Abstract
A common complication of chronic kidney disease (CKD), anemia can influence glycated hemoglobin (HbA1c) levels. In diabetic patients, anemia occurs earlier and with higher severity over the course of CKD stages. To elucidate the effect of hemoglobin (Hb) on the predictive value of HbA1c, we enrolled 1558 diabetic patients with stages 3–4 CKD, categorized according to baseline Hb and HbA1c quartiles. Linear regression revealed that higher HbA1c correlated significantly with higher Hb in the Hb < 10 g/dL group (β = 0.146, P = 0.004). A fully-adjusted Cox regression model revealed worse clinical outcomes in patients with higher HbA1c quartiles in the Hb ≥ 10 g/dL group. Hazard ratios for end-stage renal disease (ESRD), all-cause mortality, and composite endpoint (cardiovascular events and all-cause mortality) in patients with Hb ≥ 10 g/dL and the highest HbA1c quartile were 1.92 (95% confidence interval [CI], 1.17–3.15), 1.76 (95% CI, 1.02–3.03), and 1.54 (95% CI, 1.03–2.31), respectively. By contrast, HbA1c was not associated with clinical outcomes in the Hb < 10 g/dL group. In conclusion, in stages 3–4 diabetic CKD, higher HbA1c is associated with a higher risk of poor clinical outcomes in patients with Hb ≥ 10 g/dL.
Introduction
Anemia is a common feature in patients with chronic kidney disease (CKD), and it is mainly attributable to the relative decrease in erythropoietin (EPO) production by the kidneys, absolute or functional iron deficiency, and shortened red cell survival[1]. According to the National Health and Nutrition Examination Survey[2], the prevalence of anemia increases as estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2. However, in patients with diabetic kidney disease, anemia occurs earlier[3, 4] and tends to be of greater severity stratified by CKD stages and albuminuria compared with those without diabetes[5–8]. The reimbursement of erythropoietin stimulating agents (ESA), which is strictly regulated in some countries, may affect the prevalence of anemia.
In diabetes mellitus (DM), good glycemic control, which is expressed through glycated hemoglobin (HbA1c) measurements, has been confirmed to prevent or delay the occurrence of microvascular complications. Several observational studies involving diabetic patients with stages 3–4 CKD have demonstrated that a baseline HbA1c of ≥ 9% is independently associated with a higher risk of mortality and end-stage renal disease (ESRD)[9, 10]. However, it is clarified that HbA1c levels can be altered by anemia, uremic environments, or ESA administration[11]. HbA1c levels may not optimally represent the general glycemic state in CKD populations. The results of our previous study revealed a lack of prognostic value of HbA1c levels in stage 5 CKD[10]. Accordingly, the reliance on HbA1c levels to predict prognosis in CKD patients with anemia might be doubtful. By contrast, previous studies have already suggested that protein–energy malnutrition and chronic inflammation apparently influence anemia development in patients with CKD[12, 13]; lower HbA1c levels might indicate a poor nutritional status or more severe degree of inflammation, in addition to glycemic control. Therefore, the present study attempts to assess whether anemia modifies the predictive role of HbA1c levels in patients with stages 3–4 diabetic CKD, who did not receive ESA treatment.
Methods
Participants and measurements
This observational study enrolled patients with CKD from integrated or traditional CKD care programs conducted in two affiliated hospitals of Kaohsiung Medical University in Southern Taiwan from November 11, 2002, to May 31, 2009. CKD was defined according to the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative[14], and the eGFR was calculated using the equation from the four-variable Modification of Diet in Renal Disease (MDRD) study. Patients were excluded if they had acute kidney injury, defined as a decrease of > 50% in the eGFR within 3 months, or if they had undergone renal replacement therapy (RRT) before their first visit. In Taiwan, National Health Insurance regulations limit ESA administration until serum creatinine is > 6 mg/dL; therefore, patients with stages 3–4 CKD did not receive ESAs. The diagnosis of type 2 DM was defined by the World Health Organization and use of either oral hypoglycemic agents or insulin[15]. 1558 patients with diabetes and stages 3–4 CKD were included. All patients periodically received follow-ups until May 31, 2010, for serial blood exams and evaluation of CKD complications. Data were obtained with the informed consent of all participants and the approval of the Kaohsiung Medical University Hospital Institutional Review Board, in accordance with the Declaration of Helsinki.
All biochemical data were recorded at the first visit (baseline). Baseline blood samples were drawn after an overnight fast, and all laboratory values, including serum creatinine, albumin, hemoglobin (Hb), blood glucose, HbA1c, total calcium, phosphate, uric acid, total cholesterol, triglyceride, C-reactive protein (CRP), and urine protein-to-creatinine ratio (UPCR), were determined through standardized methods. The eGFR was calculated using the MDRD equation, which is generally applied in the Taiwan National Database to assess CKD prevalence and dialysis initiation[16, 17]. HbA1c values were measured as clinically indicated through standard automated cation-exchange high-performance liquid chromatography. We classified participants into two groups according to their baseline Hb levels: < 10 g/dL and ≥ 10 g/dL. Each group was subsequently divided into quartiles on the basis of HbA1c values at 6.4, 7.2 and 8.3%.
All demographic information and relevant medical histories of the participants, including data on their age, sex, blood pressure (BP), stroke, cardiovascular disease (CVD), and congestive heart failure (CHF), were collected from the medical records, at the time of enrolment. Weight and height were assessed, and the body mass index (BMI) was calculated. Hypertension was diagnosed as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg and/or by the requirement of antihypertensive treatment. CVD was defined as clinically diagnosed myocardial infarction, heart failure, ischemic heart disease, or cerebrovascular disease.
Clinical outcomes
The primary outcomes measures were ESRD, CV events, and all-cause mortality. ESRD was defined by a history of RRT (initiation of hemodialysis and peritoneal dialysis or renal transplantation). ESRD development was ascertained using catastrophic illness cards issued by the Bureau of National Health Insurance. Hospital records were analysed to identify CV events and the most responsible diagnoses of acute coronary syndrome (International Classification of Diseases, Ninth Revision, Clinical Modification: 410.x–412.x), acute cerebrovascular disease (430.x–438.x), and CHF (428.x) and death from the aforementioned causes (but only in patients with the occurrence of CV events after the index date). All-cause mortality was determined through death certificates and the National Death Index.
Statistical analysis
Descriptive statistics were summarized as the frequency and percentage for categorical data and means with standard deviations (or medians with interquartile ranges) for continuous variables with approximately normal distributions. Between-groups comparisons of the baseline characteristics were achieved by ANOVA for normally distributed variables and by chi-square tests for categorical variables. Logarithmic transformation was applied for variables with a skewed distribution (cholesterol and CRP). Multiple imputation was applied for missing data in iron and ferritin. The correlation of Hb with HbA1c levels was constructed through multivariable linear regression analysis.
To determine the variables that were independently predictive of HbA1c levels, multivariable linear regression analysis was performed. To identify the effects of baseline HbA1c (stratified by Hb) on clinical outcomes, Cox proportional regression models were employed. The models were adjusted to control for the effects of related factors including age, sex, eGFR, log-transformed UPCR, CVD, mean BP, log-transformed cholesterol, log-transformed CRP, phosphorus levels, BMI, Hb, albumin, and iron. Covariates were selected on the basis of their statistical significance or clinical relevance. We also applied several sensitivity analyses to account for the effects on clinical outcomes with different classifications divided: (1) by clinical relevance of HbA1c values: < 6%, ≥ 6% to < 7%, ≥ 7% to < 9%, and ≥ 9%, and (2) by fasting glucose levels. The results are expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). For all analyses, all tests were two-tailed and results with P < 0.05 were considered statistically significant. Statistical analyses were conducted using R 3.3.0 software (R Foundation for Statistical Computing, Vienna, Austria) and SPSS Version 21.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of diabetic patients with stages 3–4 CKD, stratified by Hb and HbA1c quartiles
Baseline characteristics of the 1558 patients classified by Hb and HbA1c quartiles are shown in Table 1. This cohort had a mean age of 64.7 ± 12.8 years, mean Hb level of 11.6 ± 2.2 g/dL, mean HbA1c level of 7.6% ± 1.8%, median UPCR of 1164 (322–3297) mg/g, and mean eGFR of 33.0 ± 11.9 mL/min/1.73 m2. To assess the effect of anemia, the participants were divided into two groups: 411 patients with Hb < 10 g/dL and 1147 with Hb ≥ 10 g/dL. In both groups, patients with higher HbA1c levels had higher levels of total cholesterol and triglyceride (all P for trend < 0.05), but not a higher percentage of CVD. Additionally, in the Hb ≥ 10 g/dL group, higher HbA1c levels exhibited higher UPCR (P for trend < 0.05). As for the iron profile evaluating the cause of anemia in both groups, there were lower iron and higher prevalence of iron deficiency in the Hb < 10 g/dL group, compared with Hb ≥ 10 g/dL group (P < 0.05). There no difference in iron profile among HbA1c groups in patients with Hb < 10 g/dL.
Table 1. Baseline characteristics of patients with stages 3–4 CKD, stratified by Hb and HbA1c quartiles.
| All | Hemoglobin < 10 g/dl | P | Hemoglobin ≥ 10 g/dl | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c quartiles | HbA1c quartiles | ||||||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | ||||
| HbA1c (%) | < 6.4 | 6.4–7.2 | 7.2–8.3 | ≥ 8.3 | < 6.4 | 6.4–7.2 | 7.2–8.3 | ≥ 8.3 | |||
| No. of patients | 1558 | 136 | 90 | 91 | 94 | 256 | 282 | 322 | 287 | ||
| Demographics and medical history | |||||||||||
| Age (years) | 64.7 (12.8) | 67.2 (12.9) | 66.0 (12.4) | 65.1 (11.6) | 62.8 (13.3) | 0.001 | 67.0 (11.9) | 63.6 (14.2) | 64.3 (13.3) | 62.9 (11.6) | 0.001 |
| Female (n[%]) | 589 (37.8%) | 81 (59.6%) | 38 (42.2%) | 50 (54.9%) | 53 (56.4%) | 0.073 | 82 (32.0%) | 89 (31.6%) | 107 (33.2%) | 89 (31.0%) | 0.944 |
| Hypertension (n[%]) | 1695 (71%) | 107 (78.7%) | 63 (70.0%) | 61 (67.0%) | 62 (66.0%) | 0.121 | 186 (72.7%) | 171 (60.6%) | 198 (61.5%) | 177 (61.7%) | 0.011 |
| CVD (n[%]) | 812 (33.8%) | 52 (38.2%) | 36 (40.0%) | 28 (30.8%) | 28 (29.8%) | 0.327 | 88 (34.4%) | 86 (30.5%) | 69 (21.4%) | 99 (34.5%) | 0.001 |
| CHF (n[%]) | 406 (16.9%) | 28 (20.6%) | 22 (24.4%) | 21 (23.1%) | 16 (17.0%) | 0.620 | 28 (10.9%) | 42 (14.9%) | 22 (6.8%) | 41 (14.3%) | 0.007 |
| Stroke (n[%]) | 492 (20.5%) | 35 (25.7%) | 21 (23.3%) | 16 (17.6%) | 13 (13.8%) | 0.125 | 68 (26.6%) | 52 (18.4%) | 48 (14.9%) | 66 (23.0%) | 0.003 |
| BMI (m2/kg) | 25.2 (3.9) | 23.4 (3.4) | 24.4 (4.8) | 24.2 (3.8) | 24.6 (3.9) | 0.037 | 25.1 (3.6) | 26.1 (4.0) | 26.0 (4.1) | 25.9 (3.7) | 0.102 |
| MAP (mmHg) | 99.3 (14.0) | 96.7 (14.1) | 96.9 (15.1) | 99.2 (14.1) | 98.8 (13.8) | 0.034 | 99.2 (13.6) | 98.7 (13.1) | 100.2 (14.0) | 101.1 (14.8) | 0.003 |
| Hb and iron profiles | |||||||||||
| Hemoglobin (g/dl) | 11.6 (2.2) | 8.9 (0.9) | 8.9 (1.1) | 8.9 (0.9) | 9.1 (0.8) | 0.012 | 12.6 (1.6) | 12.7 (1.7) | 12.5 (1.7) | 12.4 (1.6) | 0.644 |
| Iron (mg/dl) | 69.8 (27.2) | 57.6 (26.3) | 59.0 (30.0) | 54.1 (25.6) | 60.8 (30.1) | 0.617 | 83.7 (28.3) | 77.0 (26.9) | 71.5 (28.5) | 70.7 (29.6) | <0.001 |
| Ferritin (ng/ml) | 212 (110–387) |
201 (109–454) |
273 (113–481) |
240 (104–467) |
222 (114–413) |
0.761 | 201 (108–345) |
203 (96–352) |
193 (88–331) |
200 (107–387) |
0.311 |
| Iron deficiency (n[%]) a | 547 (46.2%) | 69 (67.6%) | 49 (69.0%) | 33 (62.3%) | 36 (58.1%) | 0.577 | 66 (29.9%) | 80 (35.7%) | 109 (48.7%) | 105 (46.5%) | <0.001 |
| Laboratory data | |||||||||||
| eGFR (ml/min/1.73 m2) | 33.0 (11.9) | 25.3 (8.6) | 26.6 (9.5) | 27.6 (10.7) | 28.2 (12.3) | 0.897 | 34.5 (11.5) | 36.8 (11.8) | 35.8 (11.8) | 34.0 (11.5) | 0.086 |
| UPCR (mg/g) | 1164 (322–3297) | 1714 (711–4227) |
1957 (652–5390) |
2970 (977–6135) |
3106 (1345–5787) |
0.073 | 567 (250–1757) |
545 (167–1532) |
923 (268–2885) |
1499 (452–4187) | <0.001 |
| Albumin (g/dl) | 3.8 (0.6) | 3.5 (0.7) | 3.5 (0.6) | 3.4 (0.7) | 3.4 (0.6) | 0.430 | 4.0 (0.5) | 4.0 (0.5) | 3.9 (0.6) | 3.7 (0.5) | 0.242 |
| Blood glucose (mg/dl) | 134.8 (56.4) | 105.4 (31.4) | 127.6 (46.0) | 141.2 (59.0) | 162.5 (75.6) | <0.001 | 106.4 (28.3) | 118.2 (41.2) | 139.3 (45.7) | 176.2 (71.5) | <0.001 |
| HbA1c (%) | 7.6 (1.8) | 5.7 (0.5) | 6.8 (0.2) | 7.7 (0.3) | 9.7 (1.5) | <0.001 | 5.8 (0.5) | 6.8 (0.2) | 7.7 (0.3) | 10.2 (1.5) | <0.001 |
| Total calcium (mg/dl) | 9.3 (0.7) | 9.0 (0.7) | 8.9 (0.7) | 9.1 (0.6) | 9.2 (0.8) | 0.001 | 9.3 (0.6) | 9.4 (0.6) | 9.3 (0.7) | 9.3 (0.7) | 0.003 |
| Phosphate (mg/dl) | 4.0 (0.9) | 4.4 (0.7) | 4.4 (1.0) | 4.2 (0.9) | 4.2 (0.9) | 0.155 | 3.8 (0.8) | 3.8 (0.8) | 3.9 (0.9) | 4.0 (0.9) | 0.001 |
| Uric acid (mg/dl) | 7.8 (1.9) | 7.8 (1.7) | 8.1 (2.1) | 7.5 (2.0) | 8.2 (2.9) | <0.001 | 7.9 (1.9) | 7.9 (1.8) | 7.6 (1.7) | 7.6 (1.9) | 0.003 |
| Total cholesterol (mg/dl) | 193 (164–226) | 177 (146–210) | 177 (153–216) | 187 (162–230) | 201 (167–246) | <0.001 | 187 (164–215) | 194 (164–223) | 196 (164–225) | 209 (174–250) | <0.001 |
| Triglyceride (mg/dl) | 140 (100–208) | 115 (78–166) | 119 (86–158) | 150 (96–202) | 157 (108–241) | <0.001 | 124 (92–182) | 138 (98–210) | 145 (103–212) | 180 (118–261) | <0.001 |
| CRP (mg/l) | 1.6 (0.4–10.0) | 1.6 (0.5–10.4) | 4.4 (0.7–19.7) | 3.0 (0.5–35.0) | 1.3 (0.4–13.3) | 0.184 | 1.0 (0.3–5.7) | 1.2 (0.4–7.9) | 1.5 (0.5–8.7) | 2.1 (0.5–14.6) | 0.017 |
Data expressed as the mean ± standard deviation, median (interquartile range), or count (percentage).
Abbreviations: HbA1c, glycated hemoglobin; CVD, cardiovascular disease; CHF, congestive heart failure; BMI, body mass index; MAP, mean arterial pressure; Hb, hemoglobin; eGFR, estimated glomerular filtration rate; UPCR, urine protein-to-creatinine ratio; CRP, C-reactive protein.
a. Iron deficiency was defined as iron saturation <20% or ferritin < 100 ng/ml.
During the 3-year follow-up period, 132 (32.1%) and 180 (15.7%) incident cases of ESRD were observed among the patients with Hb < 10 and Hb ≥ 10 g/dL, respectively, in Table 2. In the Hb ≥ 10 g/dL group, patients with higher HbA1c levels had higher rates of ESRD. There were 110 (26.8%) and 161 (14.0%) incident cases of all-cause mortality among the patients with Hb < 10 and Hb ≥ 10 g/dL, respectively. Furthermore, higher incidence rates of all-cause mortality and composite outcomes were associated with higher HbA1c levels in the Hb ≥ 10 g/dL group.
Table 2. Clinical outcomes of patients with stages 3–4 CKD, stratified by Hb and HbA1c quartiles.
| All | Hemoglobin < 10 g/dl | P | Hemoglobin ≥ 10 g/dl | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c quartiles | HbA1c quartiles | ||||||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | ||||
| HbA1c (%) | < 6.4 | 6.4–7.2 | 7.2–8.3 | ≥ 8.3 | < 6.4 | 6.4–7.2 | 7.2–8.3 | ≥ 8.3 | |||
| No. of patients | 1558 | 136 | 90 | 91 | 94 | 256 | 282 | 322 | 287 | ||
| Follow-up days | 1029 (607–1642) |
837 (532–1597) |
875 (520–1512) |
962 (507–1551) |
1025 (554–1765) | 0.595 | 1028 (637–1654) |
1061 (587–1633) |
1051 (581–1695) |
1171 (763–1654) |
0.216 |
| Annual eGFR decline (ml/min/1.73 m2/year) | -3.0 (-7.4 to 0.1) |
-5.4 (-9.4 to -1.6) |
-5.1 (-10.3 to -0.9) |
-5.8 (-13.2 to -1.6) |
-5.0 (-10.2 to -1.0) |
0.767 | -1.5 (-4.5 to 0.7) |
-1.3 (-4.4 to 1.4) |
-2.4 (-6.4 to 0.0) |
-4.0 (-9.6 to -0.4) |
<0.001 |
| Rapid eGFR decline a | 563 (36.1%) | 72 (52.9%) | 45 (50.0%) | 48 (52.7%) | 47 (50.0%) | 0.953 | 60 (23.4%) | 65 (23.0%) | 98 (30.4%) | 128 (44.6%) | <0.001 |
| ESRD | 312 (20.0%) | 44 (32.4%) | 27 (30.0%) | 36 (39.6%) | 25 (26.6%) | <0.001 | 32 (12.5%) | 25 (8.9%) | 48 (14.9%) | 75 (26.1%) | <0.001 |
| All-cause mortality | 271 (17.4%) | 33 (24.3%) | 30 (33.3%) | 26 (28.6%) | 21 (22.3%) | 0.320 | 23 (9.0%) | 38 (13.5%) | 42 (13.0%) | 58 (20.2%) | 0.002 |
| CV event + all-cause mortality | 390 (25.0%) | 48 (39.3%) | 35 (38.9%) | 36 (39.6%) | 31 (33.0%) | 0.653 | 43 (16.8%) | 46 (16.3%) | 59 (18.4%) | 92 (32.1%) | <0.001 |
Abbreviations: ESRD, end-stage renal disease; CV, cardiovascular.
a Annual eGFR decline more than −5 mL/min/1.73 m2/year
Variables associated with HbA1c levels
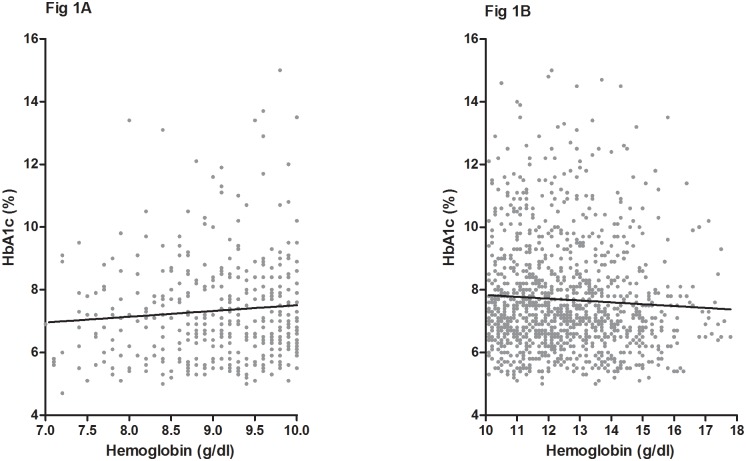
Table 3 shows the relevant covariates of the HbA1c levels in multivariable linear regression. HbA1c levels correlated with age, eGFR, log UPCR, Hb levels, and log cholesterol. However, HbA1c levels correlated significantly with Hb levels only in the Hb < 10 g/dL group (95% CI, 0.047–0.24; P = 0.004) but not in the Hb ≥ 10 g/dL group (95% CI, −0.054–0.082; P = 0.689; Table 3 and Fig 1).
Table 3. Multivariable linear regression of HbA1c levels.
| All | |||
|---|---|---|---|
| Variables | β | 95% CI | p-value |
| Age (years) | -0.009 | -0.014 to -0.003 | 0.002 |
| Male vs. female | 0.029 | -0.116 to 0.174 | 0.693 |
| CVD | 0.030 | -0.113 to 0.173 | 0.680 |
| BMI (kg/m2) | 0.002 | -0.015 to 0.020 | 0.795 |
| MAP (mmHg) | -0.002 | -0.007 to 0.003 | 0.421 |
| eGFR (ml/min/1.73 m2) | 0.012 | 0.005 to 0.018 | <0.001 |
| Log UPCR | 0.308 | 0.146 to 0.469 | <0.001 |
| Albumin (g/dl) | -0.210 | -0.343 to -0.077 | 0.002 |
| Hemoglobin (g/dl) | |||
| In total population | 0.099 | 0.058 to 0.141 | <0.001 |
| In Hb ≥ 10 g/dl* | 0.014 | -0.054 to 0.082 | 0.689 |
| In Hb < 10 g/dl* | 0.146 | 0.047 to 0.245 | 0.004 |
| Log cholesterol | 2.098 | 1.524 to 2.672 | <0.001 |
| Log CRP | 0.059 | -0.011 to 0.130 | 0.097 |
| Phosphate (mg/dl) | -0.063 | -0.133 to 0.006 | 0.072 |
Abbreviations: Hb, hemoglobin; CVD, cardiovascular disease; BMI, body mass index; MAP, mean arterial pressure; eGFR, estimated glomerular filtration rate; UPCR, urine protein-to-creatinine ratio; CRP, C-reactive protein.
* Segmental linear regression with the same variables
P < 0.05 indicates a significant association with HbA1c levels.
Fig 1. Regression diagram for HbA1c and Hb levels among subjects with (A) Hb < 10 g/dL and (B) Hb ≥ 10 g/dL.
Relation between HbA1c quartiles and clinical outcomes
In the Hb ≥ 10 g/dL group (Table 4), a fully adjusted multivariable Cox regression model revealed an increased risk of RRT in the third quartile (HR = 1.65, 95% CI, 0.99–2.76; P = 0.057), and the highest quartile (HR = 1.92, 95% CI, 1.17–3.15; P = 0.010) (P for trend = 0.042) compared with the first quartile subgroup. In addition, a trend of increased risks of composite outcomes (CV events combined with all-cause mortality) was associated with the third quartile (HR = 1.51, 95% CI, 0.98–2.32; P = 0.059), and the highest quartile (HR = 1.54, 95% CI, 1.03–2.31; P = 0.036) (P for trend = 0.171). Similarly, significantly increased risks of all-cause mortality were related to the second quartile (HR = 2.17, 95% CI, 1.23–3.84; P = 0.012) and the highest quartile (HR = 1.76, 95% CI, 1.02–3.03; P = 0.043) (P for trend = 0.063). However, in the Hb < 10 g/dL group (Table 4), the fully adjusted Cox regression model did not indicate a significant risk trend for clinical outcomes for the different HbA1c quartiles compared with the first quartile subgroup.
Table 4. Risk of outcomes among subjects with Hb < 10 g/dL and Hb ≥ 10 g/dL, stratified by HbA1c quartiles.
| All | Hemoglobin < 10 g/dl | Hemoglobin ≥ 10 g/dl | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c quartiles | HbA1c quartiles | HbA1c quartiles | ||||||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| HR (95% CI) for RRT | ||||||||||||
| Unadjusted | 1 | 0.68 (0.46–1.00)* | 1.13 (0.81–1.58) | 1.28 (0.93–1.77) | 1 | 0.80 (0.47–1.34) | 1.12 (0.69–1.79) | 0.59 (0.35–1.00) | 1 | 0.69 (0.38–1.23) | 1.42 (0.88–2.30) | 2.25 (1.44–3.54)* |
| Adjusted a | 1 | 1.05 (0.71–1.57) | 1.37 (0.96–1.93) | 1.34 (0.95–1.87) | 1 | 1.24 (0.71–2.17) | 1.32 (0.80–2.20) | 0.79 (0.45–1.36) | 1 | 1.14 (0.62–2.10) | 1.65 (0.99–2.76) | 1.92 (1.17–3.15)* |
| HR (95% CI) for all-cause mortality | ||||||||||||
| Unadjusted | 1 | 1.43 (0.98–2.11) | 1.23 (0.83–1.81) | 1.51 (1.04–2.19)* | 1 | 1.51 (0.88–2.60) | 1.07 (0.59–1.94) | 0.96 (0.53–1.71) | 1 | 1.69 (0.96–2.96) | 1.68 (0.97–2.91) | 2.34 (1.38–3.96)* |
| Adjusted a | 1 | 1.79 (1.21–2.65)* | 1.30 (0.87–1.93) | 1.49 (1.01–2.18)* | 1 | 1.62 (0.92–2.85) | 0.94 (0.50–1.76) | 1.04 (0.56–1.91) | 1 | 2.17 (1.23–3.84)* | 1.72 (0.98–3.02) | 1.76 (1.02–3.03)* |
| HR (95% CI) for CV event + all-cause mortality | ||||||||||||
| Unadjusted | 1 | 0.96 (0.70–1.33) | 1.07 (0.78–1.45) | 1.45 (1.08–1.95)* | 1 | 1.13 (0.70–1.81) | 1.05 (0.65–1.69) | 0.89 (0.55–1.44) | 1 | 1.01 (0.65–1.58) | 1.27 (0.84–1.93) | 2.07 (1.40–3.06)* |
| Adjusted a | 1 | 1.26 (0.91–1.76) | 1.28 (0.93–1.75) | 1.40 (1.03–1.89)* | 1 | 1.20 (0.73–1.97) | 1.05 (0.64–1.73) | 1.01 (0.61–1.67) | 1 | 1.29 (0.82–2.03) | 1.51 (0.98–2.32) | 1.54 (1.03–2.31)* |
a The Cox proportional hazard model was adjusted for age, sex, estimated glomerular filtration rate, log (urine protein-to-creatinine ratio), cardiovascular disease, hypertension, mean blood pressure, hemoglobin, albumin, log (cholesterol), log (C-reactive protein), phosphorus, body mass index and iron.
Abbreviations: RRT, renal replacement therapy
* p < 0.05 indicates significant differences compared with the reference group
To explore the independent effects of variables on RRT, we conducted sequential models to examine changes in parameter estimates. Cox regression model with 4 incremental levels of covariate adjustment yielded similar results to the primary analysis and is shown in S1 Table.
Sensitivity analyses
In the Hb ≥ 10 g/dL group, sensitivity analysis reclassifying patients into subgroups by clinical relevance showed similar tendency towards an increased HR for RRT, with HbA1c levels of ≥ 6% to < 7%, ≥ 7% to < 9%, and ≥ 9%, having HRs of 1.83 (95% CI, 0.92–3.65), 2.43 (95% CI, 1.26–4.68), and 3.06 (95% CI, 1.56–5.99) (P for trend = 0.008), respectively, when compared with HbA1c < 6% (S2 Table). Similarly, increased risks of composite outcomes (CV events combined with all-cause mortality) were associated with HbA1c levels with HRs of 1.41 (95% CI, 0.88–2.26) for ≥ 7% to < 9%, and 1.66 (95% CI, 1.02–2.70) for ≥ 9%, respectively (P for trend = 0.194). Also, increased risks of all-cause mortality were associated with HbA1c levels with HRs of 1.64 (95% CI, 0.92–2.95) for ≥ 7% to < 9%, and 1.88 (95% CI, 1.00–3.52) for ≥ 9%, respectively (P for trend = 0.079). By contrast, the association was not observed in patients with Hb < 10 g/dL group (S2 Table).
Furthermore, to clarify the direct effect of glucose control on RRT, we reclassified the patients into four subgroups according to their mean plasma glucose levels, with glucose levels of 125, 155, and 210 mg/dL corresponding to HbA1c levels of 6%, 7%, and 9% (S3 Table). An increased risk of RRT was associated with mean plasma glucose levels of 125–155 mg/dL (HR = 1.21, 95% CI, 0.62–2.36), 155–210 mg/dL (HR = 1.07, 95% CI, 0.55–2.07), and ≥ 210 mg/dL (HR = 2.32, 95% CI, 1.04–5.19) (P for trend = 0.164).
Discussion
In diabetic patients with stages 3–4 CKD, we found that higher HbA1c levels are associated with a trend of increased risks for RRT, all-cause mortality and composite outcomes. In the Hb ≥ 10 g/dL group, the magnitudes of association were prominent and statistically significant among those with the highest HbA1c quartile compared with those with the lowest quartile. In patients with Hb < 10 g/dL, HbA1c levels were not associated with clinical outcomes; however, mean plasma glucose levels could be more useful in predicting RRT risk.
Anemia occurs commonly in patients with CKD as serum Hb levels correlate almost linearly with eGFR[18]. In diabetic patients, the prevalence of anemia is higher even in the absence of nephropathy, and DM has been indicated as an independent determinant of Hb levels[3, 19]. Several mechanisms have been described for the link between anemia and diabetes: (1) reduced erythropoietin production attributed to splanchnic sympathetic denervation of the kidneys resulting from diabetic autonomic neuropathy[20]; (2) impairment of the hypoxia-sensing mechanism secondary to vascular and tubulointerstitial lesions[21, 22]; (3) chronic systemic inflammation contributing to erythropoietin hyporesponsiveness and functional iron deficiency through increased hepcidin levels, which are independent of uremic toxin retention[23, 24]; (4) increased urinary excretion of transferrin and erythropoietin as a result of nonselective proteinuria[25]; and (5) renin–angiotensin system blockade partly impeding the physiologic erythropoietic effects of angiotensin II[26]. Several observational studies, including our cohort study, have recognized that patients with diabetic CKD present higher prevalence of anemia than that in non-diabetic counterparts, across all ranges of kidney function (CKD stages 1–5)[4, 23].
HbA1c levels are widely used to assess the degree of glycemic control and risk prediction of future vascular complications, in addition to being a diagnostic marker of diabetes. However, the underlying challenges associated with the predictive ability of HbA1c persist in CKD environments, including the accurate reflection of glycemic control and the related outcomes[11]. In addition to glucose, HbA1c levels may be falsely elevated or decreased in CKD because a uremic environment shortens the red blood cell (RBC) lifespan, and carbamylated Hb formed in the presence of high urea interferes with glycosylation of Hb[27, 28]. Many factors present in CKD have an impact on RBC turnover, including fragile RBCs, ESA administration, iron deficiency anemia, and transfusion therapy[28–30]. Prior published reports have supported this notion. Freedman et al. reported an inverse correlation between eGFR and the glucose/HbA1c ratio in diabetic patients with stages 3–4 CKD[31]. Kim et al. also demonstrated that among diabetic patients with pre-dialysis CKD, glucose/HbA1c and glycated albumin/HbA1c ratios correlated inversely with eGFR, whereas the glucose/glycated albumin ratio did not[32]. Considering the effect of Hb, Agarwal et al. reported that among 128 patients with DM and CKD, a decline in HbA1c correlated with advancing CKD stages, but the statistical significance was removed after adjustment for Hb[33]. Taken together, HbA1c levels appear to falsely decrease with the declining eGFR in patients with DM and advanced CKD. In our cohort, we confirmed that eGFR significantly affected HbA1c independently in all stages 3–4 CKD patients through the regression model. Moreover, it was established that the positive correlation between Hb and HbA1c levels only occurred in the Hb < 10 g/dL group.
HbA1c levels might be used to predict clinical outcomes more accurately in CKD patients without anemia. Se Won Oh et al. explored that 799 diabetic patients with eGFR < 60 mL/min/1.73m2 showed increased risks of ESRD as HbA1c levels increased from 6.5%[34]. A large observational study conducted by Shurraw et al. on 23 000 patients with DM and eGFR < 60 mL/min/1.73m2 demonstrated that baseline HbA1c > 7% was associated with increased risks of all-cause mortality, myocardial infarction, and ESRD[9]. To the best of our knowledge, although HbA1c levels could be affected by Hb variability, none of these studies have examined this effect. In our diabetic patients with stages 3–4 CKD and Hb < 10 g/dL, we observed that higher HbA1c levels were not predictive of inferior clinical outcomes, and similar results were observed with different HbA1c classifications. By contrast, higher average fasting glucose levels remained associated with higher RRT risks. In this regard, we speculate that the use of direct glucose monitors or alternative markers other than HbA1c levels for evaluating glycemic state and predicting clinical outcomes might eliminate the confounding effect as kidney function declines accompanied by Hb drops. Recently, glycated albumin which is not affected by Hb appears to be a more appropriate representation of short-term glycemic control and glucose fluctuations compared with HbA1c in CKD patients[11]. Besides, cumulative evidence supports the predictive role of glycated albumin in diabetic complications [35–38]. This might provide a solution to improve the accuracy as a marker of glycemic control in diabetic CKD populations.
Anemia is also regarded as an index of chronic inflammation and poor nutrition[12, 13]. Several studies have provided the speculation that in populations with a high prevalence of inflammation and malnutrition, such as advanced CKD, HbA1c levels alone could be less predictive of clinical outcomes. Our previous analysis demonstrated that HbA1c ≥ 9% predicts ESRD and composite outcomes in CKD stages 3–4 patients but not in stage 5 patients[10]. Kalantar et al. demonstrated that in diabetic patients undergoing maintenance hemodialysis and with Hb < 11 g/dL, higher HbA1c levels were not associated with increased risks of mortality, as seen in nonanemic patients[39]. Moreover, from the post hoc analysis of the Trial to Reduce Cardiovascular Events With Aranesp Therapy in diabetic CKD patients with anemia, higher baseline CRP levels, known as inflammation biomarkers, were associated with a greater risk of future ESRD, whereas higher HbA1c levels did not present the association[40]. In our current study, in which diabetic patients with stages 3–4 CKD and without ESA therapy were recruited, one possibility might be that a lower HbA1c level identifies individuals with lower Hb instead of simply reflecting glycemic control, particularly among patients with Hb < 10 g/dL. Even though the hemoglobin adjustment was done, HbA1c was still not predictive, which might imply other mechanisms.
This study had some limitations. First, we relied on the baseline measurement of HbA1c values for analysis rather than mean values. However, because of the legacy effect of HbA1c and the influence of eGFR on HbA1c levels, it is reasonable to use baseline HbA1c as an indicator, when CKD stages were classified at simultaneously. Second, we did not have explicit surrogate nutrition markers. Therefore, residual confounding based on variables related to malnutrition may remain. Third, our laboratory measurements of serum glucose levels were performed in a fasting state. We did not take postprandial glucose levels into account; however, postprandial hyperglycemia resulting from insulin resistance may affect HbA1c levels in advanced CKD[41]. Fourth, although HbA1c values obtained with immunoassays were unaffected by carbamylation in uremic environments, we were constrained to use automated cation-exchange high-performance liquid chromatography as the HbA1c assessment method in our study. Finally, the sample size of patients with Hb < 10 g/dl was only half as many patients with Hb > 10 g/dl, which might partially explain the lack of statistically significant risks.
In conclusion, among patients with stage 3–4 diabetic CKD, higher baseline HbA1c levels correlated with higher risks for ESRD, all-cause mortality, and composite outcomes (CV events and all-cause mortality) in patients with Hb ≥ 10 g/dL, whereas the association did not exist in those with Hb < 10 g/dL. Additionally, the association reached statistical significance in the highest HbA1c quartile. Further research is required to determine alternative markers (glycated albumin and continuous glucose monitoring) that might be affected to a smaller extent in CKD conditions, and the clinical target ranges at which glycemic control can reduce outcome risks in anemic CKD.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Vanholder R, Fouque D, Glorieux G, Heine GH, Kanbay M, Mallamaci F, et al. Clinical management of the uraemic syndrome in chronic kidney disease. Lancet Diabetes Endocrinol. 2016;4(4):360–73. Epub 2016/03/08. doi: 10.1016/S2213-8587(16)00033-4 . [DOI] [PubMed] [Google Scholar]
- 2.Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2002;162(12):1401–8. Epub 2002/06/22. . [DOI] [PubMed] [Google Scholar]
- 3.Grossman C, Dovrish Z, Koren-Morag N, Bornstein G, Leibowitz A. Diabetes mellitus with normal renal function is associated with anaemia. Diabetes Metab Res Rev. 2014;30(4):291–6. Epub 2013/10/31. doi: 10.1002/dmrr.2491 . [DOI] [PubMed] [Google Scholar]
- 4.Al-Khoury S, Afzali B, Shah N, Covic A, Thomas S, Goldsmith DJ. Anaemia in diabetic patients with chronic kidney disease—prevalence and predictors. Diabetologia. 2006;49(6):1183–9. Epub 2006/04/13. doi: 10.1007/s00125-006-0254-z . [DOI] [PubMed] [Google Scholar]
- 5.Ravanan R, Spiro JR, Mathieson PW, Smith RM. Impact of diabetes on haemoglobin levels in renal disease. Diabetologia. 2007;50(1):26–31. Epub 2006/11/30. doi: 10.1007/s00125-006-0514-y . [DOI] [PubMed] [Google Scholar]
- 6.Thomas MC, MacIsaac RJ, Tsalamandris C, Molyneaux L, Goubina I, Fulcher G, et al. The burden of anaemia in type 2 diabetes and the role of nephropathy: a cross-sectional audit. Nephrol Dial Transplant. 2004;19(7):1792–7. Epub 2004/04/23. doi: 10.1093/ndt/gfh248 . [DOI] [PubMed] [Google Scholar]
- 7.El-Achkar TM, Ohmit SE, McCullough PA, Crook ED, Brown WW, Grimm R, et al. Higher prevalence of anemia with diabetes mellitus in moderate kidney insufficiency: The Kidney Early Evaluation Program. Kidney Int. 2005;67(4):1483–8. Epub 2005/03/23. doi: 10.1111/j.1523-1755.2005.00226.x . [DOI] [PubMed] [Google Scholar]
- 8.Thomas MC, Tsalamandris C, MacIsaac RJ, Jerums G. The epidemiology of hemoglobin levels in patients with type 2 diabetes. Am J Kidney Dis. 2006;48(4):537–45. Epub 2006/09/26. doi: 10.1053/j.ajkd.2006.06.011 . [DOI] [PubMed] [Google Scholar]
- 9.Shurraw S, Hemmelgarn B, Lin M, Majumdar SR, Klarenbach S, Manns B, et al. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med. 2011;171(21):1920–7. Epub 2011/11/30. doi: 10.1001/archinternmed.2011.537 . [DOI] [PubMed] [Google Scholar]
- 10.Kuo IC, Lin HY, Niu SW, Hwang DY, Lee JJ, Tsai JC, et al. Glycated Hemoglobin and Outcomes in Patients with Advanced Diabetic Chronic Kidney Disease. Sci Rep. 2016;6:20028 Epub 2016/01/29. doi: 10.1038/srep20028 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speeckaert M, Van Biesen W, Delanghe J, Slingerland R, Wiecek A, Heaf J, et al. Are there better alternatives than haemoglobin A1c to estimate glycaemic control in the chronic kidney disease population? Nephrol Dial Transplant. 2014;29(12):2167–77. Epub 2014/01/29. doi: 10.1093/ndt/gfu006 . [DOI] [PubMed] [Google Scholar]
- 12.Molnar MZ, Czira ME, Rudas A, Ujszaszi A, Haromszeki B, Kosa JP, et al. Association between the malnutrition-inflammation score and post-transplant anaemia. Nephrol Dial Transplant. 2011;26(6):2000–6. Epub 2010/12/01. doi: 10.1093/ndt/gfq690 . [DOI] [PubMed] [Google Scholar]
- 13.Locatelli F, Andrulli S, Memoli B, Maffei C, Del Vecchio L, Aterini S, et al. Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol Dial Transplant. 2006;21(4):991–8. doi: 10.1093/ndt/gfk011 . [DOI] [PubMed] [Google Scholar]
- 14.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2002;39(2 Suppl 1):S1–266. Epub 2002/03/21. . [PubMed] [Google Scholar]
- 15.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S . [DOI] [PubMed] [Google Scholar]
- 16.Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2010;25(8):2616–24. Epub 2010/06/04. doi: 10.1093/ndt/gfq308 . [DOI] [PubMed] [Google Scholar]
- 17.Hsu CC, Hwang SJ, Wen CP, Chang HY, Chen T, Shiu RS, et al. High prevalence and low awareness of CKD in Taiwan: a study on the relationship between serum creatinine and awareness from a nationally representative survey. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006;48(5):727–38. Epub 2006/10/25. doi: 10.1053/j.ajkd.2006.07.018 . [DOI] [PubMed] [Google Scholar]
- 18.Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13(2):504–10. Epub 2002/01/24. . [DOI] [PubMed] [Google Scholar]
- 19.Craig KJ, Williams JD, Riley SG, Smith H, Owens DR, Worthing D, et al. Anemia and diabetes in the absence of nephropathy. Diabetes Care. 2005;28(5):1118–23. Epub 2005/04/28. . [DOI] [PubMed] [Google Scholar]
- 20.Spallone V, Maiello MR, Kurukulasuriya N, Barini A, Lovecchio M, Tartaglione R, et al. Does autonomic neuropathy play a role in erythropoietin regulation in non-proteinuric Type 2 diabetic patients? Diabet Med. 2004;21(11):1174–80. Epub 2004/10/23. doi: 10.1111/j.1464-5491.2004.01306.x . [DOI] [PubMed] [Google Scholar]
- 21.Bosman DR, Winkler AS, Marsden JT, Macdougall IC, Watkins PJ. Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diabetes Care. 2001;24(3):495–9. Epub 2001/04/06. . [DOI] [PubMed] [Google Scholar]
- 22.Deray G, Heurtier A, Grimaldi A, Launay Vacher V, Isnard Bagnis C. Anemia and diabetes. Am J Nephrol. 2004;24(5):522–6. Epub 2004/09/29. doi: 10.1159/000081058 . [DOI] [PubMed] [Google Scholar]
- 23.Loutradis C, Skodra A, Georgianos P, Tolika P, Alexandrou D, Avdelidou A, et al. Diabetes mellitus increases the prevalence of anemia in patients with chronic kidney disease: A nested case-control study. World J Nephrol. 2016;5(4):358–66. Epub 2016/07/28. doi: 10.5527/wjn.v5.i4.358 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehdi U, Toto RD. Anemia, diabetes, and chronic kidney disease. Diabetes Care. 2009;32(7):1320–6. Epub 2009/07/01. doi: 10.2337/dc08-0779 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard RL, Buddington B, Alfrey AC. Urinary albumin, transferrin and iron excretion in diabetic patients. Kidney Int. 1991;40(5):923–6. Epub 1991/11/01. . [DOI] [PubMed] [Google Scholar]
- 26.Mohanram A, Zhang Z, Shahinfar S, Lyle PA, Toto RD. The effect of losartan on hemoglobin concentration and renal outcome in diabetic nephropathy of type 2 diabetes. Kidney Int. 2008;73(5):630–6. Epub 2007/12/21. doi: 10.1038/sj.ki.5002746 . [DOI] [PubMed] [Google Scholar]
- 27.Fluckiger R, Harmon W, Meier W, Loo S, Gabbay KH. Hemoglobin carbamylation in uremia. N Engl J Med. 1981;304(14):823–7. Epub 1981/04/02. doi: 10.1056/NEJM198104023041406 . [DOI] [PubMed] [Google Scholar]
- 28.Ly J, Marticorena R, Donnelly S. Red blood cell survival in chronic renal failure. Am J Kidney Dis. 2004;44(4):715–9. Epub 2004/09/24. . [PubMed] [Google Scholar]
- 29.Ng JM, Cooke M, Bhandari S, Atkin SL, Kilpatrick ES. The effect of iron and erythropoietin treatment on the A1C of patients with diabetes and chronic kidney disease. Diabetes Care. 2010;33(11):2310–3. Epub 2010/08/28. doi: 10.2337/dc10-0917 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansari A, Thomas S, Goldsmith D. Assessing glycemic control in patients with diabetes and end-stage renal failure. Am J Kidney Dis. 2003;41(3):523–31. Epub 2003/03/04. doi: 10.1053/ajkd.2003.50114 . [DOI] [PubMed] [Google Scholar]
- 31.Freedman BI, Shihabi ZK, Andries L, Cardona CY, Peacock TP, Byers JR, et al. Relationship between assays of glycemia in diabetic subjects with advanced chronic kidney disease. Am J Nephrol. 2010;31(5):375–9. doi: 10.1159/000287561 . [DOI] [PubMed] [Google Scholar]
- 32.Kim IY, Kim MJ, Lee DW, Lee SB, Rhee H, Song SH, et al. Glycated albumin is a more accurate glycemic indicator than hemoglobin A1c in diabetic patients with pre-dialysis chronic kidney disease. Nephrology (Carlton, Vic). 2015. Epub 2015/05/15. doi: 10.1111/nep.12508 . [DOI] [PubMed] [Google Scholar]
- 33.Agarwal R, Light RP. Relationship between glycosylated hemoglobin and blood glucose during progression of chronic kidney disease. Am J Nephrol. 2011;34(1):32–41. Epub 2011/06/11. doi: 10.1159/000328737 . [DOI] [PubMed] [Google Scholar]
- 34.Oh SW, Kim YC, Koo HS, Jin DC, Na KY, Chae DW, et al. Glycated haemoglobin and the incidence of end-stage renal disease in diabetics. Nephrol Dial Transplant. 2011;26(7):2238–44. Epub 2010/11/26. doi: 10.1093/ndt/gfq707 . [DOI] [PubMed] [Google Scholar]
- 35.Murea M, Moran T, Russell GB, Shihabi ZK, Byers JR, Andries L, et al. Glycated albumin, not hemoglobin A1c, predicts cardiovascular hospitalization and length of stay in diabetic patients on dialysis. Am J Nephrol. 2012;36(5):488–96. doi: 10.1159/000343920 . [DOI] [PubMed] [Google Scholar]
- 36.Kondaveeti SB, D K, Mishra S, Kumar RA, Shaker IA. Evaluation of glycated albumin and microalbuminuria as early risk markers of nephropathy in type 2 diabetes mellitus. J Clin Diagn Res. 2013;7(7):1280–3. doi: 10.7860/JCDR/2013/5145.3117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CW, Drechsler C, Suntharalingam P, Karumanchi SA, Wanner C, Berg AH. High Glycated Albumin and Mortality in Persons with Diabetes Mellitus on Hemodialysis. Clin Chem. 2017;63(2):477–85. doi: 10.1373/clinchem.2016.258319 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park SB, Kim SS, Kim IJ, Nam YJ, Ahn KH, Kim JH, et al. Variability in glycated albumin levels predicts the progression of diabetic nephropathy. J Diabetes Complications. 2017;31(6):1041–6. doi: 10.1016/j.jdiacomp.2017.01.014 . [DOI] [PubMed] [Google Scholar]
- 39.Kalantar-Zadeh K, Kopple JD, Regidor DL, Jing J, Shinaberger CS, Aronovitz J, et al. A1C and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30(5):1049–55. Epub 2007/03/06. doi: 10.2337/dc06-2127 . [DOI] [PubMed] [Google Scholar]
- 40.Mc Causland FR, Claggett B, Burdmann EA, Eckardt KU, Kewalramani R, Levey AS, et al. C-Reactive Protein and Risk of ESRD: Results From the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT). Am J Kidney Dis. 2016;68(6):873–81. doi: 10.1053/j.ajkd.2016.07.022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Standl E, Schnell O, Ceriello A. Postprandial hyperglycemia and glycemic variability: should we care? Diabetes Care. 2011;34 Suppl 2:S120–7. Epub 2011/05/06. doi: 10.2337/dc11-s206 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.