Abstract
Objective
This study investigated changes in fat free mass (FFM) and skeletal muscle (SM) five years after surgery in participants from the Longitudinal Assessment of Bariatric Surgery-2 trial.
Methods
A 3-compartment model assessed FFM, and whole-body magnetic resonance imaging (MRI) quantified SM mass prior to surgery (T0), 1 year (T1), 2 years (T2), and 5 years (T5) postoperatively in 93 patients (85% female, 68% Caucasian, age 44.2 ± 11.6 yrs) who underwent gastric bypass (RYGB), sleeve gastrectomy (SLEEVE), or adjustable gastric band (BAND). Repeated-measures mixed models were used to analyze the data.
Results
Significant weight loss occurred across all surgical groups in females from T0 to T1. FFM loss from T0–T1 was greater after RYGB (mean±SE:−6.9±0.6 kg) than BAND (−3.5±1.4 kg; p<0.05). RYGB females continued to lose FFM (−3.3±0.7 kg; p<0.001) from T1 to T5. A subset of RYGB males and females with MRI-measured SM showed similar initial FFM loss while maintaining FFM and SM from T1 to T5.
Conclusions
Between 1 and 5 years following common bariatric procedures, FFM and skeletal muscle are maintained or decrease minimally. The changes observed in FFM and muscle during the follow-up phase may be consistent with aging.
Keywords: bariatric surgery, fat free mass, skeletal muscle
INTRODUCTION
For people with severe obesity, bariatric surgery is now considered the most effective method of achieving significant weight loss and maintaining a reduced weight over time. The Utah Obesity Study reported body weight to be 35% lower two years after gastric bypass surgery, with average percent weight of 28% and 27% below pre-surgery weight at six and 12 years, respectively (1). This rapid and sustained weight loss resulting from what are now termed “metabolic” surgical procedures is accompanied (and even preceded) by a near-immediate improvement in metabolic co-morbidities such as type 2 diabetes mellitus (2), with sustained benefits in a majority of patients at least 8 years after surgery (3).
Non-surgical intervention studies indicate that extreme calorie restriction, lack of exercise, and losing weight rapidly result in greater relative reductions of fat-free mass (FFM) during weight loss (4). A similar observation was made in surgical interventions, where procedures that produce a greater magnitude of weight loss also result in a higher percentage loss of FFM (4). Although losing some FFM is expected with weight loss, excessive FFM loss is undesirable as it negatively impacts regulation of metabolic rate, the integrity of skeletal muscle, and preservation of functional capacity in aging (5).
Recent studies have assessed FFM in bariatric surgery patients soon after weight loss compared to weight- and age-matched controls and found the same or even greater FFM than expected within their BMI category (6, 7). While these findings appear promising for bariatric surgery patients, it is important to note that most of the studies of FFM after surgically-induced weight loss do not extend beyond the initial weight loss period (1–2 years) (4, 8). Further, many of these short-term studies rely on body composition assessment methods that have not been sufficiently validated in patients with severe obesity or in those who have lost significant weight. Key assumptions such as tissue hydration (9), for example, differ significantly in these patients when compared to normal-weight controls (10, 11), and may yield invalid results.
Essential to our understanding of both short- and long-term effects of bariatric surgery on FFM are longitudinal trials with repeated measures of body composition using reliable methods in these patients. A three-compartment (3C) model takes into account hydration-associated variance within patients with severe obesity before and after surgery, and is considered a gold standard for FFM assessment in this population (12). Skeletal muscle, a primary component of FFM, is critical to mobility and metabolic health for post-surgery patients. While dual-energy X-ray absorptiometry (DXA) estimates are well-accepted for assessing body fat, accuracy may be reduced in patients with severe obesity due to greater trunk thickness (13). Whole-body magnetic resonance imaging (MRI) is considered a gold standard for skeletal muscle quantification (14, 15).
The purpose of this study was to assess FFM using a 3-compartment model and SM using whole-body MRI in bariatric surgery patients 1, 2, and 5 years post-operatively to investigate how the lean tissue component of body weight changes with extreme weight loss, subsequent maintenance, or weight regain.
METHODS
Surgery participants
Between November 2006 and February 2009, 105 bariatric surgery patients participating in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) trial, which has been described previously (16, 17) also enrolled in an ancillary body composition study (18, 19) conducted at two of the LABS-2 collection sites: Weill Cornell Medical College (WCMC, n=53) and University of Pittsburgh Medical Center (UPMC, n=52). As reported previously (18), 100 participants proceeded to have one of four bariatric surgery procedures: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SLEEVE), adjustable gastric band (BAND); and biliopancreatic diversion with duodenal switch (SWITCH). Seven patients from the WCMC site (two males who underwent SLEEVE and one who underwent BAND, and four females who underwent SWITCH) were excluded from current analyses due to insufficient numbers to conduct comparisons by surgical procedure. LABS-2 is a prospective, observational cohort trial that did not introduce any dietary or exercise intervention beyond the standard pre- and postoperative recommendations given patients at its surgical centers. Patients self-selected surgical procedure with a LABS-certified surgeon (16). All studies were approved by the Institutional Review Boards of St. Luke’s-Roosevelt Hospital and Columbia University (where body composition of Weill Cornell patients was assessed) and the University of Pittsburgh, and written informed consent was obtained.
Body composition measures
Body weight (Weight Tronix, New York, NY; and Scale-Tronix, Wheaton, IL), height (Holtain; Crosswell, Wales-New York), and body density (Bod Pod; (Cosmed, Chicago, IL; software version 2.3) measurements were obtained. Total body water was assessed by deuterium dilution, where a ~0.1g/kg oral dose of D2O was ingested immediately following a venous blood sample drawn from an antecubital vein. A second blood sample was drawn after 3 hours. A three-compartment (3C) model was used to estimate fat mass: (20) fat (kg) = 2.122 × (BW/d) – 0.779 × TBW – 1.356 × BW, where BW is the body weight in kilograms, d is the body density derived from BodPod, and TBW is the total body water in kilograms. Fat free mass was derived as the difference between body weight and fat mass.
Skeletal muscle mass was measured using a whole-body multi-slice MRI protocol, as previously described (21, 22). A subset of participants whose body size was fully accommodated within the MRI field-of-view, from the New York and Pittsburgh sites were placed on a 1.5 T MRI scanner (GE, 6X Horizon, Milwaukee, WI) table and scanned with arms above their heads. Approximately 40 axial images with 10 mm thickness and 40 mm interslice gap were acquired across the entire body. SliceOmatic image analysis software (Tomovision, Montreal, CA) was used by a single image analyst at the New York Obesity Nutrition Research Center to tag skeletal muscle on each image. Skeletal muscle volume was converted to mass using an assumed density of 1.04 kg/L (23). The coefficient of variation for skeletal muscle obtained from a repeat blinded analysis of the same whole-body MRI by a single analyst in our lab is 2.4%.
All body composition assessments were conducted an average of 1.3 weeks prior to surgery (T0), and repeated at follow-up visits that were on average 1.1 years (T1), 2.1 years (T2), and 5.1 years (T5) after surgery.
Statistical analysis
Descriptive statistics were calculated for all variables. For continuous variables, number, mean, and standard deviation are reported and for discrete variables number and percentage are reported. The dependent variables, weight, FFM, FFM/weight, SM, SM/weight, and SM/FFM were analyzed using a mixed model analysis of variance which utilized all available data in the analysis and allows for subjects with missing data to be included in the analysis. The SAS procedure, PROC MIXED, was used to perform all calculations. Due to the distribution of gender and surgery type, two analyses were performed. The first analysis was restricted to females and considered surgery type and observation as independent variables. For this analysis, the mixed model included adjustment for surgery type, observation time, and the interaction between surgery type and observation time as fixed effects. Subjects were indicated as a random effect with an unstructured covariance matrix. The second analysis was restricted to subjects with RYGB surgery and included males and females. For this analysis, the mixed model included adjustment for gender, observation, and the interaction between gender and observation time as fixed effects. Subjects were indicated as a random effect with an unstructured covariance matrix. The LSMEANS option was used to estimate the mean and standard error of the mean at each observation for each surgery type in the first analysis, or for each gender in the second analysis and are reported as adjusted means and standard errors of the mean. The ESTIMATE statement was used to estimate the change between observations in the dependent variable for each level of the surgery type, first analysis, or gender, second analysis and to compare the changes between surgery type or gender. To assess the effect of age or total body water on these relationships, all models were repeated with either baseline age or total body water included as a covariate. All statistical calculations were performed using the SAS statistical software procedure (version 9.4). The level of significance for all statistical tests was taken as 0.05.
RESULTS
Of the 93 participants included in this study, 79 were female: 53 were Caucasian, 14 African American, 11 Hispanic, and one Asian; 10 of the 14 males were Caucasian, one was African American, and three were Hispanic. Included males had a mean age of 46±15 years, a pre-surgical BMI of 44.7±4.1 kg/m2, and all underwent RYGB. Females were age 44±11 years and pre-surgical BMI was 45.7±7.0 kg/m2.
Unadjusted means and standard deviations for body weight, FFM and skeletal muscle mass at T0, T1, T2, and T5 are presented separately for females by three surgical procedures and for males who had RYGB in Table 1. The first analysis included females only and investigated mixed model adjusted means and differences across time to compare the effects of three surgical procedures (Table 2). The mean pre-surgery body weight was not different between the RYGB and BAND groups, but was respectively (mean±SE) 22.5±6.3 kg and 29.5±8.4 kg less than the average pre-surgical weight of the SLEEVE group (p<0.001 for both). Absolute FFM did not differ between the three groups before surgery. Expressed as a percentage of pre-surgical body weight, SLEEVE had less (−3.9±1.5%, p<0.05) FFM than RYGB, but did not differ from BAND in this female sample.
Table 1.
Unadjusted Body Composition Means by Surgery Type and Sex
| Females | Males | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| RYGB | SLEEVE | BAND | RYGB | ||||||
|
| |||||||||
| Variable | Time | N | Mean±SD | N | Mean±SD | N | Mean±SD | N | Mean±SD |
| Weight (kg) | T0 | 58 | 123.1±18.9 | 11 | 145.7±28.1 | 10 | 116.1±18.2 | 14 | 143.8±17.2 |
| T1 | 55 | 79.8±16.3 | 9 | 104.3±25.2 | 9 | 102.1±21.3 | 14 | 98.8±17.8 | |
| T2 | 48 | 80.0±18.0 | 10 | 107.6±24.3 | 10 | 100.3±21.3 | 11 | 101.0±18.9 | |
| T5 | 34 | 83.3±21.8 | 8 | 108.4±17.7 | 9 | 99.2±16.7 | 7 | 101.9±11.8 | |
| TBW (kg) | T0 | 57 | 44.0±7.0 | 11 | 46.9±6.6 | 10 | 40.6±5.7 | 14 | 56.6±11.1 |
| T1 | 55 | 37.9±6.0 | 8 | 41.5±5.9 | 9 | 37.7±6.3 | 14 | 51.9±6.5 | |
| T2 | 46 | 37.1±5.6 | 9 | 41.7±5.4 | 9 | 38.9±4.6 | 9 | 52.5±4.7 | |
| T5 | 34 | 36.7±6.3 | 8 | 40.8±6.2 | 9 | 38.3±6.7 | 7 | 52.3±6.9 | |
| 3C FFM (kg) | T0 | 56 | 57.6±8.4 | 10 | 59.5±7.4 | 10 | 53.5±7.4 | 14 | 74.9±9.3 |
| T1 | 55 | 50.4±7.1 | 9 | 54.3±7.2 | 9 | 50.3±7.9 | 14 | 69.6±6.8 | |
| T2 | 46 | 49.4±7.1 | 9 | 54.4±6.7 | 10 | 51.6±5.4 | 9 | 69.5±4.6 | |
| T5 | 29 | 47.8±7.1 | 8 | 53.5±6.7 | 9 | 49.7±7.7 | 7 | 68.3±8.6 | |
| MRI SM (kg) | T0 | 21 | 25.3±3.3 | 2 | 28.6±4.8 | 2 | 23.6±4.0 | 3 | 36.8±3.3 |
| T1 | 40 | 20.5±3.6 | 5 | 21.3±3.1 | 6 | 22.5±3.6 | 10 | 30.5±5.3 | |
| T2 | 35 | 20.7±3.8 | 5 | 21.0±3.9 | 5 | 22.5±4.3 | 6 | 28.5±3.2 | |
| T5 | 21 | 19.9±4.3 | 1 | 36.1 | 5 | 19.3±3.2 | 5 | 29.5±6.1 | |
N: number of subjects with data in each category; T0: prior to surgery; T1, T2, and T5: 1, 2, and 5 years after surgery, respectively; Means±SD: unadjusted means and standard deviation for the N listed with complete data; RYGB: Roux-en-Y gastric bypass; Sleeve: sleeve gastrectomy; Band: laparoscopic adjustable gastric band; 3C FFM (kg): fat free mass estimate by 3-compartment model; MRI SM (kg): skeletal muscle mass by MRI.
Table 2.
Post-surgical Fat Free Mass Changes in Females by Surgery Type
| RYGB | SLEEVE | BAND | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Variable | Time | N | Adjusted Mean (SE) |
Adjusted Diff (SE) |
N | Adjusted Mean (SE) |
Adjusted Diff (SE) |
N | Adjusted Mean (SE) |
Adjusted Diff (SE) |
| Weight (kg) | T0 | 58 | 123.1 (2.7) | 11 | 145.7 (6.1)* | 10 | 116.1 (6.4)⁑ | |||
| T1 | 55 | 80.0 (2.3) | −43.1 (1.5)† | 9 | 105.3 (5.4)* | −40.4 (3.5)† | 9 | 101.0 (5.6)* | −15.1 (3.6)† | |
| T2 | 48 | 78.9 (2.6) | −1.1 (1.1) | 10 | 104.7 (5.8)* | −0.6 (2.5) | 10 | 100.3 (6.0)* | −0.7 (2.4) | |
| T5 | 34 | 81.3 (2.9) | +2.4 (2.2) | 8 | 105.7 (6.3)* | +1.0 (4.5) | 9 | 98.6 (6.3)* | −1.6 (4.1) | |
| 3C FFM (kg) | T0 | 56 | 57.5 (1.1) | 10 | 60.4 (2.5) | 10 | 53.5 (2.6) | |||
| T1 | 55 | 50.6 (0.9) | −6.9 (0.6)† | 9 | 53.7 (2.2) | −6.7 (1.5)† | 9 | 50.0 (2.3) | −3.5 (1.4)† | |
| T2 | 46 | 49.2 (0.9) | −1.4 (0.5)† | 9 | 54.3 (2.1)* | +0.6 (1.2) | 10 | 50.3 (2.1) | +0.2 (1.2) | |
| T5 | 29 | 47.3 (1.0) | −1.9 (0.7)†‡ | 8 | 54.2 (2.2)* | +0.1 (1.1) | 9 | 49.3 (2.3) | −0.9 (1.0) | |
| FFM/Weight (%) | T0 | 56 | 46.9 (0.6) | 10 | 43.0 (1.4)* | 10 | 46.4 (1.4) | |||
| T1 | 55 | 64.4 (1.0) | +17.5 (1.0)† | 9 | 52.0 (2.5)* | +9.1 (2.5)† | 9 | 50.2 (2.5)* | +3.8 (2.3)† | |
| T2 | 46 | 63.5 (1.2) | −0.9 (1.0) | 9 | 52.9 (2.7)* | −0.9 (2.4) | 10 | 51.0 (2.7)* | +0.8 (2.2) | |
| T5 | 29 | 59.8 (1.4) | −3.7 (1.4)†‡ | 8 | 51.7 (2.8)* | −1.2 (2.8) | 9 | 50.6 (2.7)* | −0.3 (2.6) | |
T0: prior to surgery; T1, T2, T5: 1, 2 and 5 years after surgery, respectively; N: number of subjects with data in each category; Adjusted mean (SE) and Diff (SE): adjusted mixed model means and difference estimates between each testing visit based on all subjects included in the model; RYGB: Roux-en-Y gastric bypass; SLEEVE: Sleeve gastrectomy; BAND: laparoscopic adjustable gastric band; 3C FFM (kg): fat free mass estimate by 3-compartment model; FFM/Weight (%): fat free mass expressed as a percentage of body weight;
group mean is significantly different from RYGB group (p<0.05);
group mean is significantly different from SLEEVE group (p<0.05);
significant (p<0.05) change compared to previous assessment;
significant (p<0.05) change from T1 to T5;
FFM change during post-surgical weight loss (T0 – T1)
Significant weight loss occurred in all female surgical procedure groups during the first postoperative year (Table 2). Expressed as a percentage of adjusted pre-surgical means, total body weight loss (%TBWL) at T1 was 35.0%, 27.7%, and 13.0% for RYGB, SLEEVE, and BAND groups, respectively. The magnitude of weight loss varied by procedure, with RYGB and SLEEVE losing 28.1±3.9 kg and 25.3±5.1 kg, respectively, more body weight than BAND (p<0.0001) in the first year. Concomitant FFM loss occurred from T0 to T1 in all procedure groups, although the magnitude of FFM loss was significantly greater in RYGB (−6.9±0.6 kg) compared to BAND (−3.5±1.4 kg; p<0.05). FFM expressed as a percent of total body weight increased by 17.5±1.0% in RYGB, a significantly greater change than either SLEEVE (9.1± 2.5%; p<0.005) or BAND (3.8±2.3%; p<0.0001).
FFM during weight maintenance phase (T1–T5)
No significant changes in body weight occurred for any group from T1–T5. RYGB females lost 1.4±0.7 kg (p<0.01) of FFM from T1 to T2, and an additional 1.9±0.7 kg (p<0.005) from T2 to T5, with a total FFM loss of 3.3±0.7 kg from T1 to T5 (p<0.0001), despite no significant change in body weight over the same period. FFM did not change significantly in SLEEVE or BAND from T1 to T5. FFM expressed as a percentage of total body weight in RYGB (59.8±1.4%) remained significantly higher compared to SLEEVE (51.7±2.8%, p<0.05) or BAND (50.6±2.7%, p<0.005), despite a decrease of 3.7±1.4% (p<0.05) from T1 to T5.
Skeletal muscle and FFM changes during RYGB-induced weight loss in the MRI subset
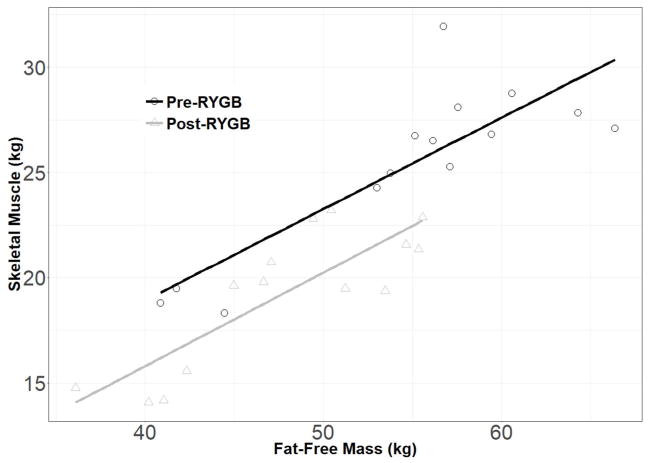
The second analysis in this study was on a subset of RYGB patients with whole-body skeletal muscle quantified by MRI on at least one visit (Table 3). Mixed models revealed a main effect of sex for all variables, but no difference in the pattern of change across time. Similar to the full sample, this subset lost significant weight (females: −37.9 kg; males: −43.0 kg; p<0.0001) and FFM (females: −6.1 kg; males: −8.7 kg; p<0.01) from T0 to T1. Skeletal muscle decreased in females and males (−5.9 kg and −8.0 kg changes, respectively; both p<0.0001) from T0 to T1. Approximately 15% of the weight loss from T0 to T1 was muscle in females, and 19% in males. In a subset of 14 females who underwent RYGB and had complete data for FFM and SM, the positive relationship between muscle and FFM remained linear and the slope of the regression line was not different after post-surgical weight loss (Figure 1). Expressed as a percent of total body weight, skeletal muscle increased in females (+4.4%, p<0.0001) and males (+5.4%, p=0.0001) from T0 to T1.
Table 3.
Male vs. Female Body Composition Changes after RYGB
| Variable | Time | Females | Males | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | Adjusted Mean (SE) |
Adjusted Diff (SE) |
N | Adjusted Mean (SE) |
Adjusted Diff (SE) |
||
| Weight (kg) | T0 | 21 | 117.4 (3.1) | 3 | 137.4 (8.0)* | ||
| T1 | 40 | 79.5 (2.5) | −37.9 (3.1)† | 10 | 94.4 (5.0)* | −43.0 (7.7)† | |
| T2 | 35 | 78.0 (2.8) | −1.5 (1.1) | 6 | 98.1 (5.8)* | +3.7 (2.6) | |
| T5 | 21 | 85.0 (3.7) | +7.1 (2.1)†‡ | 5 | 105.4 (7.5)* | +7.3 (4.5) | |
| 3C FFM (kg) | T0 | 19 | 56.3 (1.6) | 3 | 77.8 (3.0)* | ||
| T1 | 40 | 50.1 (1.1) | −6.1 (1.3)† | 10 | 69.1 (2.2)* | −8.7 (2.8)† | |
| T2 | 34 | 49.2 (1.1) | −0.9 (0.7) | 5 | 70.2 (2.5)* | +1.1 (1.7) | |
| T5 | 20 | 47.7 (1.3) | −1.5 (0.7)†‡ | 5 | 69.9 (2.5)* | −0.4 (1.6) | |
| MRI SM (kg) | T0 | 21 | 26.4 (0.7) | 3 | 39.1 (1.6)* | ||
| T1 | 40 | 20.4 (0.6) | −5.9 (0.5)† | 10 | 31.1 (1.3)* | −8.0 (1.1)† | |
| T2 | 35 | 20.3 (0.6) | −0.1 (0.2) | 6 | 31.8 (1.3)* | +0.7 (0.5) | |
| T5 | 21 | 19.9 (0.7) | −0.5 (0.3) | 5 | 31.2 (1.5)* | −0.5 (0.7) | |
| SM/Weight (%) | T0 | 21 | 21.8 (0.5) | 3 | 27.4 (1.1)* | ||
| T1 | 40 | 26.2 (0.5) | +4.4 (0.6)† | 10 | 32.8 (1.0)* | +5.4 (1.2)† | |
| T2 | 35 | 26.5 (0.5) | +0.3 (0.3) | 6 | 32.5 (1.1)* | −0.3 (0.7) | |
| T5 | 21 | 24.4 (0.7) | −2.2 (0.6)†‡ | 5 | 29.6 (1.3)* | −2.9 (1.1)†‡ | |
| SM/FFM (%) | T0 | 19 | 47.3 (0.8) | 3 | 50.0 (2.0) | ||
| T1 | 40 | 40.9 (0.7) | −6.4 (0.8)† | 10 | 45.0 (1.3)* | −5.1 (2.4)† | |
| T2 | 34 | 41.3 (0.6) | +0.4 (0.6) | 5 | 44.9 (1.4) | +0.1 (1.4) | |
| T5 | 20 | 41.5 (0.8) | −0.2 (0.7) | 5 | 43.2 (1.6) | −1.7 (1.6) | |
RYGB: Roux-en-Y gastric bypass; N: number of subjects with data in each category; T0: prior to surgery; T1, T2, T5: 1, 2 and 5 years after surgery, respectively; Adjusted mean (SE) and Diff (SE): adjusted mixed model estimates of mean and difference between current and previous assessment, based on subjects included in the model; 3C FFM (kg): fat free mass estimate by 3-compartment model; MRI SM (kg): skeletal muscle mass by MRI, limited to those with MRI data; SM/Weight (%): Skeletal muscle expressed as a percentage of body weight; SM/FFM (%): Skeletal muscle expressed as a percentage of fat-free mass;
significant (p<0.0001) mean value compared to females;
significant (p<0.05) change compared to previous assessment;
significant (p<0.05) change from T1 to T5.
Figure 1.
Pre- and post-surgery skeletal muscle (SM) and fat-free mass (FFM) values in a subset of 14 female patients who underwent Roux-en-Y gastric bypass surgery (RYGB). In these completers and also in a mixed model analysis including all data points, the slopes of the regression lines were not different before and after surgery.
Skeletal muscle and FFM changes in the RYGB MRI subset from T1–T5
Females who underwent RYGB maintained weight and FFM from T1 to T2, and gained 7.1 kg of body mass from T2 to T5 (p<0.005), with a total weight change of +5.6±2.1 kg (p<0.05) from T1 to T5. The average %TBWL for this subset at T1 was 32.3%; at T5 it was 27.6%. Among these females, FFM decreased (−2.4±0.9 kg; p<0.05) from T1 to T5. Absolute skeletal muscle mass did not change significantly after the first year. However, with a significant weight gain from T1 to T5, skeletal muscle expressed as a percentage of total body weight decreased 1.8±0.5% (p<0.005), but remained 2.5±0.7% greater than the 21.8% measured prior to surgery (p<0.05).
In males, average %TBWL at T1 was 31.3%; at T5 it was 23.3%. No significant changes in FFM or skeletal muscle occurred from T1 to T5. However, when expressed as a percentage of total body weight, skeletal muscle decreased 3.2±1.0% (p<0.01) over this period, and was no longer significantly greater than pre-surgery levels (p>0.15).
Effect of age or total body water on changes in body composition after bariatric surgery
In a post-hoc analysis, baseline age was added as a covariate in each of the models to determine whether body weight, FFM, or SM changes after surgery were significantly influenced by age. When comparing females in three surgery groups (Table 2), age was significant in body weight (p<0.05) and FFM (p<0.0001) models, but none of the contrasts differed from the original analyses. In the comparison of males and females who had MRI SM data (Table 3), age was not significant in the body weight model, but was significant in FFM (p<0.005) and the SM (p<0.01) models. Adding baseline age to the FFM model rendered the T2–T5 change non-significant (age-adjusted: −1.3 (0.7), p=0.07; compared to non-adjusted: −1.5 (0.7), p=0.04).
A similar analysis was conducted to determine the effect of TBW on changes in FFM across time. The effect of total body water was significant in the FFM model (p<0.0001). However, its inclusion only affected the BAND group, rendering the T0–T1 decrease in FFM non-significant (p>0.15) and the decline in FFM from T2–T5 became significant (TBW-adjusted: −1.0 (0.5), p<0.05; compared to non-adjusted: −0.9 (1.0)).
DISCUSSION
This study describes the unique course of FFM and skeletal muscle changes across 5 years after bariatric surgery. During the dramatic weight loss phase in the first post-operative year, FFM decreased significantly in females, regardless of surgery type. Subsequent changes in body weight and FFM were minimal, indicating general maintenance from T1 to T5. In men and women with MRI-measured muscle mass who underwent RYGB surgery, changes in skeletal muscle generally mirrored those of FFM, decreasing the first year and then maintaining during the subsequent four years.
Early observations by Forbes (24) point to a close linkage between body weight and FFM, such that some FFM loss is expected during body weight loss. Indeed, results from numerous studies have shown FFM losses of similar magnitude or greater than this study during rapid weight loss in the first 1–2 years after RYGB (7, 25, 26, 27, 28, 29, 30), SLEEVE (7, 25, 28, 29), and BAND (7, 26, 27, 31, 32, 33) procedures. The rate and amount of FFM lost during these initial post-operative years may vary by surgical procedure, extent of weight loss, and possibly assessment method. A previous study by Das et al. assessed FFM loss by 3C model in RYGB females and reported that 23% of total weight loss was FFM over 14.4 months (34). In the current study, the percent of total weight loss that was FFM during the first year ranged from 16% to 23%, with RYGB males and females averaging between 16% and 19%, and females with SLEEVE and BAND procedures at 17% and 23%, respectively. When considering the extent of weight loss occurring in the first post-operative year, the FFM loss observed was not excessive, and is well within the ranges of studies using 3C model (34) and DXA (26, 27, 29, 33, 35).
A novel aspect of this study is whole-body MRI quantification of muscle mass across five post-surgical years in male and female RYGB patients. Not surprisingly, males had greater body weight, FFM, and skeletal muscle prior to surgery and throughout the study, but initial weight loss and long-term maintenance trajectories did not differ from females. Although the average percentage of FFM that is skeletal muscle in this subset ranges between 41.5% and 50.0%, a high percentage of FFM lost in the first year was skeletal muscle for females (96.7%) and males (92.0%). This loss of skeletal muscle among fat-free tissues in the first year underscores the importance of recommendations for exercise (36), and in particular, strength training as a means of attenuating muscle atrophy during the weight loss period.
The significant increase in weight observed in RYGB women from T1–T5, coupled with a decrease in FFM over that same period, indicates that post-surgical weight gain is neither FFM nor muscle. A large Swedish population-based cross-sectional study using DXA recently reported that FFM does not appear to decline until beyond 60 years of age (37), seemingly contradicting the notion that age is related to decreases in FFM for middle-age adults. However, a weakness of cross-sectional design for aging studies is that different samples of people are used at each observation, introducing potential selection and even survival bias across age categories. Forbes’ observations of long-term FFM changes in longitudinal studies (24) establishes that although changes in FFM across time are highly related to changes in weight, adults who maintain their weight ultimately lose approximately 1.5 kg of FFM per decade, and this rate of decline is greater in sedentary individuals. While the RYGB females in our study lost FFM during a period of weight gain, the rate of FFM loss was commensurate with rates observed in longitudinal studies of sedentary individuals during the normal course of aging.
The skeletal muscle component of FFM was essentially maintained after the first postoperative year in both males and females who underwent RYGB. This observation is encouraging for bariatric surgery patients, given that a single cross-sectional study of MRI-quantified whole body skeletal muscle has reported an age-related decline in SM after age 45 (38). A post-hoc analysis of the FFM and SM changes in the current study indicate an age effect that, although significant in FFM, did not modify the pattern of change in a meaningful way. These 5-year prospective data contradict the notion that a malabsorptive bariatric surgery procedure is detrimental to skeletal muscle mass, particularly after initial weight loss. However, several intervention studies demonstrate that exercise, even without increasing muscle mass, improves strength and physical function within post-surgical patients (39, 40). Thus, the effects of exercise training may be beneficial to maintain functionality in the face of fat regain.
Although this study has several strengths, including gold-standard assessments of FFM and skeletal muscle with repeated measures extending five years after bariatric surgery in a sample with some racial/ethnic diversity, there are some limitations. First, MRI skeletal muscle was obtained on a subset of participants prior to surgery, largely due to size limitations of the MRI scanner. This limited the pre-surgical sample size, particularly in males. In addition, 5-year follow-up data were available in only 62% (58 of 93) participants. To take advantage of the information provided by all available data and to facilitate comparisons across multiple time points, we chose a mixed models approach to account for missing data. There is a possibility that our results reflect a completers bias if the missing values in the model were not representative of the entire sample. However, the similar weight and FFM changes between subjects with and without MRI skeletal muscle support the validity of study outcomes. Second, we had too few patients with MRI data who underwent procedures other than RYGB to adequately describe and compare skeletal muscle changes between surgery groups. Skeletal muscle changes in other procedures may differ from those observed in RYGB. Third, we did not measure physical activity or protein intake in these participants; both of which could have affected the time course of changes in FFM and/or skeletal muscle after surgery.
In conclusion, we found no evidence of a disproportionate FFM loss after bariatric surgery, given that FFM loss was 16–23% of the total weight loss in the first post-operative year. Skeletal muscle accounts for almost all of the FFM lost in RYGB patients during post-surgical weight loss, however. Both FFM and skeletal muscle are well-maintained after the first year, with subsequent changes either non-significant or at rates of decline commensurate with aging. Designing and implementing strategies such as increasing physical activity and incorporating muscle strengthening into the lifestyle of post-surgical patients remain important targets of research, as it may enable patients to attenuate muscle atrophy during weight loss and avoid subsequent fat gain during the maintenance phase.
What is already known about this subject?
Excessive fat free mass loss is undesirable due to its negative impact on the integrity of skeletal muscle and preservation of functional capacity in aging
Studies of fat free mass following surgically-induced weight loss have rarely extended beyond the initial weight loss period and none have used gold standard techniques for fat free mass into the weight maintenance phase
What does your study add?
No evidence of a disproportionate fat free mass loss following bariatric surgery
Both fat free mass and skeletal muscle mass are well-maintained at the end of the first post-surgical year, with subsequent changes through five years commensurate with aging
Acknowledgments
Funding: Supported by National Institutes of Health grants RO1-DK-72507, P30-DK-26687, UL1 TR000040, T32-DK007559 (supported LD, EW, and TL), T32-DK091227 (supported EW) and R21-DK099619.
Footnotes
Clinical Trial Registration: Long-term Effects of Bariatric Surgery (LABS-2) NCT00465829
Disclosure: The authors declared no conflict of interest.
Author contributions: DG designed the study. LD and JT conducted the analyses. WY, BG, JD, EW, TL, GS, AP, AC, and SL contributed to recruitment, study procedures, and database management. IJ analyzed all MRI scans. LD, DG, and JT wrote the paper. All authors were involved in writing the paper and had final approval of the submitted and published versions.
References
- 1.Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N Engl J Med. 2017;377:1143–1155. doi: 10.1056/NEJMoa1700459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Annals of surgery. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. discussion 350–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arterburn DE, Bogart A, Sherwood NE, Sidney S, Coleman KJ, Haneuse S, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obesity surgery. 2013;23:93–102. doi: 10.1007/s11695-012-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond) 2007;31:743–750. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- 5.Marks BL, Rippe JM. The importance of fat free mass maintenance in weight loss programmes. Sports medicine (Auckland, NZ. 1996;22:273–281. doi: 10.2165/00007256-199622050-00001. [DOI] [PubMed] [Google Scholar]
- 6.Skogar M, Holmback U, Hedberg J, Riserus U, Sundbom M. Preserved Fat-Free Mass after Gastric Bypass and Duodenal Switch. Obesity surgery. 2016 doi: 10.1007/s11695-016-2476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strain GW, Ebel F, Honohan J, Gagner M, Dakin GF, Pomp A, et al. Fat-free mass is not lower 24 months postbariatric surgery than nonoperated matched controls. Surg Obes Relat Dis. 2017;13:65–69. doi: 10.1016/j.soard.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browning MG, Franco RL, Cyrus JC, Celi F, Evans RK. Changes in Resting Energy Expenditure in Relation to Body Weight and Composition Following Gastric Restriction: A Systematic Review. Obesity surgery. 2016;26:1607–1615. doi: 10.1007/s11695-016-2184-2. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Heshka S, Wang J, Wielopolski L, Heymsfield SB. Magnitude and variation of fat-free mass density: a cellular-level body composition modeling study. Am J Physiol Endocrinol Metab. 2003;284:E267–273. doi: 10.1152/ajpendo.00151.2002. [DOI] [PubMed] [Google Scholar]
- 10.Leone PA, Gallagher D, Wang J, Heymsfield SB. Relative overhydration of fat-free mass in postobese versus never-obese subjects. Annals of the New York Academy of Sciences. 2000;904:514–519. doi: 10.1111/j.1749-6632.2000.tb06508.x. [DOI] [PubMed] [Google Scholar]
- 11.Brozek J, Grande F, Anderson JT, Keys A. Densitometric Analysis of Body Composition: Revision of Some Quantitative Assumptions. Annals of the New York Academy of Sciences. 1963;110:113–140. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- 12.Levitt DG, Beckman LM, Mager JR, Valentine B, Sibley SD, Beckman TR, et al. Comparison of DXA and water measurements of body fat following gastric bypass surgery and a physiological model of body water, fat, and muscle composition. J Appl Physiol. 2010;109:786–795. doi: 10.1152/japplphysiol.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valentine RJ, Misic MM, Kessinger RB, Mojtahedi MC, Evans EM. Location of body fat and body size impacts DXA soft tissue measures: a simulation study. Eur J Clin Nutr. 2008;62:553–559. doi: 10.1038/sj.ejcn.1602770. [DOI] [PubMed] [Google Scholar]
- 14.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 15.Ross R, Goodpaster B, Kelley D, Boada F. Magnetic resonance imaging in human body composition research. From quantitative to qualitative tissue measurement. Annals of the New York Academy of Sciences. 2000;904:12–17. doi: 10.1111/j.1749-6632.2000.tb06415.x. [DOI] [PubMed] [Google Scholar]
- 16.Belle SH, Berk PD, Chapman WH, Christian NJ, Courcoulas AP, Dakin GF, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis. 2013;9:926–935. doi: 10.1016/j.soard.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belle SH, Berk PD, Courcoulas AP, Flum DR, Miles CW, Mitchell JE, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3:116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toro-Ramos T, Goodpaster BH, Janumala I, Lin S, Strain GW, Thornton JC, et al. Continued loss in visceral and intermuscular adipose tissue in weight-stable women following bariatric surgery. Obesity (Silver Spring) 2015;23:62–69. doi: 10.1002/oby.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widen EM, Strain G, King WC, Yu W, Lin S, Goodpaster B, et al. Validity of bioelectrical impedance analysis for measuring changes in body water and percent fat after bariatric surgery. Obesity surgery. 2014;24:847–854. doi: 10.1007/s11695-014-1182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva AM, Shen W, Wang Z, Aloia JF, Nelson ME, Heymsfield SB, et al. Three-compartment model: critical evaluation based on neutron activation analysis. Am J Physiol Endocrinol Metab. 2004;287:E962–969. doi: 10.1152/ajpendo.00104.2004. [DOI] [PubMed] [Google Scholar]
- 21.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, et al. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr. 2009;89:807–814. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder WS, Cooke MJ, Mnassett ES, Larhansen LT, Howells GP, Tipton IH. Report of the Task Group on Reference Man. Pergamon: Oxford; 1975. [Google Scholar]
- 24.Forbes GB. Longitudinal changes in adult fat-free mass: influence of body weight. Am J Clin Nutr. 1999;70:1025–1031. doi: 10.1093/ajcn/70.6.1025. [DOI] [PubMed] [Google Scholar]
- 25.Wells J, Miller M, Perry B, Ewing JA, Hale AL, Scott JD. Preservation of Fat-free Mass after Bariatric Surgery: A Comparison of Malabsorptive and Restrictive Procedures. The American surgeon. 2015;81:812–815. [PubMed] [Google Scholar]
- 26.Tam CS, Redman LM, Greenway F, LeBlanc KA, Haussmann MG, Ravussin E. Energy Metabolic Adaptation and Cardiometabolic Improvements One Year After Gastric Bypass, Sleeve Gastrectomy, and Gastric Band. J Clin Endocrinol Metab. 2016;101:3755–3764. doi: 10.1210/jc.2016-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabl C, Rao MN, Schwarz JM, Mulligan K, Campos GM. Thermogenic changes after gastric bypass, adjustable gastric banding or diet alone. Surgery. 2014;156:806–812. doi: 10.1016/j.surg.2014.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otto M, Elrefai M, Krammer J, Weiss C, Kienle P, Hasenberg T. Sleeve Gastrectomy and Roux-en-Y Gastric Bypass Lead to Comparable Changes in Body Composition after Adjustment for Initial Body Mass Index. Obesity surgery. 2016;26:479–485. doi: 10.1007/s11695-015-1792-6. [DOI] [PubMed] [Google Scholar]
- 29.Schneider J, Peterli R, Gass M, Slawik M, Peters T, Wolnerhanssen BK. Laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass lead to equal changes in body composition and energy metabolism 17 months postoperatively: a prospective randomized trial. Surg Obes Relat Dis. 2016;12:563–570. doi: 10.1016/j.soard.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Carrasco F, Ruz M, Rojas P, Csendes A, Rebolledo A, Codoceo J, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obesity surgery. 2009;19:41–46. doi: 10.1007/s11695-008-9638-0. [DOI] [PubMed] [Google Scholar]
- 31.Galtier F, Farret A, Verdier R, Barbotte E, Nocca D, Fabre JM, et al. Resting energy expenditure and fuel metabolism following laparoscopic adjustable gastric banding in severely obese women: relationships with excess weight lost. Int J Obes (Lond) 2006;30:1104–1110. doi: 10.1038/sj.ijo.0803247. [DOI] [PubMed] [Google Scholar]
- 32.Coupaye M, Bouillot JL, Coussieu C, Guy-Grand B, Basdevant A, Oppert JM. One-year changes in energy expenditure and serum leptin following adjustable gastric banding in obese women. Obesity surgery. 2005;15:827–833. doi: 10.1381/0960892054222768. [DOI] [PubMed] [Google Scholar]
- 33.Di Renzo L, Carbonelli MG, Bianchi A, Iacopino L, Fiorito R, Di Daniele N, et al. Body composition changes after laparoscopic adjustable gastric banding: what is the role of -174G>C interleukin-6 promoter gene polymorphism in the therapeutic strategy? Int J Obes (Lond) 2012;36:369–378. doi: 10.1038/ijo.2011.132. [DOI] [PubMed] [Google Scholar]
- 34.Das SK, Roberts SB, Kehayias JJ, Wang J, Hsu LK, Shikora SA, et al. Body composition assessment in extreme obesity and after massive weight loss induced by gastric bypass surgery. Am J Physiol Endocrinol Metab. 2003;284:E1080–1088. doi: 10.1152/ajpendo.00185.2002. [DOI] [PubMed] [Google Scholar]
- 35.Coupaye M, Bouillot JL, Poitou C, Schutz Y, Basdevant A, Oppert JM. Is lean body mass decreased after obesity treatment by adjustable gastric banding? Obesity surgery. 2007;17:427–433. doi: 10.1007/s11695-007-9072-8. [DOI] [PubMed] [Google Scholar]
- 36.Coen PM, Goodpaster BH. A role for exercise after bariatric surgery? Diabetes Obes Metab. 2016;18:16–23. doi: 10.1111/dom.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson I, Lissner L, Samuelson G, Fors H, Lantz H, Naslund I, et al. Body composition through adult life: Swedish reference data on body composition. Eur J Clin Nutr. 2015;69:837–842. doi: 10.1038/ejcn.2014.268. [DOI] [PubMed] [Google Scholar]
- 38.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 39.Huck CJ. Effects of supervised resistance training on fitness and functional strength in patients succeeding bariatric surgery. Journal of strength and conditioning research/National Strength & Conditioning Association. 2015;29:589–595. doi: 10.1519/JSC.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 40.Stegen S, Derave W, Calders P, Van Laethem C, Pattyn P. Physical fitness in morbidly obese patients: effect of gastric bypass surgery and exercise training. Obesity surgery. 2011;21:61–70. doi: 10.1007/s11695-009-0045-y. [DOI] [PubMed] [Google Scholar]