Abstract
Objective
To examine the associations between 25(OH)D and biomarkers of ovarian reserve in a large community-based sample of women.
Methods
In 2010–2016, women aged 30–44 without any known fertility problems were recruited from the Chapel Hill, North Carolina area for a prospective time-to-pregnancy cohort study. At enrollment 561 women provided a blood sample that was used to measure 25(OH)D, AMH, FSH, and inhibin-B. Unadjusted associations were estimated with Spearman correlation coefficients. Multivariable linear regression was used to estimate associations of 25(OH)D with ovarian reserve biomarkers, after adjusting for age, race, body mass index, smoking history, and recent use of hormonal birth control.
Results
The mean 25(OH)D was 36 ng/ml (SD=11 ng/ml). 25(OH)D was not correlated with AMH, FSH, or inhibin-B (all r<0.03). Multivariable results with continuous hormonal outcomes were also null. For dichotomous outcomes there was a tendency for insufficient 25(OH)D (<30 ng/ml) to be associated with low AMH (<0.7 ng/ml) (Odds ratio (95% Confidence Interval): 1.8 (0.9, 4)).
Conclusions
For the most part, 25(OH)D was not associated with ovarian reserve biomarkers in a group of women trying to become pregnant. We found some evidence that low 25(OH)D (<30 ng/ml) was associated with low AMH, but this should be confirmed in studies with a higher prevalence of low 25(OH)D.
Keywords: inhibins, follicle stimulating hormone, fertility, ovary, vitamin D, vitamin D deficiency
Introduction
Diminished ovarian reserve (a low quantity of oocytes) is associated with a poor response to ovarian stimulation among women seeking fertility treatment 1. Diminished ovarian reserve with subsequent early menopause not only limits a woman’s reproductive life span but has also been linked to increased risk of osteoporosis, cardiovascular disease, and all-cause mortality 2–6.
Ovarian senescence results in a decline in both the quantity7, 8 and quality9 of ovarian follicles and oocytes. The aging ovary produces smaller amounts of the granulosa cell hormones, Anti-Mullerian Hormone (AMH)10 and inhibin B11 which leads to a rise in early follicular phase follicle stimulating hormone (FSH).12 Previous studies have shown associations between AMH, FSH, and inhibin B and ovarian aging.13–18 AMH and inhibin B decline and FSH rises as a woman moves through the menopausal transition.19–21 The levels of AMH and FSH mark later stages of reproductive aging such as early perimenopause, late perimenopause, and postmenopause.13, 22–26 AMH is predictive of time to menopause. 27
Vitamin D, which is well-known for its role in maintaining bone health 28, has also been associated with reproductive health (reviewed in 29) and menstrual function. 30–32 In a previous study of women aged 35–44, lower plasma total 25-hydroxyvitamin D (25(OH)D) was associated with increased FSH.33
The gene for AMH contains a vitamin D response element, which suggests that vitamin D may regulate AMH expression 34, 35. Treatment of isolated follicles from rhesus macaques with active vitamin D increases AMH concentrations. 36 Human studies of AMH and vitamin D (as measured by 25(OH)D) have shown mixed results. One study reported a positive correlation 37, while others report no correlation 38–41. One intervention study reported that supplementation with vitamin D prevented seasonal fluctuations in AMH 42, while another study found no seasonal variation in AMH. 38 No previous studies have examined the associations between 25(OH)D and inhibin-B.
Our objective was to examine the association between 25(OH)D and three biomarkers of ovarian reserve (FSH, AMH, and inhibin-B) in a large community-based cohort of women attempting to conceive.
Methods
Study design
Time to Conceive was a prospective, time-to-pregnancy cohort study of biomarkers of ovarian reserve (2008–2016).43 Women who were intending to become pregnant were recruited through mass emails, introductory letters, and web and radio advertising. Eligible women had to be between the ages of 30 and 44 and trying to conceive naturally for 3 months or less. Attempt time was self-reported as the amount of time they had been “having regular intercourse without doing anything to prevent pregnancy”. Women were excluded if they reported a history of infertility, polycystic ovarian syndrome, endometriosis, a partner with infertility, or current breastfeeding. Women completed a self-administered questionnaire that included demographic data, reproductive history, contraceptive history, tobacco use, and other behaviors.
Women were instructed to schedule a study visit at the beginning of their first menses after study recruitment (cycle-day 2, 3, or 4). If they missed this window, women were asked to come in at the next menstrual cycle. At the study visit, women gave informed consent and provided a venous blood sample. In 2010, the study protocol was amended to add the collection of whole blood spots at the study visit, which were dried and stored frozen.
Ethical approval
All women provided informed consent, and all study activities were approved by the University of North Carolina IRB.
25(OH)D
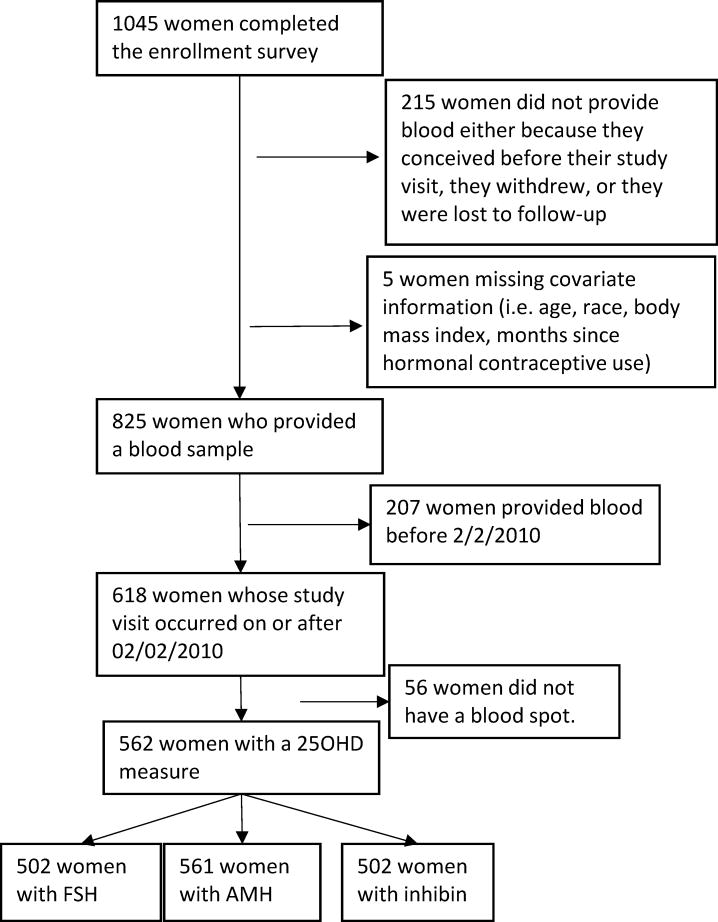
25(OH)D was extracted from 6mm punches from stored blood spots using previously described methods.44 25(OH)D3 and 25(OH)D2 were quantified through liquid chromatography-tandem mass spectrometry. 25(OH)D measured in dried blood spots shows good agreement with plasma measures.45 Blinded samples indistinguishable from test samples were also sent to the lab. Based on these samples, the intra-assay coefficient of variation was 6.3% and the inter-assay coefficient of variation was 7.7%. Of the 618 women enrolled in or after 2010, 562 women provided a blood-spot measure of vitamin D (Figure 1).
Figure 1.
Time to Conceive participants included in this analysis.
We examined 25(OH)D both as the continuous, quantified measure, and as a dichotomized category of “insufficient”, which was defined based on the Endocrine Society guidelines as <30 ng/ml.46 Finer categories of 25(OH)D were examined to determine the shape of the associations with ovarian reserve biomarkers. This model was compared with a linear fit to 25(OH)D using Akaike’s Information Criterion (AIC) and the model with the lowest AIC was chosen, which was the linear fit. It would be expected that biologic effects of 25(OH)D on ovarian reserve would accrue over time, and thus the average level of 25(OH)D over the whole previous year, averaged across seasonal fluctuations, might be associated with the measured ovarian reserve biomarkers. To investigate this, we also estimated the annual mean 25(OH)D level, according to the method of Sachs et al.47 Briefly, a model is estimated that predicts 25(OH)D based on the season of the blood draw. This model is used to predict each woman’s yearly average 25(OH)D level. To accomplish this, the difference between the woman’s date-specific predicted and actual 25(OH)D is calculated as her “residual”. The intercept of the predictive model, which is the overall annual mean across women, was added to the woman’s residual to obtain the woman’s estimated annual mean 25(OH)D.
AMH, FSH, Inhibin-B
AMH was measured in serum samples that were stored at −30°C until analysis. Samples were shipped frozen in a single batch to the University of Southern California Reproductive Endocrinology Laboratory. There they were assayed using sensitive and specific assays for FSH (Immulite analyzer, Siemens, Deerfield, IL), inhibin-B (ELISA, Ansh Labs, Webster, TX), and AMH (Ultrasensitive AMH ELISA, Ansh). Interassay coefficients of variation ranged from 4–5% for FSH, 5–8% for inhibin-B, and 9–11% for AMH. Values below the limit of detection, 0.078 ng/ml for AMH (N=10) and 9 pg/ml for inhibin-B (N=42) were replaced with the limit of detection divided by the square root of two.48
Of the 567 women with a 25(OH)D measure, 566 also had an AMH level. Samples from fifty-nine women who enrolled near the end of the study were sent to the lab for quantification of AMH, but the other hormones were not measured. Thus, of the 567 women, 507 had FSH, and 507 had inhibin-B.
Covariates
Variables examined as potential confounders were selected based on previous studies, 49, 50, and included age at the time of the blood draw, race, body mass index, smoking and number of months since the participant had used an estrogen-containing hormonal contraception (categorized as one month or less, two months, three months, or more than three months or never). The multivariate analyses were also run without controlling for race, and the results were not substantially different. Five women were excluded from the multivariable analyses because they were missing covariate information.
Statistical analysis
We calculated means and standard deviations or, where appropriate, geometric means and geometric ranges. The geometric range is bounded by two quantities: the geometric mean divided by the geometric standard deviation, and the geometric mean multiplied by the geometric standard deviation51. The geometric range will contain approximately two-thirds of the data. This is analogous to the standard confidence interval calculation.
We calculated Spearman correlation coefficients among untransformed 25(OH)D, AMH, FSH, and inhibin-B with their associated Fisher 95% confidence intervals. We used multivariable linear regression to estimate the association between 25(OH)D (not log-transformed) and each hormone measure independently while adjusting for covariates. AMH and FSH were natural log transformed to achieve normality of the regression residuals. Inhibin-B did not need log-transformation. Additionally, outliers for some of the hormonal measurements influenced model fit. We addressed this with a sensitivity analysis in which values below the limit of detection (0.078ng/ml) for AMH were excluded from the linear regression model (N=10), values less than 1.5 ng/ml were excluded from the FSH model (N=9), and values >200pg/ml were excluded from the inhibin-B linear regression (N=6) (Supplemental Table 1). In the main results, these values were included in the models. We carried out an additional sensitivity analysis in which, instead of being excluded, values of FSH under 1.5ng/ml were set to 1.5, values of AMH less than 0.078ng/ml were set to 0.078, and inhibin-B values above 200pg/ml were truncated to 200. These results are also shown in Supplemental Table 1.
We created dichotomous variables for AMH and FSH. For AMH, “low” was less than 0.7 ng/ml 43 and “high” was greater than 8.5 ng/ml (the upper 10th percentile of the cohort). For FSH, “high” was greater than 10 ng/ml. 52 Outliers that were excluded from the linear regression were included in the analyses of these dichotomous variables. We used multivariable logistic regression models to estimate the associations between 25(OH)D and high FSH (compared with normal FSH), between 25(OH)D and high AMH (compared with normal AMH), and between 25(OH)D and low AMH (compared with normal AMH), while adjusting for covariates.
All analyses were completed with SAS software, version 9.4.
Results
The median 25(OH)D was 35 ng/ml (IQR: 29, 41). Insufficient levels (<30ng/ml) were seen in 30% of the women, further deficiency was infrequent with 4% at <20 ng/ml and one woman <10ng/ml. Levels tended to be lower in women ages 31–40, in African-American women, and in women with high BMI. (Table 1). Recent users of hormonal birth control had higher 25(OH)D.
Table 1.
Ovarian reserve biomarkers and 25(OH)D, stratified by participant characteristics.
| 25(OH)D (ng/ml) | AMH (ng/ml) | Inhibin-B (pg/ml) | FSH (mIU/ml) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Variable | N | Geometric Mean |
Geometric Range a |
N | Geometric Mean |
Geometric Range a |
N | Mean | Standard Deviation |
N | Geometric Mean |
Geometric Range a |
| Age | ||||||||||||
| 29 – 30 | 112 | 35.2 | (27, 46) | 112 | 4.0 | (1.9, 8.4) | 104 | 69.6 | 38.5 | 104 | 6.0 | (3.8, 9.6) |
| 31 – 32 | 157 | 34.2 | (24, 48) | 157 | 3.1 | (1.2, 8.1) | 144 | 88.2 | 47.3 | 144 | 6.5 | (4.3, 9.8) |
| 33 – 35 | 164 | 33.5 | (26, 44) | 164 | 2.6 | (0.93, 7.3) | 140 | 73.5 | 45.7 | 140 | 6.5 | (3.8, 11) |
| 36 – 40 | 106 | 33.9 | (24, 47) | 106 | 1.6 | (0.53, 4.8) | 93 | 70.0 | 42.3 | 93 | 6.5 | (3.8, 11) |
| >40 | 22 | 36.1 | (26, 51) | 22 | 0.6 | (0.12, 3.1) | 21 | 59.0 | 51 | 21 | 8.8 | (5.5, 14) |
| BMI | ||||||||||||
| <20 | 75 | 35.5 | (27, 46) | 75 | 2.3 | (0.72, 7.4) | 66 | 90.3 | 57.4 | 66 | 7.0 | (4.4, 11) |
| 20 – 25 | 292 | 35.8 | (28, 47) | 292 | 2.7 | (0.93, 7.8) | 260 | 73.0 | 42.9 | 260 | 6.3 | (3.7, 11) |
| >25 – 30 | 110 | 32.4 | (23, 45) | 110 | 2.9 | (1.1, 7.8) | 99 | 74.9 | 42.4 | 99 | 6.4 | (4.6, 9.0) |
| >30 | 84 | 30.4 | (22, 43) | 84 | 2.0 | (0.65, 6.2) | 77 | 61.8 | 36.4 | 77 | 6.7 | (3.9, 11) |
| Race | ||||||||||||
| African-American | 49 | 26.0 | (17, 39) | 49 | 2.7 | (1, 7.3) | 45 | 75.8 | 46.8 | 45 | 6.2 | (3.1, 12) |
| White | 438 | 36.2 | (28, 47) | 438 | 2.5 | (0.86, 7.3) | 388 | 73.2 | 44.5 | 388 | 6.5 | (4.1, 10) |
| Other | 74 | 29.6 | (21, 41) | 74 | 2.8 | (0.93, 8.4) | 69 | 77.1 | 44.1 | 69 | 6.6 | (4.1, 10) |
| Smoking status | ||||||||||||
| Never | 430 | 34.1 | (24, 48) | 430 | 2.6 | (0.90, 7.5) | 383 | 76.5 | 45.6 | 383 | 6.5 | (4.1, 10) |
| Former | 123 | 34.5 | (27, 45) | 123 | 2.5 | (0.83, 7.5) | 111 | 66.7 | 40.5 | 111 | 6.2 | (3.9, 10) |
| Current | 8 | 32.9 | (25, 43) | 8 | 1.6 | (0.59, 4.3) | 8 | 55.1 | 38.4 | 8 | 8.1 | (4.8, 14) |
| Months since hormonal estrogen use | ||||||||||||
| One or less | 32 | 40.1 | (31, 52) | 32 | 2.7 | (1.1, 6.5) | 27 | 62.2 | 69.2 | 27 | 5.2 | (2.6, 10) |
| Two | 20 | 37.4 | (29, 49) | 20 | 1.4 | (0.4, 4.9) | 19 | 65.2 | 42.8 | 19 | 7.4 | (5.3, 10) |
| Three | 41 | 37.3 | (29, 48) | 41 | 2.5 | (0.76, 8.3) | 37 | 72.5 | 45.7 | 37 | 6.9 | (4.3, 11) |
| More than three | 468 | 33.5 | (24, 47) | 468 | 2.6 | (0.90, 7.5) | 419 | 75.2 | 42.6 | 419 | 6.5 | (4.1, 10) |
The geometric range is defined as: (geometric mean/geometric standard deviation, geometric mean x geometric standard deviation). Two-thirds of all observations lie within this range.
25(OH)D was not correlated with AMH, FSH, or inhibin-B (all correlations <0.05, Table 2). After adjustment for age and other covariates, women who were insufficient in 25(OH)D had 17% lower AMH levels than women who were sufficient, although this was not statistically significant. Neither 25(OH)D nor the estimated yearly average 25(OH)D was associated with the other continuous measures of any of the hormones (Table 3). Supplemental Table 1 shows the same models as in Table 3, but with the outlying values excluded or included at the range (see Table, Supplemental Digital Content 1). Season was not hypothesized to be an independent predictor of ovarian reserve and thus was not considered a potential confounder in this analysis, however, none of the associations were altered with adjustment for season (see Table, Supplemental Digital Content 2).
Table 2.
Spearman correlation coefficients between 25(OH)D and ovarian reserve biomarkers
| Mean (SD) | 25(OH)D | AMH | Inhibin-B | |
|---|---|---|---|---|
| 25(OH)D | 36 (11) | -- | -- | -- |
| AMH | 4.0 (3.6) | −0.002 (−0.08, 0.08) | -- | -- |
| Inhibin-B | 74 (45) | 0.02 (−0.07, 0.10) | 0.24 (0.16, 0.32)* | -- |
| FSH | 7.1 (3.1) | 0.01 (−0.08, 0.10) | −0.36 (−0.43, −0.28)* | 0.005 (−0.08, 0.09) |
p<0.0001
Table 3.
Adjusted associations between 25(OH)D and ovarian reserve biomarkers.a
| AMH, b Percent Difference (CI) | FSH, b Percent Difference (CI) | Inhibin-B c, Beta pg/ml (CI) | |
|---|---|---|---|
|
| |||
| N=561 | N=502 | N=502 | |
| 25(OH)D, per 10ng/ml decrease | −3.3 (−12, 4.8) | 0.53 (−3.8, 4.7) | −1.0 (−3.0, 5.1) |
| Annual mean 25(OH)D, per 10ng/ml decrease | −3.0 (−12, 5.1) | 0.54 (−3.8, 4.7) | −1.2 (−2.8, 5.2) |
| Insufficient 25(OH)Dd | −17 (−31, 0.2) | 8.7 (−0.86, 19) | 3.2 (−5.5, 12) |
Adjusted for age, race, smoking history, BMI and recent use of hormonal birth control
Natural log-transformed.
Inhibin-B is not log-transformed.
25(OH)D less than 30 ng/ml, the Endocrine Society guideline for sufficiency, compared with at least 30 ng/ml
When examined categorically, there was a tendency for decreasing 25(OH)D to be associated with decreased odds of high FSH (OR(CI): 0.81 (0.61, 1.1)), but this was not statistically significant. In contrast, women with vitamin D deficiency tended to have increased odds of high FSH, but the confidence interval was wide (Table 4). Insufficient 25(OH)D was associated with increased odds of low AMH (OR:1.8 (0.91, 3.6)), but the confidence interval was wide (Table 4). We also examined an alternative cutpoint for “high” AMH, 7.75 ng/ml (rather than 8.5ng/ml).53 When high AMH was defined as > 7.75 ng/ml (rather than >8.5) 53, the estimate was unchanged, OR (CI): 1.8 (0.9, 3.6).
Table 4.
Adjusted associations between 25(OH)D and dichotomous measures of ovarian reserve biomarkers.a
| AMH | FSH | ||
|---|---|---|---|
|
| |||
| Low (<0.7 ng/ml) (N=561) OR (CI) | High (>8.5 ng/ml) (N=561) OR (CI) | High (>10 ng/ml) (N=502) OR (CI) | |
| 25(OH)D, per 10ng/ml decrease | 1.0 (0.77, 1.4) | 0.97 (0.73, 1.3) | 0.81 (0.61, 1.1) |
| Annual mean 25(OH)D, per 10ng/ml decrease | 1.0 (0.76, 1.4) | 0.97 (0.73, 1.3) | 0.82 (0.62, 1.1) |
| Insufficient 25(OH)Db | 1.8 (0.91, 3.6) | 0.71 (0.36, 1.4) | 1.0 (0.52, 1.9) |
| Insufficient 25(OH)Dc | 1.5 (0.73, 2.9) | 0.81 (0.42, 1.6) | 0.90 (0.45, 1.8) |
All regression models adjusted for age, race, smoking history, BMI and recent use of hormonal birth control
Measured 25(OH)D less than 30 ng/ml, the Endocrine Society guideline for sufficiency, compared with at least 30 ng/ml
Annual mean 25(OH)D less than 30 ng/ml, the Endocrine Society guideline for sufficiency, compared with at least 30 ng/ml
The associations between decreasing 25(OH)D and high FSH and insufficient 25(OH)D and low AMH were unchanged with adjustment for season; for FSH, OR(CI): 0.79 (0.59, 1.1), for AMH (1.8 (0.91, 3.6). Similarly, adjustment for physical activity (which was only available for a subset of the population, N=473) also did not alter results, high FSH, 0.79 (0.58, 1.1) and low AMH, 1.7 (0.82, 3.4).
A previous study reported that the association between 25(OH)D and AMH was limited to women who were at least 40 years of age37. In our study the estimate for insufficient 25(OH)D in association with low AMH was stronger in this group, OR (CI): 5.2 (0.49, 55), but there were only 38 women in this age category.
Discussion
In this cohort of women of late reproductive age, there was little evidence of association between vitamin D and biomarkers of ovarian reserve. When the measures were categorized, there was some suggestion that insufficient 25(OH)D was associated with low AMH, but the confidence interval was broad and associations were not consistent across all analyses. There were no associations between 25(OH)D and inhibin-B.
AMH declines and FSH rises as a woman moves through the menopausal transition,19–21 and these hormones mark the later stages of reproductive aging such as early perimenopause, late perimenopause, and postmenopause.13, 22–26 The literature is mixed regarding vitamin D and ovarian reserve biomarkers. In one study, increasing vitamin D was associated with increasing AMH37 and in another study with decreasing FSH.33 However other observational studies report no association.38–41 The studies reporting no association are difficult to interpret as some do not report point estimates (only r-square or p-values) and it is not clear that age and BMI were accounted for in each analysis presented.38–41 All four of these null studies sampled women from fertility clinic populations, while the studies reporting associations33, 37 each sampled from a broader target population.
An intervention study of 33 women, reported that AMH varied across seasons, and that supplementation with cholecalciferol (vitamin D3) was associated with apparent suppression of seasonal AMH changes.42 However, seasonal fluctuations were not found in a subsequent observational study.38 In another intervention study, supplementation with active vitamin D (1,25 dihydroxyvitamin D) decreased AMH levels among women with PCOS (who typically have elevated AMH levels), but there was no effect among normally ovulatory women.54
It is possible that there is racial or ethnic effect modification of the association between vitamin D and AMH or FSH. In two of the studies that found an association between vitamin D and either AMH or FSH the majorities of the study populations (~57%) were African-American33, 37, while most of the studies that did not detect an association included primarily white women, including the results reported in the current analysis. One explanation for this could be that African-Americans have lower 25(OH)D, and it is possible that the association with AMH is strongest at low levels of 25(OH)D. Though one small study that reported no association included women with 25(OH)D levels as low as those seen in the two previously referenced studies, that study only reported the results as unadjusted correlations.40 Our analysis is the largest in the literature to date, and it was drawn from a community-based sample, but very few of the women were vitamin D deficient.
Conclusions
In this cohort of late reproductive-aged women, we did not find strong evidence of associations between 25(OH)D and biomarkers of ovarian reserve. We found some evidence that low 25(OH)D (<30 ng/ml) was associated with low AMH, but there were few women who had both of these characteristics.
Supplementary Material
Acknowledgments
Financial support: This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH) under award numbers R00HD079659 and R01HD067683. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was also supported, in part, by the intramural research program of the NIEHS, NIH (Z01ES044003-39).
Footnotes
Conflict of interest: None
Disclaimers: None
Author’s roles: AMZJ conceived of the study, designed and interpreted the analysis, and wrote the first draft of the manuscript. AJW, CRW, and DDB provided input on the design and interpretation of the study and edited the manuscript. AZS designed and implemented the parent study, Time to Conceive, and provided input on the current study design, analysis and interpretation, and edited the manuscript.
References
- 1.Nikolaou D, Templeton A. Early ovarian ageing: a hypothesis. Detection and clinical relevance. Hum Reprod. 2003;18(6):1137–9. doi: 10.1093/humrep/deg245. [DOI] [PubMed] [Google Scholar]
- 2.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13(2):265–79. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007;14(3 Pt 2):567–71. doi: 10.1097/gme.0b013e31804c793d. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen BK, Heuch I, Kvale G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol. 2003;157(10):923–9. doi: 10.1093/aje/kwg066. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. Journal of clinical epidemiology. 1999;52(4):303–7. doi: 10.1016/s0895-4356(98)00170-x. [DOI] [PubMed] [Google Scholar]
- 6.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005;162(11):1089–97. doi: 10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- 7.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342–6. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 8.Gougeon A, Chainy GB. Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil. 1987;81(2):433–42. doi: 10.1530/jrf.0.0810433. [DOI] [PubMed] [Google Scholar]
- 9.Henderson SA, Edwards RG. Chiasma frequency and maternal age in mammals. Nature. 1968;218(5136):22–8. doi: 10.1038/218022a0. [DOI] [PubMed] [Google Scholar]
- 10.Lee MM, Donahoe PK. Mullerian inhibiting substance: a gonadal hormone with multiple functions. Endocr Rev. 1993;14(2):152–64. doi: 10.1210/edrv-14-2-152. [DOI] [PubMed] [Google Scholar]
- 11.Roberts VJ, Barth S, el-Roeiy A, Yen SS. Expression of inhibin/activin subunits and follistatin messenger ribonucleic acids and proteins in ovarian follicles and the corpus luteum during the human menstrual cycle. J Clin Endocrinol Metab. 1993;77(5):1402–10. doi: 10.1210/jcem.77.5.8077341. [DOI] [PubMed] [Google Scholar]
- 12.Santoro N, Isaac B, Neal-Perry G, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88(11):5502–9. doi: 10.1210/jc.2002-021839. [DOI] [PubMed] [Google Scholar]
- 13.Backer LC, Rubin CS, Marcus M, Kieszak SM, Schober SE. Serum follicle-stimulating hormone and luteinizing hormone levels in women aged 35–60 in the U.S. population: the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994) Menopause. 1999;6(1):29–35. [PubMed] [Google Scholar]
- 14.Broekmans FJ, Scheffer GJ, Bancsi LF, Dorland M, Blankenstein MA, te Velde ER. Ovarian reserve tests in infertility practice and normal fertile women. Maturitas. 1998;30(2):205–14. doi: 10.1016/s0378-5122(98)00075-9. [DOI] [PubMed] [Google Scholar]
- 15.Kim YK, Wasser SK, Fujimoto VY, Klein NA, Moore DE, Soules MR. Utility of follicle stimulating hormone (FSH), luteinizing hormone (LH), oestradiol and FSH:LH ratio in predicting reproductive age in normal women. Hum Reprod. 1997;12(6):1152–5. doi: 10.1093/humrep/12.6.1152. [DOI] [PubMed] [Google Scholar]
- 16.Ng EH, Yeung WS, Fong DY, Ho PC. Effects of age on hormonal and ultrasound markers of ovarian reserve in Chinese women with proven fertility. Hum Reprod. 2003;18(10):2169–74. doi: 10.1093/humrep/deg404. [DOI] [PubMed] [Google Scholar]
- 17.Tufan E, Elter K, Durmusoglu F. Assessment of reproductive ageing patterns by hormonal and ultrasonographic ovarian reserve tests. Hum Reprod. 2004;19(11):2484–9. doi: 10.1093/humrep/deh448. [DOI] [PubMed] [Google Scholar]
- 18.van Rooij IA, Broekmans FJ, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979–87. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 19.de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77(2):357–62. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 20.Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351–8. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- 21.Ferrell RJ, O’Connor KA, Holman DJ, et al. Monitoring reproductive aging in a 5-year prospective study: aggregate and individual changes in luteinizing hormone and follicle-stimulating hormone with age. Menopause. 2007;14(1):29–37. doi: 10.1097/01.gme.0000227859.50473.20. [DOI] [PubMed] [Google Scholar]
- 22.Burger HG, Cahir N, Robertson DM, et al. Serum inhibins A and B fall differentially as FSH rises in perimenopausal women. Clin Endocrinol (Oxf) 1998;48(6):809–13. doi: 10.1046/j.1365-2265.1998.00482.x. [DOI] [PubMed] [Google Scholar]
- 23.Gracia CR, Sammel MD, Freeman EW, et al. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12(2):128–35. doi: 10.1097/00042192-200512020-00005. [DOI] [PubMed] [Google Scholar]
- 24.Lenton EA, Sexton L, Lee S, Cooke ID. Progressive changes in LH and FSH and LH: FSH ratio in women throughout reproductive life. Maturitas. 1988;10(1):35–43. doi: 10.1016/0378-5122(88)90129-6. [DOI] [PubMed] [Google Scholar]
- 25.Santoro N, Brockwell S, Johnston J, et al. Helping midlife women predict the onset of the final menses: SWAN, the Study of Women’s Health Across the Nation. Menopause. 2007;14(3 Pt 1):415–24. doi: 10.1097/gme.0b013e31802cc289. [DOI] [PubMed] [Google Scholar]
- 26.van Rooij IA, Tonkelaar I, Broekmans FJ, et al. Anti-mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6 Pt 1):601–6. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- 27.Depmann M, Broer SL, van der Schouw YT, et al. Can we predict age at natural menopause using ovarian reserve tests or mother’s age at menopause? A systematic literature review. Menopause. 2016;23(2):224–32. doi: 10.1097/GME.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 28.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 29.Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol. 2012;166(5):765–78. doi: 10.1530/EJE-11-0984. [DOI] [PubMed] [Google Scholar]
- 30.Jukic AM, Steiner AZ, Baird DD. Lower plasma 25-hydroxyvitamin D is associated with irregular menstrual cycles in a cross-sectional study. Reprod Biol Endocrinol. 2015;13:20. doi: 10.1186/s12958-015-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jukic AM, Upson K, Harmon QE, Baird DD. Increasing serum 25-hydroxyvitamin D is associated with reduced odds of long menstrual cycles in a cross-sectional study of African American women. Fertil Steril. 2016 doi: 10.1016/j.fertnstert.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jukic AM, Wilcox AJ, McConnaughey DR, Weinberg CR, Steiner AZ. Lower 25-hydroxyvitamin D is associated with long menstrual cycles in a prospective cohort study. Epidemiology. doi: 10.1097/EDE.0000000000000804. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jukic AM, Steiner AZ, Baird DD. Association between serum 25-hydroxyvitamin D and ovarian reserve in premenopausal women. Menopause. 2015;22(3):312–6. doi: 10.1097/GME.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merhi Z, Doswell A, Krebs K, Cipolla M. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab [Article] 2014;99(6):E1137–E45. doi: 10.1210/jc.2013-4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malloy PJ, Peng L, Wang J, Feldman D. Interaction of the vitamin D receptor with a vitamin D response element in the Mullerian-inhibiting substance (MIS) promoter: regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinology. 2009;150(4):1580–7. doi: 10.1210/en.2008-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Hennebold JD, Seifer DB. Direct vitamin D3 actions on rhesus macaque follicles in three-dimensional culture: assessment of follicle survival, growth, steroid, and antimullerian hormone production. Fertil Steril. 2016;106(7):1815–20. e1. doi: 10.1016/j.fertnstert.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merhi ZO, Seifer DB, Weedon J, et al. Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductive-aged women: Women’s Interagency HIV Study. Fertil Steril. 2012;98(1):228–34. doi: 10.1016/j.fertnstert.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearce K, Gleeson K, Tremellen K. Serum anti-Mullerian hormone production is not correlated with seasonal fluctuations of vitamin D status in ovulatory or PCOS women. Hum Reprod. 2015;30(9):2171–7. doi: 10.1093/humrep/dev167. [DOI] [PubMed] [Google Scholar]
- 39.Drakopoulos P, van de Vijver A, Schutyser V, et al. The effect of serum vitamin D levels on ovarian reserve markers: a prospective cross-sectional study. Hum Reprod. 2017;32(1):208–14. doi: 10.1093/humrep/dew304. [DOI] [PubMed] [Google Scholar]
- 40.Chang EM, Kim YS, Won HJ, Yoon TK, Lee WS. Association between sex steroids, ovarian reserve, and vitamin D levels in healthy nonobese women. J Clin Endocrinol Metab. 2014;99(7):2526–32. doi: 10.1210/jc.2013-3873. [DOI] [PubMed] [Google Scholar]
- 41.Neville G, Martyn F, Kilbane M, et al. Vitamin D status and fertility outcomes during winter among couples undergoing in vitro fertilization/intracytoplasmic sperm injection. Int J Gynaecol Obstet. 2016;135(2):172–6. doi: 10.1016/j.ijgo.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS. The level of serum anti-Mullerian hormone correlates with vitamin D status in men and women but not in boys. J Clin Endocrinol Metab. 2012;97(7):2450–5. doi: 10.1210/jc.2012-1213. [DOI] [PubMed] [Google Scholar]
- 43.Steiner AZ, Herring AH, Kesner JS, et al. Antimullerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet Gynecol. 2011;117(4):798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larkin EK, Gebretsadik T, Koestner N, et al. Agreement of blood spot card measurements of vitamin D levels with serum, whole blood specimen types and a dietary recall instrument. PloS one. 2011;6(1):e16602. doi: 10.1371/journal.pone.0016602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heath AK, Williamson EJ, Ebeling PR, Kvaskoff D, Eyles DW, English DR. Measurements of 25-hydroxyvitamin D concentrations in archived dried blood spots are reliable and accurately reflect those in plasma. J Clin Endocrinol Metab. 2014;99(9):3319–24. doi: 10.1210/jc.2014-1269. [DOI] [PubMed] [Google Scholar]
- 46.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 47.Sachs MC, Shoben A, Levin GP, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97(6):1243–51. doi: 10.3945/ajcn.112.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- 49.Bleil ME, Gregorich SE, Adler NE, Sternfeld B, Rosen MP, Cedars MI. Race/ethnic disparities in reproductive age: an examination of ovarian reserve estimates across four race/ethnic groups of healthy, regularly cycling women. Fertil Steril. 2014;101(1):199–207. doi: 10.1016/j.fertnstert.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dolleman M, Verschuren WM, Eijkemans MJ, et al. Reproductive and lifestyle determinants of anti-Mullerian hormone in a large population-based study. J Clin Endocrinol Metab. 2013;98(5):2106–15. doi: 10.1210/jc.2012-3995. [DOI] [PubMed] [Google Scholar]
- 51.Kirkwood T. Geometric means and measures of dispersion. Biometrics. 1979;35:908–9. [Google Scholar]
- 52.Jain T, Soules MR, Collins JA. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 2004;82(1):180–5. doi: 10.1016/j.fertnstert.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 53.Quinn MM, Kao CN, Ahmad AK, et al. Age-stratified thresholds of anti-Mullerian hormone improve prediction of polycystic ovary syndrome over a population-based threshold. Clin Endocrinol (Oxf) 2017 doi: 10.1111/cen.13415. [DOI] [PubMed] [Google Scholar]
- 54.Irani M, Minkoff H, Seifer DB, Merhi Z. Vitamin D increases serum levels of the soluble receptor for advanced glycation end products in women with PCOS. J Clin Endocrinol Metab. 2014;99(5):E886–90. doi: 10.1210/jc.2013-4374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.