Abstract
Introduction
The number of preventive care guidelines is rapidly increasing. It is unknown whether the number of guideline-recommended preventive services is associated with utilization.
Methods
The authors used Poisson regression of 390,778 person-years of electronic medical records data from 2008 to 2015, in 80,773 individuals aged 50–75 years. Analyses considered eligibility for 11 preventive services most closely associated with guidelines: tobacco cessation; control of obesity, hypertension, lipids, or blood glucose; influenza vaccination; and screening for breast, cervical, or colorectal cancers, abdominal aortic aneurysm, or osteoporosis. The outcome was the rate of preventive care utilization over the following year. Results were adjusted for demographics and stratified by the number of disease risk factors (smoking, obesity, hypertension, hyperlipidemia, diabetes). Data were collected in 2016 and analyzed in 2017.
Results
Preventive care utilization was lower when the number of guideline-recommended preventive services was higher. The adjusted rate of preventive care utilization decreased from 38.67 per 100 (95% CI=38.16, 39.18) in patients eligible for one guideline-recommended service to 31.59 per 100 (95% CI=31.29, 31.89) in patients eligible for two services and 25.43 per 100 (95% CI=24.68, 26.18) in patients eligible for six or more services (p-trend<0.001). Results were robust to disease risk factors and observed for all but two services (tobacco cessation, obesity reduction). However, for any given number of guideline-recommended services, patients with more disease risk factors had higher utilization rates.
Conclusions
The rate of preventive care utilization was lower when the number of guideline-recommended services was higher. Prioritizing recommendations might improve utilization of high-value services.
INTRODUCTION
Each year, about 1.1 million U.S. deaths are attributable to preventable risk factors.1–3 Optimal use of preventive care would prevent 50,000–100,000 deaths/year4 and add more than 2 million more healthy life years5,6 in the U.S. In addition, preventable disease costs between $224 billion and $315 billion for tobacco-related illness, alcohol abuse, diabetes, and heart disease and stroke.7–11 The number of guidelines is also growing quickly; Agency for Healthcare Research and Quality’s National Guideline Clearinghouse lists 115 guidelines for prevention in primary care, doubling since 2014.12 However, preventive care utilization is low. Only one quarter of middle-aged adults (aged 50–64 years) and half of elderly adults (aged 65 years and older) receive all recommended services.13,14
With a growing shift toward value-based payment, there will be increased focus on preventive care delivery. Through the Medicare Access and Child Health Insurance Program Reauthorization Act of 2015, physicians and healthcare organizations are required to report preventive care quality metrics.15 To remind physicians of services for which their patients are eligible, healthcare organizations may invest in clinical decision supports.16–18 Yet, although the benefits of particular services may vary as much as 100-fold for a given patient,19,20 for the purposes of value-based payment, all preventive care quality measures are weighted equally.15 Thus, a physician’s quality of care rating depends on the number of services completed, regardless of value. Recently, the nonprofit National Commission on Prevention Priorities provided scientific evidence for the relative health impact and cost effectiveness for evidence-based preventive services, but support for prioritizing evidence-based preventive services remains low.5,6,21
This study seeks to assess whether eligibility for more guideline-recommended preventive services is associated with lower preventive care utilization.
METHODS
For this retrospective cohort study, electronic medical records (EMRs) were reviewed from 2008 to 2015 for the Cleveland Clinic Health System, based in northeast Ohio. The Cleveland Clinic Health System consists of one large academic medical center, 13 regional hospitals, 21 family health centers, and >75 outpatient locations.22 Cleveland Clinic implemented an integrated, vendor-based EMR system (Epic™) in 2001. The EMR system leverages robust clinical decision support including reminders for disease prevention and screening. EMRs were chosen over administrative claims because clinical information, such as smoking and obesity, was needed to assess preventive care eligibility.
Study Sample
Inclusion criteria were patients aged 50–75 years who received regular primary care, defined as ≥5 years of care in the health system with visits to an internal medicine or family medicine provider at least every other year, and self-reported residence in Ohio. It was reasoned that patients with regular primary care were more likely to have complete data on receipt of preventive services. For example, if a patient had primary care visits in each year of 2009–2012 and 2014, then the patient was considered to have received no medical care in 2013 and included from 2009 to 2014 (but not earlier or later years). Results were robust to variation in these requirements. Because supporting evidence accompanying U.S. Preventive Services Task Force (USPSTF) recommendations generally considers individuals of average comorbidity, patients were excluded if they had conditions that might substantially alter the risk–benefit tradeoff of preventive services, including history of cardiovascular disease (because severity was not always known), cancer (excluding non-melanoma skin cancer), chronic obstructive pulmonary disease, or end-stage renal disease.
Measures
At the end of each year, the definitions in Table 1 were used to assess patient eligibility for the 11 preventive services most closely associated with recommendations by the USPSTF, American College of Cardiology, or American Diabetes Association: tobacco cessation23; control of obesity,24,25 hypertension,26 lipids,27,28 or blood glucose29; and screening for breast,30 cervical,31 or colorectal cancers,32 abdominal aortic aneurysm,33 or osteoporosis.34 Additionally, all patients were eligible for influenza vaccination each year. These services had consistent data availability. Patients without visits in a year were considered not to have received any preventive care, but could be up-to-date based on previous years. Similarly, smoking status, obesity, and blood pressure were only assessed during clinical encounters, not between visits. Eligibility was assessed only at the end of each year (on December 31) because of concern that physicians might have discussed different preventive services at office visits throughout the year, but according to current guidelines, they should have discussed all services at some point during each year. Year-end also was chosen as a focal point around which the organization evaluated performance and reported outcomes.35 Two preventive services were not considered: aspirin because nonprescription medications were less reliable in EMRs, and lung cancer screening because it was not recommended until 2014 (late in the study).36 Table 1 shows definitions of eligibility and utilization for each preventive service.
Table 1.
Definitions of Eligibility for and Utilization of Each Preventive Service, 2008–2015 (Individuals Aged 50–75 Years)
| Preventive service | Definition of eligibility | Definition of utilization |
|---|---|---|
| Tobacco cessation23 | Current smoker | Former smoker |
| Obesity control24,25 | BMI ≥30 kg/m2 | Weight loss of ≥5%a |
| Hypertension control26 | Blood pressure ≥140/90 mmHgb | Blood pressure <140/90 mmHgb |
| Lipids control27,28 | LDL-C ≥130 mg/dL for non-diabetics, ≥100 mg/dL for diabeticsc | LDL <130 mg/dL for non-diabetics, <100 mg/dL for diabeticsc |
| Blood glucose control29 | Diagnosis with diabetes and HbA1c ≥7% | HbA1c <7.0% |
| Breast cancer screening30 | Women with no mammogram in past 2 years, in women without bilateral mastectomy | Receipt of mammography |
| Cervical cancer screening31 | In women aged <65 years and without prior hysterectomy, no Pap test in past 3 years or no Pap+HPV co-testing in past 5 years | Pap test, with or without HPV testing |
| Colorectal cancer screening32 | Colonoscopy in past 10 years, flexible sigmoidoscopy in past 5 years, or fecal occult blood testing in past 1 year | Receipt of colonoscopy, flexible sigmoidoscopy or fecal occult blood testing |
| Abdominal aortic aneurysm screening33 | Men aged 65–75 years who ever smoked with no record of screening | Receipt of screening |
| Osteoporosis screening34 | Women aged ≥65 years | Receipt of osteoporosis screening |
| Influenza vaccination | All individuals | Receipt of influenza vaccine |
Weight loss ≥10% in sensitivity analysis.
Average of three latest visits in each calendar year, if they occurred within 90 days of each other.
Based on ATP III criteria,28 which were in place during the majority of this study.
LDL-C, low density lipoprotein cholesterol; HPV, human papilloma virus; ATP III, Adult Treatment Panel III; HbA1c, glycated hemoglobin.
A health maintenance tab in the EMR was available for physicians to view screening recommendations. The primary care provider may have been assisted by other members of the care team, but in most cases was responsible for ordering each service.
In the year after which a patient was identified as eligible, researchers examined successful utilization of the service most closely associated with each guideline (definitions, Table 1). For example, researchers examined whether a patient who was eligible for breast cancer screening on December 31, 2008 (had not obtained a mammogram in the prior 2 years) had a mammogram at any point in 2009. The definition of utilization was typically the same as the relevant USPSTF guideline (e.g., obtain mammography), with four exceptions: tobacco cessation, weight loss, hypertension control, and blood glucose control. The USPSTF recommends counseling for tobacco cessation and obesity, and screening for hypertension and diabetes. However, supporting materials accompanying these recommendations specify a rationale of helping at-risk individuals prevent negative health outcomes. Consistent with this rationale, utilization was defined to reflect common clinical goals (quitting smoking [identified by a change from current to former smoking status in EMR], ≥5% weight loss, blood pressure <140/90 mmHg, and HbA1c<7%), although experts do not always agree on targets.25,26,29 Often, an individual must utilize another service to reach an outcome (e.g., medication) but this study’s concern was the ultimate preventive care goal.
Statistical Analysis
Poisson regression modeling was conducted to estimate the association between number of guideline-recommended preventive services for which a patient was eligible (one, two, three, four, five, six or more) and number of preventive services utilized over the following year. An offset equal to the log number of eligible services allowed the coefficient to be interpreted as the rate of preventive service utilization per eligible service. Additionally, to estimate the odds of utilizing specific preventive services (e.g., tobacco cessation, colorectal cancer screening), logistic regression was conducted to assess the association between the number of guideline-recommended preventive services and utilization of that service over the following year. Both analyses were adjusted for demographics (sex, race, ethnicity, marital status, health insurance, median household income by race and Census block group, primary care site, and year), and included a random effect for each patient with SEs clustered by patient. To translate adjusted rate ratios (Poisson model) or AORs (logistic model) into an absolute measure, average marginal effects estimated the partial derivative of preventive care utilization with respect to each independent variable and averaged the results.
Several secondary analyses were undertaken to assess robustness. Because unhealthy patients generally were eligible for more preventive services, results were stratified by number of disease risk factors (defined as current smoker, BMI ≥30 kg/m2, and diagnosis of diabetes [with HbA1c ≥7%], hypertension [with blood pressure ≥140/90 mmHg] or hyperlipidemia [with low-density lipoprotein cholesterol ≥130 mg/dL for non-diabetics, ≥100 mg/dL for diabetics, based on Adult Treatment Panel III criteria which were the in place during the majority of the study28]). Results were also stratified by each disease risk factor. Additionally, because some preventive services required achievement of a goal whereas others required receipt of a test or procedure, services were classified as outcome-based or receipt-based and separately analyzed. Outcome-based services were tobacco cessation and control of obesity, hypertension, lipids, and blood glucose. All other preventive services were considered receipt-based.
Additionally, six sensitivity analyses were conducted: changing the threshold for successful weight loss from ≥5% to ≥10%, excluding influenza vaccination (because of lower data quality during 2009–2012), considering only patients with appointments ≥30 minutes at some point during the year (allowing more time to discuss recommendations), considering only patients with appointments ≤20 minutes (limited time), including all patients regardless of comorbidity (to examine sensitivity to exclusion criteria), and stratifying results by year (because prior work suggests that utilization increased over time).37
Data were collected in 2016 and analyzed in 2017. Analyses were conducted using Stata/MP, version 13.1. Significance tests were two-sided at the 5% level. This study was approved by Cleveland Clinic’s IRB.
RESULTS
The sample included 80,773 patients across 390,778 person-years. Characteristics appear in Table 2. Nearly half of patients (44%) had no disease risk factors, 35% had one risk factor, 16% had two risk factors and 5% had three or more risk factors.
Table 2.
Population Characteristics, 2008–2015 (Individuals Aged 50–75 Years)
| Characteristics | Number (%) |
|---|---|
| N person-years | 390,778 |
| N individuals | 80,773 |
| Age, years (mean, SD) | 60.0 (6.5) |
| Female | 232,361 (59.5) |
| Race | |
| White | 334,582 (85.6) |
| Black | 42,847 (11.0) |
| Other | 13,349 (3.4) |
| Hispanic | 4,747 (1.2) |
| Marital status | |
| Married or domestic partner | 273,905 (70.0) |
| Other (divorced, legally separated, single, widowed) | 112,169 (28.7) |
| Unknown | 4,704 (1.2) |
| Median household income in ZIP code (mean, SD) | $69,256 ($31,209) |
| Insurance | |
| Commercial | 209,825 (53.7) |
| Medicare | 74,754 (19.1) |
| Self-pay | 70,002 (17.9) |
| Cleveland Clinic Employee Health Plan | 32,316 (8.3) |
| Medicaid | 3,806 (1.0) |
| Other | 75 (0.0) |
| Yeara | |
| 2008 | 50,080 (12.8) |
| 2009 | 57,045 (14.6) |
| 2010 | 58,834 (15.1) |
| 2011 | 59,700 (15.3) |
| 2012 | 59,117 (15.1) |
| 2013 | 57,038 (14.6) |
| 2014 | 48,964 (12.5) |
| Number of disease risk factorsb | |
| 0 | 172,609 (44.2) |
| 1 | 135,912 (34.8) |
| 2 | 61,988 (15.9) |
| ≥3 | 20,269 (5.2) |
| Eligible for preventive service | |
| Total number of eligible services (mean, SD) | 2.6 (1.4) |
| Tobacco cessation | 34,334 (8.8) |
| Obesity reduction | 134,477 (34.4) |
| Hypertension control | 45,381 (11.6) |
| Lipids reduction | 86,605 (22.2) |
| Glycemic control | 23,110 (5.9) |
| Breast cancer screening | 57,870 (14.8) |
| Cervical cancer screening | 71,105 (18.2) |
| Colorectal cancer screening | 153,488 (39.1) |
| Abdominal aortic aneurysm screening | 13,944 (3.6) |
| Osteoporosis screening | 8,798 (2.3) |
| Influenza vaccination | 390,778 (100.0) |
| Utilization of preventive service or goal | |
| Total number of adhered services (mean, SD) | 0.7 (0.8) |
| Tobacco cessation | 2,996 (0.8) |
| Obesity reduction | 13,432 (3.4) |
| Hypertension control | 20,963 (5.4) |
| Lipids reduction | 20,752 (5.3) |
| Glycemic control | 4,503 (1.2) |
| Breast cancer screening | 19,360 (5.0) |
| Cervical cancer screening | 12,899 (3.3) |
| Colorectal cancer screening | 25,483 (6.5) |
| Abdominal aortic aneurysm screening | 659 (0.2) |
| Osteoporosis screening | 1,567 (0.4) |
| Influenza vaccination | 165,229 (42.3) |
Year in which eligibility for preventive services was assessed. Adherence to preventive services was assessed 1 year later.
Defined as current smoker, BMI ≥30 kg/m2, or diagnosis of diabetes, hypertension, or hyperlipidemia.
Patients who were eligible for more guideline-recommended preventive services utilized more services (Figure 1). However, eligibility for more guideline-recommended preventive services was strongly associated with lower utilization of each individual service over the following year. Table 3 presents the results. The adjusted rate of preventive care utilization decreased from 38.67 preventive services utilized per 100 eligible services (95% CI=38.16, 39.18) in patients eligible for one guideline-recommended service to 31.59 per 100 (95% CI=31.29, 31.89) in patients eligible for two services and 25.43 per 100 (95% CI=24.68, 26.18) in patients eligible for six or more services (p-trend<0.001). The adjusted rate ratios were 0.74 (95% CI=0.73, 0.75) in patients eligible for two services and 0.51 (95% CI=0.49, 0.52) in patients eligible for six or more services (Appendix Table 1). The pattern was similar for patients with no disease risk factors and those with one, two, or three or more disease risk factors (all p-trend<0.001). Similar results were found for each specific disease risk factor except current smoking, for which the trend was not significant (p=0.075). However, when the number of eligible services was held constant, patients with more disease risk factors had a higher rate of utilization. For example, among patients who were eligible for four guideline-recommended preventive services, the rate of preventive care utilization for individuals with no disease risk factors was 20.84 per 100 eligible services (95% CI=19.89, 21.79) whereas the rate in individuals with three or more disease risk factors was 31.50 per 100 eligible services (95% CI=30.79, 32.31).
Figure 1.
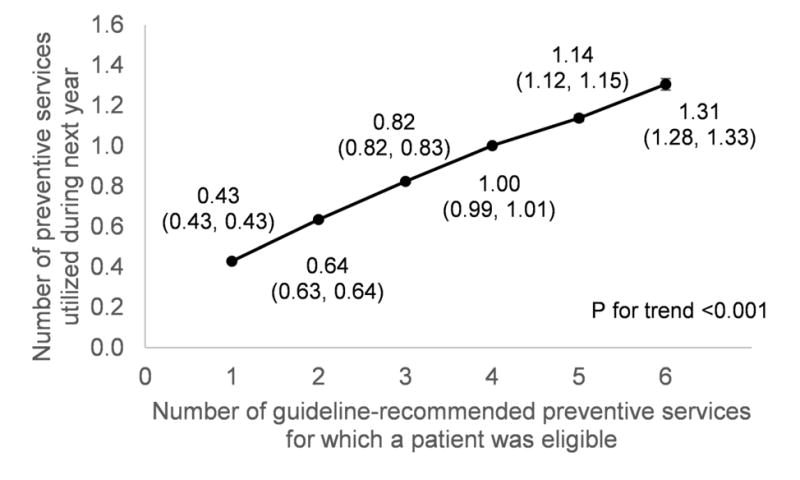
Association between number of guideline-recommended preventive services and number of preventive services utilized.
Notes: Patients who were eligible for more guideline-recommended preventive services utilized more services over the following year. N=80,773 patients across 390,778 person-years. 95% CI shown in parentheses. Results were adjusted for demographics (sex, race, ethnicity, marital status, health insurance, median household income by race in each patients’ Census block group, primary care geographic location and calendar year). Analyses included a random effect for each patient. SEs were clustered by patient.
Table 3.
Association Between Number of Guideline-recommended Preventive Services and Rate of Preventive Care Utilizationa
| Preventive care utilization | Number of guideline-recommended preventive services
|
p for trend | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ≥6 | ||
| Utilization of any single preventive service, adjusted rate per 100 eligible services (95% CI) | |||||||
| Overall | 38.67 (38.16, 39.18) |
31.59 (31.29, 31.89) |
28.43 (28.22, 28.63) |
26.91 (26.59, 27.23) |
25.48 (24.98, 25.99) |
25.43 (24.68, 26.18) |
<0.001 |
| By number of disease risk factors | |||||||
| 0 | 40.25 (39.72, 40.79) |
31.56 (31.20, 31.93) |
28.54 (27.85, 29.22) |
20.84 (19.89, 21.79) |
– | – | <0.001 |
| 1 | – | 34.61 (34.21, 35.00) |
27.02 (26.72, 27.32) |
25.61 (25.09, 26.12) |
18.78 (18.09, 19.46) |
– | <0.001 |
| 2 | – | – | 32.38 (31.98, 32.79) |
26.35 (25.97, 26.74) |
24.92 (24.29, 25.54) |
18.74 (17.96, 19.52) |
<0.001 |
| ≥3 | – | – | – | 31.50 (30.79, 32.21) |
26.06 (25.41, 26.70) |
24.61 (23.63, 25.60) |
<0.001 |
| By specific disease risk factorsb | |||||||
| Current smoker | – | 22.72 (21.56, 23.87) |
20.74 (20.19, 21.30) |
21.51 (20.97, 22.06) |
21.04 (20.20, 21.87) |
22.12 (20.87, 23.36) |
0.075 |
| Obese | – | 30.82 (30.14, 31.51) |
27.96 (27.66, 28.26) |
27.10 (26.79, 27.41) |
24.99 (24.45, 25.52) |
24.25 (23.42, 25.07) |
<0.001 |
| Diabetes | – | 42.14 (39.93, 44.35) |
33.01 (32.12, 33.91) |
29.71 (29.06, 30.35) |
27.15 (26.23, 28.07) |
25.12 (23.64, 26.60) |
<0.001 |
| Hypertension | – | 53.23 (52.07, 54.39) |
38.21 (37.59, 38.83) |
31.42 (30.82, 32.01) |
26.93 (26.21, 27.64) |
23.62 (22.78, 24.47) |
<0.001 |
| Hyperlipidemia | – | 35.75 (35.07, 36.44) |
29.63 (29.21, 30.05) |
27.23 (26.75, 27.71) |
25.38 (24.74, 26.02) |
23.82 (22.97, 24.68) |
<0.001 |
| Outcome-based servicesb | 17.03 (16.82, 17.25) |
20.67 (20.42, 20.93) |
21.79 (21.38, 22.21) |
21.35 (20.42, 22.28) |
17.98 (15.19, 20.77) |
– | <0.001 |
| All outcome-based services except obesity reduction | 26.67 (26.23, 27.12) |
25.83 (24.75, 26.91) |
24.33 (22.51, 26.15) |
18.96 (15.81, 22.11) |
– | – | 0.010 |
| Receipt-based servicesc | 42.80 (42.25, 43.34) |
31.98 (31.76, 32.22) |
30.25 (29.77, 30.72) |
22.89 (22.16, 23.62) |
16.57 (1.11, 32.03) |
– | <0.001 |
| Utilization of specific preventive services, adjusted probability, % (95% CI) | |||||||
| Tobacco cessation | – | 7.80 (6.88, 8.72) |
7.52 (6.86, 8.19) |
7.49 (6.79, 8.18) |
6.36 (5.64, 7.08) |
5.69 (4.86, 6.52) |
0.12 |
| Obesity reduction | – | 9.43 (9.09, 9.76) |
9.86 (9.58, 10.13) |
10.11 (9.78, 10.43) |
10.54 (10.10, 10.99) |
10.89 (10.26, 11.52) |
<0.001 |
| Hypertension control | – | 56.42 (54.74, 58.10) |
54.03 (52.93, 55.12) |
49.72 (48.64, 50.79) |
45.78 (44.49, 47.07) |
42.76 (41.22, 44.31) |
<0.001 |
| Lipids reduction | – | 27.69 (26.74, 28.64) |
26.23 (25.55, 26.90) |
24.39 (23.69, 25.09) |
21.46 (20.61, 22.31) |
17.46 (16.45, 18.47) |
<0.001 |
| Glycemic control | – | 22.36 (19.33, 25.39) |
19.83 (18.30, 21.35) |
19.12 (17.87, 20.38) |
17.15 (15.81, 18.50) |
14.12 (12.76, 15.48) |
<0.001 |
| Breast cancer screening | – | 55.03 (53.10, 56.96) |
46.06 (44.90, 47.22) |
35.67 (34.74, 36.59) |
30.04 (29.03, 31.04) |
25.28 (24.07, 26.48) |
<0.001 |
| Cervical cancer screening | – | 23.60 (22.48, 24.72) |
21.05 (20.31, 21.78) |
19.07 (18.42, 19.72) |
16.55 (15.81, 17.28) |
13.92 (13.03, 14.80) |
<0.001 |
| Colorectal cancer screening | – | 19.43 (18.95, 19.91) |
18.12 (17.73, 18.50) |
15.36 (14.96, 15.76) |
12.78 (12.29, 13.27) |
10.35 (9.72, 10.99) |
<0.001 |
| Abdominal aortic aneurysm screening | – | 5.33 (4.17, 6.50) |
4.53 (3.54, 5.53) |
4.28 (3.26, 5.28) |
2.71 (1.67, 3.75) |
2.00 (0.69, 3.32) |
0.055 |
| Osteoporosis screening | – | 32.19 (26.48, 37.91) |
23.52 (20.43, 26.60) |
17.02 (18.88, 19.17) |
11.49 (9.56, 13.43) |
6.95 (5.18, 8.71) |
<0.001 |
| Influenza vaccination | 32.07 (31.53, 32.61) |
40.30 (39.70, 40.91) |
37.25 (36.66, 37.85) |
34.58 (33.89, 35.28) |
31.97 (31.03, 32.91) |
29.96 (28.56, 31.35) |
<0.001 |
Notes: The rate of utilization of most preventive services was lower when the number of guideline-recommended preventive services was higher. N=80,773 patients across 390,778 person-years.
Results were adjusted for demographics (sex, race, ethnicity, marital status, health insurance, median household income by race in each patients’ Census block group, primary care geographic location and calendar year). Analyses included a random effect for each patient. SEs were clustered by patient.
Tobacco cessation; control of obesity, hypertension, lipids, and blood glucose.
Screening for breast, cervical and colorectal cancers, abdominal aortic aneurysm, and osteoporosis.
A similar pattern was observed for most individual preventive services (Table 3). For example, among patients who were eligible for hypertension control, those with five guideline-recommended preventive services had a 45.78% probability of achieving hypertension control (95% CI=44.49%, 47.07%), compared with a 56.42% probability (95% CI=52.75%, 58.10%) for patients with two guideline-recommended preventive services (AOR=0.55, 95% CI=0.53, 0.57, p<0.001; Appendix Table 1). There were two exceptions. There was no significant change in the adjusted odds of achieving tobacco cessation with additional guideline-recommended services (p-trend=0.12). The association was reversed for obesity reduction; for example, individuals with five guideline-recommended preventive services had a 24.99% probability of weight loss compared with a 30.82% probability of weight loss in individuals with two guideline-recommended services (p-trend<0.001).
A positive association was observed for outcome-based services; for example, the rate of utilization increased from 17.03 per 100 (95% CI=16.82, 17.25) in patients eligible for one outcome-based service to 21.79 per 100 (95% CI=21.38, 22.21) in patients eligible for four outcome-based services (p-trend<0.001; Table 3). However, this association was driven by obesity reduction. The association continued to be negative for other outcome-based services; for example, the rate of utilization decreased from 26.67 per 100 (95% CI=26.23, 27.12) in patients eligible for one service to 24.33 per 100 (95% CI=22.51, 26.15) in patients eligible for three services (p-trend=0.010). The slope of this association was flatter than in the overall analysis (decline of 2.34 per 100 for an increase from one to three eligible services vs 10.24 per 100 in the overall analysis). A negative association was also observed for receipt-based services (p-trend<0.001).
The rate of preventive care utilization increased substantially over the study period (Appendix Table 2). The rate of utilizing one guideline-recommended service increased from 28.95 per 100 in 2009 (95% CI=28.29, 29.63) to 51.97 per 100 in 2015 (95% CI=51.13, 52.82). However, within each year, the utilization rate was lower when the number of eligible guideline-recommended services was higher (all p-trend<0.001). Results for all other sensitivity analyses were consistent with the main results (all p-trend<0.001).
DISCUSSION
In this 8-year cohort study, eligibility for more guideline-recommended preventive services was strongly associated with lower patient utilization of each recommended service. This relationship held for nearly all major preventive services and stratification by disease risk factors. Because the value of preventive care interventions is not uniform,5,19,20,38 these findings suggest a need for prioritization of preventive care guidelines to maximize value for patients.6,21,39
Prior work has found that increased burden of prevention may result in lower utilization because of competing demands for patients’ work, family, friends, and hobbies.40–44 For example, individuals with diabetes find it difficult to take medicine, monitor blood sugar, and visit their doctor45–49 plus treat other chronic conditions, such as arthritis or asthma.50–52 This study appears to be the first to extend these findings to preventive care more broadly.
Analysis also provides perspective by quantifying the impact of guideline eligibility on utilization of each major preventive service and overall utilization. In contrast to earlier work,50–52 when the number of eligible preventive services was held constant, patients with more disease risk factors had a higher rate of utilization. Possible explanations include more primary care visits among riskier patients (allowing more time to discuss prevention) or increased recognition of preventive care needs.
Evidence suggests that preventive care interventions vary in their ability to improve longevity and quality of life.5,19 If patients are unable to utilize all recommendations, physicians and patients may do best to prioritize services that offer the greatest gain in life expectancy or quality of life. To this end, the nonprofit National Commission on Prevention Priorities recently ranked preventive services based on a combination of potential to add healthy life years to the U.S. population and cost.5,6,21 By contrast, pay-for-performance quality measures around these topics are unweighted,15 providing monetary incentive to recommend as much as possible without regard to relative benefits that could inform priorities of the different services. This study found that patients who were eligible for more preventive services also utilized more services, suggesting that prioritization (which, by definition, limits the number of services) does not occur. From the accountable care organization’s perspective, a “recommend everything” strategy maximizes compensation. However, because patients were far less likely to utilize almost every specific service—as reflected by lower odds of utilization—the current system would appear to produce suboptimal patient outcomes. If instead, physicians were to focus on prioritizing only a few services with the strongest evidence of effectiveness, value from preventive care might improve.5,6,19,21
This approach to care is patient-centered but may not conform to local quality-improvement standards. For example, in many systems, a middle-aged female who is obese, diabetic, a current smoker, has hypertension (blood pressure≥140/90 mmHg) and hyperlipidemia, and is not up-to-date with any cancer screenings would be recommended eight or more preventive services. This work suggests a better approach would be to prioritize control of hypertension, lipids, and blood glucose, which were outcome-based services with relatively little decline in the rate of utilization for each additional service and delay cancer screenings until a later date. Additionally, at every visit, providers should offer counseling on tobacco cessation (which was not associated with number of eligible preventive services) and weight loss (which had a positive association).
Limitations
For outcome-based services, broad goals (e.g., weight loss) were considered rather than specific interventions (e.g., counseling, calorie-reduction). The magnitude or sign of intervention-specific coefficients may differ from this study’s results. Second, it was unknown which preventive services were discussed in provider–patient visits, only whether the patient was eligible. To reduce potential bias, all visits over the course of an entire year were included, during which time physicians are generally expected to discuss all guideline-recommended services. Alternatively, it is possible that combining visits accidentally introduced bias, especially for patients who became eligible for and completed a preventive service within a single year. This risk largely pertains to receipt-based cancer screenings, for which patients routinely became re-eligible. Because of the model’s robustness to exclusion of receipt-based services, any resulting bias should be small. However, patients might also become eligible for and utilize some outcome-based services within a year (e.g., hypertension control). Therefore, the authors cannot rule out the possibility of more substantial bias. Results may be more relevant for patients with multiple disease risk factors (e.g., obese smokers) who by definition do not utilize all eligible services. Third, there was potential for unobserved confounding, especially because individuals with more recommended services tended to be less healthy. However, results held after stratification by number of disease risk factors. Fourth, generalizability may be limited by data for a single healthcare system with limited Medicaid patients (1%). For lower-income patients, competing demands such as multiple jobs may make utilization even more difficult. Similarly, because analysis was limited to patients with regular primary care, if this analysis were repeated in a population with less healthcare access, the observed trend might be more drastic. Fifth, waiting time for appointments could not be assessed, which may have influenced decision making. Sixth, the observed results could not be attributed to patient-, provider- or health system-related factors. Similarly, as a Poisson model, between- versus within-subject variation was not considered. Finally, only structured EMR data were captured. Physician notes on alcohol use, nutrition, physical activity, weight loss counseling, and tobacco cessation counseling were not captured.
CONCLUSIONS
In conclusion, preventive care utilization was lower when the number of eligible preventive services was higher. Although further research is necessary to confirm the results and more thoroughly address each disease risk factor, healthcare organizations and physicians interested in providing value-based care might consider ways to prioritize preventive recommendations and maximize utilization. It may be more effective to offer patients a few high priority services per year, than to offer all eligible services at once.
Supplementary Material
Acknowledgments
Dr. Taksler was supported by grants KL2TR000440 (from the National Center for Advancing Translational Sciences and the Clinical and Translational Science Collaborative of Cleveland) and R21AG052849 (from the National Institute on Aging). The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
Author contributions were as follows: conception and design, statistical expertise, and collection and assembly of data: GBT; drafting of the manuscript: GBT and EPF; and analysis and interpretation of the data, critical revision for important intellectual content, and final approval of the manuscript: all authors.
References
- 1.Rhodes HG. Measuring the Risks and Causes of Premature Death: Summary of Workshops. Washington, DC: 2015. [PubMed] [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. https://doi.org/10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270(18):2207–2212. https://doi.org/10.1001/jama.1993.03510180077038. [PubMed] [Google Scholar]
- 4.Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med. 2010;38(6):600–609. doi: 10.1016/j.amepre.2010.02.016. https://doi.org/10.1016/j.amepre.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Maciosek MV, LaFrance AB, Dehmer SP, et al. Updated priorities among effective clinical preventive services. Ann Fam Med. 2017;15(1):14–22. doi: 10.1370/afm.2017. https://doi.org/10.1370/afm.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isham G, Sanchez E, Jones WA, Teutsch S, Woolf S, Haddix A. Prevention priorities: guidance for value-driven health improvement. Ann Fam Med. 2017;15(1):6–8. doi: 10.1370/afm.2023. https://doi.org/10.1370/afm.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. U.S. DHHS; 2014. www.surgeongeneral.gov/library/reports/50-years-of-progress/. Accessed March 21, 2017. [Google Scholar]
- 8.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update a report from the American Heart Association. Circulation. 2014;129(3):E28–E292. doi: 10.1161/01.cir.0000441139.02102.80. https://doi.org/10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–1046. doi: 10.2337/dc12-2625. https://doi.org/10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41(5):516–524. doi: 10.1016/j.amepre.2011.06.045. https://doi.org/10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff (Millwood) 2009;28(5):W822–W831. doi: 10.1377/hlthaff.28.5.w822. https://doi.org/10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 12.National Guideline Clearinghouse. www.guideline.gov/search?f_Clinical_Specialty=Internal+Medicine%3bFamily+Practice&fLockTerm=Internal+Medicine&f_Guideline_Category=Prevention&page=1&f_Meets_Revised_Inclusion_Criteria=yes. Accessed February 27, 2017.
- 13.CDC, AARP, American Medical Association. Promoting Preventive Services for Adults 50–64: Community and Clinical Partnerships. Atlanta, GA: National Association of Chronic Disease Directors; 2009. www.cdc.gov/aging/pdf/promoting-preventive-services.pdf. Accessed February 15, 2018. [Google Scholar]
- 14.CDC. CDC focuses on need for older adults to receive clinical preventive services. www.cdc.gov/aging/pdf/cps-clinical-preventive-services.pdf. Published 2012. Accessed October 24, 2017.
- 15.Centers for Medicare and Medicaid Services, DHHS. Medicare Program; Merit-Based Incentive Payment System (MIPS) and Alternative Payment Model (APM) Incentive Under the Physician Fee Schedule, and Criteria for Physician-Focused Payment Models. Final rule with comment period. Fed Regist. 2016;81(214):77008–77831. [PubMed] [Google Scholar]
- 16.Hopkins DP, Community Preventive Services Task Force Clinical decision support systems recommended to prevent cardiovascular disease. Am J Prev Med. 2015;49(5):796–799. doi: 10.1016/j.amepre.2015.03.041. https://doi.org/10.1016/j.amepre.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 17.Haegerich TM, Sugerman DE, Annest JL, Klevens J, Baldwin GT. Improving injury prevention through health information technology. Am J Prev Med. 2015;48(2):219–228. doi: 10.1016/j.amepre.2014.08.018. https://doi.org/10.1016/j.amepre.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bright TJ, Wong A, Dhurjati R, et al. Effect of clinical decision-support systems: a systematic review. Ann Intern Med. 2012;157(1):29–43. doi: 10.7326/0003-4819-157-1-201207030-00450. https://doi.org/10.7326/0003-4819-157-1-201207030-00450. [DOI] [PubMed] [Google Scholar]
- 19.Taksler GB, Keshner M, Fagerlin A, Hajizadeh N, Braithwaite RS. Personalized estimates of benefit from preventive care guidelines: a proof of concept. Ann Intern Med. 2013;159(3):161–168. doi: 10.7326/0003-4819-159-3-201308060-00005. https://doi.org/10.7326/0003-4819-159-3-201308060-00005. [DOI] [PubMed] [Google Scholar]
- 20.Owens DK, Goldhaber-Fiebert JD. Prioritizing guideline-recommended interventions. Ann Intern Med. 2013;159(3):223–224. doi: 10.7326/0003-4819-159-3-201308060-00014. https://doi.org/10.7326/0003-4819-159-3-201308060-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor PJ, Sperl-Hillen JM, Margolis KL, Kottke TE. Strategies to prioritize clinical options in primary care. Ann Fam Med. 2017;15(1):10–13. doi: 10.1370/afm.2027. https://doi.org/10.1370/afm.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ernst, Young LLP. Consolidated Financial Statements and Supplementary Information. The Cleveland Clinic Foundation; 2016. [Google Scholar]
- 23.Siu AL, U.S. Preventive Services Task Force Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(8):622–634. doi: 10.7326/M15-2023. https://doi.org/10.7326/M15-2023. [DOI] [PubMed] [Google Scholar]
- 24.Moyer VA, U.S. Preventive Services Task Force Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(5):373–378. doi: 10.7326/0003-4819-157-5-201209040-00475. https://doi.org/10.7326/0003-4819-157-5-201209040-00475. [DOI] [PubMed] [Google Scholar]
- 25.American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the Obesity Expert Panel, 2013. Obesity (Silver Spring) 2014;22(suppl 2):S5–39. doi: 10.1002/oby.20821. https://doi.org/10.1002/oby.20821. [DOI] [PubMed] [Google Scholar]
- 26.Siu AL, U.S. Preventive Services Task Force Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778–786. doi: 10.7326/M15-2223. https://doi.org/10.7326/M15-2223. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Preventive Services Task Force. Bibbins-Domingo K, Grossman DC, et al. Statin use for the primary prevention of cardiovascular disease in adults: U.S. Preventive Services Task Force recommendation statement. JAMA. 2016;316(19):1997–2007. doi: 10.1001/jama.2016.15450. https://doi.org/10.1001/jama.2016.15450. [DOI] [PubMed] [Google Scholar]
- 28.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. https://doi.org/10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 29.Selph S, Dana T, Bougatsos C, Blazina I, Patel H, Chou R. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 US Preventive Services Task Force recommendation. Rockville, MD: 2015. [PubMed] [Google Scholar]
- 30.Siu AL, U.S. Preventive Services Task Force Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296. doi: 10.7326/M15-2886. https://doi.org/10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 31.Moyer VA, U.S. Preventive Services Task Force Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. https://doi.org/10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 32.Koretz RL. Evidence-based guideline: The USPSTF recommends screening for colorectal cancer in adults 50 to 75 years of age. Ann Intern Med. 2016;165(6):JC26. doi: 10.7326/ACPJC-2016-165-6-026. https://doi.org/10.7326/ACPJC-2016-165-6-026. [DOI] [PubMed] [Google Scholar]
- 33.LeFevre ML, U.S. Preventive Services Task Force Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(4):281–290. doi: 10.7326/M14-1204. https://doi.org/10.7326/M14-1204. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2011;154(5):356–364. doi: 10.7326/0003-4819-154-5-201103010-00307. https://doi.org/10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 35.2016 Cleveland Clinic Outcomes Book: Medicine Institute. Measuring Outcomes Promotes Quality Improvement Cleveland Clinic. https://my.clevelandclinic.org/-/scassets/files/org/outcomes/outcomes-medicine.ashx. Accessed January 5, 2018.
- 36.Moyer VA, U.S. Preventive Services Task Force Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. https://doi.org/10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 37.Han X, Robin Yabroff K, Guy GP, Jr, Zheng Z, Jemal A. Has recommended preventive service use increased after elimination of cost-sharing as part of the Affordable Care Act in the United States? Prev Med. 2015;78:85–91. doi: 10.1016/j.ypmed.2015.07.012. https://doi.org/10.1016/j.ypmed.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taksler GB, Braithwaite RS. Developing a composite weighted quality metric to reflect the total benefit conferred by a health plan. Am J Manag Care. 2015;21(3):221–227. [PubMed] [Google Scholar]
- 39.Satcher D. Preventive interventions: an immediate priority. Ann Fam Med. 2017;15(1):8–9. doi: 10.1370/afm.2026. https://doi.org/10.1370/afm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeRigne L, Stoddard-Dare P, Collins C, Quinn L. Paid sick leave and preventive health care service use among U.S. working adults. Prev Med. 2017;99:58–62. doi: 10.1016/j.ypmed.2017.01.020. https://doi.org/10.1016/j.ypmed.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Jaen CR, McIlvain H, Pol L, Phillips RL, Jr, Flocke S, Crabtree BF. Tailoring tobacco counseling to the competing demands in the clinical encounter. J Fam Pract. 2001;50(10):859–863. [PubMed] [Google Scholar]
- 42.Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38(2):166–171. [PubMed] [Google Scholar]
- 43.Jaen CR, Stange KC, Tumiel LM, Nutting P. Missed opportunities for prevention: smoking cessation counseling and the competing demands of practice. J Fam Pract. 1997;45(4):348–354. [PubMed] [Google Scholar]
- 44.Nutting PA, Baier M, Werner JJ, Cutter G, Conry C, Stewart L. Competing demands in the office visit: what influences mammography recommendations? J Am Board Fam Pract. 2001;14(5):352–361. [PubMed] [Google Scholar]
- 45.Bohlen K, Scoville E, Shippee ND, May CR, Montori VM. Overwhelmed patients: a videographic analysis of how patients with type 2 diabetes and clinicians articulate and address treatment burden during clinical encounters. Diabetes Care. 2012;35(1):47–49. doi: 10.2337/dc11-1082. https://doi.org/10.2337/dc11-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295(16):1935–1940. doi: 10.1001/jama.295.16.1935. https://doi.org/10.1001/jama.295.16.1935. [DOI] [PubMed] [Google Scholar]
- 47.May C. Towards a general theory of implementation. Implement Sci. 2013;8:18. doi: 10.1186/1748-5908-8-18. https://doi.org/10.1186/1748-5908-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.May CR, Eton DT, Boehmer K, et al. Rethinking the patient: using Burden of Treatment Theory to understand the changing dynamics of illness. BMC Health Serv Res. 2014;14:281. doi: 10.1186/1472-6963-14-281. https://doi.org/10.1186/1472-6963-14-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wyatt KD, Stuart LM, Brito JP, et al. Out of context: clinical practice guidelines and patients with multiple chronic conditions: a systematic review. Med Care. 2014;52(suppl 3):S92–S100. doi: 10.1097/MLR.0b013e3182a51b3d. https://doi.org/10.1097/MLR.0b013e3182a51b3d. [DOI] [PubMed] [Google Scholar]
- 50.Pentakota SR, Rajan M, Fincke BG, et al. Does diabetes care differ by type of chronic comorbidity?: An evaluation of the Piette and Kerr framework. Diabetes Care. 2012;35(6):1285–1292. doi: 10.2337/dc11-1569. https://doi.org/10.2337/dc11-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piette JD, Richardson C, Valenstein M. Addressing the needs of patients with multiple chronic illnesses: the case of diabetes and depression. Am J Manag Care. 2004;10(2 Pt 2):152–162. [PubMed] [Google Scholar]
- 52.Aung E, Donald M, Coll J, Dower J, Williams GM, Doi SA. The impact of concordant and discordant comorbidities on patient-assessed quality of diabetes care. Health Expect. 2015;18(5):1621–1632. doi: 10.1111/hex.12151. https://doi.org/10.1111/hex.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


