Abstract
CONTEXT
Cancer patients’ continued tobacco use results in poorer therapeutic outcomes including decreased quality of life and survival.
OBJECTIVE
To assess reach and impact of a free, opt-out, telephone-based tobacco cessation program for thoracic cancer center patients.
DESIGN
Observational study.
SETTING
Comprehensive Cancer Center in Western New York.
PARTICIPANTS
Current or recent (within past 30 days) tobacco using thoracic cancer center patients referred to a tobacco cessation support service between October 2010 and October 2012 at a Comprehensive Cancer Center (n=942 of 1313 referrals were eligible for cessation support).
INTERVENTION
A free, opt-out, telephone-based cessation service that was implemented as standard of care. Cessation specialists had patient guided conversations that assessed readiness to quit, methods used in the past, provided cessation strategies and worked to set up a quit date. There was an average of 35.9 days between referral and first contact.
MAIN OUTCOME MEASURES
Program reach (referral and participation rates) and impact (as self-reported cessation outcomes measured twice after referral).
RESULTS
730 of 942 (77.5%) patients referred to and called by a tobacco cessation service participated in at least one cessation support call, of which 440/730 (60.3%) were called for follow-up and 89.5% (394/440) participated. 20.2% (69/342) of current smokers at referral reported at least 7-day abstinence at follow-up. Among current smokers at referral and first contact, being married (OR=2.05, 95% CI: 1.01–4.18) and having a lower Eastern Cooperative Oncology Group (ECOG) performance score (OR=4.05, 95% CI: 1.58–10.39) was associated with quitting at follow-up, after controlling for demographic, clinical, and health behavior characteristics.
CONCLUSIONS
Our results demonstrate that 78% of thoracic center patients, if contacted, participated at least once in this cessation support service; and for current smokers at referral and first contact, being married and having a lower ECOG performance score is associated with self-reported quitting at follow-up.
Keywords: tobacco, smoking cessation, cessation program, lung cancer
INTRODUCTION
Tobacco use is the single leading preventable cause of disease in the United States.1,2 It is the most important risk factor for the development of lung cancer and COPD and is a major cardiovascular disease and stroke risk factor.1,2 It is now recognized that tobacco use has a broader impact beyond that of disease development. Tobacco contributes to a poorer prognosis, increased all-cause and cancer-specific mortality, increased risk of second primary tumors, and is thought to negatively influence cancer treatment outcomes and disease recurrence among those diagnosed with cancer.2 It is important to examine the impact of a cancer diagnosis and its potential use as a powerful teachable moment for smoking cessation interventions given the wide-ranging influence of smoking on important clinical outcomes. The impact of a cancer diagnosis, especially one related to tobacco use, can serve as an opportune teachable moment and may result in a population more receptive to a tobacco cessation intervention and more motivated to attempt to quit or stay quit.3–5
Historically, tobacco cessation efforts following a cancer diagnosis have received little attention. A small number of studies demonstrated that the smoking rates among cancer patients and survivors are similar to or higher than that of the general population.6,7 Up to approximately 60% of cancer patients continue using tobacco products after their diagnosis of cancer, with varying rates depending on the cancer site.4,6 Among lung cancer patients specifically, continued smoking after their cancer diagnosis varies greatly in the published literature, ranging from 19% to 85%.7–9
Unlike the majority of studies where newly diagnosed patients are given the opportunity to opt-in to a cessation program, this observational study describes an opt-out program where all patients are screened for tobacco use and offered cessation assistance.9–14 Opt-out designs remove self-selection into the cessation assistance programs compared to opt-in, which depends on the patient proactively calling for telephone-based cessation program. For example, the Ask-Advise-Connect model has shown to dramatically increase patient enrollment into quitline services when integrated into the primary care setting, compared to Ask-Advise-Refer.15,16 In October 2010, Roswell Park Cancer Institute (RPCI) established a policy requiring the systematic assessment of tobacco use and automated electronic referral to a dedicated tobacco cessation support service for thoracic center patients as well as patients with other tobacco-related cancers. This paper presents data from a cessation support service to examine the following: 1) the reach by examining the proportion of referred and participating thoracic cancer clinic patients; and 2) the impact by examining the change in quit rate from the first cessation support call to follow-up, as well as the characteristics of continued users compared to those who quit at follow-up.
METHODS
Data Collection
Starting in October 2010, all RPCI thoracic center patients were systematically screened for tobacco use. This included patients with thoracic malignancies, lung cancer screening patients, undiagnosed patients, and pulmonary patients. Thoracic center nurses administer a standardized tobacco use assessment using the electronic medical record (EMR) for every patient and update that information every 30 days thereafter.17 Patients who report using tobacco currently or in the past 30 days are automatically referred to the cessation support service via the EMR. Additional information on the cessation support service can be found elsewhere.17–19
Patients referred to the cessation support service are mailed a welcome packet with information about the free program, the impact of smoking on overall health and specifically on cancer treatment, pharmacologic cessation support options, and information on the New York State (NYS) Smokers’ Quitline, where immediate support is available. Due to staffing limitations, new thoracic patients that were current daily users had call priority. If a patient was not reached after nine call attempts, they were classified as “not reached” and mailed another letter describing the service, again highlighting the benefits of quitting, and inviting them to call if they are interested in cessation support. The cessation specialist tailored conversations to the needs of each patient, while discussing the impact of continued smoking on cancer treatment and outcomes, and obtaining information on tobacco use and quitting history, barriers to quitting, and willingness to quit. The cessation specialist assisted patients in making a quit plan that included techniques to reduce behaviors that promote smoking, implement strategies for coping with cravings, and obtaining pharmacologic support if eligible. If a patient recently quit, the cessation specialist used similar techniques to encourage the maintenance of a patient’s quit status.
The cessation specialist provided up to seven additional follow-up support calls with patients. As a patient-centered service, there is no specific protocol for frequency, duration, or content of the counseling calls. Cessation specialists tailored the frequency and duration of follow-up contact to individual patient needs, which are made, on average, every six weeks. Patients have an option to opt-out of the program at any time after the referral.
Sample
Patients met the following criteria for inclusion in this study: 1) classified as a new patient for the tobacco use assessment; and 2) a cessation service referral generated between October 1, 2010 and October 31, 2012 from the RPCI thoracic center. Patients directly referred to the cessation service by a clinician in the thoracic center between October 1, 2010 and October 31, 2012 (n=46) were also eligible for inclusion. Additionally, to be included in the analysis for the second objective, patients must have participated in at least two telephone calls by August 2013 (i.e. the first cessation support call and a follow-up cessation support call).
Patients seen at the RPCI thoracic center include those diagnosed with or suspected of having a thoracic malignancy, persons participating in the high-risk lung cancer screening program, as well as undiagnosed and pulmonary patients. Ineligible patients include those enrolled in hospice or end-of-life care, leaving RPCI for treatment elsewhere, referred patients for whom only family members or assisted living facility staff could be contacted, established patients screened at follow-up visits, or inaccurate referrals (i.e. quit years ago, occasional smokers). Established patients were patients who had already initiated cancer treatment and were presenting to the thoracic clinic after initial consultation for cancer care. We excluded established patients from this analysis because call priority was given to patients referred to the cessation service with the new patient screen rather than the established patient screen. Also, only new patients, rather than new and established, were included in these analyses since they received smoking cessation assistance closer to the time of diagnosis than established patients did.
Statistical Analysis
Demographic and health information was obtained from the EMR (age, pack-years, referral date, smoking status at referral, Eastern Cooperative Oncology Group (ECOG) performance status), the RPCI billing database (sex, date of birth, race, ethnicity, marital status), and the RPCI cancer registry (cancer status). Race and ethnicity were self-reported during patient registration. The main outcome variable, quit status at first and follow-up cessation support calls, was obtained from the cessation service records. A patient was considered quit if they self-reported being smoke-free for at least the last 7 days prior to the first or follow-up cessation support calls. Marital status was recoded as single or married; patients of other marital situations, such as being widowed, divorced, or separated were included in the single category. ECOG performance status was dichotomized into 0 (healthy individual with no physical restrictions) and scores of ≥ 1 (patients with limited physical restrictions to completely disabled or deceased).20 There is no comparison group for this observational study since this program was implemented as standard of care. Data were linked by the patient medical record number, compiled into the main dataset, and de-identified. The RPCI Institutional Review Board approved this study.
Descriptive and multivariate statistics were used to characterize: 1) the proportion of referred and participating thoracic center patients; and 2) the self-reported 7-day quit rates at time of referral and at the first and follow-up cessation support calls, and the characteristics of current smokers compared to those who reported quitting at follow-up. Chi-square tests were used to examine the differences in categorical demographic and health behavior variables between current users and those who quit at each time point. An exact McNemar’s test was used to determine the difference in self-reported smoking status at each contact. A multivariate logistic regression model was used to describe characteristics associated with quitting at follow-up among current users at referral and the first cessation support call. Data were analyzed using SPSS v20.21
RESULTS
1) Reach of the Cessation Support Service
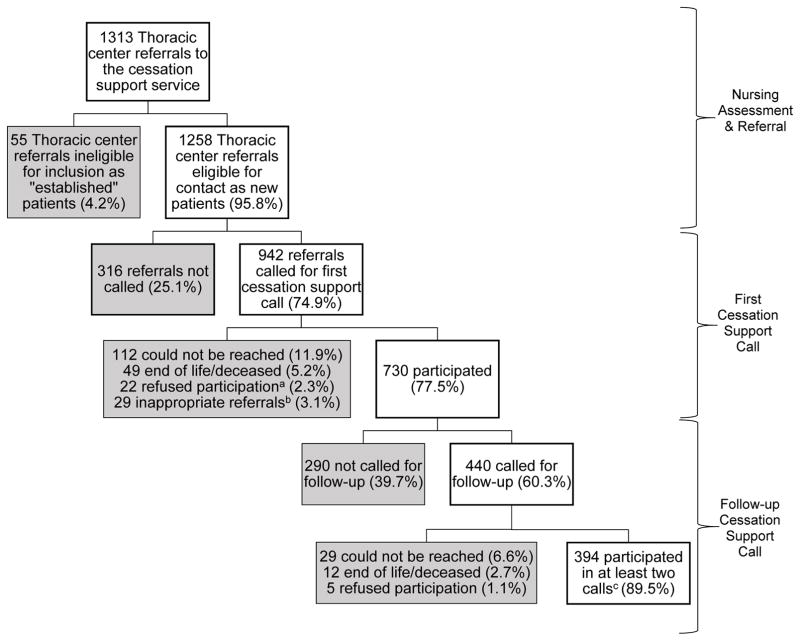
Between October 2010 and October 2012, 1313 patients were referred from the thoracic center to the cessation support service, of which 1258 referrals were eligible to be contacted by the cessation service as new patients (Figure 1). Attempts were made to contact 942/1258 (74.9%) by telephone. 316/1258 (25.1%) of the referrals were not called due to staffing limitations but were still mailed the welcome packet.
Figure 1.
Participation for patients referred to the cessation support service from the thoracic center between October 2010 and October 2012.
aThose who refused participation either hung up on the cessation support service cessation specialists or said they were not interested in participating/“do not bother me again”. n=14 answered call 1 and said do not bother me again, but completed the phone call enough for the stage of change to be assessed (n=1 had quit for at least 7 days prior to contact, n=9 were in pre-contemplation, and n=4 were in preparation). n=5 hung up before the stage of change could be assessed and n=3 said do not bother me again because they were no longer patients at RPCI.
bAmong inappropriate referrals n=25 quit years ago, n=3 were never users, and 1 was an occasional smoker.
c41.8% of patients referred and called for at least once cessation support call participated in a follow-up call (394/942). n=7 participants were not reached in the first call and proactively called the cessation service after being sent a “no reach” letter inviting them to participate in the free service, but were not included in the final analyses.
Please note: Follow-up time for the first call and follow-up was until August 2013 for those referred through October 31, 2012. The average number of days between referral and the first cessation support call for all thoracic patients is 35.9 days with a median 14 days and an average of 1.63 call attempts made. The average number of days between the first cessation support call and follow-up for all thoracic patients is 49.3 days with a median of 21 days and an average of 1.8 call attempts made.
Out of the 942 patients with at least one contact attempt, 730 (77.5%) participated in one cessation support telephone call (Figure 1). One-hundred twelve (11.9%) of the 942 patients with whom a contact was attempted could not be reached, 49/942 (5.2%) patients were in an end-of-life situation or deceased, and 22/942 (2.3%) patients actively refused participation. Twenty-nine (3.1%) of the 942 patients were considered inappropriate referrals once contacted (i.e. never or occasional tobacco users, or quit more than 30 days prior to referral). An average of 35.9 days (std. dev.=79.5, median=14 days, range: 0–812) elapsed between referral and the first cessation support call, and there was an average of 1.6 calls attempted per patient. Patients who participated in the first cessation support call tended to be female (n=384/478; 80.3%) compared to male (n=341/457; 74.6% males; p=0.036) and white (n=646/818; 79.0%) compared to any other race (n=76/117; 67.5%; p=0.005; results not shown).
Follow-up cessation support calls were attempted for 440 out of the 730 successfully reached for the first cessation support call (60.3%), of which 394/440 (89.5%) participated in the follow-up call (Figure 1). The remaining 290 out of 730 (39.7%) were not called for a follow-up due to staffing limitations. Of note, seven patients proactively called the cessation support service after they were sent a “no reach” letter explaining the program with an invitation to call for cessation support, but these patients are not included in the total of 394 for participating in both the first and follow-up cessation support calls. At follow-up, only 1.1% (n=5/440) actively refused participation in the service. The remaining 41 patients did not participate in a follow-up cessation support call due to being in hospice care or deceased (n=12/41, 2.7%), or were unable to be reached (n=29/41; 6.6%; i.e. no answer, wrong telephone number in EMR). There were no demographic, health behavior, or disease characteristics significantly associated with participating in the follow-up cessation support call (results not shown). There was an average of 49.3 days (std. dev.=98.1; median=21; range: 0–742) between the first and follow-up cessation support calls, and an average of 1.8 call attempts made.
2) Impact of the Cessation Support Service
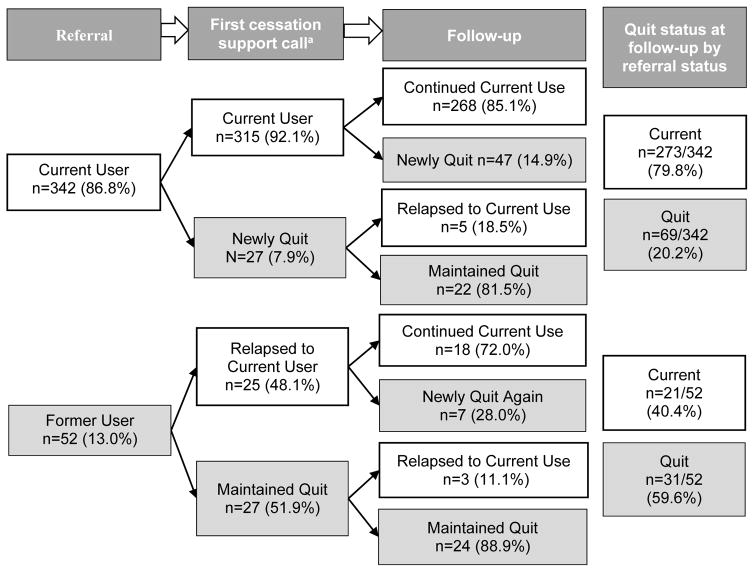
As shown in Figure 2, 86.8% (n=342/394) of patients participating in two cessation support calls had been referred as current users and 13.2% (n=52/394) had been referred as recent quitters. Among the 342 patients referred as current users, 20.2% (n=69/342) reported having quit at the follow-up cessation support call. Among current users at both referral and the first cessation support call, 14.9% (n=47/315) reported quitting at follow-up.
Figure 2.
Self-reported 7-day quit rates for thoracic center patients contacted at least twice by the cessation support service (n=394).
Please note: a “current user” is someone who reports using tobacco, at least a puff, in the past 7 days. A “newly quit” patient includes those who reported current use at the last contact, but now reports being 7 days tobacco free. “Maintained quit” is someone who reports the previously mentioned quit definition for the previous and current contacts. “Relapse” is someone who reports quitting at the previous contact, but is now reporting current tobacco use. Follow-up time was through August 2013. Among those contacted twice, the average time to the first cessation support call was 46.1 days and was 44.6 days between the first cessation support call and follow-up. The p-value for the difference between referral (n=52/394; 13.2%) and the first cessation support call (n=54/394; 13.7%) overall quit rates is p=0.834. The p-value for the difference between the first cessation support call (n=54/394; 13.7%) and follow-up (n=100/394; 25.4%) overall quit rates is p<0.001.
The quit rate was not statistically different between those reported having recently quit at the time of referral (n=52/394; 13.2%) and those who reported having quit at the first cessation support call (n=54/394; 13.7%; p=0.834; Figure 2). However, there was a significant increase in quit rate noted at the first cessation support call (n=54/394; 13.7%) compared to follow-up (n=100/394; 25.4%) for patients who participated in at least two calls (p<0.001).
After controlling for demographic and health behavior factors, marital status and ECOG performance status significantly predicted cessation at the follow-up cessation support call among current users at referral and first cessation support call (Table 1). Married patients were more likely to report cessation at follow-up compared to single patients (OR=2.05; 95% CI: 1.01–4.18). Patients with an ECOG performance score of 0 were more likely to report cessation at follow-up as compared with persons with an ECOG performance score of 1 or greater (OR=4.05, 95% CI: 1.58–10.39). No covariates were statistically significant predictors of reporting cessation at follow-up for patients referred to the cessation support service as recent quitters (results not shown).
Table 1.
Predictors of self-reported 7-day quit status at follow-up for current smokers at the time of referral and first contact (n=275).
| Continuous Covariates | Bivariate results | Multivariate results (n=275) | ||
|---|---|---|---|---|
|
|
|
|||
| Total N | Mean (sd) quit at follow-up (n=65) | OR | 95% CI | |
| Age | 275 | 57.88 (10.89) | 0.99 | 0.96–1.03 |
| Pack-years | 275 | 52.23 (28.94) | 1.00 | 0.99–1.01 |
| Days between referral & follow-up | 275 | 105.18 (150.56) | 1.001 | 0.999–1.003 |
| Number of counseling calls | 275 | 3.60 (1.75) | 1.00 | 0.81–1.23 |
|
| ||||
| Categorical Covariates | Total N | % quit at follow-up | OR | 95% CI |
|
| ||||
| Sex | ||||
| Female | 145 | 14.5 | 1.00 | Ref. |
| Male | 130 | 14.6 | 0.90 | 0.44–1.82 |
| Marital Status | ||||
| Single | 155 | 11.0 | 1.00 | Ref. |
| Married | 120 | 19.2 | 2.05 | 1.01–4.18 |
| ECOG Status | ||||
| 0 | 180 | 18.9 | 4.05 | 1.58–10.39 |
| ≥1 | 95 | 6.3 | 1.00 | Ref. |
| Patient Type | ||||
| Other thoracic center patient | 165 | 12.7 | 1.00 | Ref. |
| Lung cancer patient | 110 | 17.3 | 1.85 | 0.86–3.98 |
Please note: Statistically significant results at p<0.05 are in bold and underlined.
All variables presented were included in the multivariate model. The outcome of interest is self-reported quit status at follow-up (at least 7 days smoke-free prior to the follow-up contact), with current tobacco users as the referent group. N=40 were missing data on one or more covariates (n=21 missing pack-years and n=19 were missing marital status), therefore only 275/315 who were contacted twice and currently using tobacco at each contact are included in the stratified logistic regression analysis. Other variables that were examined bivariately but were not included in the multivariate logistic regression, and therefore are not shown here include race and ethnicity. The p-value for the chi-square test between martial status and quit status at follow-up was p=0.056. There were no statistically significant associations between these covariates and self-reporting quit at follow-up among patients referred as former smokers.
DISCUSSION
Many patients demonstrate an interest in participating in a free, opt-out, telephone-based cessation program once successfully contacted, indicated by the nearly 90% follow-up call participation rate. Opt-out programs similar to this program design have been shown to significantly improve enrollment rates into tobacco use treatment programs compared to opt-in or Ask-Advise-Refer programs.15,16,22 In the first two years of the cessation support service, only 2.3% actively refused participation at the first cessation support call and 1.1% actively refused participation at follow-up. This analysis suggests that the opt-out program experienced few patients actively refusing participation and low levels of passive refusals and that thoracic cancer center patients reached by telephone have a high level of interest in participating in the cessation service (23% did not participate in the first cessation support call and 10% did not participate in a follow-up call).
This program was designed to maximize reach and participation compared to a clinical trial design. However, issues with implementation were discovered once the program began. Human resource limitations were the biggest barrier to implementing the program with maximal reach as designed. Calls were only placed to 74.9% of new patients referred to the cessation service from the thoracic clinic; and only 60.3% of those contacted once received a follow-up call. Another barrier to maximizing reach was not being able to reach referred patients. Telephone numbers in the EMR were out of service, voicemails were full, patients never answered, or only family members could be reached for up to nine contact attempts. Patients in an end-of-life situation or already deceased was not a common reason for participation, despite being a sick population. Similarly, active refusals were a minimal barrier to participation.
Previous studies have shown that cancer screening or a diagnosis may be a teachable moment to encourage tobacco cessation.3–5 Specifically looking at current tobacco users at the time of referral, 7.9% self-reported quitting between referral and the first cessation service contact, suggesting that diagnosis or cancer screening may be a teachable, motivational moment for a quit attempt for this particular group of patients. At follow-up, 14.9% of current users at both referral and first cessation support call reported abstinence, suggesting that the cessation support service may help motivate participating thoracic center patients. However, it is difficult to attribute quitting solely to this one-time cessation support contact since we did not assess other sources of cessation support and there was not a control group to serve as a comparison. Based on the results of the multivariate regression model, the cessation support service may explore the need to tailor cessation support messaging to improve the quit rates for current tobacco using patients at the first and follow-up call. Non-married thoracic clinic patients and patients with an ECOG performance score greater than 0 were less likely to quit at follow-up, so these groups could represent ideal patient cohorts who may benefit from alternative cessation approaches.
A strength of this program is the demonstrated support for the use of a standardized clinic-based tobacco use screening program and automatic referral system to a free, opt-out, telephone-based cessation support service. The majority of the published literature relies on retrospective medical record review to extract tobacco use collected in a non-standardized way and utilizes opt-in program designs.23–25 The program described herein utilizes a standardized and systematic tobacco use questionnaire administered to all patients, reducing the uncontrolled variability of tobacco use typically found in retrospective chart review.
This program implementation study experienced operational limitations. One operational limitation is that this observational study examined the referral, participation, and quit rates of thoracic cancer center patients at one institution, which limits the generalizability of the results. Another operational limitation with this program evaluation is that the data depend on nursing adherence to complete the tobacco assessment questionnaire. The tobacco use assessment was not mandatory for the beginning of the service, resulting in a smaller than expected proportion of referrals generated for thoracic patients seen from October 2010 to October 2012. There may be fundamental differences between patients automatically referred to the cessation service following the tobacco use assessment and those directly referred by clinical staff. However, after results were examined including and excluding the 46 direct referrals and neither bivariate nor multivariate results differed, the direct referrals remained in the final sample. Limited information on other tobacco use, such as cigars and smokeless, was not available from the beginning of the service due to poor compliance with the assessment, unintentionally restricting the analyses to combustible cigarette users. However, another study at the same institute found few adult tobacco users exclusively use non-cigarette forms of tobacco.17 To address these issues, the tobacco assessment questionnaire has been modified to be time efficient and mandatory to enhance nursing compliance.
Another operational limitation is the fact that we experienced human resource limitations in the beginning of the service. As a result, it was not possible to contact all eligible referrals or follow-up with all patients successfully contacted at least once. With only 75% of referrals having a contact attempt and 60% of those contacted successfully having a follow-up attempt, there is a clear need for adequate staffing to reach all thoracic center patients referred to the service and to provide a continuum of cessation support. An immediate solution for the human resource limitations cessation specialists prioritized making a successful telephone call at least once to new patients referred from the thoracic center as current tobacco users, then focused on established patients, and those referred to the service as former smokers. The cessation support service added additional cessation specialists in 2013. We performed an intention to treat analysis with the regression model to address the missing data resulting from loss to follow-up and the inability to call every referral. Those indicating cessation at follow-up remained as quit, and those who were current or missing at follow-up were included as current users. The results were comparable to the regression results presented in this manuscript, suggesting that the missing data may not be biasing the results. The variability in follow-up time creates another operational limitation; increased time between contacts also increases the chance a participant may relapse or quit on their own, without the assistance of the cessation service.
Two important study design limitations exist with this paper. First, there was no control group to serve as a comparison for quit rates for this population since this service was designed to be a clinical program and to serve all eligible patients; however, the goal of this report is to describe participation rates and reported quit rates from this service. Second, quit status was indicated by a short-term measure (at least seven consecutive days) and was self-reported, without biochemical confirmation. Reporting at least seven consecutive days smoke-free does not demonstrate long-term quitting, but indicates that patients are attempting to quit smoking. This limitation may result in misclassification of the outcome since cancer patients may inaccurately self-report recent quit status.26 In the future, biochemical confirmation of self-reported quit status ought to be considered in order to gain insight into the accuracy of self-reported quit status in this particular population of thoracic clinic patients. Similarly, longer term quit rates should be considered as an outcome measure.
It is imperative to analyze the longer-term effectiveness of this program since the teachable moment at diagnosis or cancer screening may be a motivator for cessation efforts among thoracic center patients. To improve the cessation service, it is essential to understand program design elements that will increase patient interest in the service, enhance compliance with cessation specialists providing and patients participating in cessation counseling calls, and generate optimal quit rates in an efficient manner.
CONCLUSION
Thoracic center patients referred to and called by a cessation service demonstrated an interest in a telephone based cessation support service by participating in the telephone calls and having low opt-out rates. Self-reported quit rates increased at each contact. The results suggest that for current users at referral and first contact, being married and having a lower performance status are most likely to self-report having quit for at least 7 days at follow-up.
IMPLICATIONS FOR POLICY & PRACTICE.
Tobacco use is a significant public health problem.1
Thoracic center patients seen at an oncology center are amenable to a free, opt-out, telephone-based cessation support service, as identified by high participation rates and minimal patients refusing participation.
Thoracic cancer center patients participating in this cessation support service are attempting to quit using tobacco products.
Our study offers data other organizations may find useful while implementing a systematic way to identify tobacco-using patients as part of routine care and to improve cessation support services available to tobacco using patients.
Acknowledgments
We would like to acknowledge and thank Patricia Hysert, Robert Hysert, and Stephanie Segal for their commitment to providing cessation support; and Robert Reed, MPH, K. Michael Cummings, PhD, Michael Zevon, PhD, and James Marshall, PhD for their contributions to and support of the cessation service. We would also like to thank Linda Kahn, PhD at the University at Buffalo Primary Care Research Institute for support during the submission of this manuscript.
Funding: This work was supported in part by the Roswell Park Alliance Foundation and NCI R25CA113951.
Footnotes
Disclaimers: Dr. Mahoney serves as an expert witness for the plaintiffs in tobacco litigation cases. Dr. Mahoney is also on the speakers’ bureau of Pfizer, manufacturer of Chantix/varenicline.
References
- 1.National Cancer Institute. Tobacco Statistics Snapshot. [Accessed 6/12/2012, 2012];Cancer Topics. 2010 http://www.cancer.gov/cancertopics/tobacco/statisticssnapshot.
- 2.National Center for Chronic Disease P, Health Promotion Office on S, Health. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. Reports of the Surgeon General. [Google Scholar]
- 3.Berg CJ, Thomas AN, Mertens AC, et al. Correlates of continued smoking versus cessation among survivors of smoking-related cancers. Psycho-oncology. 2013;22(4):799–806. doi: 10.1002/pon.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gritz ER, Fingeret MC, Vidrine DJ, Lazev AB, Mehta NV, Reece GP. Successes and failures of the teachable moment: smoking cessation in cancer patients. Cancer. 2006;106(1):17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
- 5.McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: the context of cancer care and survivorship. Cancer control : journal of the Moffitt Cancer Center. 2003;10(4):325–333. doi: 10.1177/107327480301000407. [DOI] [PubMed] [Google Scholar]
- 6.Cox LS, Africano NL, Tercyak KP, Taylor KL. Nicotine dependence treatment for patients with cancer. Cancer. 2003;98(3):632–644. doi: 10.1002/cncr.11538. [DOI] [PubMed] [Google Scholar]
- 7.Cataldo JK, Dubey S, Prochaska JJ. Smoking cessation: an integral part of lung cancer treatment. Oncology. 2010;78(5–6):289–301. doi: 10.1159/000319937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver KE, Rowland JH, Augustson E, Atienza AA. Smoking concordance in lung and colorectal cancer patient-caregiver dyads and quality of life. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(2):239–248. doi: 10.1158/1055-9965.EPI-10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooley ME, Wang Q, Johnson BE, et al. Factors associated with smoking abstinence among smokers and recent-quitters with lung and head and neck cancer. Lung cancer (Amsterdam, Netherlands) 2012;76(2):144–149. doi: 10.1016/j.lungcan.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park ER, Japuntich S, Temel J, et al. A smoking cessation intervention for thoracic surgery and oncology clinics: a pilot trial. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6(6):1059–1065. doi: 10.1097/JTO.0b013e318215a4dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gritz ER, Carr CR, Rapkin DA, Chang C, Beumer J, Ward PH. A smoking cessation intervention for head and neck cancer patients: trial design, patient accrual, and characteristics. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1991;1(1):67–73. [PubMed] [Google Scholar]
- 12.Hopenhayn C, Christian WJ, Christian A, Studts J, Mullet T. Factors associated with smoking abstinence after diagnosis of early stage lung cancer. Lung cancer (Amsterdam, Netherlands) 2013;80(1):55–61. doi: 10.1016/j.lungcan.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Gosselin MH, Mahoney MC, Cummings KM, et al. Evaluation of an intervention to enhance the delivery of smoking cessation services to patients with cancer. Journal of cancer education : the official journal of the American Association for Cancer Education. 2011;26(3):577–582. doi: 10.1007/s13187-011-0221-3. [DOI] [PubMed] [Google Scholar]
- 14.Wakefield M, Olver I, Whitford H, Rosenfeld E. Motivational interviewing as a smoking cessation intervention for patients with cancer: randomized controlled trial. Nursing research. 2004;53(6):396–405. doi: 10.1097/00006199-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173(6):458–464. doi: 10.1001/jamainternmed.2013.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vidrine JI, Shete S, Li Y, et al. The Ask-Advise-Connect approach for smokers in a safety net healthcare system: a group-randomized trial. Am J Prev Med. 2013;45(6):737–741. doi: 10.1016/j.amepre.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren GW, Marshall JR, Cummings KM, et al. Automated tobacco assessment and cessation support for cancer patients. Cancer. 2014;120(4):562–569. doi: 10.1002/cncr.28440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobson Amato KA, Hyland A, Reed R, et al. Tobacco Cessation May Improve Lung Cancer Patient Survival. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015;10(7):1014–1019. doi: 10.1097/JTO.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amato K, Reid M, Bansal-Travers M, et al. Patient Cessation Activity after Automatic Referral to a Dedicated Cessation Support Service. Journal of Smoking Cessation. 2017:1–9. doi: 10.1017/jsc.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American journal of clinical oncology. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 21.IBM Corp. SPSS Statistics for Windows. Armonk, NY: Released 2013;Version 22.0. [Google Scholar]
- 22.Richter KP, Ellerbeck EF. It’s time to change the default for tobacco treatment. Addiction. 2015;110(3):381–386. doi: 10.1111/add.12734. [DOI] [PubMed] [Google Scholar]
- 23.Gritz ER, Toll BA, Warren GW. Tobacco use in the oncology setting: advancing clinical practice and research. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(1):3–9. doi: 10.1158/1055-9965.EPI-13-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. International journal of cancer Journal international du cancer. 2013;132(2):401–410. doi: 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- 25.Warren GW, Cummings KM. Tobacco and lung cancer: risks, trends, and outcomes in patients with cancer. American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting. 2013:359–364. doi: 10.14694/EdBook_AM.2013.33.359. [DOI] [PubMed] [Google Scholar]
- 26.Morales NA, Romano MA, Michael Cummings K, et al. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer causes & control : CCC. 2013;24(6):1223–1230. doi: 10.1007/s10552-013-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]