Abstract
Background:
Numerous studies have reported a strong association between temperature and mortality. Additional insights can be gained from investigating the effects of temperature on years of life lost (YLL), considering the life expectancy at the time of death.
Objectives:
The goal of this work was to assess the association between temperature and YLL at seven low-, middle-, and high-income sites.
Methods:
We obtained meteorological and population data for at least nine years from four Health and Demographic Surveillance Sites in Kenya (western Kenya, Nairobi), Burkina Faso (Nouna), and India (Vadu), as well as data from cities in the United States (Philadelphia, Phoenix) and Sweden (Stockholm). A distributed lag nonlinear model was used to estimate the association of daily maximum temperature and daily YLL, lagged 0–14 d. The reference value was set for each site at the temperature with the lowest YLL.
Results:
Generally, YLL increased with higher temperature, starting day 0. In Nouna, the hottest location, with a minimum YLL temperature at the first percentile, YLL increased consistently with higher temperatures. In Vadu, YLL increased in association with heat, whereas in Nairobi, YLL increased in association with both low and high temperatures. Associations with cold and heat were evident for Phoenix (stronger for heat), Stockholm, and Philadelphia (both stronger for cold). Patterns of associations with mortality were generally similar to those with YLL.
Conclusions:
Both high and low temperatures are associated with YLL in high-, middle-, and low-income countries. Policy guidance and health adaptation measures might be improved with more comprehensive indicators of the health burden of high and low temperatures such as YLL. https://doi.org/10.1289/EHP1745
Introduction
Ambient temperature is an important determinant of health. Many studies have reported that mortality and morbidity increase with very high and/or very low ambient temperatures (e.g., Analitis et al. 2008; Baccini et al. 2008; Basagana et al. 2015; Basu and Samet 2002; Gasparrini et al. 2015; Kenny et al. 2010). Although evidence on the temperature–health association in wealthier countries is well established, research is still emerging on this topic from low- and middle-income countries (LMICs) (Azhar et al. 2014; Burkart et al. 2014; Diboulo et al. 2012; McMichael et al. 2008; Mrema et al. 2012). Because weather-related health outcomes are dependent on local contexts, the large volume of research generated on the temperature–health association in wealthier countries may not necessarily reflect the burden from temperature exposure in LMICs. In contrast to high-income countries, the burden of disease in LMICs is often characterized by higher youth mortality rates and high prevalence of communicable diseases. Health impacts from heat and cold in LMICs may therefore occur through causal pathways other than those identified in high-income countries. For example, lower life expectancy in low-income countries might limit the pool of vulnerable elderly individuals, who suffer disproportionately from heat–health risks (Kenny et al. 2010). In LMICs, a large proportion of the population is working in physically demanding jobs on the streets, in agriculture, or on construction sites. Greater occupational exposure to environmental risk factors in these countries might lead to increased adverse health outcomes in comparison with the outcomes in wealthier countries.
A second observation of the literature regarding temperature–health associations is that most research uses the total number of daily deaths as the primary outcome (Bunker et al. 2016; Hajat and Kosatky 2010). Daily deaths are attractive to use for a number of reasons, including the accessibility of records (in some, but not all, jurisdictions), as well as high interpretability of study findings. Death counts, however, do not necessarily provide an ideal representation of the total mortality burden attributable to high and low temperatures. A major shortcoming of using daily death counts as the outcome is that this measure does not indicate the extent to which lives are shortened by exposure to heat or cold. At short time scales, such as days to weeks, many studies suggest that a fraction of the attributable mortality to heat and cold is from individuals who would have died anyway within days or weeks, a phenomenon known as mortality displacement (e.g., Hajat et al. 2005). For these decedents, it may be more appropriate instead to largely attribute their death to pre-existing ill-health condition.
Beyond short-term displacement, an additional drawback of using total death counts is that the loss of a young person’s life is equally summed in the total burden as the loss of an old or elderly person’s life. Although all lives have value, the loss of a young person’s life leads to a greater potential loss of societal contributions (Rocklöv et al. 2009).
One approach to avoid these drawbacks is to consider years of life lost (YLL) as the outcome measure instead of death counts. YLL is an indicator of premature mortality used as a global burden of disease death metric (Lopez et al. 2006). The YLL approach accounts for the age at which death occurred by giving greater weight to deaths at a younger age. YLL is a compound measure combining the number of daily deaths with age at death; it is considered a more informative and differentiated measurement for assessing premature mortality than total or age-specific mortality rates alone. A comparison of health burdens by different exposures can facilitate risk ranking for preventive interventions, public health planning, and resource allocation (Aragón et al. 2008; Gardner and Sanborn 1990; Huang et al. 2012a, 2012b; Yang et al. 2015). Although common in other risk-assessment fields, to our knowledge YLL has rarely been applied in studies investigating the temperature–health association (Baccini et al. 2013; Egondi et al. 2015; Huang et al. 2012a, 2012b; Xu et al. 2014; Yang et al. 2015).
To expand our understanding of the impact of temperature on health and to address drawbacks of existing literature, we estimated immediate and lagged effects of high and low daily temperatures on YLL across seven high-, middle- and low-income study sites. These sites were selected to cover a range of socioeconomic and climatic settings in Africa, Asia, Europe, and North America, based on available population and mortality data. Research questions motivating the analyses were: a) What is the association of heat and cold with YLL? b) How do temperature–YLL associations vary among climatically and socioeconomically diverse locations?
Methods
Study Sites
The seven study sites are a mix of urban and rural locations, including Nouna (Burkina Faso), Nairobi and Kisumu (Kenya), Vadu (India), Phoenix and Philadelphia (USA) and Stockholm (Sweden) (Figure 1). Study locations in Africa and India are Health and Demographic Surveillance Sites (HDSS), which are part of the International Network for the Demographic Evaluation of Populations and their Health (INDEPTH) (Sankoh and Byass 2012). The sites in Sweden and the United States included the total municipal population. The locations cover a range of different climates, population characteristics, degree of economic development, and urbanization. The locations in the United States and Sweden were intended to be illustrative of cities in different climate zones.
Figure 1.
Map depicting the location of study sites. The map was generated using ArcMap (version 10.5; ESRI, Inc.) with topographical basemap content from ESRI, the United States Geological Survey, and the United States National Oceanic and Atmospheric Administration.
Study periods varied from site to site, but all covered at least nine consecutive years between 1990 and 2012 (Table 1). Because daily mortality data are difficult to obtain in low-income countries, and even in high-income countries, we chose study sites for which such data were available. Sites were selected based on existing collaboration of site representatives with the Umeå Centre of Global Health Research (Rocklöv et al. 2012) and on the availability of health and weather data for the relevant study period.
Table 1.
Descriptive statistics of exposure and outcome measures across the seven study sites.
| Population characteristics | Study site (study period, location) | ||||||
|---|---|---|---|---|---|---|---|
| Kisumu (2003–2011, Villages Asembo, Gem, Karemo, rural Kenya) |
Nairobi (2003–2012, Slums Korogocho, Viwandani, urban Kenya) |
Nouna (2000–2010, Kossi province, rural Burkina Faso) |
Vadu (2003–2012, 22 villages east of Pune, rural India) | Phoenix (1997–2007, Urban Arizona, USA) | Philadelphia (1997–2007, Urban Pennsylvania, USA) | Stockholm (1990–2002, Stockholm county, urban Sweden) |
|
| Life expectancy at birth (y)a |
|
|
|
|
|
|
|
| Population sizeb | 240,633 | 63,639 | ca. 78,000 | 110,085 | 3,251,876 | 1,517,550 | 1,823,210 |
| Daily max. temperature (°C) |
|||||||
| Minimum | 15.0 | 15.0 | 22.8 | 21.1 | 7.2 | ||
| 5th percentile | 26.0 | 21.1 | 30.0 | 26.6 | 16.0 | 1.1 | |
| Median | 29.0 | 26.0 | 36.4 | 31.3 | 30.6 | 18.3 | 9.6 |
| Mean | 29.2 | 25.8 | 36.1 | 32.0 | 29.9 | 17.9 | 10.5 |
| 95th percentile | 33.0 | 29.6 | 41.7 | 38.8 | 42.8 | 32.2 | 24.6 |
| Maximum | 37.0 | 38.2 | 43.9 | 42.4 | 47.2 | 37.8 | 33.5 |
| Standard deviation | 2.2 | 2.6 | 3.6 | 3.8 | 9.0 | 10.1 | 8.6 |
| Daily deaths | |||||||
| Minimum | 0 | 0 | 0 | 0 | 24 | 16 | 19 |
| 5th percentile | 2.0 | 0.0 | 0.0 | 0.0 | 46.0 | 31.0 | 29.0 |
| Median | 7.0 | 1.0 | 1.0 | 1.0 | 62.0 | 44.0 | 40.0 |
| Mean | 8.7 | 1.3 | 1.7 | 0.9 | 61.9 | 44.5 | 40.0 |
| 95th percentile | 15.7 | 4.0 | 4.0 | 3.0 | 80.0 | 59.0 | 52.0 |
| Maximum | 124 | 57 | 32 | 24 | 117 | 79 | 74 |
| Standard deviation | 9.6 | 1.9 | 2.8 | 1.2 | 10.5 | 8.4 | 7.2 |
| Daily YLL | |||||||
| Minimum | 0.0 | 0.1 | 0.0 | 0.0 | 387.9 | 173.7 | 119.4 |
| 5th percentile | 57.7 | 0.1 | 0.0 | 0.0 | 605.0 | 452.2 | 284.2 |
| Median | 212.0 | 40.3 | 41.5 | 9.3 | 893.6 | 695.1 | 427.2 |
| Mean | 272.1 | 56.6 | 66.2 | 24.4 | 904.0 | 703.9 | 431.7 |
| 95th percentile | 1,357.7 | 179.2 | 187.3 | 91.8 | 1,227.7 | 991.1 | 598.2 |
| Maximum | 4,326.3 | 2,324.4 | 1,607.3 | 568.3 | 1,703.7 | 1,329.5 | 818.1 |
| Standard deviation | 314.6 | 82.0 | 125.5 | 38.0 | 189.9 | 164.7 | 94.7 |
| Minimum YLL temperature | |||||||
| Temperature (°C) | 30.0 | 26.6 | 27.8 | 29.8 | 29.4 | 22.2 | 27.0 |
| Percentile | 62 | 60 | 1 | 31 | 47 | 60 | 98 |
Note: YLL, years of life lost at the age at death, based on conditional life expectancies. YLL were calculated separately for each as the difference between years of age at the time of death and estimated life expectancy given the decedent’s age and gender. Daily YLL are the total YLL of all persons who died on the same day. Minimum YLL temperature is the calculated daily maximum temperature in °C at which average YLL were lowest. HDSS, Health and Demographic Surveillance Site.
aLife expectancy at birth: Site-specific data for HDSS sites in last study year. State-level or national data from official registries: Phoenix data of Arizona (1999–2001); Philadelphia data of Pennsylvania (1999–2001); Stockholm county data of Sweden (2002). Not used for YLL analyses.
bPopulation size: HDSS sites in last study year; Phoenix, Philadelphia, and Stockholm in 2000.
Population Data
Daily mortality data were collected from HDSS sites in Kisumu, Nairobi, Nouna, and Vadu. The HDSS data contain individual statistics including gender, date of birth, date of death, and dates of in- and out-migration to and from the HDSS site. Details of the data collection procedures at these INDEPTH sites are described in Sankoh and Byass (2012). Daily mortality data for Stockholm, Phoenix, and Philadelphia were obtained from state or national population registers and vital statistics offices (Hondula et al. 2015b; Rocklöv et al. 2009). For all seven study sites, information about date and age at death of all deceased individuals was available. All causes of death were included in the analyses.
As an indicator of general population health and infant mortality, life expectancy at birth was calculated for each HDSS site, and respective data for U.S. and Swedish sites were obtained from registers for State of Arizona (Phoenix site), the State of Pennsylvania (Philadelphia site) and Sweden (Stockholm site).
Calculation of Daily Years of Life Lost
A study protocol was developed and shared among coauthors to obtain standardized daily YLL for each study site. No city-specific data of conditional life expectancies were available for the three sites in the United States and Sweden. Therefore, national abridged conditional life expectancies for five-year age groups, based on age-specific mortality rates, were used for these sites. We used age-specific mortality rates for each HDSS site to generate site-specific five-year abridged life tables, stratified by gender, for estimating life expectancy. A separate age band was created for infants under 1 y of age to account for high infant mortality at the HDSS sites. For each death occurring at the different sites, we calculated individual YLL. These YLL were estimated as the site-specific remaining life expectancy on a population level at the individual age of death. For example, if the remaining life expectancy of women age 60 to 64 y was 17.9 y, the individual YLL for a woman who died at that age would be 17.9 y. All YLL values were greater than or equal to zero. Individual YLL were calculated separately for men and women due to differences in life expectancy between the sexes. Daily YLL were the sum of individual YLL occurring on each study day.
Weather Data
Daily maximum temperature data were obtained from the nearest weather station for each study site, including Kisumu: Kisumu airport; Nairobi: Moi Airbase Eastleigh weather station (if missing, data from Jomo Kenyatta International Airport); Nouna: Dedougou weather station; Vadu: Pune Airport; Phoenix: Sky Harbor International Airport; Philadelphia: Philadelphia International Airport; Stockholm: Bromma Airport. Maximum temperature was used as the exposure variable as it was the most consistently available commonly examined temperature metric available across all sites.
Statistical Analyses
The effect of daily maximum temperature on YLL was estimated using a distributed lag nonlinear modelling (DLNM) framework (Gasparrini 2014b). The DLNM is a flexible strategy that incorporates both the lag as well as the exposure dimension simultaneously in the model. This is performed through the specification of a cross-basis function defining the maximum lag of the exposure variable with a basis function for both the exposure variable and the lag dimension. The basis function can be defined to model the effect of the exposure variable as a nonlinear or linear function.
In our analyses, we constructed a cross-basis function of daily maximum temperature with a 2-degree b-spline basis with knot placements at the second and 98th percentile. We considered lags 0 to 14 d with knot placements for the lags at three equally spaced positions. The lag dimension was modeled with a natural cubic spline basis.
To control for trend and seasonality of YLL, we included a natural cubic spline function of time with 6 degrees of freedom per year. Models also included indicator variables for day of week. In addition, models for the HDSS sites included an indicator for “heaping days,” which are dates that are customarily assigned to deaths for which the actual date of death is unknown, specifically, the 15th of each month for Kisumu and Nairobi, the ninth of each month for Nouna, and the first of January for Vadu. The sites in Sweden and the United States did not require control for heaping.
The complete model included a cross-basis function of maximum temperature, spline function of time trend to control for seasonality, the day of week and heaping day in the HDSS sites. We modeled daily YLL using a censored Gaussian distribution with Tobit (McDonald and Moffitt 1980; Tobin 1958) to keep days with (which were common at the four HDSS sites; see Figures S1 and S2) in the model.
The degree of freedom for the trend function and the knot placement for maximum temperature were chosen after testing different combinations of values. We evaluated alternative options for placement of the knots for the b-spline of maximum daily temperature (second and 98th; fifth, 25th, and 95th; 10th, 70th, 90th; second, 70th, 98th; second, 25th, 70th, 90th; and fifth, 50th, 70th, and 95th percentiles, respectively) and for the degrees of freedom (df) for the seasonal trend function (4, 8, 6, or 10 df per year). To facilitate the interpretation of effect estimates across sites, we chose the parameters for the final models (b-spline knots at the second and 98th percentiles and 6 df for the seasonal trend) that produced the lowest total AIC summed over the seven sites (see Table S1). Although this metric does not necessarily yield the optimal model at each specific site, holding model parameters consistent across the sites facilitates interpretation of effect estimates.
We estimated differences in YLL with lower or higher daily maximum temperatures relative to the YLL at the daily maximum temperature with the smallest YLL value. To determine the reference temperature for each site, we predicted the YLL for each percentile of the daily maximum temperature distribution, and identified the percentile with the lowest YLL value.
Mortality comparison.
Associations between daily maximum temperature and mortality were estimated using a quasi-Poisson model with the same cross-basis function for maximum temperature as the Gaussian model for YLL. As for the YLL models, we included a cubic spline trend function for seasonality, indicator variables for day of week, and an indicator for heaping days at the HDSS sites. Associations with higher and lower temperatures were estimated relative to the reference temperature used for the YLL analyses at each site.
Sensitivity analysis
We performed several different sensitivity analyses by varying the degrees of freedom for the trend function and the percentile for the knot placement for the different sites and compared how the YLL maximum temperature relationship changed. In addition, we reran the analysis using a natural cubic spline basis for maximum temperature instead of a b-spline, applied similar knot percentile positions, and compared the exposure response relationship.
We used STATA software (versions 11 to 13; StataCorp) for data management and descriptive statistics. The R statistical software (R Development Core Team 2015) was used for all analyses, the DLNM package (Gasparrini 2011, 2014a) for DLNM modeling and the VGAM package for the Tobit regression models.
Results
Summary statistics of the seven study sites are presented in Table 1. Among the seven study sites, Nouna had the highest average daily maximum temperature (36.1°C), and Stockholm was the coldest location (average daily maximum temperature of 10.5°C). At the HDSS sites, which are located in equatorial regions, variation of temperature was considerably smaller than in Sweden and the United States. The standard deviation of temperature was lowest in Kisumu (2.2°C) and highest in Philadelphia (10.1°C). Table 1 shows also the site-specific temperature and temperature percentile at which the lowest YLL was observed (“minimum YLL temperature”).
The numbers of deaths per day varied among the sites, with HDSS sites frequently having days with zero deaths, consistent with their smaller population sizes (Table 1). Daily YLL as a consequence of daily deaths also varied among the study sites and within each site.
Seasonal Variations in Temperature and Daily YLL
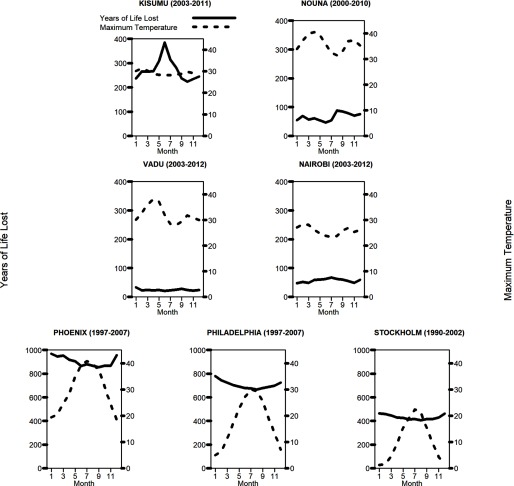
Average daily YLL varied over the year in all locations, with colder months tending to have higher average daily YLL (see Figure 2 for monthly averages at each site, and Figure S1 for time-series data). However, seasonal patterns were less pronounced for Nouna and Vadu than for the other sites. Seasonal variations in daily death counts were similar to those for YLL at each site (data not shown).
Figure 2.
Average daily maximum temperature and YLL per month at seven study sites. The YLL range vary between the sites in the HDSS and in high income sites. YLL, years of life lost at the age at death, based on conditional life expectancies. YLL were calculated separately for each site as the difference between years of age at the time of death and estimated life expectancy given the decedent’s age and gender. Complete time-series for each site are shown in Figure S1.
Association between Daily Maximum Temperature and YLL
Low- and middle-income sites.
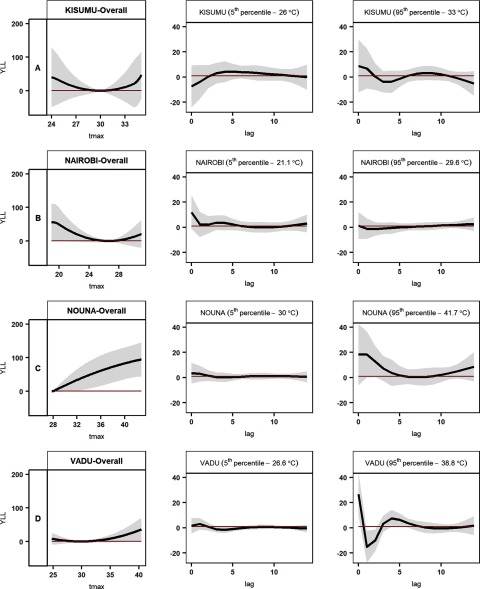
At the Kenyan sites (Kisumu and Nairobi), associations between YLL and daily maximum temperature were U-shaped (Figures 3A, B). The point of minimum YLL temperatures (and thus the lowest estimated YLL) was near the middle of the temperature range for each site (30°C and 27°C, the 62nd and 60th percentiles, respectively); estimated YLL for maximum daily temperatures were higher above and below these values. Estimates were imprecise, but differences in YLL relative to the minimum YLL were greater for daily maximum temperatures at the fifth percentile than temperatures at the 95th percentile (Table 2).
Figure 3.
Association between daily maximum temperature and years of life lost at low- and middle-income study sites. The overall estimates for each location (first column of panels A, B, C, and D, respectively) show YLL by temperature, cumulative over 14 days. The second and third columns show estimated differences in the YLL at the 5th or 95th percentile of maximum daily temperature, respectively, relative to the YLL at the reference temperature at each site, on the day of death () and up to 14 days prior to death. Tmax, daily maximum temperature; YLL, years of life lost. YLL estimates indicate the difference in years of life lost (the difference between life expectancy at the age of death and the age of death) relative to the YLL at the reference temperature for each site (the daily maximum temperature associated with the lowest average daily YLL). Gray shadings represent 95% confidence interval bands of estimates. Note: (A) Kisumu, (B) Nairobi, (C) Nouna, and (D) Vadu.
Table 2.
Estimated cumulative difference in YLL (14-day lag) at different percentiles of maximum daily temperature relative to the YLL at the reference temperature.
| Study site | Kisumu | Nairobi | Nouna | Vadu | Phoenix | Philadelphia | Stockholm |
|---|---|---|---|---|---|---|---|
| Minimum YLL temperature | |||||||
| Temperature (°C) | 30.0 | 26.6 | 27.8 | 29.8 | 29.4 | 22.2 | 27.0 |
| Percentile | 62 | 60 | 1 | 31 | 47 | 60 | 98 |
| 5th percentile | |||||||
| Temp. (°C) | 26.0 | 21.1 | 30.0 | 26.6 | 16.0 | 1.1 | |
| YLL (CI) |
|
|
|
|
|
|
|
| 25th percentile | |||||||
| Temp. (°C) | 28.0 | 24.1 | 33.3 | 29.2 | 22.2 | 10.0 | 3.6 |
| YLL (CI) |
|
|
|
|
|
|
|
| 50th percentile | |||||||
| Temp. (°C) | 29.0 | 26.0 | 36.4 | 31.3 | 30.6 | 18.3 | 9.6 |
| YLL (CI) |
|
|
|
|
|
|
|
| 75th percentile | |||||||
| Temp. (°C) | 30.4 | 27.6 | 38.9 | 35.1 | 37.8 | 27.2 | 17.5 |
| YLL (CI) |
|
|
|
|
|
|
|
| 95th percentile | |||||||
| Temp. (°C) | 33.0 | 29.6 | 41.7 | 38.8 | 42.8 | 32.2 | 24.6 |
| YLL (CI) |
|
|
|
|
|
|
|
Note: The reference temperature is the maximum daily temperature with the lowest predicted YLL at each site (“Minimum YLL temperature,” first row). YLL, years of life lost at the age at death, based on conditional life expectancies. YLL were calculated separately for each site as the difference between years of age at the time of death and estimated life expectancy given the decedent’s age and gender. YLL, years of life lost; CI, 95% confidence interval; temp, daily maximum temperature.
The minimum YLL temperature for Nouna, the hottest site, was set at the first percentile of the daily maximum temperature distribution (28°C) (Table 1). Estimated YLL at temperatures above this value increased continuously (Figure 3C). Of all sites, Nouna showed the strongest association between YLL and temperature at the 95th percentile (42°C: 90 YLL; 95% CI: 42, 139) (Table 2). At Vadu, the second-hottest site, the minimum YLL temperature was at the 31st percentile of the daily maximum temperature distribution (30°C). In comparison with this reference value, YLL were notably higher at higher temperatures only (26 YLL; 95% CI: , 52 at the 95th percentile, 39°C) (Table 2, Figure 3D).
Although lag-specific estimates at the four HDSS sites were imprecise due to small population sizes, they hinted generally at immediate effects of high temperatures, and at the Kenyan sites for delayed responses (Figure 3A and 3B and Figure S3). YLL increased with heat in Nouna on the day of exposure and remained elevated up to day 5, but rose again after lag 10. At Kisumu, cold-related YLL increased at lag 6 to 10; in Nairobi, a small increase of YLL at low temperatures was observed with a two-week delay.
Patterns of associations between daily maximum temperatures and mortality (daily deaths) were generally similar to the patterns of associations with YLL at each HDSS site (Figure S4).
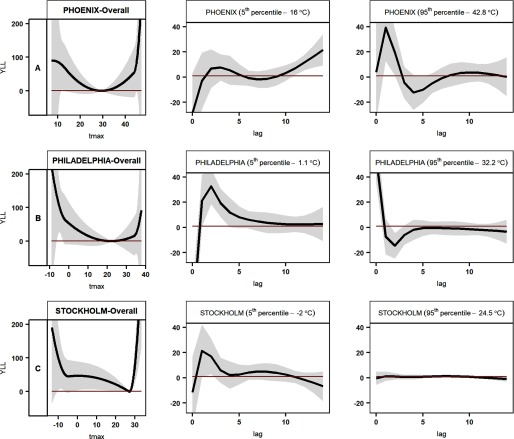
High-income sites.
At all three high-income sites, YLL increased at both high and low temperatures (Figure 4), relative to the temperature of minimum YLL. Philadelphia, the cooler of the two sites in the United States, had the widest range of temperatures ( to 38°C), 22°C (60th percentile) being the point of minimum YLL. At this site, the increase in YLL at the fifth percentile of temperature (1.1°C) and below was more pronounced than that at the 95th percentile and above (Figure 4B, Table 2). Estimated cold effects lasted for about one week (Figure S3). In Phoenix, the minimum YLL temperature was at the 47th percentile of the temperature range (29°C). Despite its hotter climate, estimated cold effects at the fifth percentile (16.0°C) were similar to heat effects at the 95th percentile (42.8°C) (Table 2). Estimated cold effects were most discernible at lag 12–14 d, whereas heat effects appeared to occur within the first four days (Figure S3).
Figure 4.
Association between daily maximum temperature and YLL at high-income study sites. The overall estimates for each location (first column of panels A, B, and C, respectively) show YLL by temperature, cumulative over 14 days. The second and third columns show estimated differences in the YLL at the 5th or 95th percentile of daily maximum temperature, respectively, relative to the YLL at the reference temperature at each site, on the day of death () and up to 14 days prior to death. Tmax, daily maximum temperature; YLL, years of life lost. YLL estimates indicate the difference in years of life lost (the difference between life expectancy at the age of death and the age of death) relative to the YLL at the reference temperature for each site (the daily maximum temperature associated with the lowest average daily YLL). Gray shadings represent 95% confidence interval bands of estimates. Note: (A) Phoenix, (B) Philadelphia, and (C) Stockholm.
Stockholm, the coolest site in our study, with a minimum YLL temperature of 27°C (98th percentile), had a sharp increase in YLL at high temperatures, but the association was stronger for temperatures below the fifth percentile () (Figure 4C). Both high and low temperatures were associated with increased YLL during the first two days (Figure S3).
The 14-d cumulative temperature-mortality associations (Figure S5) were also U-shaped, although for Philadelphia, estimated increases of mortality at low temperatures appeared weaker than those of YLL (Figure S5B). In Phoenix, YLL appeared more sensitive to heat than mortality (Figures 4A and S5A), and patterns for YLL and mortality were rather similar in Stockholm (Figures 4C and S5C).
The relationships described above did not substantially change when we varied the degrees of freedom used for the seasonal term, adjusted the knot placements to different temperature percentiles, or used a natural cubic spline basis instead of a b-spline (Figures S6-15). As expected, there were some small differences in the estimated associations on a site-by-site basis with different model parameters, especially the increase in the complexity of the modeled associations when we used more the three knots for the exposure term. However, the general nature of the differences between concerning the association between temperature and YLL remained consistent across the sensitivity analysis.
Discussion
This study investigated the association of daily maximum temperature with YLL at seven low- to high-income sites. We observed an association between high and low temperatures and YLL at many of the seven study sites, contributing to a very limited evidence base regarding the effect of heat and cold on the burden of heat beyond daily death counts.
Increasing knowledge of the role of temperature as a determinant of health, combined with projections of a warmer future (particularly in cities), has elevated public health concerns about the impacts of heat and cold (Hondula et al. 2015a; Huang et al. 2012a; Jones et al. 2015; Sheridan and Allen 2015). Enhancement of the evidence base regarding temperature–health effects that most effectively informs planning and policy in all geographic locations where heat and cold are of concern is an important goal for environmental health researchers.
The seven locations that we examined featured diverse climatic and demographic profiles, and patterns of associations between daily maximum temperature and YLL varied substantially among the sites. In HDSS sites with a hot climate—Nouna and Vadu—heat was associated with YLL, but there was no evidence of an association with cold. For Nouna, the hottest study site, YLL increased steadily beyond the reference temperature (first percentile of the temperature distribution). Patterns of associations were similar for daily temperature and mortality. The observed association was generally consistent with the study by Diboulo et al. (2012), who showed a linear association between mortality and temperature in Nouna. Those authors also reported the largest relative risks of high temperature exposure on young children, which is consistent with our observation of large temperature effects on YLL, given that children have higher life expectancy.
Of all sites, Nairobi showed the strongest association between YLL and temperatures at the fifth percentile. This HDSS site, an African city with relatively mild, subtropical climate, had the lowest estimated YLL at 27°C. In Guangzhou, China, another large, subtropical and rapidly developing city, Yang et al. (2015) estimated an immediate increase in YLL with high temperature and cold effects that lasted for one week. Overall, associations with YLL were larger for low than for high temperatures, particularly for those less than 75 years of age. We found similar results for temperature extremes in Nairobi, although unlike findings in Guangzhou, cold effects appeared to last longer than one week.
Phoenix experienced summer temperatures as high as those observed in the HDSS sites Vadu and Nouna, but much colder winters in which temperatures can fall below 10°C. In this high-income city, low temperature was associated with increased YLL. Estimated cold effects, however, were weaker than heat effects—unlike associations found in Nairobi, where YLL appeared to increase more at the lower end of the temperature range. Heat was also associated with higher YLL in Phoenix. In Brisbane, Australia, Huang et al. (2012a) investigated the association between daily mean temperature and YLL and found evidence consistent with immediate heat effects on YLL, whereas associations with cold were lagged by two days or longer—a pattern usually seen also in weather–mortality studies (Armstrong 2006; Braga et al. 2001; Guo et al. 2014; Pattenden et al. 2003). YLL from cold exposure outnumbered those from heat in Brisbane (Huang et al. 2012b). In Phoenix (the study location most like Brisbane in terms of socioeconomic and climatic features), we observed a U-shaped association, although estimated cold effects occurred over longer lags (12–14 d) than they did in Brisbane.
At the two coldest locations, Stockholm and Philadelphia, heat posed a health risk. High temperature was associated with higher YLL in Philadelphia at shorter lags, as well as in Stockholm, although to a lesser extent. One possible explanation is that summer temperatures rarely exceed a critical level for public health risks in Sweden; in Stockholm, the lowest YLL was observed at the 98th percentile of temperature, making it difficult to detect adverse effects of extreme heat on YLL. Another possible explanation is that the majority of temperature-related deaths in Stockholm occur among individuals age 65 or above, individuals who are more likely to suffer from chronic health conditions, and therefore are more vulnerable to heat effects (Oudin Åström et al. 2015; Åström et al. 2013). This hypothesis is supported by Rocklöv et al. (2011) who found short-term heat-related mortality risks in the very old, but not in younger persons. Many elderly people amounting to fewer YLL in Stockholm resulted in a low number of heat-related YLL, consistent with our results.
Interestingly, in Nouna (the hottest site) and Stockholm (the coldest site), the minimum YLL temperatures were at opposite ends of the spectrum (first and 98th percentile, respectively). However, effects of low temperature could not be estimated for Nouna due to its small population size and low death counts, whereas for Stockholm, heat above the 98th percentile was associated with a substantial increase in YLL.
In Stockholm and Philadelphia, where winter temperatures can reach as low as , YLL increased with cold, but to a lesser degree than in Nairobi. Experimental and observational studies have reported cardiovascular risk factors and diseases to be associated with cold (Mercer 2003; Mercer et al. 1999; Wolf et al. 2009). Wolf et al. (2009) report that the risk of fatal cardiac events in Germany increased with low-temperature exposure even in summer, and associations were stronger during milder years than colder ones. Heightened sensitivity in populations not adapted to low ambient temperature might explain the J- or U-shaped associations we found in warmer locations, such as Phoenix and Nairobi. Furthermore, influenza contributes to excess mortality during winter in temperate regions. In tropical and subtropical countries, however, high humidity during the rainy season rather than low temperature is the main driver of influenza incidence and mortality (Reichert et al. 2004; Tamerius et al. 2013). Although we lack cause-specific data, we assume that cardiovascular diseases are the main causes of cold-related mortality in high-income sites, whereas patterns for influenza and other respiratory diseases might differ between hot and cold regions.
The observed contrasts in estimated health effects across sites might be explained by differences in climate, socioeconomic status, demographic characteristics, and health profiles (although data-related bias cannot be excluded). Nairobi, which has mild winters with the coldest climate of all HDSS sites, was the only HDSS site with a pronounced association of low temperatures with YLL. A similar association was observed at Kisumu, but estimated effects lacked statistical precision. In a previous study, Egondi et al. (2015) described that in Nairobi YLL increased during cold waves, but not during heat waves. In our study, a modest increase in YLL was found at higher temperatures at this site, which hints at heat as the cause of relatively few deaths among people at young ages, resulting in low YLL. Physiological and behavioral adaptation as well as poverty-related factors might explain high vulnerability to cold in Nairobi, in comparison with U.S. and Swedish sites. Nairobi’s urban slum setting is characterized by high infant mortality, and infectious diseases are the dominant cause of death (Streatfield et al. 2014), both of which are related to poverty and adverse living conditions. Our study site in Vadu, on the other hand, is a setting with higher incomes in comparison with the other HDSS sites, and infant mortality is low (Streatfield et al. 2014). At Vadu, we did not find strong evidence of cold effects. Socioeconomic deprivation might therefore reinforce vulnerability to cold, even with modest reduction in temperature (as in Nairobi), making cold an underestimated public health concern in low-income settings. This speculation is supported by Yang’s study in Guangzhou, China, where larger risks were associated with heat and cold among people with lower education and lower income levels, which may be serving as a proxy for behavioral factors, exposure differences (both temperature and air quality), and/or housing quality (Yang et al. 2015).
Xu et al. (2014) described that in addition to heat, short-term variation in temperature was associated with YLL in Brisbane, Australia. This finding hinted at the importance of physiological adaptation to heat and cold and might explain observed differences in the magnitude of effect estimate among our seven study sites, where the local range and temporal fluctuation of temperature varied considerably. Building materials and indoor facilities may also play an important role in these settings to protect occupants indoor from extreme ambient temperatures.
It is likely that varying profiles of cause of death and preexisting morbidity are underlying mechanisms contributing to different temperature–health associations across sites. It is known that adaptation to extreme temperature is impaired in individuals with chronic illnesses, such as coronary heart disease (Kenny et al. 2010; Zanobetti et al. 2012), and death occurs often with short delay (Gasparrini et al. 2015). In Vadu, in contrast with the other HDSS sites of our study, noncommunicable diseases account for more than half of all deaths in the population age 12 or older, and heat-related mortality risk is largest for these causes of death (Ingole et al. 2015). A high number of fragile elderly people dying from noncommunicable diseases might therefore explain the larger heat-related YLL in Vadu, in comparison with such deaths in Nouna and Kisumu. Further inspection of lagged heat effects in Vadu (reduction and stabilization of YLL following the increase in YLL at lag 0) suggests the advancement of deaths in frail individuals.
We observed some variations in the lag structure of estimated effects at locations with a clear cold signal (Nairobi, Phoenix, Philadelphia, and Stockholm). In Philadelphia and Stockholm, associations of low temperature with YLL were larger at short lags and declined afterwards, whereas in Phoenix and Nairobi, YLL appeared to increase only in the second week. Considering that acclimatization and adaptive behavior contribute to vulnerability, subgroups affected by cold may have different characteristics in terms of behavior, physiology, and housing conditions at different locations. A closer look at cause-specific mortality related to low temperature could provide insights into the mechanisms behind differing lag structures.
Strengths and Limitations
To our knowledge, this is the first study to estimate the effects of temperature on YLL across low-, middle-, and high-income countries. Quantifying health outcomes as YLL instead of death counts has the advantage of accounting for individual life expectancy at the time of death, and as such, the magnitude of the dependent variable is disproportionately driven by death among younger people. The seven study sites cover a wide range of climates and populations with specific demographic and socioeconomic features. The INDEPTH HDSS sites provide unique data from countries where collection of regular nationwide health and demographic information is rare. These sites, however, comprise small areas and populations, reducing the occurrence of daily deaths and YLL, which could limit the statistical power and precision of results. The small sample sizes also led to imprecision of estimated life expectancies and individual YLL, adding uncertainty to the analyses. Furthermore, in particular at Vadu HDSS, the heaping day indicator might not fully control for missing dates of birth.
Our analyses accounted for nonlinear effects of temperature, such as the U-shaped association observed in Phoenix. Simultaneously, delayed effects on YLL were modeled (Armstrong 2006; Gasparrini et al. 2010). By applying a distributed lag nonlinear model, we were able to estimate the effects of both low and high temperature and differences in their lag structure to compare across sites.
Kinney (2012) noted that heat victims are likely to be more frail than others in the same age group, which implies that their remaining life expectancy might be much lower. Baccini et al. (2013) showed in a European multisite study that the magnitude of harvesting (advancement of deaths that were likely to occur within days or weeks due to frailty) is considerable, giving support for the hypothesis of differential life expectancies. Our study did not adjust for harvesting. In traditional temperature–mortality studies, failure to adjust for harvesting can result in an overestimate of the public health burden of heat or cold. We expect that the results of our study based on YLL are less sensitive to the displacement phenomena because the deaths of elderly people (who are more likely to have had deaths advanced forward in time by heat or cold by days to weeks due to higher frailty) are given less weight in calculating the total burden than the deaths of younger people. However, we recommend additional exploration of how the controlling for mortality displacement and calculations based on YLL might overlap.
For all analyses, daily maximum temperature was used as the exposure variable. The extent to which exposure-variable choice affects temperature–mortality relationships has been the subject of investigation of several recent studies in high-income countries (Barnett et al. 2010; Rodopoulou et al. 2015); sensitivity to exposure variable appears to vary on a city-by-city basis. It is possible in some of our locations that the true cold effect, especially in winter and at locations with large intradiurnal temperature variability, might have been underestimated by the use of daily maximum temperature. We expect that the general pattern of our results would hold with the choice of a different exposure variable but could not test their sensitivity because of the lack of data availability at all sites. We also examined the sensitivity of our conclusions to different modeling assumptions concerning degrees of freedom and the nature of the spline function used to represent the exposure–response relationship. By holding model parameters consistent across sites, we likely used site-specific models that were not individually optimized; this choice facilitated interpretation for this first cross-regional comparison of temperature–YLL effects. However, we encourage more research refining the appropriate statistical and theoretical frameworks to understand better how and why the exposure–response relationship varies from place to place.
Policy implications/recommendations.
Individual and environmental factors such as adaptive behavior, gender, socioeconomic status, access to health care, and building structure have been shown to contribute to the adverse health effects of heat (Dutta et al. 2015; Eisenman et al. 2016; Hondula et al. 2012; Laverdière et al. 2016; Näyhä et al. 2016; Smith et al. 2014; Son et al. 2016; Vandentorren et al. 2004). We still know little, however, about the role of these factors in exacerbating vulnerability to cold in low- and middle-income countries, where low temperature usually is not given priority as a health risk.
We found evidence of an effect of low and high temperature on YLL, an indicator of premature mortality, in climatically and socioeconomically diverse locations. By providing policy makers with an absolute number of YLL as a measure of health burden, the results are readily useful to policy makers for setting priorities to reduce heat and cold exposure of individuals in their populations. Many individual and environmental factors contributing to increased vulnerability from heat and cold can certainly be affected by simple measures (Delgado Cortez 2009; Dodman et al. 2010; WHO 2012). Apart from poverty reduction, interventions targeting improved housing conditions and awareness campaigns may help to reduce temperature-related health risks.
In high-income locations (United States and Sweden), it is important to increase public health awareness about the risks associated with temperature extremes. Even for the hot desert city of Phoenix, we found evidence of an adverse effect of low temperature, a risk factor that might be underrated by residents and health care providers. We also observed associations between low temperature and YLL in Philadelphia and Stockholm, where cold winters are common. In these locations, efforts to prevent cold-related deaths are likely already underway and should continue to be supported.
Conclusions
This study investigated the effect of temperature on daily YLL in seven locations with different socio-economic and climatic characteristics. Both heat and cold were associated with YLL in most locations, although effect sizes and lag structures varied. Our findings, using YLL as the outcome, are in line with studies showing weather effects on mortality in which the dependent variable is the number of daily deaths. Insights from this study can help to identify places and populations where burden of heat and cold is highest from a YLL perspective, which we hope can guide the improvement of local, national, and global-scale adaptation and mitigation policies and programs.
Supplemental Material
Acknowledgments
This work was undertaken within the Umeå Centre for Global Health Research at Umeå University, with support from FORTE/FAS, the Swedish Council for Working Life and Social Research (grant number 2006_1512). M.O.S. conducted this work partly during his research stay at New York University, funded by the Graduate School in Population Dynamics and Public Policy, Umeå University. D.M.H. was partially supported by the Virginia G. Piper Health Policy Informatics Initiative at Arizona State University. A.B. was funded by the Klaus-Tschira Stiftung gGmbH (grant number 00.128.2008). T.E. conducted this work during his doctoral studies, which were partly supported through the generous core funding to APHRC by the Swedish International Development Cooperation Agency (SIDA) (grant ref. number 2011–001578) and the William and Flora Hewlett Foundation (Grant No. 2012–7612). We thank Dr. A. Sié for providing data from the Nouna HDSS.
References
- Analitis A, Katsouyanni K, Biggeri A, Baccini M, Forsberg B, Bisanti L. 2008. Effects of cold weather on mortality: results from 15 European cities within the PHEWE project. Am J Epidemiol 168(12):1397–1408, PMID: 18952849, 10.1093/aje/kwn266. [DOI] [PubMed] [Google Scholar]
- Aragón TJ, Lichtensztajn DY, Katcher BS, Reiter R, Katz MH. 2008. Calculating expected years of life lost for assessing local ethnic disparities in causes of premature death. BMC Public Health 8:116, PMID: 18402698, 10.1186/1471-2458-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong B. 2006. Models for the relationship between ambient temperature and daily mortality. Epidemiology 17(6):624–631, PMID: 17028505, 10.1097/01.ede.0000239732.50999.8f. [DOI] [PubMed] [Google Scholar]
- Åström DO, Forsberg B, Edvinsson S, Rocklöv J. 2013. Acute fatal effects of short-lasting extreme temperatures in Stockholm, Sweden: evidence across a century of change. Epidemiology 24(6):820–829, PMID: 24051892, 10.1097/01.ede.0000434530.62353.0b. [DOI] [PubMed] [Google Scholar]
- Azhar GS, Mavalankar D, Nori-Sarma A, Rajiva A, Dutta P, Jaiswal A, et al. 2014. Heat-related mortality in India: excess all-cause mortality associated with the 2010 Ahmedabad heat wave. PLoS One 9(3):e91831, PMID: 24633076, 10.1371/journal.pone.0091831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccini M, Kosatsky T, Biggeri A. 2013. Impact of summer heat on urban population mortality in Europe during the 1990s: an evaluation of years of life lost adjusted for harvesting. PLoS One 8(7):e69638, PMID: 23894516, 10.1371/journal.pone.0069638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccini M, Biggeri A, Accetta G, Kosatsky T, Katsouyanni K, Analitis A, et al. 2008. Heat effects on mortality in 15 European cities. Epidemiology 19(5):711–719, PMID: 18520615, 10.1097/EDE.0b013e318176bfcd. [DOI] [PubMed] [Google Scholar]
- Barnett AG, Tong S, Clements AC. 2010. What measure of temperature is the best predictor of mortality? Environ Res 110(6):604–611, PMID: 20519131, 10.1016/j.envres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Basagana X, Escalera-Antezana JP, Dadvand P, Llatje O, Barrera-Gomez J, Cunillera J, et al. 2015. High ambient temperatures and risk of motor vehicle crashes in Catalonia, Spain (2000-2011): a time–series analysis. Environ Health Perspect 123:1309–1316, 10.1289/ehp.1409223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Samet JM. 2002. Relation between elevated ambient temperature and mortality: a review of the epidemiologic evidence. Epidemiol Rev 24(2):190–202,PMID: 12762092, 10.1093/epirev/mxf007. [DOI] [PubMed] [Google Scholar]
- Braga AL, Zanobetti A, Schwartz J. 2001. The time course of weather-related deaths. Epidemiology 12(6):662–667, PMID: 11679794. [DOI] [PubMed] [Google Scholar]
- Bunker A, Wildenhain J, Vandenbergh A, Henschke N, Rocklöv J, Hajat S, et al. 2016. Effects of air temperature on climate-sensitive mortality and morbidity outcomes in the elderly; a systematic review and meta-analysis of epidemiological evidence. EBioMedicine 6:258–268, PMID: 27211569, 10.1016/j.ebiom.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart K, Breitner S, Schneider A, Khan MM, Kramer A, Endlicher W. 2014. An analysis of heat effects in different subpopulations of Bangladesh. Int J Biometeorol 58(2):227–237, PMID: 23689928, 10.1007/s00484-013-0668-5. [DOI] [PubMed] [Google Scholar]
- Delgado Cortez O. 2009. Heat stress assessment among workers in a Nicaraguan sugarcane farm. Glob Health Action 2(1):2069, PMID: 20052378, 10.3402/gha.v2i0.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diboulo E, Sié A, Rocklöv J, Niamba L, Yé M, Bagagnan C, et al. 2012. Weather and mortality: a 10 year retrospective analysis of the Nouna Health and Demographic Surveillance System, Burkina Faso. Glob Health Action 5:6–13, PMID: 23195510, 10.3402/gha.v5i0.19078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodman D, Mitlin D, Co JR. 2010. Victims to victors, disasters to opportunities: Community-driven responses to climate change in the Philippines. Int Dev Plan Rev 32(1):1–26, 10.3828/idpr.2009.10. [DOI] [Google Scholar]
- Dutta P, Rajiva A, Andhare D, Azhar GS, Tiwari A, Sheffield P. 2015. Perceived heat stress and health effects on construction workers. Indian J Occup Environ Med 19(3):151–158, PMID: 26957814, 10.4103/0019-5278.174002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egondi T, Kyobutungi C, Rocklöv J. 2015. Temperature variation and heat wave and cold spell impacts on years of life lost among the urban poor population of Nairobi, Kenya. Int J Environ Res Public Health 12(3):2735–2748, PMID: 25739007, 10.3390/ijerph120302735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman DP, Wilhalme H, Tseng CH, Chester M, English P, Pincetl S, et al. 2016. Heat Death Associations with the built environment, social vulnerability and their interactions with rising temperature. Health Place 41:89–99, PMID: 27583525, 10.1016/j.healthplace.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Gardner JW, Sanborn JS. 1990. Years of potential life lost (YPLL) - what does it measure? Epidemiology 1(4):322–329, PMID: 2083312. [DOI] [PubMed] [Google Scholar]
- Gasparrini A. 2011. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw 43(8):1–20, PMID: 22003319. [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. 2014a. Distributed lag linear and non-linear models: the R the package dlnm. London, UK:London School of Hygiene and Tropical Medicine. [Google Scholar]
- Gasparrini A. 2014b. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat Med 33(5):881–899, PMID: 24027094, 10.1002/sim.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. 2010. Distributed lag non-linear models. Stat Med 29(21):2224–2234, PMID: 20812303, 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, et al. 2015. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 386(9991):369–375, PMID: 26003380, 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Gasparrini A, Armstrong B, Li S, Tawatsupa B, Tobias A, et al. 2014. Global variation in the effects of ambient temperature on mortality: a systematic evaluation. Epidemiology 25(6):781–789, PMID: 25166878, 10.1097/EDE.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat S, Armstrong BG, Gouveia N, Wilkinson P. 2005. Mortality displacement of heat-related deaths: a comparison of Delhi, Sao Paulo, and London. Epidemiology 16:613–620, PMID: 16135936, 10.1097/01.ede.0000164559.41092.2a. [DOI] [PubMed] [Google Scholar]
- Hajat S, Kosatky T. 2010. Heat-related mortality: a review and exploration of heterogeneity. J Epidemiol Community Health 64(9):753–760, PMID: 19692725, 10.1136/jech.2009.087999. [DOI] [PubMed] [Google Scholar]
- Hondula DM, Balling RC Jr Vanos JK, Georgescu M. 2015a. Rising temperatures, human health, and the role of adaptation. Curr Clim Change Rep 1:144–154, PMID: 22408589, 10.3390/ijerph8124563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondula DM, Davis RE, Saha MV, Wegner CR, Veazey LM. 2015b. Geographic dimensions of heat-related mortality in seven U.S. cities. Environ Res 138:439–452, PMID: 25791867, 10.1016/j.envres.2015.02.033. [DOI] [PubMed] [Google Scholar]
- Hondula DM, Davis RE, Leisten MJ, Saha MV, Veazey LM, Wegner CR. 2012. Fine-scale spatial variability of heat-related mortality in Philadelphia County, USA, from 1983–2008: a case-series analysis. Environ Health 11:16, PMID: 22443423, 10.1186/1476-069X-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CR, Barnett AG, Wang XM, Tong SL. 2012a. The impact of temperature on years of life lost in Brisbane, Australia. Nat Clim Chang 2:265–270, PMID: 24487220, 10.1097/EDE.0000000000000066. [DOI] [PubMed] [Google Scholar]
- Huang CR, Barnett AG, Wang XM, Tong SL. 2012b. Effects of extreme temperatures on years of life lost for cardiovascular deaths: a time series study in Brisbane, Australia. Circ Cardiovasc Qual Outcomes 5:609–614, PMID: 22991346, 10.1161/CIRCOUTCOMES.112.965707. [DOI] [PubMed] [Google Scholar]
- Ingole V, Rocklöv J, Juvekar S, Schumann B. 2015. Impact of heat and cold on total and cause-specific mortality in Vadu HDSS—a rural setting in Western India. Int J Environ Res Public Health 12(12):15298–15308, PMID: 26633452, 10.3390/ijerph121214980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, O’Neill BC, McDaniel L, McGinnis S, Mearns LO, Tebaldi C. 2015. Future population exposure to US heat extremes. Nature Climate Change 5(7):652–655, 10.1038/nclimate2631. [DOI] [Google Scholar]
- Kenny GP, Yardley J, Brown C, Sigal RJ, Jay O. 2010. Heat stress in older individuals and patients with common chronic diseases. CMAJ 182(10):1053–1060,PMID: 19703915, 10.1503/cmaj.081050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney PL. 2012. Health: a new measure of health effects. Nature Climate Change 2(4):233, 10.1038/nclimate1460. [DOI] [Google Scholar]
- Laverdière E, Payette H, Gaudreau P, Morais JA, Shatenstein B, Généreux M. 2016. Risk and protective factors for heat-related events among older adults of Southern Quebec (Canada): the NuAge study. Can J Public Health 107(3):e258–e265, PMID: 27763840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. 2006. Global Burden of Disease and Risk Factors. Washington, D.C., USA:World Bank. [PubMed] [Google Scholar]
- McDonald JF, Moffitt RA. 1980. The uses of Tobit analysis. Rev Econ Stat: 62(2):318–321, 10.2307/1924766. [DOI] [Google Scholar]
- McMichael AJ, Wilkinson P, Kovats RS, Pattenden S, Hajat S, Armstrong B, et al. 2008. International study of temperature, heat and urban mortality: the ‘ISOTHURM’ project. Int J Epidemiol 37(5):1121–1131, PMID: 18522981, 10.1093/ije/dyn086. [DOI] [PubMed] [Google Scholar]
- Mercer J. 2003. Cold—an underrated risk factor for health. Environ Res 92(1):8–13,PMID: 12706750, 10.1016/S0013-9351(02)00009-9. [DOI] [PubMed] [Google Scholar]
- Mercer JB, Osterud B, Tveita T. 1999. The effect of short-term cold exposure on risk factors for cardiovascular disease. Thromb Res 95(2):93–104, PMID: 10418798, 10.1016/S0049-3848(99)00028-6. [DOI] [PubMed] [Google Scholar]
- Mrema S, Shamte A, Selemani M, Masanja H. 2012. The influence of weather on mortality in rural Tanzania: a time-series analysis 1999-2010. Glob Health Action 5:33–43, PMID: 23195507, 10.3402/gha.v5i0.19068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näyhä S, Rintamäki H, Donaldson G, Hassi J, Jousilahti P, Laatikainen T, et al. 2016. The prevalence of heat-related cardiorespiratory symptoms: the vulnerable groups identified from the National FINRISK 2007 Study. Int J Biometeorol, PMID: 27658672, 10.1007/s00484-016-1243-7. [DOI] [PubMed] [Google Scholar]
- Oudin Åström D, Schifano P, Asta F, Lallo A, Michelozzi P, Rocklöv J, et al. 2015. The effect of heat waves on mortality in susceptible groups: a cohort study of a Mediterranean and a northern European city. Environ Health 14:30, PMID: 25889290, 10.1186/s12940-015-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattenden S, Nikiforov B, Armstrong BG. 2003. Mortality and temperature in Sofia and London. J Epidemiol Community Health 57(8):628–633, PMID: 12883072, 10.1136/jech.57.8.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. 2004. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol 160(5):492–502, PMID: 15321847, 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- Rocklöv J, Forsberg B, Meister K. 2009. Winter mortality modifies the heat-mortality association the following summer. Eur Respir J 33(2):245–251, PMID: 18799511, 10.1183/09031936.00037808. [DOI] [PubMed] [Google Scholar]
- Rocklöv J, Ebi K, Forsberg B. 2011. Mortality related to temperature and persistent extreme temperatures: a study of cause-specific and age-stratified mortality. Occup Environ Med 68(7):531–536, PMID: 20962034, 10.1136/oem.2010.058818. [DOI] [PubMed] [Google Scholar]
- Rocklöv J, Sauerborn R, Sankoh O. 2012. Weather conditions and population level mortality in resource-poor settings – understanding the past before projecting the future. Glob Health Action 5, 10.3402/gha.v5i0.20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodopoulou S, Samoli E, Analitis A, Atkinson RW, de'Donato FK, Katsouyanni K. 2015. Searching for the best modeling specification for assessing the effects of temperature and humidity on health: a time series analysis in three European cities. Int J Biometeorol 59(11):1585–1596, PMID: 25638489, 10.1007/s00484-015-0965-2. [DOI] [PubMed] [Google Scholar]
- Sankoh O, Byass P. 2012. The INDEPTH network: filling vital gaps in global epidemiology. Int J Epidemiol 41(3):579–588, PMID: 22798690, 10.1093/ije/dys081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan SC, Allen MJ. 2015. Changes in the frequency and intensity of extreme temperature events and human health concerns. Curr Clim Change Rep 1(3):155–162, 10.1007/s40641-015-0017-3. [DOI] [Google Scholar]
- Smith KR, Woodward A, Campbell-Lendrum D, Chadee DD, Honda Y, Liu QY, et al. 2014. Human Health: Impacts, Adaptation, and Co-Benefits. In: Climate Change 2014: Impacts, Adaptation, and Vulnerability, Pt A: Global and Sectoral Aspects: Working Group II Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY, USA:Cambridge University Press, 709–754. [Google Scholar]
- Son JY, Gouveia N, Bravo MA, de Freitas CU, Bell ML. 2016. The impact of temperature on mortality in a subtropical city: effects of cold, heat, and heat waves in São Paulo, Brazil. Int J Biometeorol 60(1):113–121, PMID: 25972308, 10.1007/s00484-015-1009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streatfield PK, Khan WA, Bhuiya A, Alam N, Sié A, Soura AB, et al. 2014. Cause-specific mortality in Africa and Asia: evidence from INDEPTH health and demographic surveillance system sites. Glob Health Action 7(1):25362, PMID: 25377324, 10.3402/gha.v7.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamerius JD, Shaman J, Alonso WJ, Bloom-Feshbach K, Uejio CK, Comrie A, et al. 2013. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog 9(3):e1003194, PMID: 23505366, 10.1371/journal.ppat.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin J. 1958. Estimation of relationships for limited dependent variables. Econometrica 26(1):24–36, 10.2307/1907382. [DOI] [Google Scholar]
- Vandentorren S, Suzan F, Medina S, Pascal M, Maulpoix A, Cohen JC, et al. 2004. Mortality in 13 French cities during the August 2003 heat wave. Am J Public Health 94(9):1518, PMID: 15333306, 10.2105/AJPH.94.9.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Schneider A, Breitner S, von Klot S, Meisinger C, Cyrys J, et al. 2009. Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation 120(9):735–742, PMID: 19687361, 10.1161/CIRCULATIONAHA.108.815860. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2012. Regional Strategy for Protecting Health from Climate Change. South-East Asia Regional Office:New Delhi, India. [Google Scholar]
- Xu Z, Hu W, Wang X, Huang C, Tong S. 2014. The impact of temperature variability on years of life lost. Epidemiology 25(2):313–314, PMID: 24487220, 10.1097/EDE.0000000000000066. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu Q-Y, Ou C-Q, Li L, Guo C, Chen P-Y, et al. 2015. The burden of ambient temperature on years of life lost in Guangzhou, China. Sci Rep 5:12250, PMID: 26247571, 10.1038/srep12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, O'Neill MS, Gronlund CJ, Schwartz JD. 2012. Summer temperature variability and long-term survival among elderly people with chronic disease. Proc Natl Acad Sci USA U S A 109(17):6608–6613, PMID: 22493259, 10.1073/pnas.1113070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.