Abstract
Diverticular bleeding is the most common cause of lower gastrointestinal bleeding with nearly 200,000 admissions in the United States annually. Less than 5% of patients with diverticulosis present with diverticular bleeding and present usually as painless, intermittent, and large volume of lower gastrointestinal bleeding. Management algorithm for patients presenting with diverticular bleeding includes resuscitation followed by diagnostic evaluation. Colonoscopy is the recommended first-line investigation and helps in identifying the stigmata of recent hemorrhage and endoscopic management of the bleeding. Radionuclide scanning is the most sensitive but least accurate test due to low spatial resolution. Angiography is helpful when patients are actively bleeding and therapeutic interventions are performed with angioembolization. Surgery for diverticular bleeding is necessary when associated with hemodynamic instability and after failed endoscopic or angiographic interventions. When the bleeding site is localized preoperatively, partial colectomy is sufficient, but subtotal colectomy is necessary when localization is not possible preoperatively.
Keywords: lower gastrointestinal bleeding, diverticular bleeding, angiography, embolization
Lower gastrointestinal (LGI) bleeding is a relatively common clinical problem with an annual incidence of 0.03% among the adult population in the United States. The rate of hospitalization for LGI bleeding is lower than that for upper gastrointestinal bleeding. Acute LGI bleeding accounts for approximately 20% of all gastrointestinal bleeding. 1 2
Colonic diverticular bleeding is the most common cause of acute LGI bleeding, accounting for 20.8 to 41.6% of LGI bleeding in the western world. 1 Diverticular disease as the etiology for patients with LGI bleeding increases with age, with prevalence as high as 85% among 85-year-old patients. There were 64,222 hospital discharges with a median length of stay of 3.0 days at a median cost of $5,818.0 per patient and an aggregate cost of $552 million in the United States in 2012. 3 Diverticular disease is also the most common cause of massive gastrointestinal bleeding. 4 Diverticular disease also accounts for 50% of readmissions for rebleeding. 5
Diverticulosis could occur along the entirety of colon but commonly affects sigmoid colon. Although the etiology of diverticular bleeding is considered to be multifactorial, its association with sigmoid colon is attributed to its anatomical and biomechanical reasons. 6 7 Diverticular bleeding occurs in 3 to 5% of patients with diverticulosis, with more than two-thirds of diverticular bleeding proven by demonstrating stigmata of recent bleeding on colonoscopy occurrence at or proximal to splenic flexure of the colon with a significant predilection to the right colon. 8 9 10 11 Increased risk of right colon diverticular disease is more common in Asian countries. Frequency of both right-sided, bilateral, and multiple diverticula has increased with time among Japanese population. 12 13 Patients with diverticular disease and obesity have a higher incidence of LGI bleeding compared with those who are not obese.
Pathophysiology
Diverticula of the colon are well-defined areas of weakness and correspond to areas where vasa recta penetrate the circular muscle layer of the colon. Diverticular bleeding is arterial and occurs from asymmetric rupture of the intramural branches of the marginal artery at the dome or neck of the diverticulum. 14 Trauma from mechanical or chemical causes within the lumen of the diverticulum leads to thinning of the mucosa most commonly along the fundus or neck of the diverticula resulting in injury to the penetrating vessels and bleeding. Repeated microtrauma in the above manner would lead to segmental weakness of the vasa recta predisposing them to bleeding. 15 Histopathological examination of diverticular bleeding sites has shown asymmetric thickening of involved small nutrient arteries often with thinning of the media and duplication of the internal elastic lamina at and near the bleeding point and general absence of diverticulitis. 15 16
Right-sided diverticular disease is associated with higher incidence of bleeding complications. This has been attributed to the right colon having diverticulum with wider necks and domes. Their vasa recta are exposed over a greater length to injurious factors arising from the colon. 15 16 17
Clinical Presentation
Diverticular bleeding typically presents as painless, intermittent, and large volume LGI bleeding. The nature/color of the bleeding varies with the intensity of the bleeding with right-sided diverticular bleeding known to present clinically as dark, maroon-colored blood to melena like. The differential diagnosis for painless LGI bleeding should include other common causes of colon cancer, angiodysplasia, postpolypectomy bleeding, and internal hemorrhoids. Other differential diagnoses to be considered during evaluation should include inflammatory bowel disease, infectious colitis, ischemic colitis, and radiation proctitis. The source of LGI bleeding could be from small bowel in approximately 5% of cases.
Initial focused history and evaluation should direct toward hemodynamic instability identifying the source and etiology of the bleeding. Focused history should include nature and duration of bleeding and any abdominal pain. Evaluation of diarrhea (colitis), altered bowel habits, and weight loss (malignancy) would help rule out other common causes of LGI bleeding. Patient history should also include previous history of GI bleeding, inflammatory bowel disease, previous abdominal or vascular surgeries, family history of colon cancer or IBD, and use of NSAIDS, antiplatelet agents, and anticoagulant medications. Patients with associated cardiac, pulmonary, renal, and hepatic disease are at a higher risk of complications with resuscitation during massive GI bleeding and during interventions at the time of evaluation and management. Upper and mid gastrointestinal bleeding are the sources of bleeding in up to 20% of patients presumed to have LGI bleeding and this must be considered during its evaluation. 36 This is more likely when associated with hemodynamic instability.
Physical examination should include evaluation for hemodynamic status and volume status with measurement of vital signs and signs of postural hypotension, and cardiopulmonary, abdominal, and anorectal exam. Laboratory investigations should include complete blood count, renal function, liver function tests, and coagulation studies with blood type including crossmatch in patients with life threatening to bleed. Nasogastric (NG) suctioning should be performed to rule out gastric bleeding.
Diverticular hemorrhage spontaneously stops in 70 to 80% patients with rebleeding rate reported to range between 22 and 38%. 8 10 11 16
Risk Stratification of Lower Gastrointestinal Bleeding
Hemodynamic instability characterized by hypotension, tachycardia, and postural hypotension is a strong predictor of severity of LGI bleeding, and the association is similar to that observed with upper GI bleeding. 18 19 20 21 22 Ongoing bleeding presenting as rectal bleeding during the first 4 hours of evaluation is associated with poor prognosis. The bleed criteria have been validated to predict adverse outcomes after both upper and LGI bleeding. High-risk features include ongoing bleeding, low systolic blood pressure (SBP), elevated prothrombin time, erratic mental status, and unstable comorbid disease. Patients who present with LGI bleeding but are stable hemodynamically, with SBP > 115 mm Hg, hemoglobin > 13 g/dL, and are not anticoagulated, could be treated from home, thus avoiding nearly 30% of potential admissions. 23 These patients were followed up as outpatients and flexible sigmoidoscopy was performed electively after 6 weeks. Full colonic evaluation should be performed in a timely fashion.
A nontender abdominal exam predicts severe bleeding as vascular disorders (diverticular bleeding and angioectasias) are associated with no abdominal pain. Use of aspirin and NSAIDS is associated with a higher incidence of diverticular bleeding. 24 Age more than 60 years, associated cardiovascular comorbidities, and raised creatinine levels are also associated with poor outcomes after LGI bleeding. Overall, the risk of adverse outcome is higher with the presence of an increasing number of risk factors. 22
Management
The general goals of management of diverticular bleeding are resuscitation, diagnosis, hemostasis, and prevention of recurrent bleeding.
Resuscitation
Vascular sources of bleeding as seen in diverticular bleeding could result in large-volume blood loss and require immediate resuscitation and control of bleeding. Transfusion strategy specific toward LGI bleeding has not yet been developed, and hence, guidelines in the management of upper GI bleeding are being followed for the management of LGI bleeding. Restrictive transfusion strategy with a threshold of less than 7 g/dL was associated with improved survival and decreased rebleeding. Patients with associated medical comorbidities, ongoing massive bleeding, and instances where therapeutic interventions are delayed would benefit from a more lenient blood transfusion threshold. 25 26
Although coagulopathy complicating LGI bleeding from intake of anticoagulant medications forms a significant clinical problem, guidelines for management have to be extracted from studies on upper GI bleeding due to the limited literature on management of LGI bleeding. When the International Normalized Ratio (INR) is more than 2.5, it is recommended that it be corrected before endoscopy. When between 1.5 and 2.5, it could be corrected before or along with endoscopic intervention. 27 28 29 Newer anticoagulants, such as apixaban, dabigatran, and rivaroxaban, are associated with an increased risk of GI bleeding, and there is no direct evidence to guide the management of these agents during active GI bleeding as of now. Reversal agent for these newer anticoagulants are in development; hence, a multidisciplinary approach, including consultation with a hematologist, is recommended for the management of these agents during active GI bleed.
Guidelines for the management of low platelets complicating LGI bleed do not exist; hence, recommendations on platelet transfusion during massive bleeding from any source are generally followed. Current recommendations are for platelet transfusion during a LGI bleed when platelet counts are less than 50 × 10 9 /L and for patients receiving massive packed red blood cell (pRBC) transfusion. 25
Diagnosis
Diagnostic modalities available to evaluate diverticular bleeding include colonoscopy/flexible sigmoidoscopy, angiography, radionuclide scintigraphy or tagged red blood cell scanning, and cross-sectional imaging techniques.
Colonoscopy
Colonoscopy is the recommended first-line investigation in patients for evaluation of LGI bleeding. 30 31 The advantages of colonoscopy include visualizing and localizing the bleeding accurately and the potential for control of bleeding with interventions. The challenges associated with the use of colonoscopy include an inability to visualize bleeding in an unprepared colon and the need for an experienced endoscopist for therapeutic intervention. The diagnostic yield of colonoscopy ranges from 42 to 100% in identifying the source of bleeding in patients with acute LGI bleeding. 32 33 34
Colonoscopy, when performed within 12 to 24 hours of onset of bleeding, has been shown to have a higher yield for stigmata of recent hemorrhage (SRH) identification. Identification of SRH was higher at 42% when performed within 12 hours of onset of bleeding compared with 22% when performed within 74 hours, although its role in preventing subsequent bleeding is not clear. 34 35 36 The American Society for Gastrointestinal Endoscopy guidelines recommend that colonoscopy for patients with significant LGI bleeding be performed within 24 hours of admission after rapid bowel preparation, although other studies have shown no changes in outcome with urgent colonoscopy. 37 Colonoscopy without bowel preparation is associated with low cecal intubation rate (55–70%), but studies have reported higher accuracy with the liberal use of suction and irrigation to evaluate the colon. Adequate preparation before urgent colonoscopy would lead to delay in intervention but multiple studies have shown to be of significant importance in improving diagnostic accuracy with the American College of Gastroenterologists recommending a quick prep with 4 to 6L of a polyethylene glycol-based solution over 3 to 4 hours. 14 34 37 This would require placing an NG tube to have the best outcome in some of the patients. 38
Colonic mucosa should be inspected both during insertion and withdrawal, as underlying lesions are known to bleed intermittently. Use of colonoscope with a large working channel (at least 3.3 mm) and using water-jet irrigation are recommended to facilitate removal of stool and blood clots and identify the underlying pathology.
Disadvantages of urgent colonoscopy include logistical difficulty in coordinating the procedure after hours, risks associated with sedation, including aspiration pneumonia, risks of high-volume purges, particularly in patients with altered mental status, and the risk of perforation ranging from 0.3 to 0.6%. 39 A national inpatient sample study in 2010 evaluated the differences between early and delayed colonoscopy for LGI bleeding (performed within 24 hours or after 24 hours of hospital admission) and found no difference in mortality between the two groups (0.3 vs. 0.4%, p = 0.24) but the early colonoscopy group had shorter length of hospital stay (2.9 vs. 4.6 days, p < 0.001), decreased need for blood transfusion (44.6 vs. 53.8%, p < 0.001), and lower hospitalization costs ($22,142 vs. $28,749, p < 0.001). These results were unchanged with subgroup analysis of patients with LGI bleeding and diverticular disease. 40
Identification of Stigmata of Recent Hemorrhage in Diverticular Bleeding
Forest criteria have been used to classify endoscopic SRH in upper gastrointestinal bleeding. SRH has also been established to be of prognostic significance in peptic ulcer-related bleeding from the upper gastrointestinal tract. Similar criteria to stratify endoscopic stigmata associated with diverticular hemorrhage do not exist and endoscopic management of diverticular hemorrhage do not have a standardized protocol. This is largely because of difficulty to identify SRH due to intermittent nature of diverticular bleeding, a large surface area of the colon, residual stool, and blood and smaller number of aggregate cases from individual centers. Identification of diverticular SRH in those who undergo colonoscopy varies from 6 to 42%. 14 41 42 43 44 45 Colonoscopy performed by an expert colonoscopist, cap attachment, and use of water jet with endoscope were also shown to be predictors of a higher percentage of identification of SRH. Adequate preparation of the colon is also associated with higher SRH, and unprepped colonoscopy is associated with lower completion rate and higher perforation rate. 42 43 44 45 46
Studies have used Forest criteria to similarly stratify diverticular-related SRH into three major bleeding categories: actively bleeding lesions (ABLs), nonbleeding visible vessel (NBVV), and adherent clots (ACs). All the three categories have a higher risk of rebleeding without intervention, and the risk appears to show a stepwise increase in the risk of rebleeding from an adherent clot to NBVV to actively bleeding diverticulum. 47
When no endoscopic interventions were performed, 30-day rebleeding rates were 84% for ABLs, 60% for NBVV, and 43% for adherent clots, which remained after jet irrigation (Jensen et al 2016). 48 The use of through-the-scope Doppler ultrasound probe (DEP) has been used as an adjunct in studies to confirm the diagnosis and adequacy of colonoscopic hemostasis. It consists of a flexible linear probe passed through the accessory channel of the colonoscope. SRH are identified initially and the probe tip is placed directly on the lesion, and the angle and signal depth are varied to detect the flow. Use of DEP has shown that vessel depth tended to be shallow (<4 mm), and the vessels appear to course across the entire base of the diverticulum. It has also demonstrated the validity of using stigmata of recent hemorrhage (SRH) in diverticular bleeding. 48
Flat spots are considered as minor stigmata of recent hemorrhage and are associated with low rebleeding rate, but this does not entirely translate into the practice. Rebleeding rates in patients with presumed diverticular bleeding and minor stigmata are likely to be higher due to misclassified or missed SRH during colonoscopy. Diverticular disease with significant bleeding should have SRH at some point but are likely to have resolved when colonoscopy is delayed leading to low sensitivity in detecting SRH. Patients with diverticular bleeding and found to have SRH on colonoscopy are more likely to be males, with NSAID use and lower hematocrit on presentation, and are likely to receive a blood transfusion than those with minor or no SRH.
Various therapeutic endoscopic interventions available include injection therapy with diluted epinephrine, electrocoagulation therapy (monopolar, bipolar, or heat probe), argon plasma electrocoagulation, through-the-scope clipping devices, and band ligation. Each of these options could be used either as monotherapy or multimodal therapeutic interventions. Coaptive coagulation has the potential to perforate thin-walled bleeding diverticulum, and endotherapy with through-the-scope hemoclips has been shown to be as an alternate option to control the bleeding ( Fig. 1 ). Hemoclip application could be performed by either targeted application over the bleeding vessel if identified or could be used to close the diverticular orifice in a “zipper-like” fashion resulting in bleeding tamponade. Complete closure of the diverticulum has been shown to achieve good immediate and long-term hemostasis (100 and 89%, respectively) with no reported episodes of diverticulitis from the closure of the entire diverticulum with the hemoclip. Application of hemoclips can be combined with injection of epinephrine locally to control the bleeding, improve visibility, and facilitate accurate clip placement. A translucent cap placed at the tip of the colonoscope has been shown to help evert small or deeper diverticulum and help with identifying the bleeding point and placement of clips. Hemoclips retention rate varies with different models and could vary from less than 7 days to more than 4 weeks. Hemoclips application has also been touted as a marker to identify the site of bleeding during reintervention in cases where bleeding recurs or persists. Endoscopic clip application with or without epinephrine injection has been shown to be effective in achieving hemostasis in 88% cases with no early rebleeding and 24% rebleeding at 30 days. 41
Fig. 1.
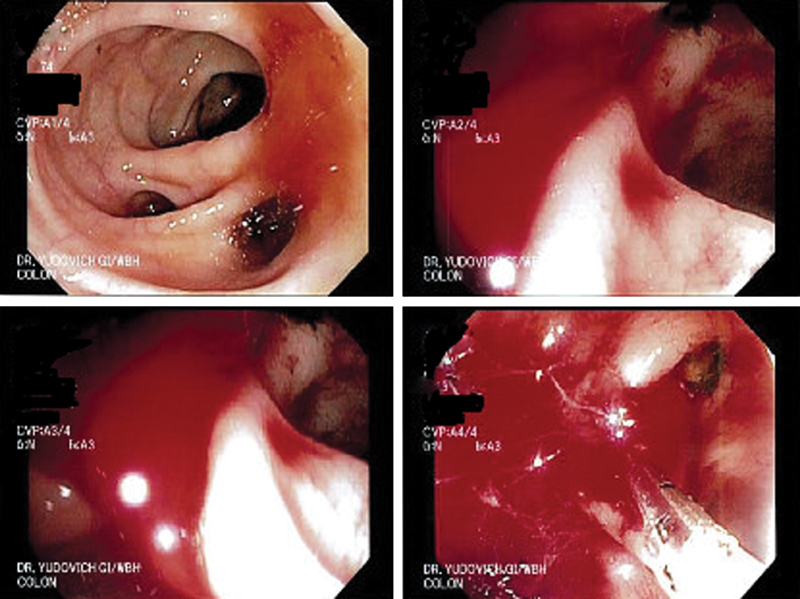
Endoscopic appearance of diverticular bleeding with closure of the bleeding diverticulum with hemoclip application.
Endoscopic Band Application
Endoscopic banding is also used to manage diverticular bleeding with SRH. Endoscopic banding requires prior identification of the culprit diverticulum and marking the site with India ink or with a through-the-scope hemoclip. The colonoscope is withdrawn, and banding is performed like banding esophageal varices by using pediatric colonoscope or a gastroscope. 49 50 51 The above-mentioned hood method of application of endoscopic banding has been shown to be effective in achieving hemostasis in 96.3% of patients with features of active SRH and presumptive bleeding. 52 Right-sided colon is thin walled than left-sided colon, and application of endoscopic band could involve more of the muscular wall on the right side than on the left side. This has to be considered in deciding the mode of therapy used for diverticular bleeding on the right- versus left-sided diverticular lesions. This approach requires an advanced skill level.
Nonendoscopic Interventions
Radionuclide Scanning
Radionuclide scanning with Technetium-99m-labeled RBC (99m Tc-labeled RBC) is the most sensitive test available to detect LGI bleeding. The tagged red blood cell scan requires the autologous red blood cells to be initially labeled with the radioisotope, then the tagged red cells are injected back into the patient. The test detects bleeding as slow as 0.1 to 0.5 mL/min with a reported sensitivity of ranging from 39 to 90% in confirming LGI bleeding. 53 The tagged red cells remain in the patient's circulation, and when bleeding is intermittent, the study could be repeated within 24 hours. 54 55 The study does require a 60-minute delay to get the autologous red cells tagged and takes approximately 2 hours to perform. The spatial resolution with this study is low, and the tagged cells within the lumen could move either antegrade or retrograde with distortion in the accuracy of localization of the bleeding. Thus, it is the least accurate method for localizing GI bleeding.
Similar to 99m Tc-labeled RBC, sulfur colloid scan can also be accomplished quickly and detects bleeding as minimal as 0.1 mL/min. The radiolabeled sulfur colloid is cleared quickly by the liver and spleen, which may obscure the bleeding site if it is located in the hepatic or splenic flexure. The test is completed within 20 minutes of administration of the radionuclide.
The use of radionuclide scanning requires the patient to be relatively hemodynamically stable for the duration it takes to complete the procedure. It is recommended before angiography to localize LGI bleeding. This may help avoid further investigations or interventions in patients with negative scintigram and would help increase the likelihood of a positive subsequent angiogram. 56 57 58
Computed Tomography Angiography
When the hemodynamics do not permit endoscopic evaluation and/or when patients are unable to tolerate a bowel preparation, noncolonoscopic interventions are relevant. The triple phase multidetector row computed tomography (CT) scan shows bleeding as extravasation of contrast, which increases through the arterial to portal venous phase. CT angiography has a baseline sensitivity to pick up LGI bleeding as low as 0.3 mL/min, with a sensitivity to diagnose bleeding ranging from 79 to 100% and a specificity of 85 to 100%. 59 60 61 62 63 64 65 But, the intermittent nature of diverticular bleeding reduces the sensitivity of CT angiography in diagnosing the diverticular bleeding. Studies by Obana et al showed the detection rate of diverticular bleeding by CT angiogram to be 15.4 against 38.5%. 66 The time from the last episode of hematochezia to the time of performing the CT scan and past history of diverticular bleeding appear to be significant factors associated with its sensitivity. CT angiography would be helpful in confirming the diagnosis and localizing the site of bleeding prior to angiography so that directed angiography could be performed.
Angiographic Therapies
Angiography detects bleeding rates of 0.5 to 1.0 mL/min but only if the patient is actively bleeding. When a bleeding site is identified, the angiographic appearance may provide further insight into the cause of the bleeding and help to confirm the diagnosis. Diverticular bleeding is often seen as an extravasation of contrast; vascular ectasias may be identified by a vascular tuft or early filling vein. Patients who require angiographic therapy have been persistently bleeding, with episodes of hemodynamic instability with ongoing resuscitation efforts, and are at higher risk for contrast-induced nephropathy. Thus, optimally, patients should have a creatinine of < 1.5 mg/dL, estimated glomerular filtration rate of > 60 mL/1.73 m 2 , and have their underlying coagulation status optimized with INR of less than 1.5 and platelet count of above 50,000/mm 3 . Evaluation for LGI bleeding typically involves interrogation of superior mesenteric artery, inferior mesenteric artery, and the order of interrogation is based on clinical suspicion on the site of bleeding. Selective catheterization of the offending vessel is performed using micro catheters. Contrast extravasation into the lumen or filling of spaces outside of bowel lumen (diverticula) is typically seen with diverticular bleeding.
Therapeutic intervention after angiographic localization could be performed with embolization or infusion of vasoconstrictive agents to decrease blood flow to the bleeding site. Materials used for embolization are either temporary (such as Gelfoam, absorbable gelatin sponge) or permanent (such as coils, particles, glue, ethylenevinyl alcohol polymer). Biodegradable gelatin sponge could be made into powder or small blocks. Use of gelatin sponge is associated with lower bowel infarction rate compared with the powdered form. 67 Use of permanent embolic agents is associated with expectedly higher bowel infarction rates.
Vasopressin is used when bleeding is diffuse or when super selective catheterization is not technically possible. Vasopressin could be infused by intra-arterial or intravenous route, with intra-arterial injection associated with a success rate of 80 to 90% in controlling LGI bleeding. 68 69 70 Intravenous vasopressin is seldom used clinically in LGI bleeding, and rebleeding rate after discontinuing vasopressin therapy (both intravenous and intraarterial) is higher. 71 72 73
The choice between angiographic embolization versus infusion of vasopressin has to be individualized to the patient and the expertise available locally. Although the results of embolization and vasopressin therapy are comparable, embolization is preferred, as vasopressin therapy is associated with a need for more complex post procedure monitoring, a longer complexity of treatment associated with infusion therapy, and increased likelihood of systemic complications. 74 Vasopressin therapy is also associated with peripheral vasoconstriction and should be used with caution in patients with coronary artery disease, congestive cardiomyopathy, severe hypertension, or severe peripheral vascular disease.
Angiographic embolization carries a higher complication risk (up to 15%) including bowel wall ischemia and stricture and thus should be reserved for patients who are at poor operative risks.
Therapeutic Barium Enema for Diverticular Bleeding
Patients with multiple bleeding diverticula, failed interventions, and/or those who are poor candidates for endoscopic or interventional radiology procedures, have been managed with highly concentrated (200%) barium sulfate impaction therapy. This has been described in the literature as early as 1970 by Adam et al. 75 This intervention involving a use of 200 mL of barium sulfate has since been reported in multiple case reports and case series with all the barium terminating the bleeding of the reported cases. 76 77 78 79 80 81 These studies have no control group and are likely to suffer from publication bias. Barium impaction therapy has been reported to be well tolerated by patients with comorbid conditions and elderly patients. Barium impaction has been reported to be as effective as endoscopic management of diverticular bleeding. 78
Recurrent Diverticular Bleeding
LGI bleeding from vascular causes as seen with diverticular bleeding and colonic angioectasias are associated with a higher risk of recurrent bleeding. The recurrent bleeding with diverticular bleeding is known to recur within a short period, with a reported recurrence rate to be as high as 47% during a median follow-up of 8.1 months. The index and recurrent bleeding have been shown to be identical in 84 and 5% patients from a different site and undetermined in the remaining 11% patients. Surgical resection was eventually required in 97% of the patients, with sigmoidectomy (43%), left colectomy (27%), subtotal colectomy (22%), and right colectomy (5%) as the required procedures. Elderly patients associated with CT-proven diverticulitis, peripheral vascular disease, and chronic renal disease are associated with an increased risk of recurrent bleeding. 82
Surgery
Surgery for diverticular bleeding is indicated when the bleeding does not stop spontaneously and could not be controlled with endoscopic and angiographic interventions. Hemodynamic instability despite adequate efforts at resuscitation also mandates emergent surgical intervention. Surgery is indicated electively in patients with recurrent diverticular bleeding. The extent of colectomy depends on preoperative localization of bleeding site. If localized, segmental colectomy is sufficient even in patients with an extensive diverticular disease, if the resection removes the bleeding site. Preoperative localization is typically performed with either colonoscopy or with angiography. Tagged red cell scan localization is not considered accurate enough to guide segmental colectomy and is to be avoided. Even with preoperative localization, segmental colectomy carries a rebleeding rate ranging from 0 to 14%. 67 83 Patients without preoperative localization require subtotal colectomy. Partial colectomy after preoperative localization is associated with perioperative morbidity of 8.6% compared with 37% when emergent subtotal colectomy is performed for those without preoperative localization. Exploratory laparotomy aided by intraoperative colonoscopy has been shown to be effective in identifying the source of LGI bleeding in 78% cases. 84 If preoperative or intraoperative localization is not possible or if the patient is hemodynamically unstable, the surgical procedure of choice is subtotal colectomy with end ileostomy. Blind segmental colectomy is contraindicated and is associated with unacceptably high morbidity, mortality, and the rebleeding rate at 83, 57, and 40%, respectively. 83 Although subtotal colectomy is associated with virtually zero rebleeding rates for diverticular bleeding, the morbidity and mortality are higher at 37% and 11 to 33%, respectively. 69 84 85 86
References
- 1.Hreinsson J P, Gumundsson S, Kalaitzakis E, Björnsson E S. Lower gastrointestinal bleeding: incidence, etiology, and outcomes in a population-based setting. Eur J Gastroenterol Hepatol. 2013;25(01):37–43. doi: 10.1097/MEG.0b013e32835948e3. [DOI] [PubMed] [Google Scholar]
- 2.Nagata N, Niikura R, Aoki T et al. Impact of discontinuing non-steroidal antiinflammatory drugs on long-term recurrence in colonic diverticular bleeding. World J Gastroenterol. 2015;21(04):1292–1298. doi: 10.3748/wjg.v21.i4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peery A F, Dellon E S, Lund Jet al. Burden of gastrointestinal disease in the United States: 2012 update Gastroenterology 2012143051179–1187..e1–e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams J B, Margolin D A. Management of diverticular hemorrhage. Clin Colon Rectal Surg. 2009;22(03):181–185. doi: 10.1055/s-0029-1236163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gayer C, Chino A, Lucas Cet al. Acute lower gastrointestinal bleeding in 1,112 patients admitted to an urban emergency medical center Surgery 200914604600–606., discussion; 606–607 [DOI] [PubMed] [Google Scholar]
- 6.Wedel T, Böttner M. Anatomy and pathogenesis of diverticular disease [Article in German] Chirurg. 2014;85(04):281–288. doi: 10.1007/s00104-013-2617-6. [DOI] [PubMed] [Google Scholar]
- 7.Ilyas M I, Zangbar B, Nfonsam V N et al. Are there differences in outcome after elective sigmoidectomy for diverticular disease and for cancer? A national inpatient study. Colorectal Dis. 2017;19(03):260–265. doi: 10.1111/codi.13461. [DOI] [PubMed] [Google Scholar]
- 8.Stollman N, Raskin J B.Diverticular disease of the colon Lancet 2004363(9409):631–639. [DOI] [PubMed] [Google Scholar]
- 9.Reinus J F, Brandt L J. Vascular ectasias and diverticulosis. Common causes of lower intestinal bleeding. Gastroenterol Clin North Am. 1994;23(01):1–20. [PubMed] [Google Scholar]
- 10.McGuire H H, Jr, Haynes B W., Jr Massive hemorrhage for diverticulosis of the colon: guidelines for therapy based on bleeding patterns observed in fifty cases. Ann Surg. 1972;175(06):847–855. doi: 10.1097/00000658-197206010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuckerman G R, Prakash C. Acute lower intestinal bleeding. Part II: etiology, therapy, and outcomes. Gastrointest Endosc. 1999;49(02):228–238. doi: 10.1016/s0016-5107(99)70491-8. [DOI] [PubMed] [Google Scholar]
- 12.Kang J Y, Melville D, Maxwell J D. Epidemiology and management of diverticular disease of the colon. Drugs Aging. 2004;21(04):211–228. doi: 10.2165/00002512-200421040-00001. [DOI] [PubMed] [Google Scholar]
- 13.Miura S, Kodaira S, Shatari T, Nishioka M, Hosoda Y, Hisa T K. Recent trends in diverticulosis of the right colon in Japan: retrospective review in a regional hospital. Dis Colon Rectum. 2000;43(10):1383–1389. doi: 10.1007/BF02236634. [DOI] [PubMed] [Google Scholar]
- 14.Jensen D M, Machicado G A, Jutabha R, Kovacs T O. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342(02):78–82. doi: 10.1056/NEJM200001133420202. [DOI] [PubMed] [Google Scholar]
- 15.Meyers M A, Alonso D R, Baer J W. Pathogenesis of massively bleeding colonic diverticulosis: new observations. AJR Am J Roentgenol. 1976;127(06):901–908. doi: 10.2214/ajr.127.6.901. [DOI] [PubMed] [Google Scholar]
- 16.McGuire H H., Jr Bleeding colonic diverticula. A reappraisal of natural history and management. Ann Surg. 1994;220(05):653–656. doi: 10.1097/00000658-199411000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong S K, Ho Y H, Leong A P, Seow-Choen F. Clinical behavior of complicated right-sided and left-sided diverticulosis. Dis Colon Rectum. 1997;40(03):344–348. doi: 10.1007/BF02050427. [DOI] [PubMed] [Google Scholar]
- 18.Kollef M H, O'Brien J D, Zuckerman G R, Shannon W. BLEED: a classification tool to predict outcomes in patients with acute upper and lower gastrointestinal hemorrhage. Crit Care Med. 1997;25(07):1125–1132. doi: 10.1097/00003246-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Rockall T A, Logan R F, Devlin H B, Northfield T C; National Audit of Acute Upper Gastrointestinal Haemorrhage.Selection of patients for early discharge or outpatient care after acute upper gastrointestinal haemorrhage Lancet 1996347(9009):1138–1140. [DOI] [PubMed] [Google Scholar]
- 20.Bordley D R, Mushlin A I, Dolan J G et al. Early clinical signs identify low-risk patients with acute upper gastrointestinal hemorrhage. JAMA. 1985;253(22):3282–3285. [PubMed] [Google Scholar]
- 21.Blatchford O, Murray W R, Blatchford M.A risk score to predict need for treatment for upper-gastrointestinal haemorrhage Lancet 2000356(9238):1318–1321. [DOI] [PubMed] [Google Scholar]
- 22.Strate L L, Orav E J, Syngal S. Early predictors of severity in acute lower intestinal tract bleeding. Arch Intern Med. 2003;163(07):838–843. doi: 10.1001/archinte.163.7.838. [DOI] [PubMed] [Google Scholar]
- 23.Patel R, Clancy R, Crowther E, Vannahme M, Pullyblank A. A rectal bleeding algorithm can successfully reduce emergency admissions. Colorectal Dis. 2014;16(05):377–381. doi: 10.1111/codi.12524. [DOI] [PubMed] [Google Scholar]
- 24.Laine L, Connors L G, Reicin A et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003;124(02):288–292. doi: 10.1053/gast.2003.50054. [DOI] [PubMed] [Google Scholar]
- 25.Strate L L, Gralnek I M. ACG Clinical Guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(05):755. doi: 10.1038/ajg.2016.155. [DOI] [PubMed] [Google Scholar]
- 26.Villanueva C, Colomo A, Bosch A. Transfusion for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(14):1362–1363. doi: 10.1056/NEJMc1301256. [DOI] [PubMed] [Google Scholar]
- 27.Jairath V, Kahan B C, Stanworth S J et al. Prevalence, management, and outcomes of patients with coagulopathy after acute nonvariceal upper gastrointestinal bleeding in the United Kingdom. Transfusion. 2013;53(05):1069–1076. doi: 10.1111/j.1537-2995.2012.03849.x. [DOI] [PubMed] [Google Scholar]
- 28.Shingina A, Barkun A N, Razzaghi A, Martel M, Bardou M, Gralnek I; RUGBE Investigators.Systematic review: the presenting international normalised ratio (INR) as a predictor of outcome in patients with upper nonvariceal gastrointestinal bleeding Aliment Pharmacol Ther 201133091010–1018. [DOI] [PubMed] [Google Scholar]
- 29.Wolf A T, Wasan S K, Saltzman J R. Impact of anticoagulation on rebleeding following endoscopic therapy for nonvariceal upper gastrointestinal hemorrhage. Am J Gastroenterol. 2007;102(02):290–296. doi: 10.1111/j.1572-0241.2006.00969.x. [DOI] [PubMed] [Google Scholar]
- 30.Eisen G M, Dominitz J A, Faigel D O et al. An annotated algorithmic approach to acute lower gastrointestinal bleeding. Gastrointest Endosc. 2001;53(07):859–863. doi: 10.1016/s0016-5107(01)70306-9. [DOI] [PubMed] [Google Scholar]
- 31.Zuccaro G, Jr; American College of Gastroenterology. Practice Parameters Committee.Management of the adult patient with acute lower gastrointestinal bleeding Am J Gastroenterol 199893081202–1208. [DOI] [PubMed] [Google Scholar]
- 32.Strate L L, Saltzman J R, Ookubo R, Mutinga M L, Syngal S. Validation of a clinical prediction rule for severe acute lower intestinal bleeding. Am J Gastroenterol. 2005;100(08):1821–1827. doi: 10.1111/j.1572-0241.2005.41755.x. [DOI] [PubMed] [Google Scholar]
- 33.Davila R E, Rajan E, Adler D G et al. ASGE Guideline: the role of endoscopy in the patient with lower-GI bleeding. Gastrointest Endosc. 2005;62(05):656–660. doi: 10.1016/j.gie.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 34.Green B T, Rockey D C, Portwood G et al. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol. 2005;100(11):2395–2402. doi: 10.1111/j.1572-0241.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 35.Angtuaco T L, Reddy S K, Drapkin S, Harrell L E, Howden C W. The utility of urgent colonoscopy in the evaluation of acute lower gastrointestinal tract bleeding: a 2-year experience from a single center. Am J Gastroenterol. 2001;96(06):1782–1785. doi: 10.1111/j.1572-0241.2001.03871.x. [DOI] [PubMed] [Google Scholar]
- 36.Jensen D M, Machicado G A. Diagnosis and treatment of severe hematochezia. The role of urgent colonoscopy after purge. Gastroenterology. 1988;95(06):1569–1574. doi: 10.1016/s0016-5085(88)80079-9. [DOI] [PubMed] [Google Scholar]
- 37.Bloomfeld R S, Rockey D C, Shetzline M A. Endoscopic therapy of acute diverticular hemorrhage. Am J Gastroenterol. 2001;96(08):2367–2372. doi: 10.1111/j.1572-0241.2001.04048.x. [DOI] [PubMed] [Google Scholar]
- 38.Parra-Blanco A, Ruiz A, Alvarez-Lobos M et al. Achieving the best bowel preparation for colonoscopy. World J Gastroenterol. 2014;20(47):17709–17726. doi: 10.3748/wjg.v20.i47.17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lhewa D Y, Strate L L. Pros and cons of colonoscopy in management of acute lower gastrointestinal bleeding. World J Gastroenterol. 2012;18(11):1185–1190. doi: 10.3748/wjg.v18.i11.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navaneethan U, Njei B, Venkatesh P GK, Sanaka M R. Timing of colonoscopy and outcomes in patients with lower GI bleeding: a nationwide population-based study. Gastrointest Endosc. 2014;79(02):297–3.06E14. doi: 10.1016/j.gie.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Kaltenbach T, Watson R, Shah J et al. Colonoscopy with clipping is useful in the diagnosis and treatment of diverticular bleeding. Clin Gastroenterol Hepatol. 2012;10(02):131–137. doi: 10.1016/j.cgh.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 42.Yamada A, Sugimoto T, Kondo S et al. Assessment of the risk factors for colonic diverticular hemorrhage. Dis Colon Rectum. 2008;51(01):116–120. doi: 10.1007/s10350-007-9137-8. [DOI] [PubMed] [Google Scholar]
- 43.Niikura R, Nagata N, Aoki T et al. Predictors for identification of stigmata of recent hemorrhage on colonic diverticula in lower gastrointestinal bleeding. J Clin Gastroenterol. 2015;49(03):e24–e30. doi: 10.1097/MCG.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 44.Tada M, Shimizu S, Kawai K. Emergency colonoscopy for the diagnosis of lower intestinal bleeding. Gastroenterol Jpn. 1991;26 03:121–124. doi: 10.1007/BF02779279. [DOI] [PubMed] [Google Scholar]
- 45.Chaudhry V, Hyser M J, Gracias V H, Gau F C. Colonoscopy: the initial test for acute lower gastrointestinal bleeding. Am Surg. 1998;64(08):723–728. [PubMed] [Google Scholar]
- 46.Ohyama T, Sakurai Y, Ito M, Daito K, Sezai S, Sato Y. Analysis of urgent colonoscopy for lower gastrointestinal tract bleeding. Digestion. 2000;61(03):189–192. doi: 10.1159/000007756. [DOI] [PubMed] [Google Scholar]
- 47.Wang C Y, Won C W, Shieh M J. Aggressive colonoscopic approaches to lower intestinal bleeding. Gastroenterol Jpn. 1991;26 03:125–128. doi: 10.1007/BF02779280. [DOI] [PubMed] [Google Scholar]
- 48.Jensen D M, Ohning G V, Kovacs T O et al. Natural history of definitive diverticular hemorrhage based on stigmata of recent hemorrhage and colonoscopic Doppler blood flow monitoring for risk stratification and definitive hemostasis. Gastrointest Endosc. 2016;83(02):416–423. doi: 10.1016/j.gie.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farrell J J, Graeme-Cook F, Kelsey P B. Treatment of bleeding colonic diverticula by endoscopic band ligation: an in-vivo and ex-vivo pilot study. Endoscopy. 2003;35(10):823–829. doi: 10.1055/s-2003-42611. [DOI] [PubMed] [Google Scholar]
- 50.Setoyama T, Ishii N, Fujita Y. Enodoscopic band ligation (EBL) is superior to endoscopic clipping for the treatment of colonic diverticular hemorrhage. Surg Endosc. 2011;25(11):3574–3578. doi: 10.1007/s00464-011-1760-8. [DOI] [PubMed] [Google Scholar]
- 51.Ishii N, Setoyama T, Deshpande G A et al. Endoscopic band ligation for colonic diverticular hemorrhage. Gastrointest Endosc. 2012;75(02):382–387. doi: 10.1016/j.gie.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 52.Shibata S, Shigeno T, Fujimori K, Kanai K, Yoshizawa K. Colonic diverticular hemorrhage: the hood method for detecting responsible diverticula and endoscopic band ligation for hemostasis. Endoscopy. 2014;46(01):66–69. doi: 10.1055/s-0033-1344890. [DOI] [PubMed] [Google Scholar]
- 53.Olds G D, Cooper G S, Chak A, Sivak M V, Jr, Chitale A A, Wong R C. The yield of bleeding scans in acute lower gastrointestinal hemorrhage. J Clin Gastroenterol. 2005;39(04):273–277. doi: 10.1097/01.mcg.0000155131.04821.f3. [DOI] [PubMed] [Google Scholar]
- 54.Gunderman R, Leef J A, Lipton M J, Reba R C. Diagnostic imaging and the outcome of acute lower gastrointestinal bleeding. Acad Radiol. 1998;5 02:S303–S305. doi: 10.1016/s1076-6332(98)80338-3. [DOI] [PubMed] [Google Scholar]
- 55.Funaki B, Kostelic J K, Lorenz J et al. Superselective microcoil embolization of colonic hemorrhage. AJR Am J Roentgenol. 2001;177(04):829–836. doi: 10.2214/ajr.177.4.1770829. [DOI] [PubMed] [Google Scholar]
- 56.Sanlı Y, Ozkan Z G, Kuyumcu S et al. Role of red blood cell scintigraphy for determining the localization of gastrointestinal bleeding. Ulus Travma Acil Cerrahi Derg. 2012;18(03):225–230. doi: 10.5505/tjtes.2012.55553. [DOI] [PubMed] [Google Scholar]
- 57.Al Qahtani A R, Satin R, Stern J, Gordon P H. Investigative modalities for massive lower gastrointestinal bleeding. World J Surg. 2002;26(05):620–625. doi: 10.1007/s00268-001-0279-x. [DOI] [PubMed] [Google Scholar]
- 58.Gunderman R, Leef J, Ong K, Reba R, Metz C. Scintigraphic screening prior to visceral arteriography in acute lower gastrointestinal bleeding. J Nucl Med. 1998;39(06):1081–1083. [PubMed] [Google Scholar]
- 59.Kuhle W G, Sheiman R G. Detection of active colonic hemorrhage with use of helical CT: findings in a swine model. Radiology. 2003;228(03):743–752. doi: 10.1148/radiol.2283020756. [DOI] [PubMed] [Google Scholar]
- 60.García-Blázquez V, Vicente-Bártulos A, Olavarria-Delgado A, Plana M N, van der Winden D, Zamora J; EBM-Connect Collaboration.Accuracy of CT angiography in the diagnosis of acute gastrointestinal bleeding: systematic review and meta-analysis Eur Radiol 201323051181–1190. [DOI] [PubMed] [Google Scholar]
- 61.Martí M, Artigas J M, Garzón G, Alvarez-Sala R, Soto J A. Acute lower intestinal bleeding: feasibility and diagnostic performance of CT angiography. Radiology. 2012;262(01):109–116. doi: 10.1148/radiol.11110326. [DOI] [PubMed] [Google Scholar]
- 62.Sun H, Jin Z, Li X et al. Detection and localization of active gastrointestinal bleeding with multidetector row computed tomography angiography: a 5-year prospective study in one medical center. J Clin Gastroenterol. 2012;46(01):31–41. doi: 10.1097/MCG.0b013e31823337ee. [DOI] [PubMed] [Google Scholar]
- 63.Wu L M, Xu J R, Yin Y, Qu X H. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16(31):3957–3963. doi: 10.3748/wjg.v16.i31.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kennedy D W, Laing C J, Tseng L H, Rosenblum D I, Tamarkin S W. Detection of active gastrointestinal hemorrhage with CT angiography: a 4(1/2)-year retrospective review. J Vasc Interv Radiol. 2010;21(06):848–855. doi: 10.1016/j.jvir.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 65.Chua A E, Ridley L J. Diagnostic accuracy of CT angiography in acute gastrointestinal bleeding. J Med Imaging Radiat Oncol. 2008;52(04):333–338. doi: 10.1111/j.1440-1673.2008.01964.x. [DOI] [PubMed] [Google Scholar]
- 66.Obana T, Fujita N, Sugita R et al. Prospective evaluation of contrast-enhanced computed tomography for the detection of colonic diverticular bleeding. Dig Dis Sci. 2013;58(07):1985–1990. doi: 10.1007/s10620-013-2629-6. [DOI] [PubMed] [Google Scholar]
- 67.Rösch J, Keller F S, Kozak B, Niles N, Dotter C T. Gelfoam powder embolization of the left gastric artery in treatment of massive small-vessel gastric bleeding. Radiology. 1984;151(02):365–370. doi: 10.1148/radiology.151.2.6608749. [DOI] [PubMed] [Google Scholar]
- 68.Clark R A, Colley D P, Eggers F M. Acute arterial gastrointestinal hemorrhage: efficacy of transcatheter control. AJR Am J Roentgenol. 1981;136(06):1185–1189. doi: 10.2214/ajr.136.6.1185. [DOI] [PubMed] [Google Scholar]
- 69.Browder W, Cerise E J, Litwin M S. Impact of emergency angiography in massive lower gastrointestinal bleeding. Ann Surg. 1986;204(05):530–536. doi: 10.1097/00000658-198611000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cherian M P, Mehta P, Kalyanpur T M, Hedgire S S, Narsinghpura K S. Arterial interventions in gastrointestinal bleeding. Semin Intervent Radiol. 2009;26(03):184–196. doi: 10.1055/s-0029-1225661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eckstein M R, Kelemouridis V, Athanasoulis C A, Waltman A C, Feldman L, van Breda A. Gastric bleeding: therapy with intraarterial vasopressin and transcatheter embolization. Radiology. 1984;152(03):643–646. doi: 10.1148/radiology.152.3.6611562. [DOI] [PubMed] [Google Scholar]
- 72.Sherman L M, Shenoy S S, Cerra F B. Selective intra-arterial vasopressin: clinical efficacy and complications. Ann Surg. 1979;189(03):298–302. doi: 10.1097/00000658-197903000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker T G, Waltman A C. Vasoconstrictive infusion therapy for management of arterial gastrointestinal hemorrhage. Semin Intervent Radiol. 1988;5:18. [Google Scholar]
- 74.Darcy M. Treatment of lower gastrointestinal bleeding: vasopressin infusion versus embolization. J Vasc Interv Radiol. 2003;14(05):535–543. doi: 10.1097/01.rvi.0000064862.65229.8a. [DOI] [PubMed] [Google Scholar]
- 75.Adams J T. Therapeutic barium enema for massive diverticular bleeding. Arch Surg. 1970;101(04):457–460. doi: 10.1001/archsurg.1970.01340280009003. [DOI] [PubMed] [Google Scholar]
- 76.Niikura R, Nagata N, Yamano K, Shimbo T, Uemura N. High-dose barium impaction therapy is useful for the initial hemostasis and for preventing the recurrence of colonic diverticular bleeding unresponsive to endoscopic clipping. Case Rep Gastrointest Med. 2013;2013:365954. doi: 10.1155/2013/365954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pausawasdi N, Al-Hawary M, Higgins P D. Therapeutic high-density barium enema in a case of presumed diverticular hemorrhage. Case Rep Gastroenterol. 2011;5(01):88–94. doi: 10.1159/000322911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fujimoto A, Sato S, Kurakata H, Nakano S, Igarashi Y. Effectiveness of high-dose barium enema filling for colonic diverticular bleeding. Colorectal Dis. 2011;13(08):896–898. doi: 10.1111/j.1463-1318.2010.02350.x. [DOI] [PubMed] [Google Scholar]
- 79.Iwamoto J, Mizokami Y, Shimokobe K, Matsuoka T, Matsuzaki Y. Therapeutic barium enema for bleeding colonic diverticula: four case series and review of the literature. World J Gastroenterol. 2008;14(41):6413–6417. doi: 10.3748/wjg.14.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsuhashi N, Akahane M, Nakajima A. Barium impaction therapy for refractory colonic diverticular bleeding. AJR Am J Roentgenol. 2003;180(02):490–492. doi: 10.2214/ajr.180.2.1800490. [DOI] [PubMed] [Google Scholar]
- 81.Nagata N, Niikura R, Shimbo T et al. High-dose barium impaction therapy for the recurrence of colonic diverticular bleeding: a randomized controlled trial. Ann Surg. 2015;261(02):269–275. doi: 10.1097/SLA.0000000000000658. [DOI] [PubMed] [Google Scholar]
- 82.Aytac E, Stocchi L, Gorgun E, Ozuner G. Risk of recurrence and long-term outcomes after colonic diverticular bleeding. Int J Colorectal Dis. 2014;29(03):373–378. doi: 10.1007/s00384-013-1804-8. [DOI] [PubMed] [Google Scholar]
- 83.Parkes B M, Obeid F N, Sorensen V J, Horst H M, Fath J J. The management of massive lower gastrointestinal bleeding. Am Surg. 1993;59(10):676–678. [PubMed] [Google Scholar]
- 84.Wagner H E, Stain S C, Gilg M, Gertsch P. Systematic assessment of massive bleeding of the lower part of the gastrointestinal tract. Surg Gynecol Obstet. 1992;175(05):445–449. [PubMed] [Google Scholar]
- 85.Browder W, Cerise E J, Litwin M S. Impact of emergency angiography in massive lower gastrointestinal bleeding. Ann Surg. 1986;204(05):530–536. doi: 10.1097/00000658-198611000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Setya V, Singer J A, Minken S L. Subtotal colectomy as a last resort for unrelenting, unlocalized, lower gastrointestinal hemorrhage: experience with 12 cases. Am Surg. 1992;58(05):295–299. [PubMed] [Google Scholar]


