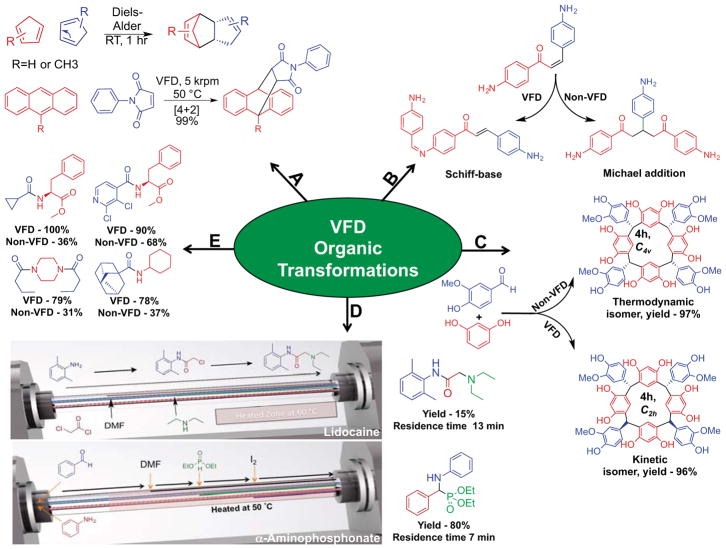
Figure 2.
Examples of VFD-mediated synthetic transformations. A) The [4+2] Diels-Alder reaction of two cyclopentadiene molecules as well as the [4+2] Diels-Alder between anthracene and N-phenylmaleimide; no conversion results from identical conditions in the non-VFD control. [10] [13] B) Processing under high mass and heat transfer in the VFD favors the Michael-addition product; by contrast, in a non-VFD control, the Schiff base product is favored.[19] C) The stereoselective synthesis of resorcin[4]arenes and pyrogallo[4]arenes in the VFD, with the VFD favoring the kinetic isomer over the thermodynamic isomer.[20] D) Assembly line synthesis of lidocaine through sequential, spatially segregated transformations and the assembly line-inspired synthesis of an α-aminophosphonate utilizing in situ solvent exchange to drive a multi-step transformation. [21] [8] E) The synthesis of amides through the coupling of acyl chlorides with amines. Products were isolated through silica column chromatography with >95% purity observed by 1H NMR analysis.