Abstract

A method for transition metal-free 1,2-carboboration of unactivated alkenes with bis(catecholato)diboron as the boron source in combination with alkyl halides as the alkyl component is introduced. The three-component reaction proceeds via a radical pathway on a broad range of unactivated alkenes, and the 1,2-carboboration products serve as valuable synthetic building blocks. Density functional theory calculations provide insights into the mechanism.
Difunctionalization of carbon–carbon double bonds, forming two new C–X (X = C or other atoms) bonds in a single operation, is a very powerful and valuable transformation in organic chemistry.1 Along these lines, alkene difunctionalization comprising a borylation step in combination with another C–X bond-forming process2 is highly attractive due to the fact that the installed C–B bond offers a range of highly useful follow-up transformations.3
In recent decades, transition metal catalyzed carboboration of alkenes has matured to an efficient approach for the construction of functionalized alkylboranes,2 which serve as important reagents and building blocks in chemical synthesis. In contrast, only few reports on transition metal-free 1,2-carboboration of alkenes have appeared to date. For example, 1,2-carboboration of allenyl ketones and esters using the strong Lewis acid B(C6F5)3 was disclosed in which B(C6F5)3 regioselectively reacts with the allene moiety (Scheme 1A).4,5 Woerpel reported a carboboration of seven-membered cyclic alkenes with triethylborane, which was suggested to proceed via a concerted process (Scheme 1B).6 A radical pathway, albeit less likely, could not be fully excluded. To our knowledge, a general transition metal-free intermolecular radical 1,2-carboboration of unactivated alkenes is unknown.7 We present herein highly regioselective three-component alkene 1,2-carboboration with a commercial diboron reagent as a chain carrying boron source and alkyl halides as radical precursors (Scheme 1C).
Scheme 1. Metal-Free 1,2-Carboboration of C=C Bonds.

Encouraged by our recent work on transition metal-free 1,2-bifunctionalization of alkenes through radical processes,8 we decided to study unprecedented intermolecular alkene 1,2-carboboration employing alkyl halides in combination with commercial diboron reagents. Tetraalkoxydiboron reagents are known to react with reactive aryl radicals9 and vinyl radicals10 via homolytic substitution at boron, and more recently, it was shown that alkyl radicals engage in radical borylation with this type of reagent.11,12
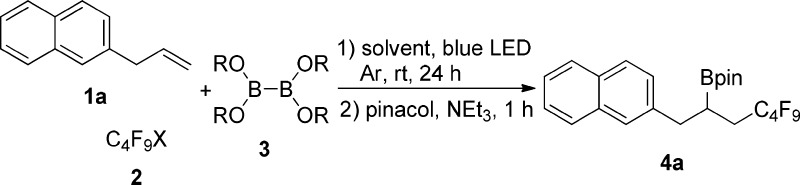
At first, we examined the reaction of 2-allylnaphthalene (1a), perfluorobutyl iodide (2a) as a C-radical precursor, and bis(catecholato)diboron (3a) as a trapping reagent at room temperature for 24 h in different solvents (Table 1, entries 1–8). Since alkyl iodides are known to generate C-radicals upon irradiation, initiation was attempted with a 10 W blue LED. In acetonitrile, the methanol and tetrahydrofuran reaction did not work (Table 1, entries 1–3). In 1,3-dimethyl-2-imidazolidinone (DMI) after transesterification with pinacol, the carboboration product 4a was formed in 19% yield (Table 1, entry 4), and dimethylacetamide (DMAc) provided a slightly lower yield (Table 1, entry 5). Since the corresponding catechol boronate was not stable during purification, the crude product was transesterified with pinacol to the pinacol boronate 4a that could be readily isolated. In hexamethylphosphoramide (HMPA) and 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU) as solvents the product 4a was obtained in 21% and 40% yield, respectively (Table 1, entries 6 and 7). Pleasingly, upon switching to dimethylformamide (DMF), yield significantly increased to 70% (Table 1, entry 8). Other diboron reagents, such as bis(pinacolato)diboron (3b) and bis(neopentyl glycolato)diboron (3c), did not provide the corresponding carboboration products (Table 1, entries 9 and 10). Perfluorobutyl bromide (2b) as a radical precursor afforded a worse result (Table 1, entry 11). Decreasing the amount of 1a or 3a to 1.0 equiv each led to lower yields (Table 1, entries 12 and 13) and yield dropped to 6% in the absence of blue LED irradiation indicating the importance of the light for the initiation step (Table 1, entry 14). Irradiation for 1 h followed by stirring for 23 h in the dark afforded 4a in only 16%, as expected for a radical chain reaction that needs continuous initiation. Using tetrahydrofuran as solvent, we tested several Lewis bases as additives (Table 1, entries 16–18). In the presence of pyridine (2.0 equiv), 4a was isolated in 40% yield (Table 1, entry 16), and 1,4-diazabicyclo[2.2.2]octane (DABCO) or potassium methoxide (MeOK) as additives did not provide any product (Table 1, entries 17 and 18).
Table 1. Optimization of Reaction Conditionsa.
| entry | X | 3 | solvent | yield (%)b |
|---|---|---|---|---|
| 1 | I | 3a | MeCN | 0 |
| 2 | I | 3a | MeOH | 0 |
| 3 | I | 3a | THF | 0 |
| 4 | I | 3a | DMI | 19 |
| 5 | I | 3a | DMAc | 14 |
| 6 | I | 3a | HMPA | 21 |
| 7 | I | 3a | DMPU | 40 |
| 8 | I | 3a | DMF | 70 |
| 9 | I | 3b | DMF | 0 |
| 10 | I | 3c | DMF | 0 |
| 11 | Br | 3a | DMF | 29 |
| 12c | I | 3a | DMF | 43 |
| 13d | I | 3a | DMF | 44 |
| 14e | I | 3a | DMF | 6 |
| 15f | I | 3a | DMF | 16 |
| 16g | I | 3a | THF | 40 |
| 17h | I | 3a | THF | 0 |
| 18i | I | 3a | THF | 0 |
Reaction condition: 1a (0.50 mmol, 2.5 equiv), 2 (0.20 mmol, 1.0 equiv), 3 (0.40 mmol, 2.0 equiv), solvent (0.40 mL), 10 W blue LED, Ar, rt, 24 h; pinacol (0.80 mmol, 4.0 equiv), NEt3 (0.70 mL), 1 h.
Isolated yields.
1a (0.20 mmol, 1.0 equiv).
3a (0.20 mmol, 1.0 equiv).
In the dark.
1 h irradiated by 10 W blue LED followed by 23 h in the dark.
2.0 equiv of pyridine was used.
2.0 equiv of DABCO was used.
2.0 equiv of MeOK was used.
To document substrate scope, various alkenes 1b–1aj were tested under optimized conditions keeping perfluorobutyl iodide as the C-radical precursor (Table 2). Allyl arenes bearing para-, ortho-, and meta-substituents at the arene ring performed well in the carboboration (4b–k). The reaction of 1-naphthyl- and 2-thienyl-substituted alkenes 1l and 1m provided 4l and 4m in 71% and 60% yields, respectively. Transformations with linear, branched, and cyclic aliphatic alkenes proceeded in good yields (4n–r). Ester- (4s–v), imide- (4w), sulfonamide- (4x), nitrile- (4y), and ether-functionalities (4z) were compatible with the reaction conditions. The alkene 1aa containing a tosyloxy group participated in the reaction to give 4aa. However, iodide 4aa′ derived from iodination of 4aa was formed as a major product. Furthermore, alkenes containing heterocyclic rings, such as carbazole, coumarin, and chromone, could be converted to the corresponding products 4ab–ae in moderate to good yields. O-Alkyl d-glucose derivative 1af provided 4af in a good yield. With 1,5-diene 1ag containing both an internal and a terminal double bond, the carboboration occurred regioselectively at the terminal double bond to give 4ag in 65% yield. The reaction of norbornene (1ah) afforded 4ah in good yield and excellent diastereoselectivity. Internal unstrained alkenes such as cyclohexene and (E)-4-octene gave the carboboration products 4ai and 4aj in significantly lower yields. Unfortunately, styrene did not react under optimized conditions.
Table 2. Varying the Alkene Radical Acceptora.
Reaction conditions: 1 (0.50 mmol, 2.5 equiv), 2a (0.20 mmol, 1.0 equiv), 3a (0.40 mmol, 2.0 equiv), DMF (0.40 mL), 10 W blue LED, Ar, rt, 24 h; pinacol (0.80 mmol, 4.0 equiv), NEt3 (0.70 mL), 1 h, isolated yields.
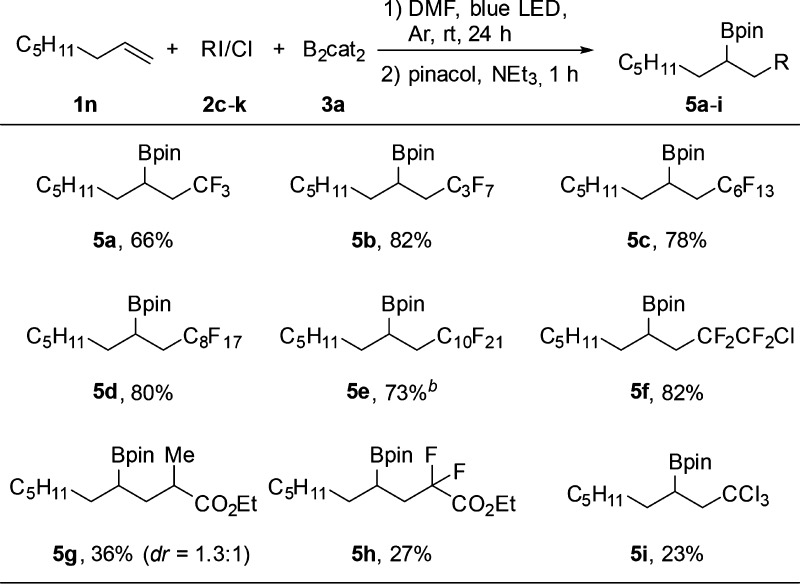
Variation of the C-radical precursor was studied next with 1-octene (1n) as the radical acceptor (Table 3). Using perfluoroalkyl iodides (CF3(CF2)n–I) with n = 0, 2, 5, 7, and 9, the corresponding pinacolboronates 5a–e were obtained in good yields. 1-Chlorotetrafluoro-2-iodoethane reacted chemoselectively by cleavage of the C–I bond to give 5f in 82% yield. Lower yields for the carboboration were achieved with ethyl 2-iodopropanoate and ethyl difluoroiodoacetate as substrates (5g, 5h). Carbon tetrachloride also engaged in the carboboration, albeit a lower yield was achieved (5i).
Table 3. Varying the Radical Precursora.
Reaction conditions: 1n (0.50 mmol, 2.5 equiv), 2 (0.20 mmol, 1.0 equiv), 3a (0.40 mmol, 2.0 equiv), DMF (0.40 mL), 10 W blue LED, Ar, rt, 24 h; pinacol (0.80 mmol, 4.0 equiv), NEt3 (0.70 mL), 1 h, isolated yields.
1 mL of DMF was used.
To address the mechanism, radical clock experiments were conducted (Scheme 2A). Reaction of vinyl cyclopropane 1ak with perfluorobutyl iodide (2a) and 3a gave exclusively the ring-opening product 6, indicating the radical nature of the carboboration.13 The radical mechanism was further supported by the carboboration of 1,6-heptadiene 1al, which afforded the cyclic boronate 7 in 78% yield.
Scheme 2. Mechanistic Studies and Suggested Mechanism.
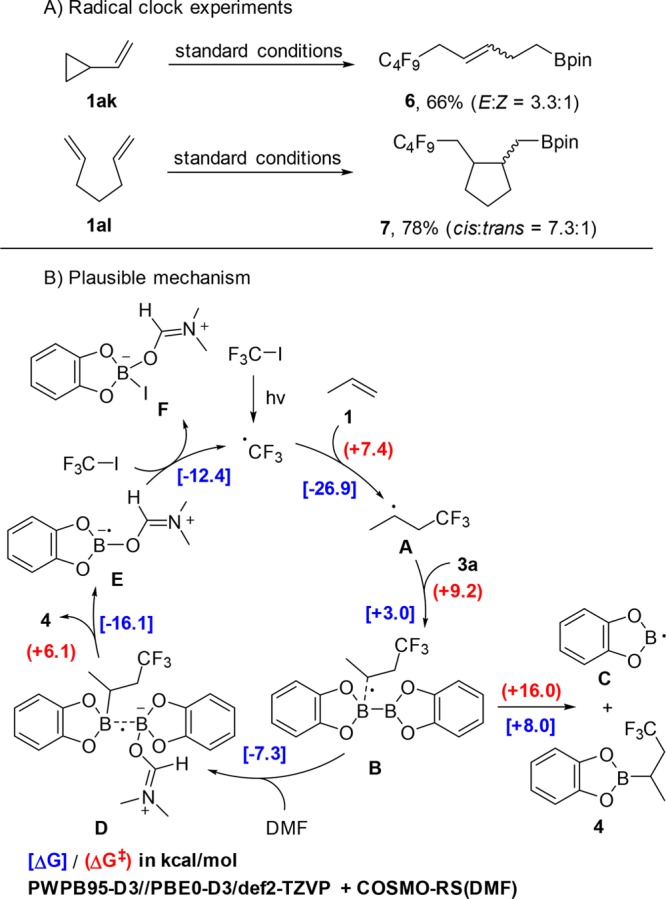
A plausible mechanism exemplified for the reaction of propene, CF3I, and bis(catecholato)diboron, which is supported by DFT calculations, is depicted in Scheme 2 (for details on the DFT calculations, see SI). The trifluoromethyl radical, generated by light-mediated C–I bond homolysis, adds with low barrier (7.4 kcal/mol) to propene to give the secondary alkyl radical A. The critical step is the subsequent formal homolytic substitution at boron of bis(catecholato)diboron (3a). Our DFT calculations reveal that alkyl radical A adds with a low barrier of 9.2 kcal/mol to the boron atom in 3a to generate the adduct radical B. Addition is endothermic by 3.0 kcal/mol. The adduct B could then fragment with a barrier of 16.0 kcal/mol to the product boronic ester 4 along with the reactive boryl radical C. C would then be immediately trapped by the solvent DMF in a strongly exothermic step (−31.4 kcal/mol) to provide the DMF-stabilized boryl radical E.11c However, considering the endothermic (+8 kcal/mol) fragmentation of B to C and 4, radical B would rather fragment back to 3a and A in a degenerate process. We therefore looked for alternative decomposition paths of intermediate B and found that it can be readily trapped without an enthalpic barrier by DMF to provide D (ΔG = −7.3 kcal/mol). Intermediate D is an interesting structure where the B/B interaction is best described as a B–B one-electron σ-bond (Figure 1).14 Fragmentation of D by cleavage of this B–B one-electron σ-bond has a low barrier of 6.1 kcal/mol and leads to product 4 and the DMF-complexed boryl radical E, which further reacts via SET reduction15 of CF3I to F and the chain carrying trifluoromethyl radical.16 Hence, the radical cascade belongs to an electron-catalyzed process.17
Figure 1.
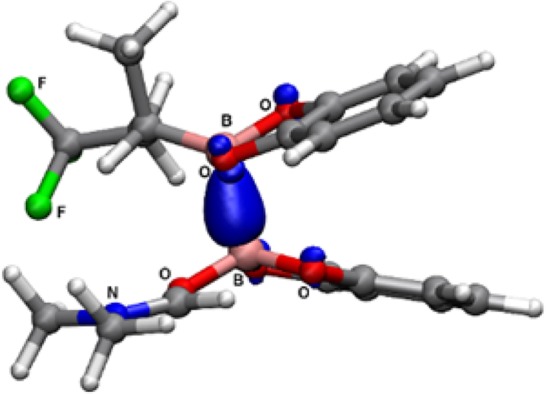
Spin density (PBE0/def2-TZVP, isosurface value = 0.02 au) of radical D, revealing the B–B single electron bond.
Finally, to demonstrate the synthetic value of the difunctionalized adducts, we investigated follow-up chemistry using alkylboronate 4a as a substrate (Scheme 3). Oxidation of 4a afforded hydroxylated product 8 in 95% yield. Further oxidation of 8 with Dess–Martin periodinane and subsequent HF elimination gave the fluorinated enone 9(18) in excellent yield. Silver-catalyzed radical deboronofluorination of 4a in aqueous solution provided the alkyl fluoride 10.19 Other successful transformations of 4a include homologation,20 oxidative coupling with thiophene,21 and amination22 to afford functionalized products 11–13. Treatment of 4a with an aqueous KHF2 solution gave the trifluoroborate salt 14 in 77% yield.
Scheme 3. Follow-up Chemistry.
In summary, a new method for transition metal-free radical carboboration of unactivated alkenes was introduced. The three-component reaction proceeds on a broad range of unactivated alkenes under mild conditions and is experimentally easy to conduct. The boron reagent and the starting alkyl iodides are commercially available. Control experiments and DFT calculations support the suggested radical mechanism. Importantly, the synthetic potential of the novel method was convincingly documented by a series of valuable follow-up transformations.
Acknowledgments
We thank the WWU Münster and the European Research Council (ERC Advanced Grant Agreement no. 692640) for financial support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b03333.
Experimental procedures and analytical data for all compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- For reviews, see:; a McDonald R. I.; Liu G.; Stahl S. S. Chem. Rev. 2011, 111, 2981. 10.1021/cr100371y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zeng X. Chem. Rev. 2013, 113, 6864. 10.1021/cr400082n. [DOI] [PubMed] [Google Scholar]; c Greenhalgh M. D.; Jones A. S.; Thomas S. P. ChemCatChem 2015, 7, 190. 10.1002/cctc.201402693. [DOI] [Google Scholar]; d Hoffmann R. W. Chem. Soc. Rev. 2016, 45, 577. 10.1039/C5CS00423C. [DOI] [PubMed] [Google Scholar]; e Coombs J. R.; Morken J. P. Angew. Chem., Int. Ed. 2016, 55, 2636. 10.1002/anie.201507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews, see:; a Shimizu Y.; Kanai M. Tetrahedron Lett. 2014, 55, 3727. 10.1016/j.tetlet.2014.05.077. [DOI] [Google Scholar]; b Neeve E. C.; Geier S. J.; Mkhalid I. A. I.; Westcott S. A.; Marder T. B. Chem. Rev. 2016, 116, 9091. 10.1021/acs.chemrev.6b00193. [DOI] [PubMed] [Google Scholar]; c Cuenca A. B.; Shishido R.; Ito H.; Fernández E. Chem. Soc. Rev. 2017, 46, 415. 10.1039/C6CS00692B. [DOI] [PubMed] [Google Scholar]; d Fyfe J. W. B.; Watson A. J. B. Chem. 2017, 3, 31. 10.1016/j.chempr.2017.05.008. [DOI] [Google Scholar]; e Collins B. S. L.; Wilson C. M.; Myers E. L.; Aggarwal V. K. Angew. Chem., Int. Ed. 2017, 56, 11700. 10.1002/anie.201701963. [DOI] [PubMed] [Google Scholar]; For recent examples, see:; f Daini M.; Suginome M. J. Am. Chem. Soc. 2011, 133, 4758. 10.1021/ja200856t. [DOI] [PubMed] [Google Scholar]; g Yoshida H.; Kageyuki I.; Takaki K. Org. Lett. 2013, 15, 952. 10.1021/ol4001526. [DOI] [PubMed] [Google Scholar]; h Semba K.; Nakao Y. J. Am. Chem. Soc. 2014, 136, 7567. 10.1021/ja5029556. [DOI] [PubMed] [Google Scholar]; i Smith K. B.; Logan K. M.; You W.; Brown M. K. Chem. - Eur. J. 2014, 20, 12032. 10.1002/chem.201404310. [DOI] [PubMed] [Google Scholar]; j Jia T.; Cao P.; Wang B.; Lou Y.; Yin X.; Wang M.; Liao J. J. Am. Chem. Soc. 2015, 137, 13760. 10.1021/jacs.5b09146. [DOI] [PubMed] [Google Scholar]; k Logan K. M.; Smith K. B.; Brown M. K. Angew. Chem., Int. Ed. 2015, 54, 5228. 10.1002/anie.201500396. [DOI] [PubMed] [Google Scholar]; l Yang K.; Song Q. Org. Lett. 2016, 18, 5460. 10.1021/acs.orglett.6b02527. [DOI] [PubMed] [Google Scholar]; m Yang K.; Song Q. J. Org. Chem. 2016, 81, 1000. 10.1021/acs.joc.5b02564. [DOI] [PubMed] [Google Scholar]; n Semba K.; Ohtagaki Y.; Nakao Y. Org. Lett. 2016, 18, 3956. 10.1021/acs.orglett.6b01675. [DOI] [PubMed] [Google Scholar]; o Huang Y.; Smith K. B.; Brown M. K. Angew. Chem., Int. Ed. 2017, 56, 13314. 10.1002/anie.201707323. [DOI] [PMC free article] [PubMed] [Google Scholar]; p Logan K. M.; Brown M. K. Angew. Chem., Int. Ed. 2017, 56, 851. 10.1002/anie.201609844. [DOI] [PMC free article] [PubMed] [Google Scholar]; q Chen B.; Cao P.; Yin X.; Liao Y.; Jiang L.; Ye J.; Wang M.; Liao J. ACS Catal. 2017, 7, 2425. 10.1021/acscatal.7b00300. [DOI] [Google Scholar]; r Kim N.; Han J. T.; Ryu D. H.; Yun J. Org. Lett. 2017, 19, 6144. 10.1021/acs.orglett.7b03022. [DOI] [PubMed] [Google Scholar]; s Kageyuki I.; Osaka I.; Takaki K.; Yoshida H. Org. Lett. 2017, 19, 830. 10.1021/acs.orglett.6b03820. [DOI] [PubMed] [Google Scholar]; t Logan K. M.; Sardini S. R.; White S. D.; Brown M. K. J. Am. Chem. Soc. 2018, 140, 159. 10.1021/jacs.7b12160. [DOI] [PMC free article] [PubMed] [Google Scholar]; u Liu Z.; Ni H.; Zeng T.; Engle K. M. J. Am. Chem. Soc. 2018, 140, 3223. 10.1021/jacs.8b00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews, see:; a Leonori D.; Aggarwal V. K. Angew. Chem., Int. Ed. 2015, 54, 1082. 10.1002/anie.201407701. [DOI] [PubMed] [Google Scholar]; b Sandford C.; Aggarwal V. K. Chem. Commun. 2017, 53, 5481. 10.1039/C7CC01254C. [DOI] [PubMed] [Google Scholar]
- For transition metal-free 1,2-carboboration of alkynes, see:; a Cade I. A.; Ingleson M. J. Chem. - Eur. J. 2014, 20, 12874. 10.1002/chem.201403614. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Devillard M.; Brousses R.; Miqueu K.; Bouhadir G.; Bourissou D. Angew. Chem., Int. Ed. 2015, 54, 5722. 10.1002/anie.201500959. [DOI] [PubMed] [Google Scholar]; c Roscales S.; Csákÿ A. G. Org. Lett. 2015, 17, 1605. 10.1021/acs.orglett.5b00517. [DOI] [PubMed] [Google Scholar]; d Yamazaki A.; Nagao K.; Iwai T.; Ohmiya H.; Sawamura M. Angew. Chem., Int. Ed. 2018, 57, 3196. 10.1002/anie.201712351. [DOI] [PubMed] [Google Scholar]
- Melen R. L.; Wilkins L. C.; Kariuki B. M.; Wadepohl H.; Gade L. H.; Hashmi A. S. K.; Stephan D. W.; Hansmann M. M. Organometallics 2015, 34, 4127. 10.1021/acs.organomet.5b00546. [DOI] [Google Scholar]
- Sanzone J. R.; Hu C. T.; Woerpel K. A. J. Am. Chem. Soc. 2017, 139, 8404. 10.1021/jacs.7b03986. [DOI] [PubMed] [Google Scholar]
- 1,2-Carboboration involving boryl radical addition and subsequent cyclization onto alkenes and alkynes was recently reported, see:; a Ren S.-C.; Zhang F.-L.; Qi J.; Huang Y.-S.; Xu A.-Q.; Yan H.-Y.; Wang Y.-F. J. Am. Chem. Soc. 2017, 139, 6050. 10.1021/jacs.7b01889. [DOI] [PubMed] [Google Scholar]; b Watanabe T.; Hirose D.; Curran D. P.; Taniguchi T. Chem. - Eur. J. 2017, 23, 5404. 10.1002/chem.201700689. [DOI] [PubMed] [Google Scholar]
- a Hartmann M.; Li Y.; Studer A. J. Am. Chem. Soc. 2012, 134, 16516. 10.1021/ja307638u. [DOI] [PubMed] [Google Scholar]; b Li Y.; Studer A. Angew. Chem., Int. Ed. 2012, 51, 8221. 10.1002/anie.201202623. [DOI] [PubMed] [Google Scholar]; c Li Y.; Hartmann M.; Daniliuc C. G.; Studer A. Chem. Commun. 2015, 51, 5706. 10.1039/C5CC00591D. [DOI] [PubMed] [Google Scholar]; d Hartmann M.; Li Y.; Mück-Lichtenfeld C.; Studer A. Chem. - Eur. J. 2016, 22, 3485. 10.1002/chem.201504852. [DOI] [PubMed] [Google Scholar]; e Hartmann M.; Li Y.; Studer A. Org. Biomol. Chem. 2016, 14, 206. 10.1039/C5OB02210J. [DOI] [PubMed] [Google Scholar]; f Kischkewitz M.; Okamoto K.; Mück-Lichtenfeld C.; Studer A. Science 2017, 355, 936. 10.1126/science.aal3803. [DOI] [PubMed] [Google Scholar]; g Tang X.; Studer A. Chem. Sci. 2017, 8, 6888. 10.1039/C7SC02175E. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Tang X.; Studer A. Angew. Chem., Int. Ed. 2018, 57, 814. 10.1002/anie.201710397. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Gerleve C.; Kischkewitz M.; Studer A. Angew. Chem., Int. Ed. 2018, 57, 2441. 10.1002/anie.201711390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Mo F.; Jiang Y.; Qiu D.; Zhang Y.; Wang J. Angew. Chem., Int. Ed. 2010, 49, 1846. 10.1002/anie.200905824. [DOI] [PubMed] [Google Scholar]; b Yu J.; Zhang L.; Yan G. Adv. Synth. Catal. 2012, 354, 2625. 10.1002/adsc.201200416. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Qiu D.; Jin L.; Zheng Z.; Meng H.; Mo F.; Wang X.; Zhang Y.; Wang J. J. Org. Chem. 2013, 78, 1923. 10.1021/jo3018878. [DOI] [PubMed] [Google Scholar]; d Qiu D.; Zhang Y.; Wang J. Org. Chem. Front. 2014, 1, 422. 10.1039/C4QO00009A. [DOI] [Google Scholar]; e Qiu D.; Meng H.; Jin L.; Tang S.; Wang S.; Mo F.; Zhang Y.; Wang J. Org. Synth. 2014, 91, 106. 10.1002/0471264229.os091.10. [DOI] [Google Scholar]; f Ahammed S.; Nandi S.; Kundu D.; Ranu B. C. Tetrahedron Lett. 2016, 57, 1551. 10.1016/j.tetlet.2016.02.097. [DOI] [Google Scholar]; g Chen K.; Zhang S.; He P.; Li P. Chem. Sci. 2016, 7, 3676. 10.1039/C5SC04521E. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Mfuh A. M.; Doyle J. D.; Chhetri B.; Arman H. D.; Larionov O. V. J. Am. Chem. Soc. 2016, 138, 2985. 10.1021/jacs.6b01376. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Mfuh A. M.; Nguyen V. T.; Chhetri B.; Burch J. E.; Doyle J. D.; Nesterov V. N.; Arman H. D.; Larionov O. V. J. Am. Chem. Soc. 2016, 138, 8408. 10.1021/jacs.6b05436. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Zhang L.; Jiao L. J. Am. Chem. Soc. 2017, 139, 607. 10.1021/jacs.6b11813. [DOI] [PubMed] [Google Scholar]; k Candish L.; Teders M.; Glorius F. J. Am. Chem. Soc. 2017, 139, 7440. 10.1021/jacs.7b03127. [DOI] [PubMed] [Google Scholar]; l Liu W.; Yang X.; Gao Y.; Li C.-J. J. Am. Chem. Soc. 2017, 139, 8621. 10.1021/jacs.7b03538. [DOI] [PubMed] [Google Scholar]; m Zhang L.; Jiao L. Chem. Sci. 2018, 9, 2711. 10.1039/C8SC00008E. [DOI] [PMC free article] [PubMed] [Google Scholar]; With Ir-catalysis:; n Jiang M.; Yang H.; Fu H. Org. Lett. 2016, 18, 5248. 10.1021/acs.orglett.6b02553. [DOI] [PubMed] [Google Scholar]; With Zn-catalysis:; o Bose S. K.; Deißenberger A.; Eichhorn A.; Steel P. G.; Lin Z.; Marder T. B. Angew. Chem., Int. Ed. 2015, 54, 11843. 10.1002/anie.201505603. [DOI] [PubMed] [Google Scholar]; p Qi X.; Jiang L.-B.; Zhou C.; Peng J.-B.; Wu X.-F. ChemistryOpen 2017, 6, 345. 10.1002/open.201700036. [DOI] [PMC free article] [PubMed] [Google Scholar]; For a review, see:; q Mo F.; Qiu D.; Zhang Y.; Wang J. Acc. Chem. Res. 2018, 51, 496. 10.1021/acs.accounts.7b00566. [DOI] [PubMed] [Google Scholar]
- a Yoshimura A.; Takamachi Y.; Han L.-B.; Ogawa A. Chem. - Eur. J. 2015, 21, 13930. 10.1002/chem.201502425. [DOI] [PubMed] [Google Scholar]; b Yoshimura A.; Takamachi Y.; Mihara K.; Saeki T.; Kawaguchi S.-i.; Han L.-B.; Nomoto A.; Ogawa A. Tetrahedron 2016, 72, 7832. 10.1016/j.tet.2016.06.040. [DOI] [Google Scholar]
- For cyclization of an alkyl radical onto boron, see; a Batey R. A.; Smil D. V. Angew. Chem., Int. Ed. 1999, 38, 1798.. [DOI] [PubMed] [Google Scholar]; Intermolecular trapping of alkyl radicals with diboron compounds:; b Bose S. K.; Fucke K.; Liu L.; Steel P. G.; Marder T. B. Angew. Chem., Int. Ed. 2014, 53, 1799. 10.1002/anie.201308855. [DOI] [PubMed] [Google Scholar]; c Fawcett A.; Pradeilles J.; Wang Y.; Mutsuga T.; Myers E. L.; Aggarwal V. K. Science 2017, 357, 283. 10.1126/science.aan3679. [DOI] [PubMed] [Google Scholar]; d Hu D.; Wang L.; Li P. Org. Lett. 2017, 19, 2770. 10.1021/acs.orglett.7b01181. [DOI] [PubMed] [Google Scholar]; Mn-catalyzed:; e Atack T. C.; Cook S. P. J. Am. Chem. Soc. 2016, 138, 6139. 10.1021/jacs.6b03157. [DOI] [PubMed] [Google Scholar]; Cu-mediated radical borylation, see:; f Yang C.-T.; Zhang Z.-Q.; Tajuddin H.; Wu C.-C.; Liang J.; Liu J.-H.; Fu Y.; Czyzewska M.; Steel P. G.; Marder T. B.; Liu L. Angew. Chem., Int. Ed. 2012, 51, 528. 10.1002/anie.201106299. [DOI] [PubMed] [Google Scholar]; g Ito H.; Kubota K. Org. Lett. 2012, 14, 890. 10.1021/ol203413w. [DOI] [PubMed] [Google Scholar]; h Kim J. H.; Chung Y. K. RSC Adv. 2014, 4, 39755. 10.1039/C4RA05999A. [DOI] [Google Scholar]; i Bose S. K.; Brand S.; Omoregie H. O.; Haehnel M.; Maier J.; Bringmann G.; Marder T. B. ACS Catal. 2016, 6, 8332. 10.1021/acscatal.6b02918. [DOI] [Google Scholar]; Ni-mediated radical borylation, see:; j Dudnik A. S.; Fu G. C. J. Am. Chem. Soc. 2012, 134, 10693. 10.1021/ja304068t. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Yi J.; Liu J.-H.; Liang J.; Dai J.-J.; Yang C.-T.; Fu Y.; Liu L. Adv. Synth. Catal. 2012, 354, 1685. 10.1002/adsc.201200136. [DOI] [Google Scholar]; l Li C.; Wang J.; Barton L. M.; Yu S.; Tian M.; Peters D. S.; Kumar M.; Yu A. W.; Johnson K. A.; Chatterjee A. K.; Yan M.; Baran P. S. Science 2017, 356, eaam7355. 10.1126/science.aam7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Review on boron in radical chemistry:Ollivier C.; Renaud P. Chem. Rev. 2001, 101, 3415. 10.1021/cr010001p. [DOI] [PubMed] [Google Scholar]
- Griller D.; Ingold K. U. Acc. Chem. Res. 1980, 13, 317. 10.1021/ar50153a004. [DOI] [Google Scholar]
- a Hoefelmeyer J. D.; Gabbai F. G. J. Am. Chem. Soc. 2000, 122, 9054. 10.1021/ja001739b. [DOI] [Google Scholar]; b Hübner A.; Diehl A. M.; Diefenbach M.; Endeward B.; Bolte M.; Lerner H.-W.; Holthausen M.; Wagner M. Angew. Chem., Int. Ed. 2014, 53, 4832. 10.1002/anie.201402158. [DOI] [PubMed] [Google Scholar]
- Alternatively, the DMF-boryl radical adduct E can react with CF3I via I-abstraction, see; a Tehfe M.-A.; Monot J.; Makhlouf Brahmi M.; Bonin-Dubarle H.; Curran D. P.; Malacria M.; Fensterbank L.; Lacote E.; Laleveé J.; Fouassier J.-P. Polym. Chem. 2011, 2, 625. 10.1039/C0PY00296H. [DOI] [Google Scholar]; b Pan X.; Lacôte E.; Lalevée J.; Curran D. P. J. Am. Chem. Soc. 2012, 134, 5669. 10.1021/ja300416f. [DOI] [PubMed] [Google Scholar]; Review:; c Curran D. P.; Solovyev A.; Makhlouf Brahmi M.; Fensterbank L.; Malacria M.; Lacote E. Angew. Chem., Int. Ed. 2011, 50, 10294. 10.1002/anie.201102717. [DOI] [PubMed] [Google Scholar]
- We also looked at the possible reaction of the DMF complexed diborane 3a–DMF with the secondary alkyl radical A. However, we could not locate an intermediate with a covalent DMF-boron bond but only a noncovalent complex, which is formed with a positive ΔG.
- a Studer A.; Curran D. P. Nat. Chem. 2014, 6, 765. 10.1038/nchem.2031. [DOI] [PubMed] [Google Scholar]; b Studer A.; Curran D. P. Angew. Chem., Int. Ed. 2016, 55, 58. 10.1002/anie.201505090. [DOI] [PubMed] [Google Scholar]
- a Kurykin M. A.; Vol’pin I. M.; German L. S. J. Fluorine Chem. 1996, 80, 9. 10.1016/S0022-1139(96)03451-3. [DOI] [Google Scholar]; b Sato K.; Higashinagata M.; Yuki T.; Tarui A.; Omote M.; Kumadaki I.; Ando A. J. Fluorine Chem. 2008, 129, 51. 10.1016/j.jfluchem.2007.08.013. [DOI] [Google Scholar]; c Sato K.; Yamazoe S.; Akashi Y.; Hamano T.; Miyamoto A.; Sugiyama S.; Tarui A.; Omote M.; Kumadaki I.; Ando A. J. Fluorine Chem. 2010, 131, 86. 10.1016/j.jfluchem.2009.10.012. [DOI] [Google Scholar]
- Li Z.; Wang Z.; Zhu L.; Tan X.; Li C. J. Am. Chem. Soc. 2014, 136, 16439. 10.1021/ja509548z. [DOI] [PubMed] [Google Scholar]
- Sonawane R. P.; Jheengut V.; Rabalakos C.; Larouche-Gauthier R.; Scott H. K.; Aggarwal V. K. Angew. Chem., Int. Ed. 2011, 50, 3760. 10.1002/anie.201008067. [DOI] [PubMed] [Google Scholar]
- Bonet A.; Odachowski M.; Leonori D.; Essafi S.; Aggarwal V. K. Nat. Chem. 2014, 6, 584. 10.1038/nchem.1971. [DOI] [PubMed] [Google Scholar]
- Hupe E.; Marek I.; Knochel P. Org. Lett. 2002, 4, 2861. 10.1021/ol0262486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.