factors (iGoodrich et al. 1996; Ingham and McMahon 2001; Marigo et al. 1996; Varjosalo and Taipale 2008; Fuse et al. 1999). After exiting mitosis, differentiated granular neurons migrate inward, past the PC layer, where they populate the internal granular layer (IGL). CGNPs are the presumed cell-of-origin for the Shh-driven subset of the malignant pediatric brain tumor medulloblastoma; thus understanding the cellular and molecular factors that govern their proliferation is critical.
Neuronal progenitors including CGNPs are often in close contact with glial cells (Shiga, Ichikawa, and Hirata 1983; Buffo and Rossi 2013), however relatively little attention has been given to the function of neuron-glial interactions in the cerebellum. Specialized, unipolar astrocytes called Bergmann glia (BG) are present in the cerebellum and originate from radial glia of the cerebellar ventricular zone. As early postnatal cerebellar development proceeds, BGs migrate behind PCs, ultimately aligning their cell bodies in the same single-celled layer. Their characterized functions in the cerebellum are three-fold. First, BG radial fibers extend to the pial surface shortly after birth where their endfeet contact the basement membrane (Rakic 1971; Yamada et al. 2000). The endfeet adhere to one another to form a glia limitans over the cerebellum (Das 1976), providing structural support as the cerebellar plate expands (Hausmann and Sievers 1985; Sievers and Pehlemann 1986; Sievers et al. 1981). Second, as granular neurons differentiate and begin to migrate inwards to form the internal granular layer, BG radial fibers function as guides to the closely aligned CGNP cell bodies (Rakic 1971). And last, BG radial fibers synapse on PC dendrites; it has been proposed that BGs contribute to PC dendritic elaboration (Lippman et al. 2008; Yamada et al. 2000) and stabilization of neuronal synaptic connections (Iino 2001; Yue 2005).
BGs belong to a select group of specialized astroglia that retain radial glial-like morphology postnatally and into adulthood; members of this group include BGs, Muller cells in the retina, and tanycytes of the hypothalamus (Rakic 2003). Importantly, while both Muller cells and tanycytes have been demonstrated to have neurogenic potential and contribute to neurogenesis in their respective regions (Haan et al. 2013; Surzenko et al. 2013; Robins et al. 2013), BGs have not been found to display such features. Rather, mice with BG defects during development exhibit altered cerebellar layering, neuronal migration, synaptic connectivity, and a disrupted pial membrane (Belvindrah et al. 2006; Graus-Porta et al. 2001; Wang et al. 2011; Eiraku et al. 2005; Komine et al. 2007; Weller et al. 2006). The contribution of BGs to neuronal specification and proliferation in the cerebellum has not been extensively studied.
Many signaling pathways are important for the formation and maintenance of BGs. Genetic studies using mice that lack Notch pathway components have demonstrated the pathway to be integral for BG specification, maturation, and monolayer formation (Eiraku et al. 2005; Komine et al. 2007; Weller et al. 2006). Other studies have shown that PTEN and integrin-linked kinase play roles in BG differentiation (Yue 2005; Belvindrah et al. 2006), whereas APC maintains BG morphology (Wang et al. 2011) and the guanine nucleotide exchange factor Ric-8a regulates BG basement membrane adhesion (Ma, Kwon, and Huang 2012). Transcriptional profiling studies of BGs also identified the Wnt and TGFβ signaling pathways as developmentally upregulated in BG though their function in BG has yet to be identified (Koirala and Corfas 2010). Interestingly, BGs have been shown to be capable of responding to Purkinje-derived Shh signals in postnatal stages through adulthood (Corrales et al. 2004; Corrales et al. 2006). It has been observed that Shh signaling induces the glial differentiation of immature postnatal mouse astroglia in vitro (Dahmane and Ruiz i Altaba 1999). In addition, inhibition of Shh activity using 5E1 hybridoma cells injected into chick embryos at early stages resulted in massive perturbations of cerebellar development, including a concomitant reduction in BLBP+ BG (Dahmane and Ruiz i Altaba 1999) (Dahmane and Ruiz i Altaba 1999). However, the role of Shh signaling activity in BG in vivo and its consequences for cerebellar development are not well understood. Understanding how BG contribute to CGNP proliferation and thus overall architecture of the cerebellum can shed light on basic developmental processes and have implications for cerebellar diseases that derive from aberrant Shh signaling and neuronal-glial relationships.
In this study, we spatially and temporally alter Shh signaling activity in postnatal BG. Mice in which Shh activator Smoothened (Smo) is postnatally ablated in BG demonstrate an obvious reduction in cerebellar size within two days of ablation of Shh signaling. Surprisingly, mutant CGNPs exhibit severely reduced proliferation and increased differentiation accompanied by a loss of Shh activity, suggesting a novel role for the BG-CGNP interaction in promoting CGNP precursor proliferation. Interestingly, Wnt signaling is ectopically elevated in mutant CGNPs concomitant with a reduction in EGL area, suggesting that this pathway is involved in cross-talk with the Shh pathway in regulating CGNP proliferation. In addition, loss of Shh signaling in BG leads to disrupted PC laminar organization and dendritic arborization as well as BG fiber morphology, indicating that BG-Shh signaling activity contributes to the maintenance of proper cerebellar laminar formation. Collectively, these data show a previously unappreciated role for BG Shh signaling activity in the proliferation of CGNPs and preservation of cerebellar architecture, thus leading to a new level of understanding of the neuronal-glial relationship in the cerebellum.
MATERIALS AND METHODS
Animals and Tamoxifen Administration
Mice of the following genetic lines, of either sex, were used in the study: Gli1nlacZ (Bai et al. 2002), TNCYFP-CreER (Fleming et al. 2013), SmoF/F (Long et al. 2001), Bat-gal (Maretto et al. 2003), Math1CreER (Machold and Fishell 2005), R26ReYFP (Srinivas et al. 2001), tdTomato (Madisen et al. 2009), L7-Cre (Lewis et al. 2004) and ShhF/F (Lewis et al. 2001). Tamoxifen (Sigma) was dissolved to a final concentration of 2 mg/ ml in corn oil (Sigma). Postnatal TNCYFP-CreER; tdTomato, TNCYFP-CreER; R26ReYFP, TNCYFP-CreER; SmoF/− (Smocko), Math1CreER; SmoF/−, and WT littermates received 50 uL of tamoxifen by intraperitoneal injection on P1 and P2 or on P4 and P5 where noted.
Tissue processing and Immunohistochemistry
For animals younger than P30, brains were dissected and fixed in 4% paraformaldehyde for either 4–6 hours or O/N at 4°C. Animals P30 and older received 50 uL intraperitoneal injections of Ketamine and received ice-cold PBS via transcardial perfusion followed by 4% paraformaldehyde. Brains were collected and submersion fixed in 4% paraformaldehyde O/N at 4°C. These tissues were either processed for frozen embedding in OCT compound or processed for paraffin embedding. Frozen tissues were sectioned on a Leica cryostat at 10 um, paraffin embedded tissues were cut at 5 um. Immunohistochemistry were performed as previously described (X. Huang et al. 2009; X. Huang et al. 2010). The following primary antibodies were used on frozen and/or paraffin tissue sections: chicken α-β-Gal (ICL), rabbit α-β-Gal (ICL), rabbit α-BLBP (Abcam), rabbit α-GFAP (Abcam), chicken α-GFP (Aves), guinea pig α-Gli2 (Qin et al., 2011), rabbit α-Calbindin (Swant), rabbit α-phospho-Histone-3 (Upstate Cell Signaling), mouse α-NeuN (Millipore), mouse α-Parvalbumin (Sigma), rabbit α-3-PGDH (Thermo-Scientific), rabbit α-Sox2 (Millipore), mouse α-Laminin (Thermo-Scientific), rabbit α-p27Kip1 (BD Transduction Labs). For bright-field staining, species-specific HRP-conjugated secondary antibodies (Invitrogen) were used followed by incubation in DAB reaction (Invitrogen) or alkaline-phosphatase (Invitrogen). Double-labeling fluorescence immunohistochemistry was performed using species-specific, AlexaFluor-tagged secondary antibodies Alexa 488, Alexa 568, and Alexa 647 (Invitrogen) followed by counterstaining with To-pro3 iodide (Invitrogen).
X-Gal and In Situ Hybridization
X-Gal staining for β-Galactosidase activity was performed on post-fixed, frozen sections according to standard protocols. Section in situ hybridizations were performed using digoxygenin-labeled riboprobes as previously described (Li et al. 2006; Li et al. 2008). Riboprobes were synthesized using the digoxygenin RNA labeling kit (Roche). The following cDNAs were used as templates for synthesizing digoxygenin-labeled riboprobes: Shh and Sfrp1 (gift of Paula Bovolenta, Centro de Biologia Molecular Universidad Autonoma Madrid, Madrid, Spain).
CGNP and Cerebellar Isolation and Western Blotting
For CGNP isolation, P4 or P5 cerebella from CD1 or SmoBG mice were dissected into calcium-free Hanks buffered saline solution (Mediatech) supplemented with 6g/L D-glucose. The meninges were stripped and pooled cerebella dissociated with Accutase (Gibco) and trituration. Cells were pelleted and resuspended in Neurobasal A-medium containing 250 µM KCl, 500µL 100× GlutaMAX I, 500µL 100× penicillin-streptomycin, and 10% FBS. Cells were passed through a 70µm filter and incubated for two times 20 minutes on poly-d-lysine coated plates. Following the settling step, the cells remaining in the media were considered the CGNP fraction and were collected, pelleted, and ready for lysis. For cerebellar isolation, P4 or P5 cerebella from CD1 or SmoBG mice were dissected and tissue mechanically dissociated by trituration. Cell or tissue lysis was performed in RIPA buffer containing 2.5 mM EDTA, 1mM PMSF, 10mM NEM, 0.1 mM sodium orthovanadate, 0.2 mM sodium fluoride, and EDTA-free complete mini protease inhibitor tablets (Roche), for thirty minutes, followed by boiling in SDS, and resolution on 10% SDS-polyacrylamide gels. For cerebellar Shh ligand detection, P4 or P5 cerebella from CD1 or Smocko mice were dissected and immediately boiled in SDS for 5 minutes.
Primary antibodies used for Western blotting were mouse α-Sfrp1 (Abcam, 1:500), rabbit α-Gli1 (Cell Signaling Technology #2534, 1:2000), guinea pig α-Gli2 (gift of Jonathan Eggenschwiler, 1:500), rabbit α-Shh (H160) (Santa Cruz Biotechnology, 1:500), mouse anti-α-tubulin (Hybridoma Bank, 1:10,000), mouse α-β-actin (ThermoScientific BA3R, 1:10,000).
Flow cytometry, RNA isolation, reverse transcription, and RT-PCR
Identical methods to those described previously (Fleming et al. 2013) were employed in this study. Briefly, following dissociation of neonatal cerebella, YFP fluorescence intensity was used to purify Bergmann glial cells from the TNCYFP-high fraction. Total RNA was purified from sorted cells using the RNeasy mini kit (QIAGEN) and cell homogenization was performed using QIAshredder columns (QIAGEN). cDNAs were synthesized with 300 ng total RNA input for all samples using a high-capacity cDNA reverse transcription kit (Applied Biosciences). PCR was performed with primers for Smo and Gapdh.
Quantification and statistical analyses
Stained slides for quantification were scanned with the Leica SCN400 Slide Scanner and quantification was performed using the Leica Ariol Software. Statistical analyses were performed using Prism software (GraphPad).
Author Contributions
F.C. and C.C. designed and wrote the manuscript. J.F. and F.C. performed experiments. J.F., F.C. and C.C. analyzed data.
Additional Information
The authors declare no competing financial interests.
RESULTS
Smocko mutants display a hypoplastic cerebellum
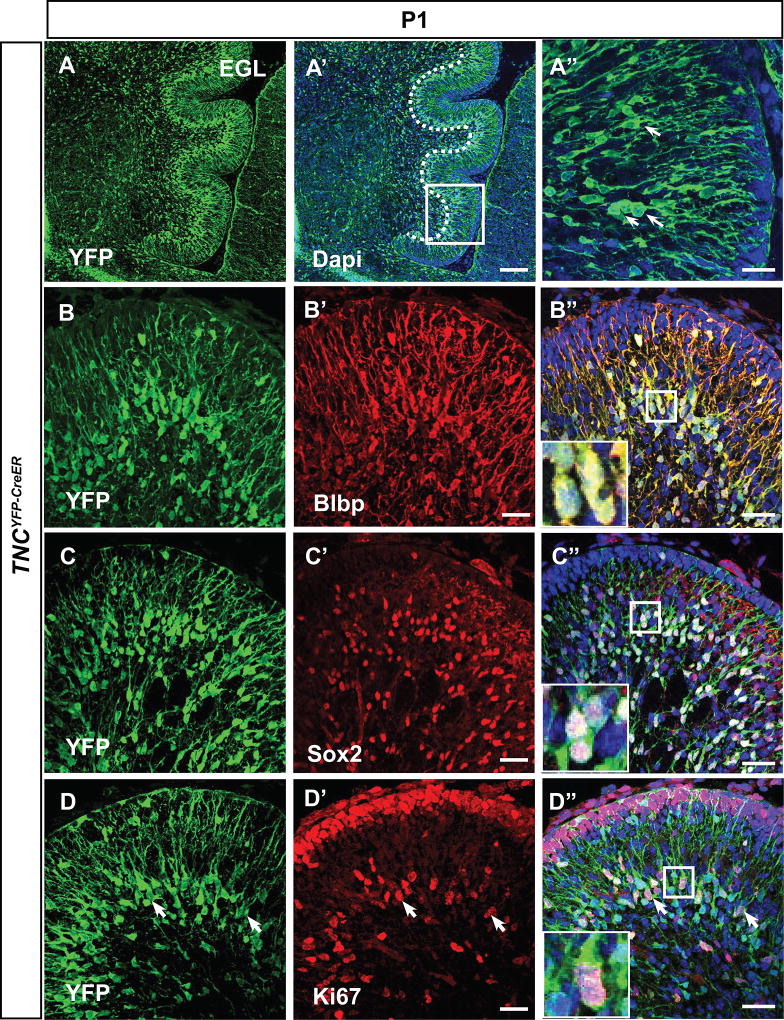
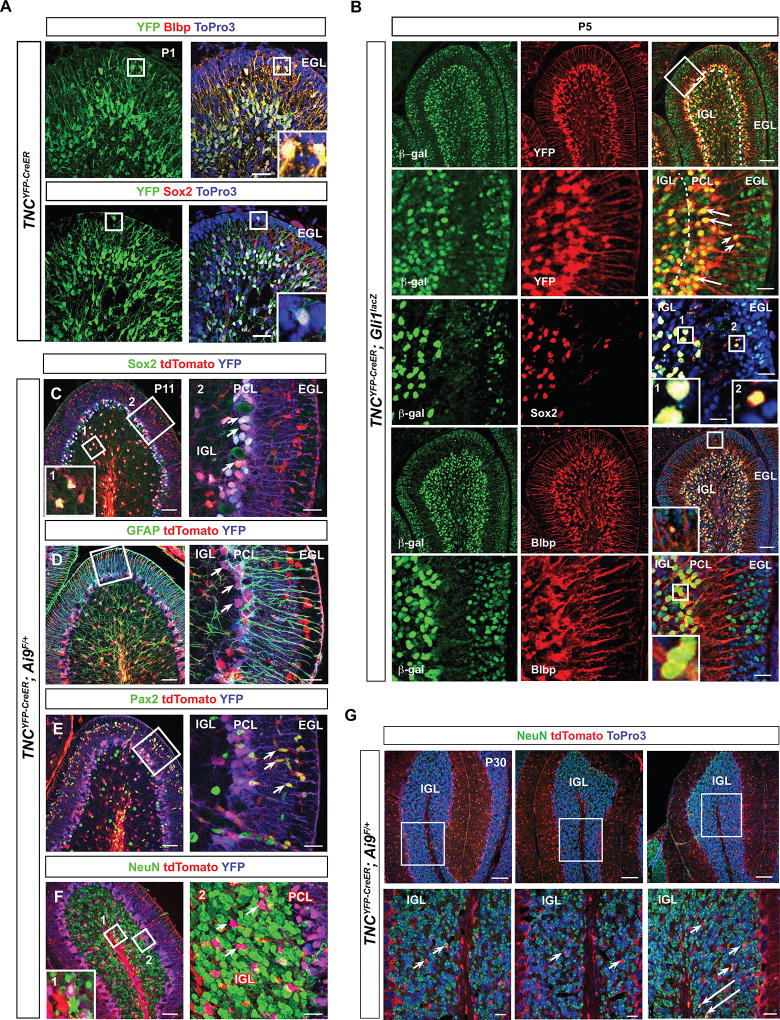
In our experiments, we used a tamoxifen-inducible Cre line, Tenascin-C(TNC)YFP-CreER in order to ablate Shh signaling in BG. TNCYFP-CreER mice express CreER and YFP as a bicistronic message from the endogenous TNC locus (Fleming et al. 2013); thus YFP can be used to mark TNC expressing cells. We first wanted to verify that TNC-YFP+ was indeed localized to BG cells, as previously described (Bartsch et al. 1992; Yuasa 1996). Using antibodies against YFP and radial glial markers Sox2 and Blbp (Feng, Hatten, and Heintz 1994; Yamada et al. 2000) at postnatal day 1, we confirmed that TNC-YFP was expressed by Sox2+, Blbp+ BG (Figure 1A–D”), whose cell bodies are seen in the developing cerebellar cortex with radial processes extending towards the pia matter. Notably, these TNC-expressing cells were proliferative as many of them expressed proliferative marker Ki67 (Figure 1E–E”, inset and arrowheads).
Figure 1. TNC-YFP-expressing cells are Bergmann glia.
(A–A”) YFP immunohistochemistry on sagittal sections demonstrates pattern of TNC expressing cells. Boxed region denotes enlarged area in (B’). TNC-YFP expression is observed in cells that extend long processes to the pial surface (A”). (B–C”) TNC-YFP expressing cells are Blbp+ (B–B”) and Sox2+ (C–C”), indicating that they express astroglial markers. Inset shows example of co-labeled cell (B”, C”). (D–D”) TNC-YFP expressing cells are ki67+, indicating that they are proliferative. Inset shows example of co-labeled cell (D”). Abbreviation: EGL, external granular layer. Scale bars are 20 µm except A”, B”, C” and D” (100 µm).
In order to determine the role for Shh signaling in BG, we crossed TNCYFP-CreER mice with SmoF/− mice to generate TNCYFP-CreER; SmoF/− (Smocko mutant) mice in which Shh effector protein Smoothened (Smo) is removed from TNC-expressing BG (Yuasa 1996). We chose to affect the time period when BG are closely apposed to their final destination between PC bodies, thus we injected one dose each of tamoxifen at P1 and P2 (Yuasa et al. 1991). We began our analysis of tamoxifen-injected animals at P3 and also analyzed later stages at P4, P5, P7 and P30.
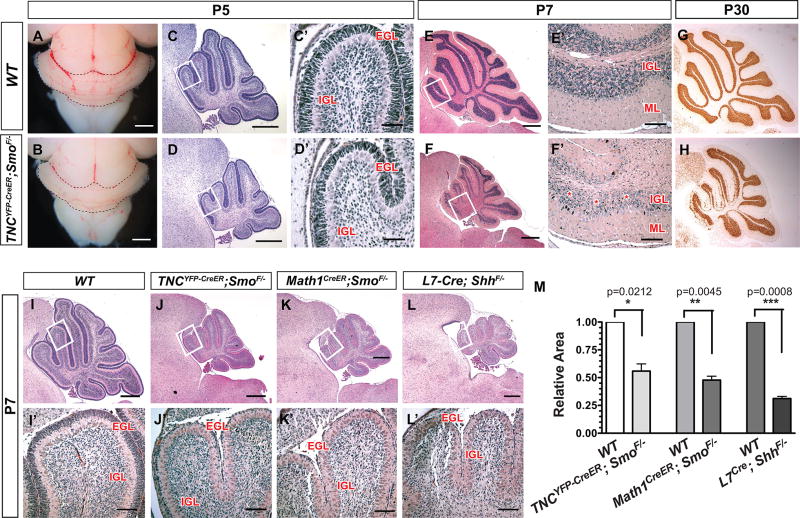
Interestingly, Smocko mutant mice at P5 revealed a noticeably hypoplastic cerebellum (Figure 2A–B). H&E staining of sagittal sections demonstrated a reduction in cerebellar area and folia size (Figure 2C–D”). By P7, a 35.8% (n=3, p=0.0282) reduction in cerebellar area was observed (Figure 2E–F”, M). At P30, the smaller cerebellar size was accompanied by a profound decrease in internal granular layer (IGL) density (Figure 2G,H).
Figure 2. TNCYFP-CreER;SmoF/− (Smocko) mutants display a hypoplastic cerebellum.
Tamoxifen was injected at P1 and P2 in WT and Smocko mutants. Mice were analyzed as early as P3 and as late as P30. (A–B) External view of Smocko mutants and WT littermates at P5 demonstrates the reduction in overall cerebellar size. (C–F’) H&E staining of mid-sagittal sections of Smocko mutants (D, D’ F, F’) and WT (C, C’, E, E’) littermates at P5 (C–D’) and P7 (E–F’). (G–H) NeuN staining at P30 demonstrates that mutants have significant cerebellar hypoplasia. (I–L’) H&E staining of mid-sagittal sections of WT (I, I’), Smocko (J, J’), Math1CreER;SmoF/− (K, K’) and L7Cre;ShhF/− (L, L’) mutants at P7. (M) Quantification of cerebellar area of WT littermates and Smocko mutants, Math1CreER;SmoF/− mutants, and L7Cre;ShhF/− mutants. Data are mean of n=3 WT and littermate pairs for each genotype. Boxed region shows enlarged region in adjacent panel. Abbreviations: EGL, external granular layer. ML, molecular layer. PCL. Purkinje cell layer. IGL, internal granular layer. Scale bar: 20 µm.
We note that the phenotype is not as severe as other mutants with aberrant cerebellar Shh signaling, L7-Cre;ShhF/− (Lewis et al. 2004) mice, in which Shh ligand is deleted from PCs, and Math1CreER; SmoF/− mice in which Smo is temporally ablated in CGNPs using tamoxifen at P1 and P2 (Machold and Fishell 2005). As shown in Figure 2I–M at P7, both the L7-Cre;ShhF/− and the Math1CreER;SmoF/− mutants displayed more severe cerebellar hypoplasia as compared to Smocko mutants, with a 49.7% (n=3, p=0.0033) and 49.8% decrease (n=3, p=0.0008) in cerebellar size compared to WT littermates, respectively. In addition, L7-Cre; ShhF/− and Math1CreER; SmoF/− mutants lacked an EGL as would be expected since Shh signaling is required for expansion of CGNPs (Figure 2I–L’)(Dahmane and Ruiz i Altaba 1999; Wechsler-Reya and Scott 1999). Taken together, these studies demonstrate that ablation of Shh signaling in TNC-expressing BG results in severe reduction in cerebellar size.
Smocko EGL is largely agranular due to severely reduced CGNP proliferation
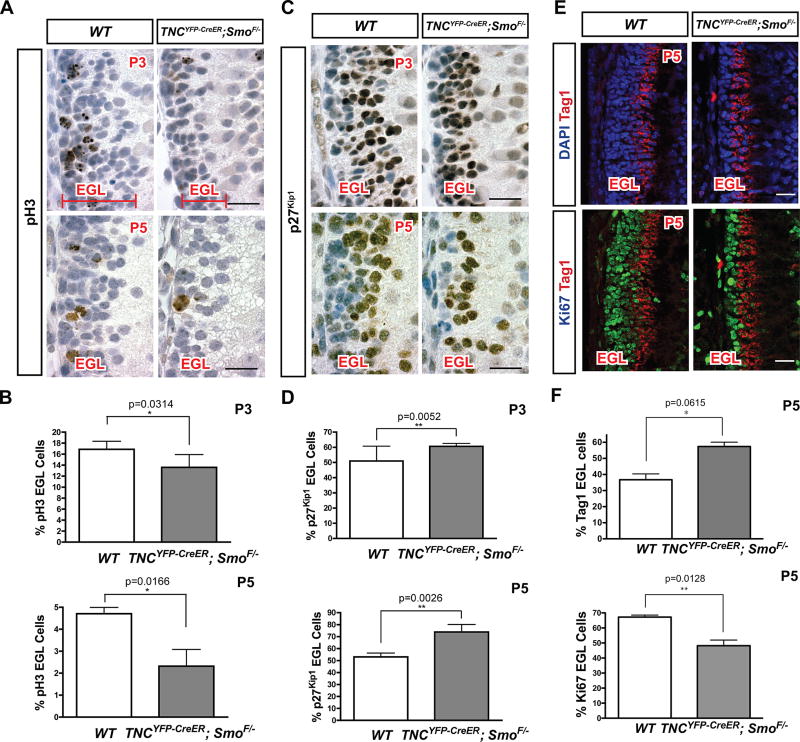
One striking feature noted in the Smocko mutant was that, despite all cortical layers being present at early postnatal stages, the EGL was noticeably reduced in thickness at P7 (Figure 2C). In order to determine the earliest time point at which EGL area reduction could be observed, we collected SmoBG mutants at P3 and P5 and quantified EGL area. At P3, the EGL in Smocko mutants was significantly reduced in area by 24.8% (n=3, p=0.0014) (Figure 3A, Figure S1). By P5, the reduction in EGL area was 50.3% (n=3, p<0.0001) (Figure 3A, Figure S1).
Figure 3. Mutant EGL is largely agranular due to severely reduced CGNP proliferation.
(A) Immunohistochemistry for pH3 (brown) at P3 and P5 in WT and Smocko mutants. (B) Quantification of pH3 staining as a percentage of total EGL cells. At P3, a 19.5% decrease (n=3, p=0.0314) in pH3 was observed. At P5, a 51.1% decrease in pH3 (n=3, p=0.0166) was observed. (C) Immunohistochemistry for p27Kip1 (brown) at P3 and P5 in WT and Smocko mutants. (D) Quantification of p27Kip1 staining as a percentage of total EGL cells. At P3, an 18.9% increase (n=3, p=0.0052) in p27Kip1-positive cells was observed. At P5, a 39.2% increase (n=3, p=0.0026) in p27Kip1-positive cells was observed. Sections in A and C were counter stained with hematoxylin to highlight the nuclei (blue). (E) Immunofluorescence staining of Ki67 (green) and Tag1 (red) at P5 in WT and Smocko mutants. (F) Quantification of Tag1 staining as a percentage of total EGL cells. A 56% increase (n=3, p=0.0615) in Tag1-positive cells was observed. In contrast, a 28.3% decrease (n=3, p=0.0128) in Ki67-positive cells was observed. Abbreviations: EGL, external granular layer. Scale bar: 20 µm.
In order to determine whether reduction in EGL area was due to changes in proliferation, differentiation, or apoptosis of CGNPs, we performed immunohistochemistry for mitotic marker phospho-histone H3 (pH3), differentiated neural marker p27Kip1, and cleaved caspase 3, respectively. At P3, we observed a 19.5% decrease in pH3 (n=3, p=0.0314) (Figure 3A, B) and an 18.9% increase in p27Kip1-positive cells (n=3, p=0.0052) (Figure 3C, D). At P5, we observed a 51.1% decrease in pH3 (n=3, p=0.0166) (Figure 3A, B) and a 39.2% increase in p27Kip1-positive cells (n=3, p=0.0026) (Figure 3C, D). Similar results were obtained with Ki67 and Tag1, which marks proliferating and differentiating CGNPs, respectively. At P5, we observed a 56% increase (n=3, p=0.0615) in Tag1-positive cells and a 28.3% decrease (n=3, p=0.0128) in Ki67-positive cells (Figure 3 E, F). No changes in apoptotic marker cleaved-caspase 3 were observed (data not shown). These results suggest that ablation of Shh signaling in BG results in a profound reduction in CGNP proliferation with a concomitant increase in differentiation. As the current understanding of BG function during early postnatal development has been limited to roles in CGNP migration and PC synaptogenesis (Rakic 1971; Yamada et al. 2000; Lordkipanidze and Dunaevsky 2005), these findings represent a novel role for BG in the regulation of cerebellar development. Specifically, these data demonstrate that Shh signaling in BG serves to maintain the proliferative capacity of CGNPs.
Loss of Shh signaling is profound in CGNPs
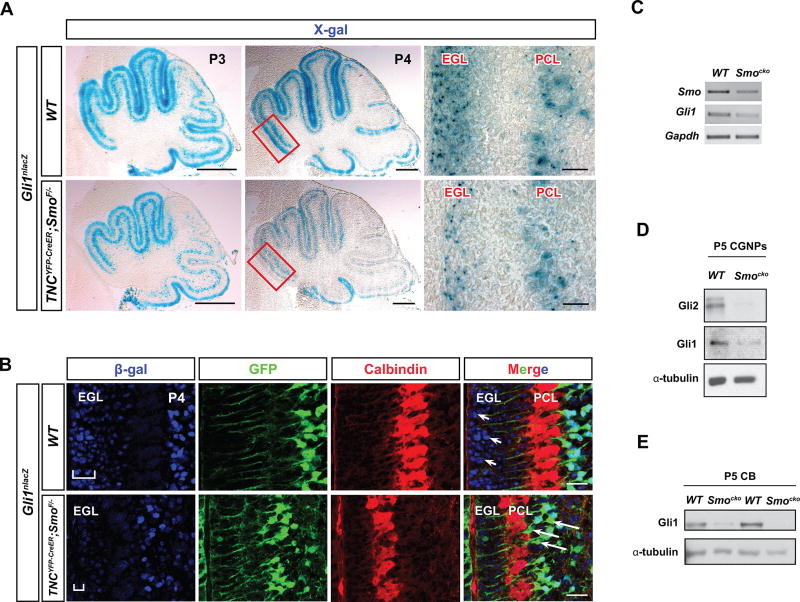
It is now well-established that CGNP expansion in the EGL relies on Shh signaling activity from PC as a proliferative signal (Dahmane et al. 1997; Wallace 1999; Wechsler-Reya and Scott 1999; Pons et al. 2001). In the absence of Shh, CGNPs cease proliferating, withdraw from the cell cycle, and differentiate (Dahmane and Ruiz i Altaba 1999; Wallace 1999; Wechsler-Reya and Scott 1999). As Smocko mutants display a profound reduction in CGNP proliferation in the EGL and an increase in differentiation, we next sought to determine whether Shh signaling activity was specifically affected in this progenitor population. Therefore, we crossed Smocko mutant and WT mice with the Gli1nlacZ knock-in mouse (Bai et al. 2002). In WT animals, X-gal staining was localized to the EGL and PC layer as previously reported (Corrales et al. 2004; Lewis et al. 2004) (Figure 4A), indicating the presence of Shh responsive cell types in those layers. As expected, in Smocko mutants, X-gal staining was reduced in the PC layer, indicating a decrease in Shh signaling in BG (Figure 4A). RT-PCR analysis for Smo and Gli1 mRNA confirmed mosaic deletion of Smo and attenuation of Shh signaling of purified TNC-YFP cells (Figure 4B). In addition to BG, X-gal staining was noticeably reduced in the EGL at P4 (Figure 4A). Likewise, β-gal antibody staining was visibly reduced in CGNPs in the EGL in addition to the BG (Figure 4C). We corroborated these results by isolating fresh CGNPs from the cerebellum and blotting for Gli1 protein. We found that CGNPs isolated from Smocko mutant cerebella demonstrated a decrease in Gli1 and Gli2 protein levels as compared with those from WT cerebella (Figure 4D). Smocko mutant cerebellar lysates at P5 also demonstrated a decrease in Gli1 levels compared to WT cerebellar lysates (Figure 4E), indicating an overall reduction in Shh signaling in mutant cerebella. These results demonstrate that reduction in proliferation of the EGL and subsequent differentiation of CGNPs in Smocko mutants occurs as a result of attenuated Shh signaling activity.
Figure 4. Loss of Shh signaling is profound in CGNPs.
(A) X-gal staining for β-galactosidase in Gli1lacZ and TNCYFP-CreER;SmoF/−;Gli1lacZ mutants shows that X-gal is reduced in the PCL and EGL of mutants at P3 and P4. Rectangular region in P4 panel shows enlarged area in adjacent panel. (B) β-galactosidase immunohistochemistry in WT and mutants reveals a decrease in antibody staining in the mutant EGL at P4. GFP and Calbindin staining demonstrates location of BGs and PCs, respectively. Arrowheads indicate presence of β-gal+ cells in WT EGL, note that β-gal expression is absent in the mutant EGL. Arrows indicate GFP+ BGs that no longer express β-gal+, indicating a reduction in Gli1 expression in the mutant. (C) RT-PCR of Smo and Gli1 from purified TNC-YFP population shows reduced Shh signaling in Smocko mutants. (D) Western blotting of P5 WT and mutant CGNPs shows a decrease in Gli1 and Gli2 protein levels in the mutant. (E) Western blotting of P5 cerebellar lysates shows a reduction in Gli1 protein levels in the mutant. Abbreviations: EGL, external granular layer. PCL. Purkinje cell layer. CGNPs, cerebellar granular neuron precursors. CB, cerebellum. Scale bar: 100 µm and 20 µm.
Resident astroglial cells in the EGL do not contribute significantly to the granular neuron population
In our characterization of the TNCYFP-CreER mouse, we observed a small subset of sparsely dispersed TNC-YFP expressing cells in the EGL (Figure 5A). In order to investigate their identity, we used Sox2 and Blbp to determine if they were astroglial cells because a small subset of astroglia have been described in the external granular layer (EGL) that express Sox2 and Blbp and are capable of giving rise to CGNPs (Silbereis et al. 2010; Sievers et al. 1981). We found the TNC-YFP-expressing EGL cells to be both Sox2+ and Blbp+ (Figure 5A), suggesting that TNC-YFP marks these previously described astroglial cells (Silbereis et al. 2010).
Figure 5. Resident astroglial cells in the EGL do not contribute significantly to the granular neuron population.
(A) YFP immunohistochemistry of TNCYFP-CreER at P1 on sagittal sections demonstrates β-gal+-expressing cells in the EGL. These are Blbp+ and Sox2+, indicating that they express astroglial markers. Inset shows example of co-labeled cell. (B) β-galactosidase immunohistochemistry of TNCYFP-CreER; Gli1lacZ sagittal sections at P5 demonstrates that YFP+ cells in the EGL and PCL are β-gal+. Arrows and arrowheads indicate cells that are co-labeled with YFP and β-gal in the PCL and EGL, respectively. β-gal+ cells in the PCL and EGL are Sox2+ and Blbp+. Boxed regions indicate examples of co-labeled cells. (C–F) Lineage tracing using TNCYFP-CreER; tdTomato sagittal sections at P11. (C) The majority of tdTomato+ cells are in the PCL and are Sox2+. Boxed region labeled “1” demonstrates example of Sox2+ TNC-lineage cell in IGL. Boxed region labeled “2” shows enlarged region in adjacent panel. Arrowheads indicate co-labeled cells in PCL, indicating that TNC-lineage cells are BGs. (D) The tdTomato+ cells in the EGL show GFAP+ fibers, boxed region shows enlarged region in adjacent panel. Arrowheads indicate tdTomato+ BGs associated with GFAP+ fibers. (E) Pax2 immunohistochemistry demonstrates that TNC-lineage cells found adjacent to the EGL are immature GABAergic interneurons. Arrowheads indicate co-labeled cells. (F) NeuN immunohistochemistry demonstrates that the majority of TNC-lineage cells are not tdTomato+, indicating that TNC-lineage cells do not contribute significantly to the granular neuron population. Boxed region labeled “1” shows the only example of co-labeled cell. Boxed region labeled “2” shows enlarged region in adjacent panel, and arrowheads indicate tdTomato+ cells that are not NeuN+. (G) Lineage tracing using TNCYFP-CreER; tdTomato sagittal sections at P30 supports the data at P11 and shows that TNC-lineage cells do not contribute significantly to the granular neuron population. Arrowheads indicate tdTomato+ cells that are not NeuN+ and arrows indicate the only two examples of co-labeled cells that were observed in three lobes. Abbreviations: EGL, external granular layer. IGL, internal granular layer. PCL. Purkinje cell layer. Scale bar: Scale bar: 100 µm and 20 µm.
We next determined whether these cells, in addition to BG, were Shh signaling-responsive, because if so, these cells would also undergo deletion of Smo, and could explain the reduction of CGNPs in our mutants. Using β-gal and YFP double immunohistochemistry in TNCYFP-CreER;Gli1lacZ mice at P5, we found that YFP-expressing BG located in the PC monolayer co-labeled with β-gal (Figure 5B), as has been previously described (Corrales et al. 2004). In addition, YFP-expressing astroglial cells in the EGL co-labeled with β-gal (Figure 5B), indicating that both subsets are capable of responding to Shh signaling.
Because the major defect observed in Smocko mutants was reduced CGNP proliferation, we wanted to determine whether the TNC-YFP+ population residing in the EGL could contribute significantly to CGNPs, similar to what has been reported (Silbereis et al. 2010). Fate-mapping of the TNC lineage using TNCYFP-CreER; tdTomato mice indicated that the majority of tdTomato+ cells in the P11 cerebellum were largely confined to Sox2+ BG (Figure 5C) with GFAP+ fibers (Figure 5D). There was a subset of Pax2+, tdTomato+ cells apposed to the EGL (Figure 5E), which were immature GABAergic interneurons that have not yet begun to express Parvalbumin (Fleming et al. 2013). In order to determine whether tdTomato+ cells contributed to the mature granular neuron population, we co-labeled with granular neuron marker NeuN (Figure 5F). Approximately one or two cells per lobe co-labeled with NeuN, demonstrating that TNC-expressing cells can contribute to the granular neuron population, but this number remained very small (no more than 1–2 per lobe, or <0.1% of total NeuN+ cells). In fact, the majority of tdTomato+ cells in the IGL were Sox2+ glial cells (Figure 5C). These results were corroborated by NeuN, tdTomato double immunohistochemistry in TNCYFP-CreER; tdTomato mice at P30, a stage when the EGL has completely disappeared. In all lobes examined, no more than 2 NeuN, tdTomato double positive cells could be found (Figure 5G, arrows), and the majority of tdTomato cells in the IGL were NeuN-negative (Figure 5G, arrowheads). These results demonstrate that TNC-lineage cells do not contribute significantly to the adult granular neuron population. Therefore, it is unlikely that the Smocko mutant EGL phenotype occurs as a consequence of attenuated Shh signaling in the EGL astroglial population rather than in BG.
Smocko mutants display altered BG arrangement and cytoarchitecture
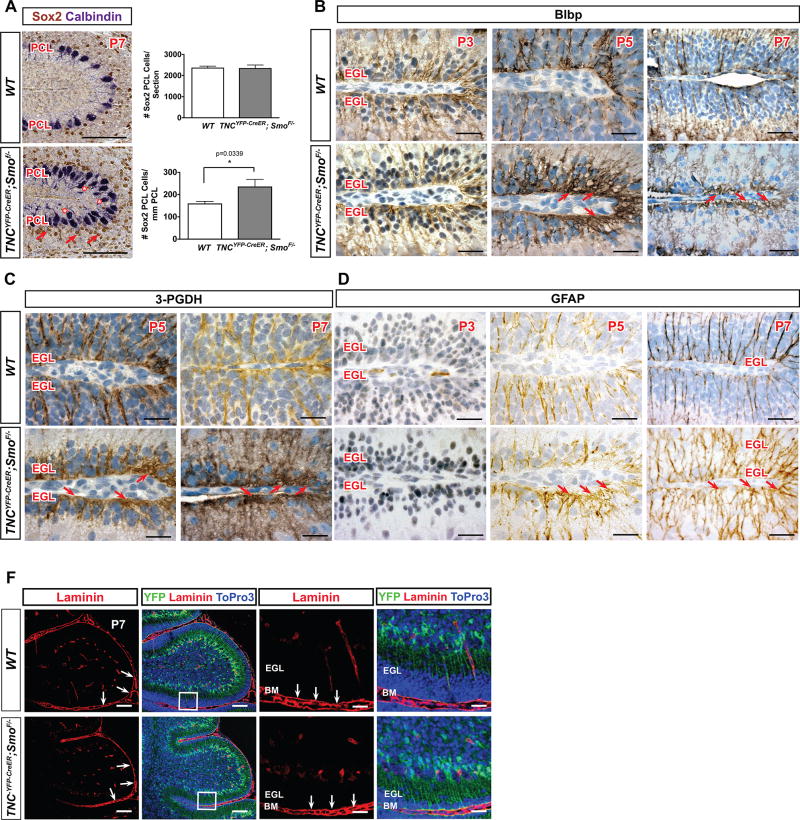
In order to further study whether loss of Shh signaling in BG affected the cytoarchitecture of BG and other cerebellar cell types, we analyzed PC and BG arrangement, density, and morphology. At P7, Calbindin+ PCs in WT cerebella formed the single-celled layer in between the molecular layer and internal granular layer that is characteristic of normal cerebellar architecture, but, in P7 Smocko mutant cerebella, PC soma localization was disrupted with many bodies out of alignment from the single-cell layer (Figure 6A, starred). BG fibers normally form a well-organized scaffold and extend processes towards the pial membrane where their endfeet contact the pial surface, and cell bodies of BG are uniformly arranged between PC bodies in the PC monolayer. We observed that BG cell soma size, as labeled by BG cell marker Sox2, were similar between WT and mutant; however positioning of cell bodies in Smocko mutants was irregular and aberrantly disorganized such that there was a significantly increased number of Sox2+ BG per mm of PCL due to smaller cerebella (68.4%, n=3, p=0.0339) (Figure 6A, arrows and graph). Rather than being localized in between PC soma, mutant BG cell bodies were displaced behind PC soma.
Figure 6. Smocko mutants display altered BG arrangement and cytoarchitecture.
(A) Sox2 labeling (brown) in P7 Smocko mutants demonstrate positioning of BG cell bodies in Smocko mutants is irregular and aberrantly disorganized (arrowheads). Calbindin labeling (purple) indicates disrupted PC soma localization (starred). Graph shows a significantly increased number of Sox2+ BG per mm of PCL and absolute numbers of BG are not different. Data are mean of n=3 WT and littermate pairs for each genotype. (B–D) Blbp, 3-PGDH, and GFAP staining (brown) at P3, P5, and P7 demonstrate an increased complexity of BG fibers and expansion of endfeet at the pial surface starting at P5 that increased in severity at P7. Sections are counter stained with hematoxylin to highlight the nuclei. (E) Laminin staining for the basement membrane did not reveal any differences Smocko mutants, indicating the basement membrane of mutant cerebella is intact. Abbreviations: EGL, external granular layer. ML, molecular layer. IGL, internal granular layer. Scale bar, 100µm and 20 µm.
In order to more extensively study Smocko mutant BG fibers, we used immunohistochemistry against BLBP, GFAP, and 3-phosphoglycerate dehydrogenase (3-PGDH) (Furuya et al. 2000) to label BG at P3, P5, and P7. BLBP is strongly expressed in BG cell bodies and fibers at early postnatal stages and is increasingly downregulated into adulthood (Feng, Hatten, and Heintz 1994). On the other hand, GFAP expression cannot be detected in BG fibers until P5 (Piper et al. 2011) and is upregulated throughout the next two weeks as BG mature (Giménez Y Ribotta et al. 2000). The enzyme 3-phosphoglycerate dehydrogenase (3-PGDH) strongly labels BG soma and fibers throughout postnatal development and adulthood (Furuya et al. 2000).
Mutant BG were positive for Blbp (Figure 6B, S2), indicating that differentiation along a glial lineage was preserved in the mutant. However, beginning at P5 we observed an expansion of the endfeet upon contact with the pial surface, which was particularly pronounced at the fissures in BG lacking Shh signaling (Figure 6B, arrows). In addition, in WT cerebella, BG fibers were uniformly rod-like and parallel to one another as they stretched from cell body to pial surface. However, in mutant cerebella the rod-like domain was severely disrupted and tortuous with an increase in lateral branching and complexity (Figure 6B). 3-PGDH and GFAP expression likewise demonstrated an increased complexity of BG fibers and expansion of endfeet at the pial surface (Figure 6C, D, arrows), which increased in severity over time.
Defects in BG fibers are also associated with aberrant formation or maintenance of the meningeal basement membrane, as has been demonstrated in several genetic mouse mutants with BG fiber defects (Belvindrah et al. 2006; Graus-Porta et al. 2001). Therefore, we assayed for laminin expression, which revealed in WT cerebella that the cerebellar cortex was covered with a continuous laminin+ layer that separated cerebellar folia (Figure 6E). We did not see any differences in laminin staining in Smocko mutants, indicating the basement membrane of mutant cerebella is intact (Figure 6E).
Smocko mutants have disrupted PC alignment and dendritic arborization
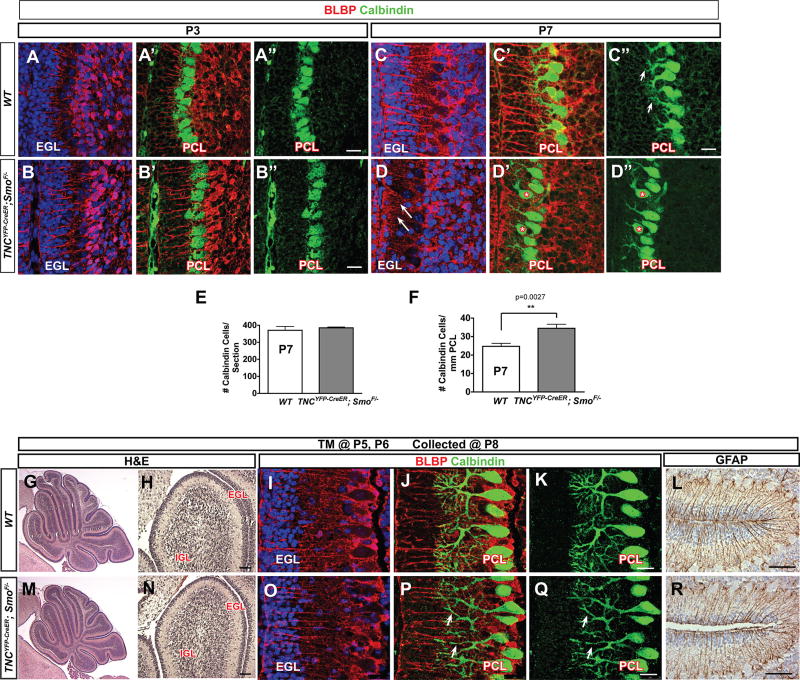
As the radial processes of BG provide a scaffold for the directed vertical growth of PC dendrites (Lordkipanidze and Dunaevsky 2005), we examined PC morphology and dendritogenesis using immunohistochemistry for the PC-cell specific marker Calbindin (Lordkipanidze and Dunaevsky 2005). At P3, the earliest time-point collected, we did not see a difference in PC morphology, layering, or dendritogenesis (Figure 7A and 7B–B”). However, as mentioned above, at P7, Calbindin+ PC soma localization was disrupted (Figure 6A and 7D–D”, starred). During early postnatal cerebellar development, clustered PCs disperse into the characteristic monolayer seen in the adult cerebellum in response to Reelin secreted by the EGL (Miyata et al. 1997; Miyata et al. 1996; Schiffmann, Bernier, and Goffinet 1997). As the EGL is severely disrupted in Smocko mutants, PC soma mislocalization is likely due to a consequence of loss of CGNPs and subsequently reduced CGNP-derived Reelin signaling. Consistent with this, we found that the absolute number of Calbindin+ PCs were comparable between the WT and mutant (Figure 7E), however there was a significant increase in the number of PCs per mm of PCL in Smocko mutants (28.3%, n=3, p=0.0027) (Figure 7F).
Figure 7. Smocko mutants have disrupted alignment and dendritic arborization of PCs.
(A–B”) At P3, Calbindin immunohistochemistry shows no difference in Smocko mutant (B–B”) PC morphology, layering, or dendritogenesis. (C–C”) At P7, Calbindin immunohistochemistry shows that PC soma localization is disrupted and have a severely disrupted fiber network, with stunted, thinned dendrites and poorly branched arbors. (E) The absolute number of Calbindin+ PCs were comparable between the WT and mutant. (F) There was a significant increase in the number of PCs per mm of PCL in Smocko mutants. Data are mean of n=3 WT and littermate pairs for each genotype. (G–R) Tamoxifen injection scheme in Smocko mutant at later timepoints. One dose of tamoxifen was injected at P5 and P6 in WT (G–L) and Smocko mutants (M–R). Mice were analyzed at P8. (G, H, M, N) H&E staining shows that changes in cerebellar size and EGL area were less severe when BG Shh signaling was ablated at P5 and P6 compared to ablation at P1 and P2. (I–L, O–R) PC dendrites in later timepoint-injected Smocko mutants (O–Q) lacked secondary branching structures as revealed by Calbindin immunohistochemistry (arrowheads) and no appreciable differences in BG fibers can be observed using Blbp (I–K, O–Q) and GFAP (L, R) immunohistochemistry. Abbreviations: EGL, external granular layer. IGL, internal granular layer. PCL. Purkinje cell layer. Scale bar: 100µm and 20 µm.
While PC dendritic arborization is immature at P7 (Sotelo and Dusart 2009), dendritic tips have begun to ascend vertically and enter the base of the EGL (Yamada et al. 2000), which was observed in our WT cerebella (Figure 7C). In contrast, mutant PC had a severely disrupted fiber network, with stunted, thinned dendrites and poorly branched arbors (Figure 7D, arrowheads). PC dendritic outgrowth depends on electrical activity (Schilling et al. 1991; Baptista et al. 1994) and neurotrophins secreted from CGNPs, including thyroid hormone (Heuer and Mason 2003), neurotrophin-3 (Lindholm et al. 1993), and BDNF (Shimada, Mason, and Morrison 1998). Thus, the disruption in PC arborization seen in Smocko mutants could be attributed to the loss of the EGL.
However, neuron dendritogenesis can also depend on factors derived from glial cells (Martin, Brown, and Balkowiec 2012; Procko and Shaham 2010), and it has been proposed that BGs contribute to PC dendritic elaboration (Lippman et al. 2008; Yamada et al. 2000). Therefore a possibility remained that BG-Shh signaling may play a role in PC dendrite formation that was unable to be appreciated due to the severity of the reduction in EGL area in Smocko mutants. In order to investigate BG Shh signaling contribution to PC dendritogenesis independent of CGNP proliferative effects, we ablated Shh signaling in BG at later developmental stages by injecting one dose each of tamoxifen at P5 and P6 and analyzed at P8. This later time point enabled us to examine the cerebellar phenotype after the first wave of CGNP proliferation has taken place. Changes in cerebellar size and EGL area were less severe when BG Shh signaling was ablated at P5 and P6 compared to ablation at P1 and P2 (Figure 7G–I, M–O). Notably, the PC dendrites still lacked secondary branching structures as revealed by Calbindin immunohistochemistry (Figure 7J–K and 7P–Q, arrows), though a BG fiber defect was not observed as determined by GFAP immunohistochemistry (Figure 7L and 7R). Because we continued to observe a PC dendrite defect in the absence of a severe EGL phenotype or BG fiber morphology defects, and in the absence of severe cerebellar hypoplasia, our results suggest that proper outgrowth of PC dendrites depends on Shh signaling activity to BG. However, we cannot rule out the possibility that minor defects in granule cell development may still contribute to PC dendritic defects.
Smocko mutants exhibit aberrant Wnt signaling
Next, we wished to determine downstream signaling pathways that could be influenced by depletion of BG-Shh signaling in Smocko mutant cerebella, and thus augmenting the reduction in CGNP proliferation. Several studies have implicated that antagonism of the Wnt pathway is important for the maintenance and proliferation of CGNPs. First, Wnt signaling is active in the rhombic lip and early migratory CGNPs at E14.5 but not in later stages of CGNP development (Fiacco and McCarthy 2006; Perea and Araque 2007; Pascual 2005). However, while it is not detected in the EGL, Wnt signaling is active postnatally in the PC layer in S100β+ BG (Selvadurai and Mason 2011) and at least one Wnt ligand, Wnt3, is expressed by BGs (GENSAT Mouse Brain Atlas).
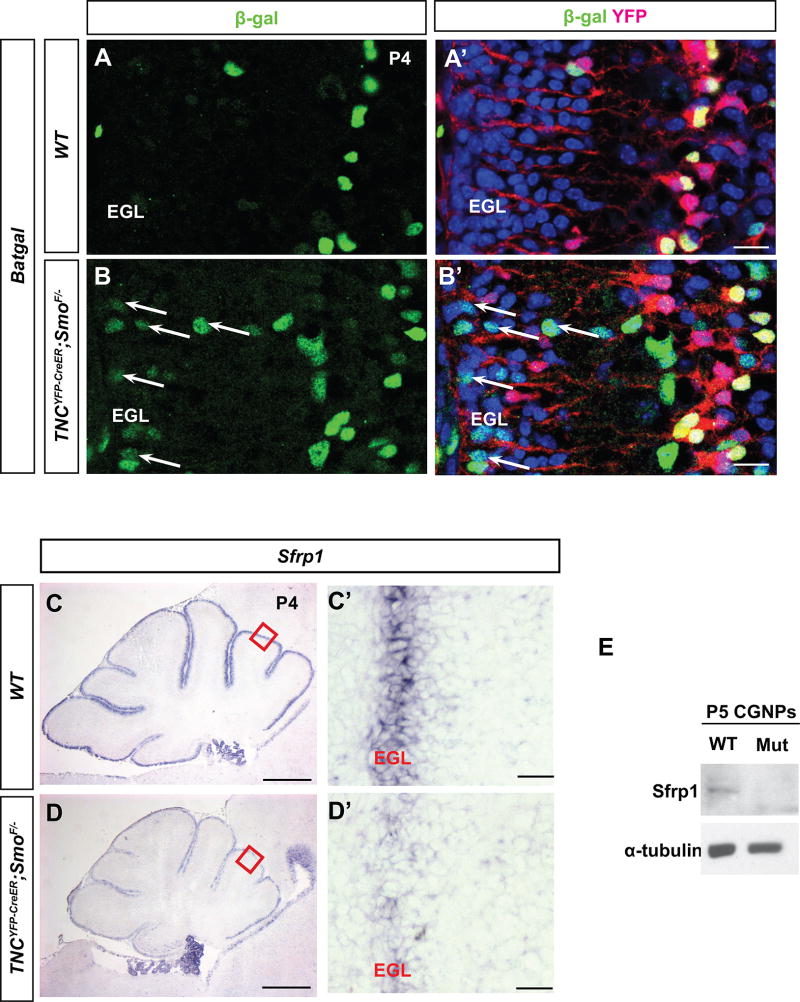
Additionally, CGNP-specific deletion of Wnt inhibitor APC resulted in severely inhibited CGNP proliferation and premature differentiation (Lorenz et al. 2011) and conditional activation of Wnt signaling using a dominant active form of β-catenin in neural precursors impaired CGNP proliferation (Pöschl et al. 2013). These studies suggest that ectopic Wnt signaling to the EGL may inhibit CGNP proliferation. Since we observed impaired CGNP proliferation in Smocko mutants, we hypothesized that Wnt signaling could be ectopically activated in the EGL. To test this hypothesis, we used a BAT-Gal transgenic reporter strain that expresses a lacZ gene under the control of β-catenin/T-cell factor responsive elements (Maretto et al., 2003) and has been widely used as a general reporter of Wnt/β-catenin activity. By crossing these mice with our mutants to obtain TNCYFP-CreER; SmoF/−; BAT-Gal mice, Wnt signaling activity could be examined in the EGL. In WT mice examined at P4, we observed very few cells expressing β-gal positivity (Figure 8A), corroborating studies demonstrating that Wnt signaling is not normally active in CGNPs at that stage (Selvadurai and Mason 2011). However, in the Smocko mutant we observed the presence of ectopic β-gal+ cells in the EGL of mutant mice (Figure 8B, arrows). These results indicate that Smocko mutant mice have enhanced Wnt signaling in CGNPs, suggesting that BG-derived Shh signaling may regulate Wnt signaling activity to the EGL.
Figure 8. Smocko mutants exhibit aberrant Wnt signaling.
(A–B’) β-galactosidase immunohistochemistry in P4 TNCYFP-CreER; SmoF/−; BAT-Gal mice (B, B’) shows the presence of ectopic β-gal+ cells in the EGL of mutant mice (arrows), indicating that Smocko mutant mice have enhanced Wnt signaling in CGNPs. (C–D’) In situ hybridization for Sfrp1 in Smocko mutants (D, D’) at P4 indicates a downregulation of the mRNA in the EGL. (E) Western blotting of lysates of freshly isolated CGNPs indicates a downregulation of Sfrp1 expression. Abbreviations: EGL, external granular layer. CGNPs, cerebellar granular neuron precursors. Scale bar, 200µm and 20 µm.
As several extracellular modulators of the Wnt pathway are expressed in BG and CGNPs, including Wnt-inhibitory factor 1 (Wif1) and Shh target gene secreted frizzled receptor protein-1 (Sfrp1), we hypothesized that the enhanced Wnt signaling observed in Smocko mutants may be due to reduced levels of Wnt antagonist expression. Sfrp1 is a 30-kDa secreted glycoprotein that acts as an antagonist of Wnt signaling (Finch et al.1997). It has been identified as a Shh signaling target gene, frequently upregulated in Hh-activated tumors (Romer et al. 2004), and suggested to serve as a molecular link for Shh-mediated Wnt inhibition (Katoh and Katoh 2006). Therefore, we examined gene expression of Sfrp1 in Smocko mutant cerebella using in situ hybridization. As seen in Figure 8C, Sfrp1 is localized to the EGL in the P4 WT cerebellum. In Smocko mutants, there was a striking decrease in Sfrp1 expression (Figure 8D), a finding we confirmed by probing lysates of freshly isolated CGNPs via Western blotting (Figure 8E). This data indicates a downregulation of Sfrp1 expression in the EGL of Smocko mutants, suggesting one possible downstream target of BG-derived Shh signaling activation.
DISCUSSION
In this study, we provide genetic evidence that Shh signaling in BG is required for proper CGNP proliferation and subsequent cerebellar cortical expansion. Our study provides a novel role for Shh signaling in the cerebellum, the non-cell-autonomous regulation of CGNP proliferation by BG. Interestingly, Smocko mutants have profound reductions in EGL area that can be observed starting 24 hours after the last tamoxifen injection (Figure 3A, S1) and prior to observable defects in BG fiber formation (Figure 6). This rapid time course from initiation of Cre-mediated recombination to EGL size reduction in mutants demonstrates the remarkable sensitivity of CGNP proliferation to BG-Shh activity, underscoring its relevance for the maintenance of overall cerebellar size and architecture.
BG belong to a select group of astroglia. They are astrocytic derivatives of radial glia that retain radial-glial like morphology into adulthood. While the other members of this group, retinal Muller cells and hypothalamic tanycytes, have been found to be neurogenic (Haan et al. 2013; Surzenko et al. 2013; Robins et al. 2013), BG have not been described as such and our lineage tracing studies demonstrate that TNC-YFP cells contribute very minimally to the granular neuron population (Figure 5C, D). Thus, it is unlikely that BG neurogenesis plays a significant role during normal development. Rather, our study indicates that, in addition to their well-characterized role in providing guidance cues for the inward migration of CGNPs (Rakic 1971), BG have a previously undescribed postnatal function in modulating proliferation of a cerebellar neuronal precursor population. This is consistent with a role for astrocytes in providing factors that support neuronal growth (Vernadakis 1988) and adds to our understanding of the neuronal-glial relationship in the cerebellum.
Although a select few other Shh-responsive cell types express TNC-YFP in the cerebellum, we conclude that the phenotype we observed is mainly due to Shh signaling ablation in BG. Several lines of evidence support this conclusion. First, our lineage tracing studies in TNCYFP-CreER; tdTomato mice indicate that the contribution of TNC-expressing cells to the granular neuron population in the IGL is insignificant, with no more than one or two per lobe in the cerebellum at P30 (Figure 5C, D). Thus, while the small TNC-expressing Sox2+/Blbp+ astroglial population in the EGL has been demonstrated to contribute to CGNPs (Silbereis et al. 2010) and is Shh responsive (Figure 5B), their contribution to the total number of granular neurons is too small to cause the severe phenotype we observe in Smocko mutants. However, we cannot exclude the possibility that these Sox2+/Blbp+ EGL cells secrete an inhibitory factor that modulates CGNP responsiveness to Shh ligand. The difficulty of studying these cells in isolation of other radial glial cells limits our ability to draw conclusions regarding this hypothesis, as currently there is no specific marker for this cell population and their function is unknown. Nevertheless, our lineage tracing studies and the sheer number of BG cells compared to these sparsely populated EGL cells, as well as the ability of the BG fiber to reach to the pial surface and contact all CGNPs, all support our conclusion that BG Shh signaling ablation is responsible for the phenotype of Smocko mutants. Additionally, the small population of TNC-expressing astroglial cells in the white matter does not contribute to GCP lineage (Fleming et al. 2013). However, in the absence of BG-specific Cre line, we cannot rule out the possibility that these astroglial cells secret a factor that contributes to granule cell precursor development.
It has been speculated that Shh signaling promotes differentiation of BG (Dahmane and Ruiz i Altaba 1999). These studies were performed in vitro and also through the use of blocking antibodies in vivo at early stages in the chick embryo when Shh is important in development of the entire neural tube. Thus, it has been difficult to distinguish between Shh regulation of radial glial cells and Shh signaling regulation of BG development without a BG-specific blockade of Shh signaling. Using the TNCYFP-CreER driver line to ablate Shh signaling in postnatal BG cells, we demonstrate that BG cell number is unchanged between the WT and mutant (Figure 6A). Indeed, BG mitotic activity peaks between P1 and P6 (Yuasa 1996) and our analysis following tamoxifen injection at P1 and P2 showed no difference in absolute numbers of BG at P7. Our findings suggest that Shh signaling in BG is not necessary for their postnatal differentiation but do not rule out the possibility that Shh signaling regulates their specification prior to birth.
The question remains as to how BG-Shh signaling alters CGNP Shh-responsiveness. One possibility is that BG Shh signaling serves to modulate levels of inhibitory secreted factors, allowing for full activation of the Shh pathway in CGNPs. Several signaling pathways have been identified that are antagonistic to Shh signaling in regulating CGNP proliferation. For example, extracellular matrix glycoproteins (Pons et al. 2001) and FGFs (Wechsler-Reya and Scott 1999) are able to differentially modulate, but not completely suppress, Shh-driven proliferation of CGNPs. However, we did not find differences in gene expression of FGF transcriptional target Etv5 in Smo mutants compared to WT cerebella (data not shown), suggesting that FGF signaling does not occur downstream of Shh signaling in BG. BMP2 and BMP4 significantly reduce CGNP proliferation in organotypic slice cultures (Rios et al. 2004) via the Smad signaling pathway (Zhao et al. 2008), however we did not find differences in protein localization and intensity of BMP signaling readout phospho-Smad1 (Ser463/465) (data not shown), suggesting that this pathway is also not regulated by Shh signaling in BG.
Notably, our data using BAT-gal reporter mice demonstrate that Wnt signaling activity is upregulated in the EGL of Smo mutants (Figure 8A), concomitant with a reduction in EGL area. While we cannot rule out the possibility that the upregulation in Wnt activity is a consequence rather than a cause of BG-Shh signaling ablation, several studies have implicated that antagonism of the Wnt pathway is important for the maintenance and proliferation of CGNPs. First, Wnt signaling is active in the rhombic lip and early migratory CGNPs at E14.5 but not in later stages of CGNP development (Selvadurai and Mason 2011). Additionally, CGNP-specific deletion of Wnt inhibitor APC resulted in severely inhibited CGNP proliferation and premature differentiation (Lorenz et al. 2011) and conditional activation of Wnt signaling using a dominant active form of β-catenin in neural precursors impaired CGNP proliferation (Pöschl et al. 2013). Interestingly, the latter study observed a comparably severe cerebellar phenotype in their mutant mice as that seen when blocking Shh in vivo, with cerebellar hypoplasia with a thinned EGL, abnormally positioned Purkinje cells, and reduced Bergmann glia (Pöschl et al. 2013). Thus, Wnt signaling to the EGL is likely inhibited during normal cerebellar development to allow for complete Shh pathway activation. Our results indicate that BG Shh signaling is involved in Wnt signaling antagonism. The source of the Wnt signal to the EGL may very well be BG as Wnt3 is expressed in BG (Gensat Brain Atlas). While we do not find differences in Wnt3 gene expression in Smocko mutants (data not shown), several extracellular modulators of the Wnt pathway are expressed in BG and CGNPs, including Wif1 and Shh target gene Sfrp1. Notably, we detect a substantial downregulation of EGL Sfrp1expression in Smocko mutants, suggesting at least one possible mediator of Wnt inhibition that may be downstream of BG-Shh signaling.
Our study of BG fiber morphology demonstrates that Shh signaling in BG is required for proper BG fiber expansion, as BGs of Smocko mutants display expanded end-feet and increased lateral branching (Figure 6B–D). However, an EGL area reduction is observed prior to detection of BG fiber defects, which are noticeable at P5. Thus, we conclude that the aberrant growth of BG fibers is secondary to the reduction in EGL area and subsequent hypoplasia. Consistent with this hypothesis, we observe a similar increase in BG lateral branching and thickening of fibers in Math1CreER;SmoF/− mice which lack an EGL (Figure 7D).
Our results also indicate that Shh signaling in BG is necessary for proper PC soma localization and dendritic arborization. Post-mitotic PC migrate from the ventricular zone to form clusters in the postnatal cerebellum, and cluster dispersal depends on EGL-derived Reelin signaling (Miyata et al. 1997). As the EGL in our mutants is severely reduced, the disorganization of PC cell bodies is most likely due to the secondary effect of decreased Reelin secretion. These results are corroborated by similarly disrupted PC soma localization in L7Cre;ShhF/− and Math1CreER;SmoF/− mutants where the EGL is completely absent. PC dendritogenesis is a dynamic process, with constant restructuring, retraction, and regrowth of fibers. It is speculated that PC dendrites eventually use BG fibers as a guide for synaptogenesis and subsequent arborization (Wang et al. 2011; Lordkipanidze and Dunaevsky 2005; Yue 2005). We find that PC dendrite arborization in Smocko mutants is reduced compared to PC in WT mice (Figure 7A). While the PC dendrite phenotype we observed could be a consequence of disrupted BG fiber morphology, a second possibility is that PC dendritic arborization is dependent on BG Shh signaling directly. Our studies in Smocko mutant animals with later stage tamoxifen injections indicate that the latter explanation is more likely. In these mutants, we find that cerebellar hypoplasia, EGL reduction, and BG fiber morphology are comparable to WT, whereas PC dendritic arborization defects are strikingly apparent (Figure 7G, H) indicating that this PC phenotype precedes the observed disruption of BG fiber morphology. The mechanism by which Shh signaling in BG regulates PC dendritogenesis requires further study.
Because CGNPs are the presumed cell of origin for medulloblastoma, determining factors that inhibit their proliferation are central to the investigation of medulloblastoma development and may elucidate pathways that can be exploited in the development of future therapies. Our study suggests that BG play a role in providing modulatory factors, likely involving Wnt signaling, that positively regulate Shh signaling in the postnatal EGL. Thus, their potential contribution to medulloblastoma pathogenesis should not be ignored. Further studies are needed to determine the molecular mechanism by which this regulation of CGNP proliferation occurs. In addition, as Shh signaling is still active in BG in the adult (Corrales et al. 2004), its function in that context may be very different from that utilized in early postnatal development and warrants further investigation. Ultimately, our Smocko mutant mouse model demonstrates a requirement for Shh signaling in BG for proper proliferation of CGNPs and multiple aspects of cerebellar architecture and lamination and therefore sheds light on the importance of neuron-glia communication in the cerebellum.
Supplementary Material
Highlights.
Early postnatal ablation of Shh signaling in Bergmann glial cells causes cerebellar hypoplasia.
Shh signaling in Bergmann glia is required for CGNP proliferation.
The absence of Shh signaling in Bergmann glia induces ectopic Wnt signaling in the CGNP.
Acknowledgments
We thank the following individuals for their contributions: Wenjuan He (University of California-San Francisco, San Francisco, CA) and Chuanming Hao (Fudan University, Shanghai, China) for the gift of the TNCYFP-CreER mouse, Jonathan Eggenschwiler, PhD (University of Georgia, Atlanta, GA) for the generous gift of polyclonal antibodies against Gli2 and Paula Bovolenta, PhD (Centro de Biologia Molecular Universidad Autonoma Madrid, Madrid, Spain) for the generous gift of the Sfrp1 probe. For their insightful feedback and discussion we thank members of the Chiang laboratory. Additionally, we are grateful to Joseph Roland and the Vanderbilt University Medical Center Epithelial Biology Digital Histology Shared Resource for assistance with slide scanning and quantification, and Sean Schaffer and the Vanderbilt University Cell Imaging Shared Resource for assistance with confocal imaging. We thank the DSHB that was developed with support from the NIH. This work is supported by National Institutes of Health (NIH) T32 GM007347, National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Number F31NS074638, and NIH NS 097898.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avanesov Andrei, Honeyager Shawn M, Malicki Jarema, Blair Seth S. The Role of Glypicans in Wnt Inhibitory Factor-1 Activity and the Structural Basis of Wif1'S Effects on Wnt and Hedgehog Signaling. In: Perrimon Norbert., editor. PLoS Genet. 2. Vol. 8. 2012. Feb 23, p. e1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C Brian, Auerbach Wojtek, Lee Joon S, Stephen Daniel, Joyner Alexandra L. Gli2, but Not Gli1, Is Required for Initial Shh Signaling and Ectopic Activation of the Shh Pathway. Development. 2002 Oct 1;129(20):4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Baptista CA, Hatten ME, Blazeski R, Mason CA. Cell-Cell Interactions Influence Survival and Differentiation of Purified Purkinje Cells in Vitro. Neuron. 1994 Feb;12(2):243–260. doi: 10.1016/0896-6273(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Bartsch S, Bartsch U, Dörries U, Faissner A, Weller A, Ekblom P, Schachner M. Expression of Tenascin in the Developing and Adult Cerebellar Cortex. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 1992 Mar;12(3):736–749. doi: 10.1523/JNEUROSCI.12-03-00736.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvindrah Richard, Nalbant Perihan, Ding Sheng, Wu Chuanyue, Bokoch Gary M, Müller Ulrich. Integrin-Linked Kinase Regulates Bergmann Glial Differentiation During Cerebellar Development. Molecular and Cellular Neuroscience. 2006 Oct;33(2):109–125. doi: 10.1016/j.mcn.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Buffo Annalisa, Rossi Ferdinando. Origin, Lineage and Function of Cerebellar Glia. Progress in Neurobiology. 2013 Aug 25; doi: 10.1016/j.pneurobio.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Corrales JMD, Rocco GL, Blaess S, Guo Q, Joyner AL. Spatial Pattern of Sonic Hedgehog Signaling Through Gli Genes During Cerebellum Development. Development. 2004;131(22):5581. doi: 10.1242/dev.01438. [DOI] [PubMed] [Google Scholar]
- Corrales JMD, Blaess S, Mahoney EM, Joyner AL. The Level of Sonic Hedgehog Signaling Regulates the Complexity of Cerebellar Foliation. Development-Cambridge. 2006;133(9):1811. doi: 10.1242/dev.02351. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Ruiz i Altaba A. Sonic Hedgehog Regulates the Growth and Patterning of the Cerebellum. Development. 1999 Jun;126(14):3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Lee J, Robins P, Heller P, i Altaba AR. Activation of the Transcription Factor Gli1 and the Sonic Hedgehog Signalling Pathway in Skin Tumours. Nature. 1997;389(6653):876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- Das GD. Differentiation of Bergmann Glia Cells in the Cerebellum: a Golgi Study. Brain Research. 1976 Jul 9;110(2):199–213. doi: 10.1016/0006-8993(76)90397-8. [DOI] [PubMed] [Google Scholar]
- Eiraku Mototsugu, Tohgo Akira, Ono Katsuhiko, Kaneko Megumi, Fujishima Kazuto, Hirano Tomoo, Kengaku Mineko. DNER Acts as a Neuron-Specific Notch Ligand During Bergmann Glial Development. Nature Neuroscience. 2005 Jun 19;8(7):873–880. doi: 10.1038/nn1492. [DOI] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N. Brain Lipid-Binding Protein (BLBP): a Novel Signaling System in the Developing Mammalian CNS. Neuron. 1994 Apr;12(4):895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Finch PW, He X, Kelley MJ, Uren A, Schaudies RP, Popescu NC, Rudikoff S, Aaronson SA, Varmus HE, Rubin JS. Purification and Molecular Cloning of a Secreted, Frizzled-Related Antagonist of Wnt Action. Proceedings of the National Academy of Sciences of the United States of America. 1997 Jun 24;94(13):6770–6775. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JT, He W, Hao C, Ketova T, Pan FC, et al. The Purkinje Neuron Acts as a Central Regulator of Spatially and Functionally Distinct Cerebellar Precursors. Developmental Cell. 2013;27(3):278–292. doi: 10.1016/j.devcel.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S, Tabata T, Mitoma J, Yamada K, Yamasaki M, Makino A, Yamamoto T, Watanabe M, Kano M, Hirabayashi Y. L-Serine and Glycine Serve as Major Astroglia-Derived Trophic Factors for Cerebellar Purkinje Neurons. Proceedings of the National Academy of Sciences of the United States of America. 2000 Oct 10;97(21):11528–11533. doi: 10.1073/pnas.200364497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse N, Maiti T, Wang B, Porter JA, Hall TMT, Leahy DJ, Beachy PA. Sonic Hedgehog Protein Signals Not as a Hydrolytic Enzyme but as an Apparent Ligand for Patched. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(20):10992. doi: 10.1073/pnas.96.20.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez Y Ribotta M, Langa F, Menet V, Privat A. Comparative Anatomy of the Cerebellar Cortex in Mice Lacking Vimentin, GFAP, and Both Vimentin and GFAP. Glia. 2000 Jul;31(1):69–83. doi: 10.1002/(sici)1098-1136(200007)31:1<69::aid-glia70>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the Hedgehog/Patched Signaling Pathway From Flies to Mice: Induction of a Mouse Patched Gene by Hedgehog. Genes & Development. 1996;10(3):301. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Müller U. Beta1-Class Integrins Regulate the Development of Laminae and Folia in the Cerebral and Cerebellar Cortex. Neuron. 2001 Aug 16;31(3):367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, El Agha E, Bellusci S, Hajihosseini MK. Fgf10-Expressing Tanycytes Add New Neurons to the Appetite/Energy-Balance Regulating Centers of the Postnatal and Adult Hypothalamus. Journal of Neuroscience. 2013 Apr 3;33(14):6170–6180. doi: 10.1523/JNEUROSCI.2437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann B, Sievers J. Cerebellar External Granule Cells Are Attached to the Basal Lamina From the Onset of Migration Up to the End of Their Proliferative Activity. The Journal of Comparative Neurology. 1985 Nov 1;241(1):50–62. doi: 10.1002/cne.902410105. [DOI] [PubMed] [Google Scholar]
- Heuer Heike, Mason Carol Ann. Thyroid Hormone Induces Cerebellar Purkinje Cell Dendritic Development via the Thyroid Hormone Receptor Alpha1. Journal of Neuroscience. 2003 Nov 19;23(33):10604–10612. doi: 10.1523/JNEUROSCI.23-33-10604.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Liu J, Ketova T, Fleming JT, Grover VK, Cooper MK, Litingtung Y, Chiang C. Transventricular Delivery of Sonic Hedgehog Is Essential to Cerebellar Ventricular Zone Development. Proceedings of the National Academy of Sciences. 2010 May 4;107(18):8422–8427. doi: 10.1073/pnas.0911838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Ketova T, Fleming JT, Wang H, Dey SK, Litingtung Y, Chiang C. Sonic Hedgehog Signaling Regulates a Novel Epithelial Progenitor Domain of the Hindbrain Choroid Plexus. Development. 2009 Jul 10;136(15):2535–2543. doi: 10.1242/dev.033795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Glia-Synapse Interaction Through Ca2+-Permeable AMPA Receptors in Bergmann Glia. Science. 2001 May 4;292(5518):926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog Signaling in Animal Development: Paradigms and Principles. Genes & Development. 2001;15(23):3059. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Katoh Yuriko, Katoh Masaru. WNT Antagonist, SFRP1, Is Hedgehog Signaling Target. International Journal of Molecular Medicine. 2006 Jan;17(1):171–175. [PubMed] [Google Scholar]
- Kenney Anna, Rowitch David. Sonic Hedgehog Promotes G1 Cyclin Expression and Sustained Cell Cycle Progression in Mammalian Neuronal Precursors. Molecular and Cellular Biology. 2000 Dec 1;20(23):9055. doi: 10.1128/MCB.20.23.9055-9067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koirala Samir, Corfas Gabriel. Identification of Novel Glial Genes by Single-Cell Transcriptional Profiling of Bergmann Glial Cells From Mouse Cerebellum. In: Reh Thomas A., editor. PLoS ONE. 2. Vol. 5. 2010. Feb 12, p. e9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine Okiru, Nagaoka Mai, Watase Kei, Gutmann David H, Tanigaki Kenji, Honjo Tasuku, Radtke Freddy, Saito Toshiki, Chiba Shigeru, Tanaka Kohichi. The Monolayer Formation of Bergmann Glial Cells Is Regulated by Notch/RBP-J Signaling. Developmental Biology. 2007 Nov;311(1):238–250. doi: 10.1016/j.ydbio.2007.08.042. [DOI] [PubMed] [Google Scholar]
- Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, Mcmahon AP. Cholesterol Modification of Sonic Hedgehog Is Required for Long-Range Signaling Activity and Effective Modulation of Signaling by Ptc1. Cell. 2001 Jun 1;105(5):599–612. doi: 10.1016/s0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic Hedgehog Signaling Is Required for Expansion of Granule Neuron Precursors and Patterning of the Mouse Cerebellum. Developmental Biology. 2004;270(2):393–410. doi: 10.1016/j.ydbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Li Yina, Zhang Huimin, LItingtung Ying, Chiang Chin. Cholesterol Modification Restricts the Spread of Shh Gradient in the Limb Bud. Proceedings of the National Academy of Sciences of the United States of America. 2006 Apr 25;103(17):6548–6553. doi: 10.1073/pnas.0600124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Yina, Gordon Julie, Manley Nancy R, LItingtung Ying, Chiang Chin. Bmp4 Is Required for Tracheal Formation: a Novel Mouse Model for Tracheal Agenesis. Developmental Biology. 2008 Oct;322(1):145–155. doi: 10.1016/j.ydbio.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Castrén E, Tsoulfas P, Kolbeck R, da P Berzaghi M, Leingärtner A, Heisenberg CP, et al. Neurotrophin-3 Induced by Tri-Iodothyronine in Cerebellar Granule Cells Promotes Purkinje Cell Differentiation. The Journal of Cell Biology. 1993 Jul;122(2):443–450. doi: 10.1083/jcb.122.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman Jocelyn J, Lordkipanidze Tamar, Buell Margaret E, Yoon Sung Ok, Dunaevsky Anna. Morphogenesis and Regulation of Bergmann Glial Processes During Purkinje Cell Dendritic Spine Ensheathment and Synaptogenesis. Glia. 2008 Oct;56(13):1463–1477. doi: 10.1002/glia.20712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F, Zhang XM, Karp S, Yang Y, Mcmahon AP. Genetic Manipulation of Hedgehog Signaling in the Endochondral Skeleton Reveals a Direct Role in the Regulation of Chondrocyte Proliferation. Development. 2001 Dec;128(24):5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- Lordkipanidze Tamar, Dunaevsky Anna. Purkinje Cell Dendrites Grow in Alignment with Bergmann Glia. Glia. 2005;51(3):229–234. doi: 10.1002/glia.20200. [DOI] [PubMed] [Google Scholar]
- Lorenz A, Deutschmann M, Ahlfeld J, Prix C, Koch A, Smits R, Fodde R, Kretzschmar HA, Schüller U. Severe Alterations of Cerebellar Cortical Development After Constitutive Activation of Wnt Signaling in Granule Neuron Precursors. Molecular and Cellular Biology. 2011 Jul 27;31(16):3326–3338. doi: 10.1128/MCB.05718-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Kwon HJ, Huang Z. Ric-8a, a Guanine Nucleotide Exchange Factor for Heterotrimeric G Proteins, Regulates Bergmann Glia-Basement Membrane Adhesion During Cerebellar Foliation. Journal of Neuroscience. 2012 Oct 24;32(43):14979–14993. doi: 10.1523/JNEUROSCI.1282-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold Rob, Fishell Gord. Math1 Is Expressed in Temporally Discrete Pools of Cerebellar Rhombic-Lip Neural Progenitors. Neuron. 2005 Oct 6;48(1):17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Madisen Linda, Zwingman Theresa A, Sunkin Susan M, Oh Seung Wook, Zariwala Hatim A, Gu Hong, Ng Lydia L, et al. A Robust and High-Throughput Cre Reporting and Characterization System for the Whole Mouse Brain. Nature Neuroscience. 2009 Dec 20;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto Silvia, Cordenonsi Michelangelo, Dupont Sirio, Braghetta Paola, Broccoli Vania, Hassan A Bassim, Volpin Dino, Bressan Giorgio M, Piccolo Stefano. Mapping Wnt/Beta-Catenin Signaling During Mouse Development and in Colorectal Tumors. Proceedings of the National Academy of Sciences of the United States of America. 2003 Mar 18;100(6):3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo V, Johnson RL, Vortkamp A, Tabin CJ. Sonic Hedgehog Differentially Regulates Expression of GLI and GLI3 During Limb Development. Dev. Biol. 1996;180(1):273–283. doi: 10.1006/dbio.1996.0300. [DOI] [PubMed] [Google Scholar]
- Martin JL, Brown AL, Balkowiec A. Glia Determine the Course of Brain-Derived Neurotrophic Factor-Mediated Dendritogenesis and Provide a Soluble Inhibitory Cue to Dendritic Growth in the Brainstem. Neuroscience. 2012 Apr;207:333–346. doi: 10.1016/j.neuroscience.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Nakajima K, Aruga J, Takahashi S, Ikenaka K, Mikoshiba K, Ogawa M. Distribution of a Reeler Gene-Related Antigen in the Developing Cerebellum: an Immunohistochemical Study with an Allogeneic Antibody CR-50 on Normal and Reeler Mice. The Journal of Comparative Neurology. 1996 Aug 19;372(2):215–228. doi: 10.1002/(SICI)1096-9861(19960819)372:2&lt;215::AID-CNE5&gt;3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Miyata T, Nakajima K, Mikoshiba K, Ogawa M. Regulation of Purkinje Cell Alignment by Reelin as Revealed with CR-50 Antibody. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 1997 May 15;17(10):3599–3609. doi: 10.1523/JNEUROSCI.17-10-03599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper Michael, Harris Lachlan, Barry Guy, Evelyn Heng Yee Hsieh, Plachez Celine, Gronostajski Richard M, Richards Linda J. Nuclear Factor One X Regulates the Development of Multiple Cellular Populations in the Postnatal Cerebellum. The Journal of Comparative Neurology. 2011 Sep 27;519(17):3532–3548. doi: 10.1002/cne.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S, Trejo JL, Martinez-Morales JR, Marti E. Vitronectin Regulates Sonic Hedgehog Activity During Cerebellum Development Through CREB Phosphorylation. Development. 2001;128(9):1481. doi: 10.1242/dev.128.9.1481. [DOI] [PubMed] [Google Scholar]
- Porter JA, Young KE, Beachy PA. Cholesterol Modification of Hedgehog Signaling Proteins in Animal Development. Science. 1996 Oct 11;274(5285):255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- Pöschl Julia, Grammel Daniel, Dorostkar Mario M, Kretzschmar Hans A, Schüller Ulrich. Constitutive Activation of B-Catenin in Neural Progenitors Results in Disrupted Proliferation and Migration of Neurons Within the Central Nervous System. Developmental Biology. 2013 Feb;374(2):319–332. doi: 10.1016/j.ydbio.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Procko Carl, Shaham Shai. Assisted Morphogenesis: Glial Control of Dendrite Shapes. Current Opinion in Cell Biology. 2010 Oct;22(5):560–565. doi: 10.1016/j.ceb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Neuron-Glia Relationship During Granule Cell Migration in Developing Cerebellar Cortex. a Golgi and Electronmicroscopic Study in Macacus Rhesus. The Journal of Comparative Neurology. 1971 Mar;141(3):283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Rakic Pasko. Developmental and Evolutionary Adaptations of Cortical Radial Glia. Cerebral Cortex (New York, N.Y. : 1991) 2003 Jun;13(6):541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- Rios I, Alvarez-Rodríguez R, Martí E, Pons S. Bmp2 Antagonizes Sonic Hedgehog-Mediated Proliferation of Cerebellar Granule Neurones Through Smad5 Signalling. Development. 2004;131(13):3159. doi: 10.1242/dev.01188. [DOI] [PubMed] [Google Scholar]
- Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, et al. A-Tanycytes of the Adult Hypothalamic Third Ventricle Include Distinct Populations of FGF-Responsive Neural Progenitors. Nature Communications. 2013 Jun 27;4 doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, Connelly M, Stewart CF, Gould S, Rubin LL. Suppression of the Shh Pathway Using a Small Molecule Inhibitor Eliminates Medulloblastoma in Ptc1+/−P53−/−Mice. Cancer Cell. 2004;6(3):229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Bernier B, Goffinet AM. Reelin mRNA Expression During Mouse Brain Development. The European Journal of Neuroscience. 1997 May;9(5):1055–1071. doi: 10.1111/j.1460-9568.1997.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Schilling K, Dickinson MH, Connor JA, Morgan JI. Electrical Activity in Cerebellar Cultures Determines Purkinje Cell Dendritic Growth Patterns. Neuron. 1991 Dec;7(6):891–902. doi: 10.1016/0896-6273(91)90335-w. [DOI] [PubMed] [Google Scholar]
- Selvadurai Hayden J, Mason John O. Wnt/B-Catenin Signalling Is Active in a Highly Dynamic Pattern During Development of the Mouse Cerebellum. In: Gottardi Cara., editor. PLoS ONE. 8. Vol. 6. 2011. Aug 8, p. e23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga T, Ichikawa M, Hirata Y. A Golgi Study of Bergmann Glial Cells in Developing Rat Cerebellum. Anatomy and Embryology. 1983;167(2):191–201. doi: 10.1007/BF00298510. [DOI] [PubMed] [Google Scholar]
- Shimada A, Mason CA, Morrison ME. TrkB Signaling Modulates Spine Density and Morphology Independent of Dendrite Structure in Cultured Neonatal Purkinje Cells. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 1998 Nov 1;18(21):8559–8570. doi: 10.1523/JNEUROSCI.18-21-08559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers J, Pehlemann FW. Influences of Meningeal Cells on Brain Development. Findings and Hypothesis. Die Naturwissenschaften. 1986 Apr;73(4):188–194. doi: 10.1007/BF00417722. [DOI] [PubMed] [Google Scholar]
- Sievers J, Mangold U, Berry M, Allen C, Schlossberger HG. Experimental Studies on Cerebellar Foliation. I. a Qualitative Morphological Analysis of Cerebellar Fissuration Defects After Neonatal Treatment with 6-OHDA in the Rat. The Journal of Comparative Neurology. 1981 Dec 20;203(4):751–769. doi: 10.1002/cne.902030412. [DOI] [PubMed] [Google Scholar]
- Silbereis John, Heintz Tristan, Taylor Mary Morgan, Ganat Yosif, Ment Laura R, Bordey Angelique, Vaccarino Flora. Astroglial Cells in the External Granular Layer Are Precursors of Cerebellar Granule Neurons in Neonates. Molecular and Cellular Neuroscience. 2010 May 19;:1–12. doi: 10.1016/j.mcn.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C, Dusart I. Intrinsic Versus Extrinsic Determinants During the Development of Purkinje Cell Dendrites. Neuroscience. 2009 Sep;162(3):589–600. doi: 10.1016/j.neuroscience.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre Reporter Strains Produced by Targeted Insertion of EYFP and ECFP Into the ROSA26 Locus. BMC Developmental Biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surzenko N, Crowl T, Bachleda A, Langer L, Pevny L. SOX2 Maintains the Quiescent Progenitor Cell State of Postnatal Retinal Muller Glia. Development. 2013 Mar 12;140(7):1445–1456. doi: 10.1242/dev.071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J. Hedgehog: Functions and Mechanisms. Genes & Development. 2008;22(18):2454. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- Vernadakis A. Neuron-Glia Interrelations. International Review of Neurobiology. 1988;30:149–224. [PubMed] [Google Scholar]
- Wallace VA. Purkinje-Cell-Derived Sonic Hedgehog Regulates Granule Neuron Precursor Cell Proliferation in the Developing Mouse Cerebellum. Current Biology. 1999;9(8):445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- Wang Xiaohong, Imura Tetsuya, Sofroniew Michael V, Fushiki Shinji. Loss of Adenomatous Polyposis Coli in Bergmann Glia Disrupts Their Unique Architecture and Leads to Cell Nonautonomous Neurodegeneration of Cerebellar Purkinje Neurons. Glia. 2011 Jun;59(6):857–868. doi: 10.1002/glia.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of Neuronal Precursor Proliferation in the Cerebellum by Sonic Hedgehog. Neuron. 1999;22(1):103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Weller Mathias, Krautler Nike, Mantei Ned, Suter Ueli, Taylor Verdon. Jagged1 Ablation Results in Cerebellar Granule Cell Migration Defects and Depletion of Bergmann Glia. Developmental Neuroscience. 2006;28(1–2):70–80. doi: 10.1159/000090754. [DOI] [PubMed] [Google Scholar]
- Yamada K, Fukaya M, Shibata T, Kurihara H, Tanaka K, Inoue Y, Watanabe M. Dynamic Transformation of Bergmann Glial Fibers Proceeds in Correlation with Dendritic Outgrowth and Synapse Formation of Cerebellar Purkinje Cells. The Journal of Comparative Neurology. 2000 Feb 28;418(1):106–120. doi: 10.1002/(SICI)1096-9861(20000228)418:1<106::AID-CNE8>3.0.CO;2-N/asset/8_ftp.pdf?v=1&t=h968d4qo&s=33a3e210756ae9bcd89a4e2e0b1af794908c861f. [DOI] [PubMed] [Google Scholar]
- Yuasa S. Bergmann Glial Development in the Mouse Cerebellum as Revealed by Tenascin Expression. Anatomy and Embryology. 1996 Sep;194(3):223–234. doi: 10.1007/BF00187133. [DOI] [PubMed] [Google Scholar]
- Yuasa S, Kawamura K, Ono K, Yamakuni T, Takahashi Y. Development and Migration of Purkinje Cells in the Mouse Cerebellar Primordium. Anatomy and Embryology. 1991;184(3):195–212. doi: 10.1007/BF01673256. [DOI] [PubMed] [Google Scholar]
- Yue Q. PTEN Deletion in Bergmann Glia Leads to Premature Differentiation and Affects Laminar Organization. Development. 2005 Jul 15;132(14):3281–3291. doi: 10.1242/dev.01891. [DOI] [PubMed] [Google Scholar]
- Zhao H, Ayrault O, Zindy F, Kim JH, Roussel MF. Post-Transcriptional Down-Regulation of Atoh1/Math1 by Bone Morphogenic Proteins Suppresses Medulloblastoma Development. Science's STKE. 2008;22(6):722. doi: 10.1101/gad.1636408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.