Abstract
Purpose
To present the evolution of intravitreal therapy for retinal diseases and its impact on clinical practice.
Design
Retrospective literature review and personal perspective.
Methods
Retrospective literature review and personal perspective.
Results
Pharmacotherapeutic advances in retinal disease have been remarkable over the last 25 years. Almost all of the new drugs developed have required intravitreal administration to be highly effective, leading to an exponential increase in the annual number of intravitreal injections given. The use of intravitreal antibiotic injections to treat endophthalmitis, usually on a one-time basis, first familiarized ophthalmologists with this method of drug delivery. Ganciclovir was the first widely available, relatively inexpensive, compounded drug that was used for repeat intravitreal injection to treat a chronic retinal disease, followed by triamcinolone for diabetic macular edema and bevacizumab for neovascular AMD. Ganciclovir was formulated for sustained-release drug delivery to avoid frequent intravitreal injections, a goal that has been more elusive for anti-VEGF drugs. Political obstacles encountered while conducting some of the trials to evaluate these treatments were substantial. Addressing the issues they raised led to important national policy changes that will impact the conduct of future clinical trials. The first comparative efficacy trial of intravitreal therapies was the Comparison of AMD Treatments Trials (CATT). The primary results from CATT and the many publications that followed continue to shape the use of intravitreal therapy today.
Conclusion
Intravitreal therapy has proven highly effective for the treatment of many retinal diseases. The treatment burden for patients from numerous injections, the cost to health care systems, and the impact on workflows in clinical practice have been substantial. Efforts to develop effective intravitreal therapies with reduced treatment burden and cost are ongoing.
Introduction
It is an extraordinary honor to present the 74th Jackson Memorial Lecture. One of the more difficult tasks after learning you have been selected to give this lecture is choosing the topic. Most people who know me would expect me to discuss the Comparison of AMD Treatments Trials (CATT) and do not worry, I will not disappoint! But CATT was just one trial that occurred during a fundamental paradigm shift in the treatment of retinal disease that began more than 25 years ago. I have had the opportunity to serve in a leadership role for many of the trials that contributed to this paradigm shift, a series of drug discoveries that have fundamentally altered outcomes for diseases once thought untreatable and dramatically changed how we practice. The common denominator for these new drugs is that they are all intravitreally delivered.
This lecture is a narrative that includes my own personal reflection on the clinical trials that shaped this treatment revolution, the data and new concepts they revealed and the challenges that were encountered while attempting to execute these studies. It is important to note that I am only one of the hundreds of physicians and scientists who made these clinical trials possible. Clinical trials are a team sport, a true democratic effort for which the leaders frequently receive much more credit than they are due. That has never been more true than at this very moment. Almost everything I know or have contributed has been the result of teamwork with a group of mentors and colleagues who are among the greatest luminaries of our generation. These include Stuart Fine, Maureen Maguire, Rick Ferris, Robert Machemer, Tom Aaberg Sr, Travis Meredith, Paul Sternberg, Robert Nussenblatt, Matthew Davis, my collaborators in CATT and now the Diabetic Retinopathy Clinical Research (DRCR) Network, and all of my colleagues at the Cleveland Clinic Cole Eye Institute. I have been profoundly shaped by their brilliance and by their friendship and I share this honor with them. The collegial and collaborative ophthalmic community in which I have spent my career is one that I am sure would have made Edward Jackson proud.
Over the last 25 years, the greatest rate of growth of any procedure in all of medicine is CPT code 67028, intravitreal injection of a pharmacologic agent. In 1993, the total number of CPT-67028 claims paid by fee-for-service Medicare was 2,382. In 2016, this number had exploded to 3,272,800 (Figure 1)1,2 When one includes the 35% of patients in Medicare Advantage Programs, the number of injections jumps to 4,418,280 and when the best estimates for Medicaid and commercial insurance are added, the estimated number of intravitreal injections in the United States in 2016 is 7 million. Worldwide, the estimated number of intravitreal injections in 2016 is over 20 million. Assuming that there are approximately 2500 retina specialists who deliver the majority of these injections in the US and assuming about 180 clinic days per physician per year, the typical retina specialist who might have given 1 or 2 intravitreal injections in an entire year 25 years ago now administers more than 15 per day. This stunning increase is a direct reflection of the numerous drug discoveries for retinal pharmacotherapy that have taken place over the last few decades and the recognition that these drugs are most effective when intravitreally delivered.
Figure 1.
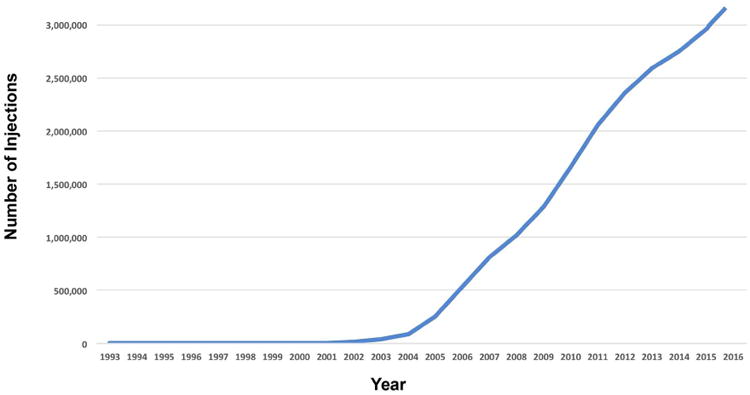
(A) Number of intravitreal injection claims paid by fee-for-service Medicare between 1993 and 2016.
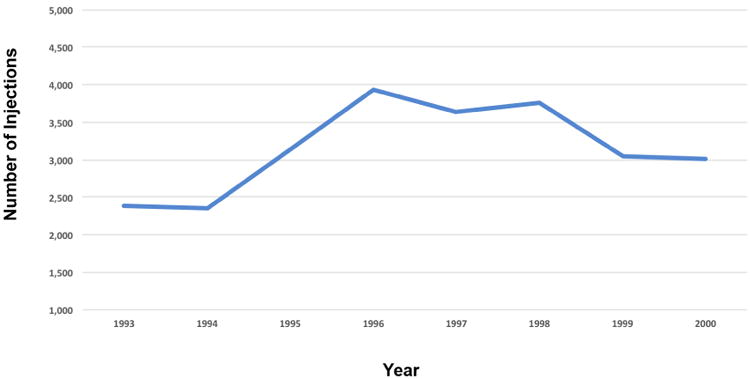
(B) Increase and decline in intravitreal injections between 1994 and 2000, presumably due to the initial increase and subsequent decline in CMV retinitis during this time period. Patients with CMV patients were usually too young to qualify for Medicare unless they were disabled and some were. The absolute number of injections between 1994 and 2000 was much higher but the trend was still captured in this Medicare database.
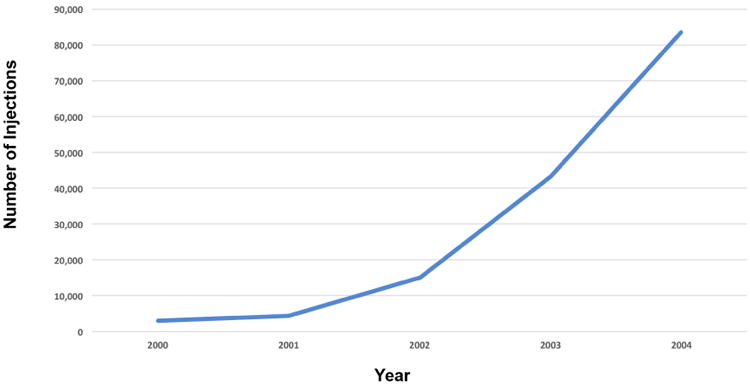
(C) Increase in number of injections beween 2000 and 2004 presumably due to the substantial increase in the use of intravitreal triamcinolone.
Throughout the first half of the 20th century, the vitreous cavity was a sacred space, one that was rarely entered. It was not until the late 1960's when Robert Machemer and Tom Aaberg began developing the surgical principles and instrumentation for vitrectomy, that the idea of regularly accessing this space for both surgical and pharmacotherapeutic purposes began to evolve.3 In the 1970's, Gholam Peyman and others injected a variety of antibacterial, anti-neoplastic, and anti-inflammatory compounds into the vitreous cavity in an animal model to assess safety, and many of these drugs were later used in humans.4-7 However, the initial use of these drugs was infrequent and usually limited to a single dose. The more common mechanism for delivering drugs to the posterior segment at the time was systemic administration. Several studies demonstrated that measurable intraocular drug levels could be achieved by intravenous or oral drug administration.8,9 However, these levels were modest at best and frequently below the minimum inhibitory concentration needed to clear an acute infection or render a disease inactive.10,11 There are two reasons for these low drug levels: the blood-ocular barrier and the size and avascular nature of the vitreous cavity. The blood-ocular barrier is highly effective at limiting the penetration of systemically administered drugs into the eye. Any amount of drug that does enter the eye does so along the circumference of the vitreous cavity and then has to diffuse at least half way across a 1 inch avascular sphere, making it difficult to achieve therapeutic levels of drug across the entire volume of the vitreous cavity.
In the 1980's, intravitreal antibiotics began to be used with increasing frequency to treat intraocular infection.12 The Endophthalmitis Vitrectomy Study subsequently established intravitreal antibiotics as standard of care for the treatment of acute post-operative endophthalmitis.13 These single-dose intravitreal injections proved to be highly effective and ophthalmologists became very familiar with their administration. However, the idea of repeatedly bypassing the blood-ocular barrier to treat a chronic posterior segment disease with intravitreal injections or sustained-release intravitreal therapy did not exist. The disease that would change all of that was cytomegalovirus (CMV) retinitis.
CMV Retinitis and Intravitreal Ganciclovir
Prior to the advent of the acquired immunodeficiency syndrome (AIDS), CMV retinitis was an uncommon disease that occurred almost exclusively in patients who had rare forms of immune compromise or were pharmacologically immunosuppressed for organ transplantation or treatment of severe autoimmune disease.14,15 As the AIDS epidemic unfolded in the 1980's, a large pool of patients emerged who were profoundly immunosuppressed (CD4 < 50 cells/mm3) and at risk for developing CMV disease.16,17 By the early 1990's, CMV infection and its most obvious clinical presentation, CMV retinitis, had become the most common opportunistic infection in patients with AIDS.18 At the same time, mean survival of CMV retinitis patients had increased over a 10 year period from 6 weeks19 to more than 1 year20,21 due to improved opportunistic infection prophylaxis and a modest level of HIV suppression from a growing number of nucleoside reverse-transcriptase inhibitors.22,23 The net effect was an exponential increase in the number of CMV retinitis patients who needed treatment and a pressing need for therapy that could more effectively control their disease.
With the advent of zidovudine (AZT) in 1986 for the treatment of human immunodeficiency virus (HIV) infection24 and intravenous ganciclovir for the treatment of CMV retinitis in 1988,25,26 patients with AIDS and CMV retinitis were confronted with a vexing clinical problem. Both drugs were myelosuppressive and patients were already severely immunocompromised. As a result, some patients were forced to choose between treating their underlying HIV disease or the viral retinitis that would inevitably render them blind. In addition, the therapeutic effect of intravenous ganciclovir was limited, with a median time to progression of retinitis of only 47 days,26 and chronic intravenous ganciclovir adminstration required an indwelling catheter that was associated with an increased risk for sepsis and an adverse impact on quality life. In an effort to overcome these treatment limitations, several physicians began to investigate the use of intravitreal injections of ganciclovir for CMV retinitis.27, 28 The intravitreal half-life was short and it became obvious that injections needed to be given weekly. This strategy proved to be highly effective and became an important therapeutic option for the treatment of patients who had failed intravenous ganciclovir or foscarnet. In a small number of clinics in the United States, Europe, and Australia, intravitreal ganciclovir was adopted as first-line therapy.29-31 As such, ganciclovir became the first widely available, relatively inexpensive, compounded drug that was employed for repeat intravitreal injection to treat a chronic retinal disease (Figure 1). It was also the first time that retina specialists began to manage clinical workflows to accommodate the many injections they were performing and that the treatment burden for patients from frequent intravitreal injections became obvious.
As patients' AIDS-related co-morbidities increased in frequency and severity, clinic visits and the associated injections were sometimes missed leading to progression of retinitis. To address this problem, a sustained-release ganciclovir implant that could be surgically placed in the vitreous cavity was developed (Figure 2A-C).32-34 The initial experience was promising and a series of randomized clinical trials followed. In the first study, patients with peripheral CMV retinitis were randomly assigned to the ganciclovir implant or initial observation. The median time to progression of retinitis for ganciclovir implant treated patients was 226 days versus 15 days for patients who were initially observed (p<0.001) (Figure 3).35 In a second trial that compared the ganciclovir implant to intravenous ganciclovir, the median time to progression of retinitis was 221 days for implant-treated patients versus 71 days for patients treated with intravenous ganciclovir (p<0.001) (Figure 4).36 In the third and largest trial, the median time to progression of retinitis was extended to more than 365 days when the implant was paired with oral ganciclovir (4.5 gm/day) versus 66 days for patients treated with intravenous ganciclovir (p<0.001) (Figure 5).37 I had the privilege of leading all three of these trials and the therapeutic efficiency of intravitreal treatment was obvious. The ganciclovir implant contained 5-6 mg of ganciclovir that was released directly into the vitreous cavity over an 8-month period and produced a mean vitreous drug level of 4 mcg/ml, well above the concentration required to achieve 50% and 90% inhibition of viral plaque formation of most clinical isolates of CMV.35 In contrast, standard induction/maintenance dosing of intraveneous ganciclovir over the same 8-month period would result in administration of over 100,000 mg of ganciclovir and would achieve a mean vitreous drug level of only 1 mcg/ml, a level that is well below the concentration needed for 50% or 90% inhibition of viral plaque formation for most strains of CMV.10,11 These subtherapeutic drug levels allow active CMV replication to occur in the presence of drug and likely explains the frequent development of ganciclovir resistance within 6 months after initiating intraveneous ganciclovir.38 In contrast, ganciclovir resistance was almost never seen in patients who were initially and continuously treated with the ganciclovir implant (personal observation – resistance never seen in over 300 cases of newly diagnosed CMV retinitis treated with the ganciclovir implant by me).
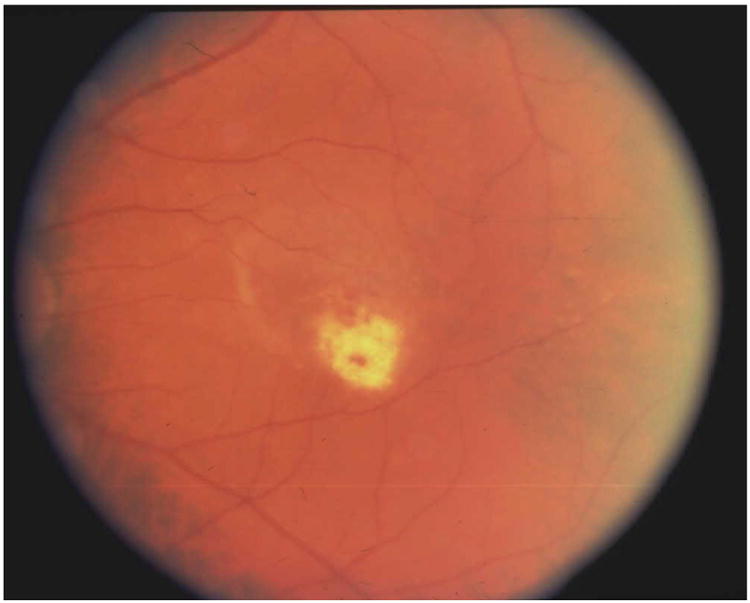
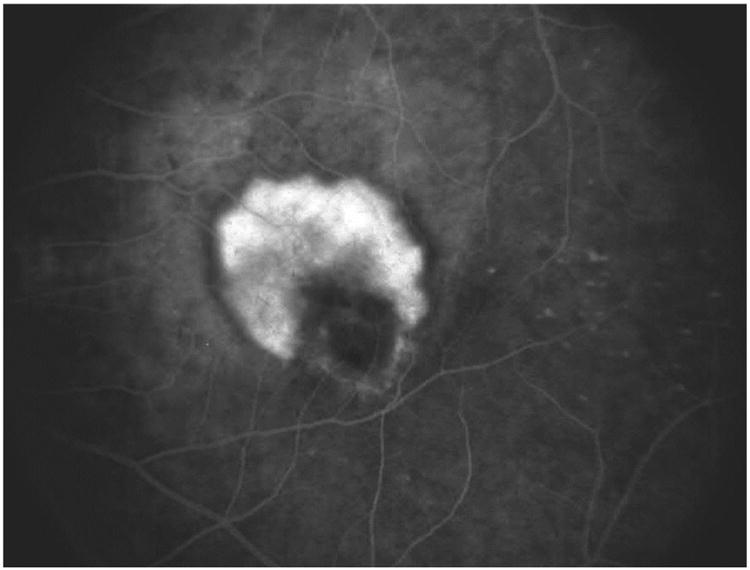
Figure 2.
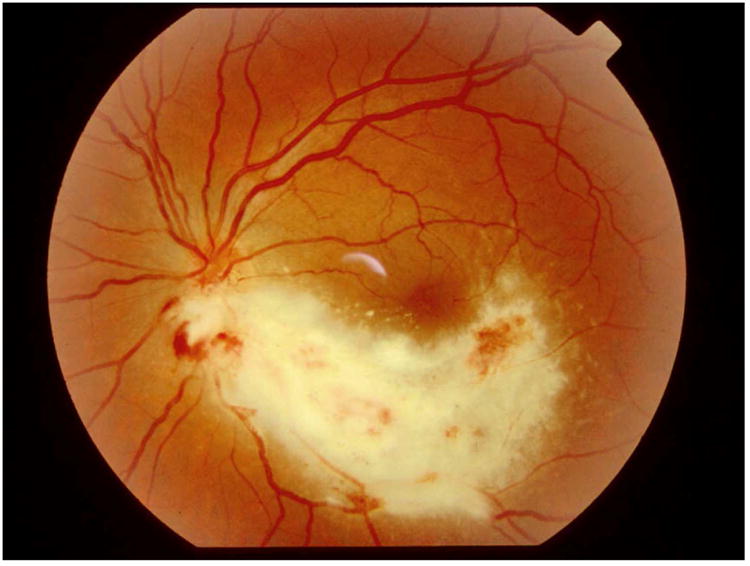
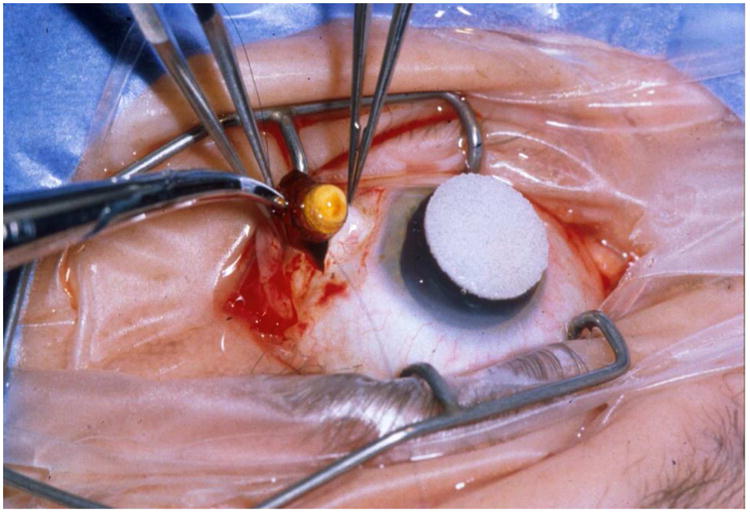
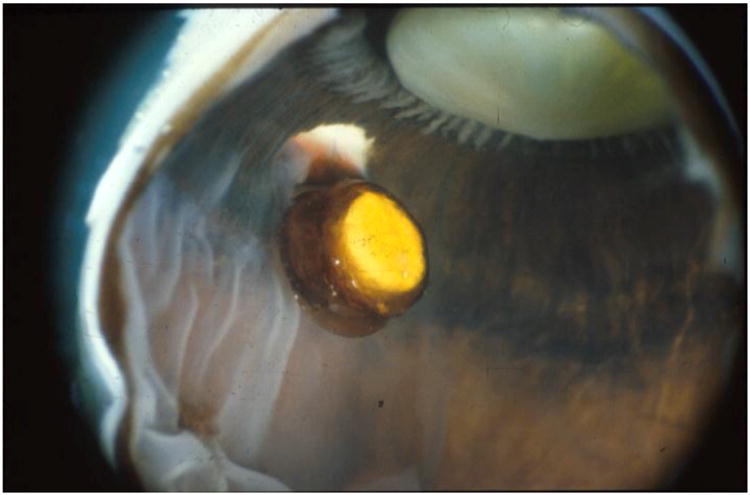
A) Typical case of CMV retinitis. B) Surgical insertion of ganciclovir implant. C) Location of ganciclovir implant as seen in a post-mortem eye.
Figure 3.
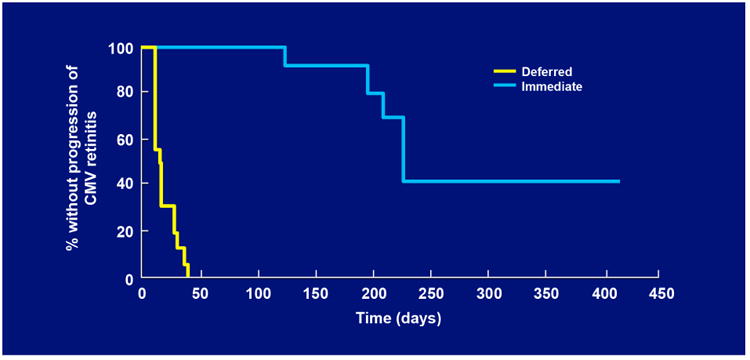
Kaplan-Meier curves showing the time to progression of cytomegalovirus (CMV) retinitis among eyes assigned to immediate treatment with a ganciclovir implant or deferred treatment, as determined by the Fundus Photograph Reading Center.
Figure 4.
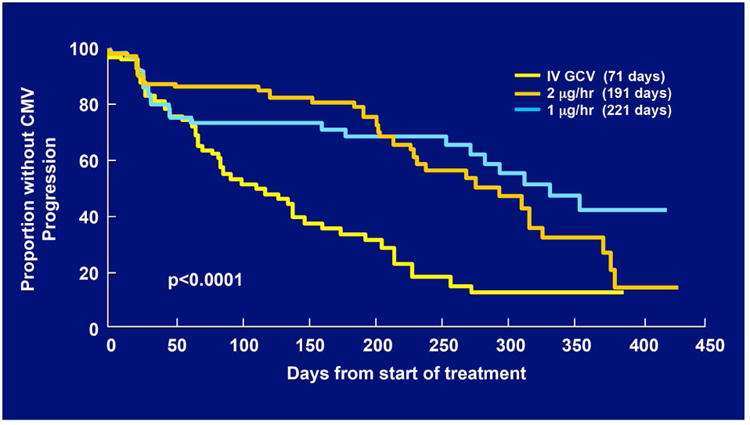
Kaplan-Meier curves showing the time to progression of cytomegalovirus (CMV) retinitis among eyes assigned to treatment with one of two doses of a ganciclovir implant or intravenous ganciclovir, as determined by the Fundus Photograph Reading Center.
Figure 5.
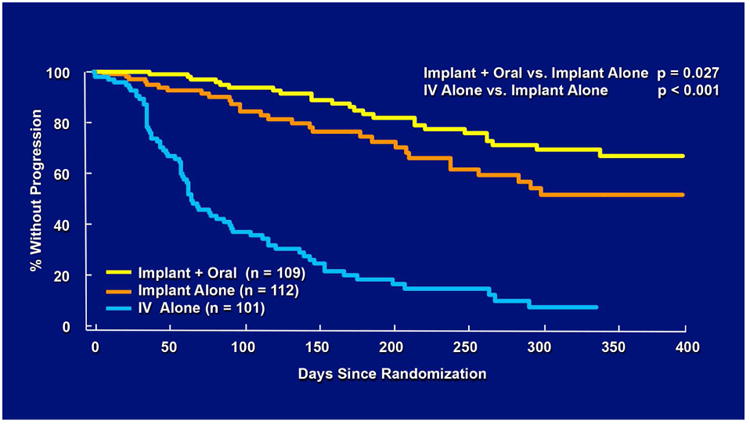
Kaplan-Meier curves showing the time to progression of cytomegalovirus (CMV) retinitis among eyes assigned to treatment with a ganciclovir implant alone, ganciclovir implant and oral ganciclovir (4.5 gm/day) or intravenous ganciclovir, as determined by the Fundus Photograph Reading Center.
The ganciclovir implant received Food and Drug Administration (FDA) approval in March 1996 and immediately became a standard of care. At about the same time, three protease inhibitors (saquinavir,39 ritonavir,40 and indinavir41,42) were FDA approved to treat HIV infection. The combination of one of these protease inhibitors with two nucleoside reverse transcriptase inhibitors produced Highly Active Antiretroviral Therapy (HAART). For the first time in the AIDS epidemic, HIV replication could be effectively suppressed, which allowed immune recovery to occur in many cases.43 As a result, the incidence of CMV retinitis dropped dramatically throughout the latter half of the 1990's (Figure 6).37, 44 In addition, the fundamental assumptions that drove the need for the ganciclovir implant, specifically the inevitability of progression of retinitis and the need for long-term constant levels of ganciclovir in the vitreous cavity, were altered and the need for the ganciclovir implant declined.45 Today, most patients who are diagnosed with CMV retinitis need only short term anti-CMV treatment until their HIV can be effectively treated and immune recovery occurs. Valganciclovir, an oral prodrug of ganciclovir, has been shown to be highly effective for providing short term anti-CMV coverage and has become the standard of care.46 The ganciclovir implant is no longer manufactured but its place in history remains important. It was the first FDA approved sustained-release drug delivery device for any disease in any organ and it firmly established that local therapy, specifically bypassing the blood-ocular barrier, was not only well tolerated but much more effective than systemic therapy for a retinal disease.
Figure 6.
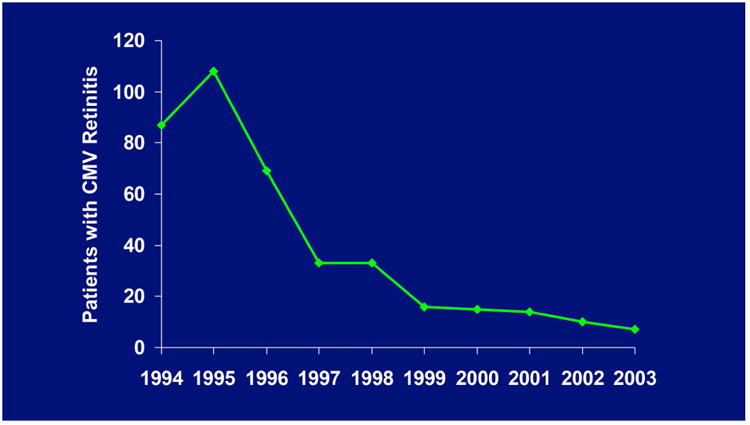
New cases of CMV retinitis referred to me between 1994 and 2001.
Inflammation, Diabetic Macular Edema and Intravitreal Steroids
The ganciclovir implant paved the way for the fluocinolone implant. This sustained-release drug delivery device contains fluocinolone, a lipophilic steroid that was ideal for formulation into an implant that would slowly release drug over several years.47,48 Clinical trials showed that it was highly effective for controlling intraocular inflammation and it was subsequently FDA approved for severe posterior uveitis.49-51 However, it was use of the fluocinolone implant in a small number of patients with recalcitrant diabetic macular edema (DME) that precipitated the next wave of intravitreal injections for retinal disease. At the annual meeting of the American Academy of Ophthalmology in 2000, Dr. Andrew Pearson presented a small series of patients with severe recalcitrant diabetic macular edema that he treated with a fluocinolone implant. In all cases, there was immediate and complete resolution of the edema, a therapeutic effect that had not been observed before in DME.52 The fluocinolone implant was not yet clinically available but another steroid, triamcinolone was available in every retina office where it was commonly employed for subTenon's injection. Almost immediately, physicians began injecting triamcinolone into the vitreous cavity hoping to replicate the beneficial steroid effects of the fluocinolone implant. These injections resulted in dramatic improvement in retinal thickening, as seen on the newly available optical coherence tomography (OCT), and led to an exponential increase in intravitreal steroid use (Figure 1) without any studies demonstrating a clear functional benefit when compared to focal laser treatment. Multiple publications followed, first case series53,54 and then randomized clinical trials.55,56 These studies confirmed that intravitreal triamcinolone reduced or eliminated diabetic macular edema on OCT in most patients and was associated with improved visual acuity in many patients, but not all. The therapeutic indication for intravitreal triamcinolone expanded to include neovascular AMD, where it was combined with photodynamic therapy (PDT),57,58 and macular edema from any cause such as retinal vein occlusion,59 uveitis,60 and pseudophakic cystoid macular edema.61 Intravitreal triamcinolone produced the first substantial pharmacotherapeutic effect for many of these diseases and accounted for the continued increase in the number of intravitreal injection between 2000 and 2005, despite the near disappearance of CMV retinitis from clinical practice. It was the second inexpensive and widely available drug for intravitreal injection that was rapidly adopted as standard of care despite the absence of any randomized clinical trial data to support its use. It was a foreshadowing of what was to come a few years later with the introduction of drugs to block vascular endothelial growth factor (VEGF).
Neovascular AMD and Intravitreal Anti-VEGF Treatment
During the 1990s, there were a number of studies that identified VEGF as a potential target for blockade to treat choroidal neovascularization. VEGF and VEGF receptors were expressed at high levels in areas of CNV in primates,62 VEGF was present at high levels in surgically excised neovascular membranes from patients with AMD,63 and VEGF was expressed in choroidal neovascular membranes of autopsied eyes with AMD.64 Two anti-VEGF drugs were initially developed: a 28-nucleotide aptamer (with attached polyethylene glycol moiety) that was designed to selectively bind to VEGF-A165,65 and a recombinant humanized Fab fragment (rhuFAB) of a monoclonal antibody that blocked all isoforms of VEGF-A.66,67 In January 1999, the first intravitreal anti-VEGF injection in humans was given. I had the privilege of giving that first injection and the compound was the 28-nucleotide aptamer, then known as NX1838. The patient had a disciform scar and was injected as part of the phase 1 trial to determine drug safety. However, a subsequent patient in the study had active recurrent subfoveal CNV following previous laser photocoagulation. One month after injection, there was decreased leakage on fluorescein angiography and no additional lesion growth. Three months after the injection, at a time when the drug would have been fully cleared from the eye based on its half-life, there was substantial lesion growth (Figure 7A-C). This was the first clinical suggestion that suppressing VEGF-A might have a therapeutic effect on neovascular AMD.
Figure 7.
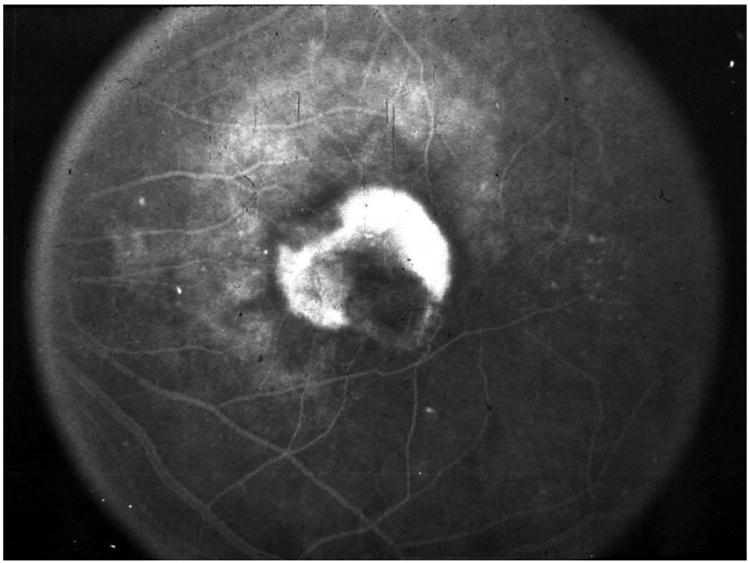
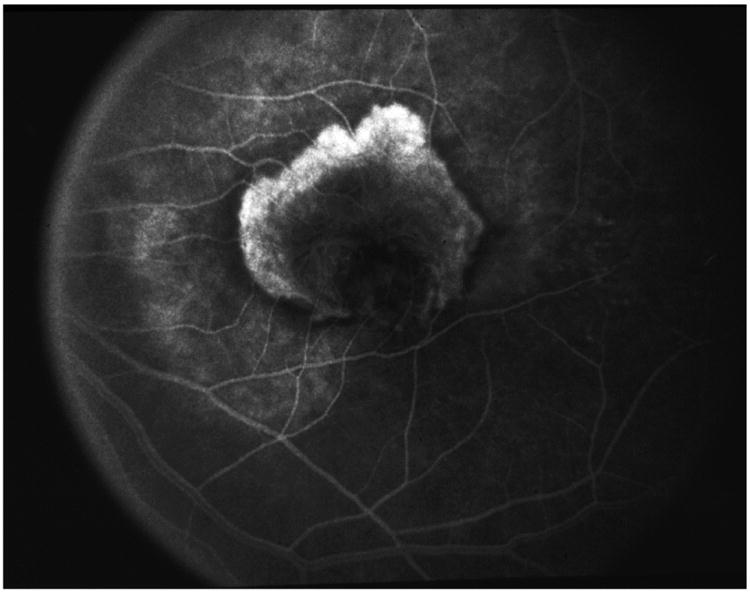
A&B) Color photo and fluorescein angiography of an active recurrent subfoveal CNV following previous laser photocoagulation. C) One month after intravitreal injection of NX1838. There was decreased leakage on fluorescein angiography, mild contraction of the CNV and no additional lesion growth. D) Three months after the injection, at a time when the drug would have been fully cleared from the eye based on its half-life, there was substantial lesion growth.
During the Phase 1 trial, NexStar, the company developing the drug, was acquired by Gilead. Gilead was focused on developing antiviral drugs (CMV, HIV, influenza), was mostly interested in NexStar's drug discovery technology, and had little interest in their anti-VEGF compound. At the time, Gilead's only FDA approved drug was cidofovir, a drug for CMV retinitis and as a result, I knew the company well. When asked who would be interested in an anti-VEGF compound, my recommendation to Gilead was to sell to a company who could formulate the anti-VEGF drug in a sustained-release drug delivery device, much as we had done with the ganciclovir implant, to avoid the need for frequent injections. The retina community was already unhappy with every three month PDT retreatments for neovascular AMD and there was little appetite by physicians or patients for a treatment that required office visits and injections every month. At the time, Bausch and Lomb had the largest portfolio of patents covering sustained-release drug delivery technology and I facilitated the initial meetings between Gilead and Bausch & Lomb. Near the end of negotiations between the two companies, EyeTech emerged and acquired NX1838. In subsequent discussions with EyeTech, as well as with Genentech who was developing rhuFab, I was asked several times if patients and clinicians would really tolerate monthly intravitreal injections. In 2001, the only group of clinicians in the US who had experience with thousands of intravitreal injections and had run clinics to accommodate these frequent injections was a small group of us who had treated large numbers of patients with CMV retinitis. We were accustomed to an injection frequency of every week and had developed the injection protocols and work flows that are widely used today. An every 4 week schedule would be better than every week, but still less desirable than the development of a sustained-release device capable of delivering anti-VEGF therapy.
Between 2001 and 2005, NX1838 became pegaptanib (Macugen) and rhuFab became ranibizumab (Lucentis). Eyetech completed two randomized clinical trials that established pegaptanib as modestly effective for slowing vision loss, but ineffective for improving vision in most patients.68,69 It was enough for FDA approval in December 2004 and the drug was launched in January 2005. Soon thereafter, Genentech completed its two pivotal clinical trials for ranibizumab.70,71 It was these results along with the emergence of bevacizumab that would profoundly affect the future course of treatment of neovascular AMD and many other retinal diseases.
Ranibizumab and Bevacizumab
In July 2005, results from the MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab In the treatment of Neovascular AMD) clinical trial showed that intravitreal ranibizumab was highly effective for the treatment of occult and minimally classic choroidal neovascularization secondary to AMD.70 Previous treatments had only been able to slow the rate of vision loss and visual acuity improvement had been rare. Instead, monthly ranibizumab in both MARINA and later in ANCHOR (Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in AMD), a clinical trial that compared ranibizumab to PDT in patients with predominantly classic CNV,71 resulted in a mean visual acuity improvement of 7 and 11 letters, respectively. Forty percent of patients in both trials retained visual acuity of 20/40 or better at one year.
At the same national meeting in July 2005 where the MARINA clinical trial results were presented, Dr. Philip Rosenfeld presented a single case report of the use of intravitreal bevacizumab to treat neovascular AMD.72 In a previous study, bevacizumab had been given intravenously to treat neovascular AMD.73 Most patients had experienced improvement in visual acuity and marked reduction in retinal thickness, a therapeutic effect not seen with previous treatments. However, given the known risk of hypertension and systemic thromboembolic events associated with the use of intravenous bevacizumab in cancer patients, additional study was not pursued. Instead, an intravitreal injection of bevacizumab was administered in one patient and there was immediate and marked resolution of fluid on OCT, similar to what was assumed to have occurred with ranibizumab. Bevacizumab had target specificity that was similar to ranibizumab, was already FDA approved for colorectal cancer and therefore available for off-label use, and had a low cost given the small volume required for intraocular treatment.74 Following this single case report, ophthalmologists began using off-label intravitreal bevacizumab as a surrogate for ranibizumab to treat neovascular AMD. The beneficial effect was obvious and within months, bevacizumab had been adopted as first-line therapy throughout the United States despite the absence of any randomized clinical trial data to support its use. By the time ranibizumab was FDA approved in June 2006, bevacizumab had become standard of care and it was estimated that more half a million injections had been given worldwide. In addition, PRN (pro re nata) dosing had become the most common treatment strategy employed. When ophthalmologists began using bevacizumab, its intraocular safety and duration of therapeutic effect were unknown. Ophthalmologists had proceeded cautiously, treating only when there was evidence of disease activity. PRN dosing appeared to produce good visual acuity results, and monthly injections had not been required for many patients.74-76 A small study of PRN dosing with ranibizumab was performed and yielded encouraging results.77 It was assumed that when ranibizumab was approved, it too would be administered mostly on a PRN basis even though there was no clinical trial data that confirmed PRN dosing as equivalent to monthly dosing. The need for a head-to-head trial comparing the two drugs as well as monthly versus PRN dosing was obvious and the National Eye Institute approved funding for the Comparison of AMD Treatments Trials (CATT). Although the cost of ranibizumab was unknown at the time CATT was funded, once the drug was FDA approved and its price established, the trial took on a whole new dimension. At a cost of $2,000 per dose for ranibizumab and $50 per dose for bevacizumab, the estimated annual savings to patients and payers in the US if bevacizumab was used instead of ranibizumab was more than $3 billion. With such large amounts of money at stake, one would assume that obtaining additional federal support for conducting a comparative effectiveness study would be easy. It was not.
Obstacles for Comparative Effectiveness Studies
The total cost of CATT was estimated to be $50 million. The National Eye Institute (NEI) granted the CATT Research Group $16.2 million over a four-year period to fund the infrastructure of the trial. However, the remaining $34 million in patient care cost was not covered. In our discussions prior to applying to NEI for funding, Center for Medicare and Medicaid Services (CMS) leaders were uncertain about their authority to cover the cost of ranibizumab in CATT even though it was FDA approved and Medicare would be responsible for the drug cost if the patient was not participating in the trial. A Clinton Executive Memorandum in 2000 allowed Medicare to cover routine care costs for Medicare patients participating in a clinical trial. However, the memorandum also specified that the investigational agent in a clinical trial could not be covered. By the time the grant for CATT was awarded, CMS lawyers had chosen to place precedence on the prohibition of paying for a drug under investigation and ignore the part about covering standard care, even though it was clear at that point that routine care included the use of ranibizumab. The fact that CMS stood to save more than $24 million due to the fact that half of patients would be assigned to bevacizumab and stood to save an additional $8 million from having patients assigned to PRN instead of monthly dosing, had no impact on their considerations. CATT leadership proposed covering the drug cost through Coverage with Evidence Development (CED), a CMS program authorized in 2003, that appeared ideal for our circumstance. However, at the time of our discussions in 2006, the program had never been utilized and it was not clear to anyone how it might be implemented. What was needed was an amendment to Medicare Clinical Trial Policy that clarified coverage of drug costs for clinical trial participants. CATT leadership spent more than a year working with CMS, which culminated in the Revised Medicare Clinical Trial Policy in July 2007. This policy revision explicitly extended coverage to drugs under investigation if they were normally covered outside of the trial. With this policy in place, 80% of the drug cost was covered.
Obtaining coverage for the remaining 20% of the drug cost, the standard Medicare co-pay, presented a new set of obstacles. Approximately 15% of Medicare beneficiaries did not have supplemental insurance. If these patients were assigned to monthly ranibizumab, they would become personally responsible for more than $5000 in drug cost per year versus less than $100 a year if they were assigned to PRN bevacizumab. This large cost difference could have led patients who were assigned to receive the more expensive drug to refuse treatment as their bills accumulated, possibly leading to differential drop out and erosion of the scientific integrity of the study. We first asked CMS if they could waive the co-pay, which we estimated to be $1 million for the entire study. The agency stood to save at least $24 million as previously described, but CMS had no statutory authority to alter co-pay requirements. Next, we worked closely with CMS to create a Medicare Demonstration Project. At different stages in the development of the project, we proposed a variety of creative mechanisms to cover the total cost of the drug. Despite positive reviews, each version of the project was declined. Finally, we explored whether or not another federal agency, in this case the National Eye Institute, could pay the co-pay for a Medicare beneficiary participating in an NIH-sponsored trial. There was no precedent for this, but after legal review it was determined that the co-pay could be covered by a federal grant and the NEI committed to do so when supplemental insurance was not available. This ruling has benefited several subsequent NIH sponsored trials including, most recently, trials conducted by the Diabetic Retinopathy Clinical Reasearch Network (DRCR.net).
The next challenge was obtaining and masking the identity of the drugs. When there is a pharmaceutical company partner in a clinical trial, the drugs under investigation are typically provided at no charge to the patient. In addition, the company usually supplies matching study drugs, thus facilitating drug administration in masked fashion. CATT leadership invited Genentech to partner with us on several occasions and at one point, the company agreed to participate. After several months of joint planning, the company decided not to support the trial. CATT was able to supply bevacizumab for the study and the cost of purchase, repackaging, and distribution was covered by the grant. However, ranibizumab had to be obtained commercially and that presented a number of challenges. Purchasing the drug while keeping the identity of the drug masked to the patient and at the same time relieving the patient of making payments while their insurance was billed proved to be impossible under the laws and policies then in place. CATT leadership worked with CMS for more than a year to develop a Demonstration Project that would address these questions. The final proposal was approved by CMS, but several months later, the Office of General Counsel of the Department of Health and Human Services inexplicably refused to give final approval. In our earliest meetings with CMS, we were told that “we would need an act of Congress” to execute the trial in the way that we thought was most scientifically rigorous. CATT leadership proposed the initial language for an amendment that granted statutory authority to CMS through the Secretary of Health and Human Services to develop alternative payment mechanisms that are needed to support the scientific integrity of NIH-sponsored trials. The bill was passed on July 15, 2008 as part of the 2008 Medicare Improvements for Patients and Providers Act (MIPPA).
Our goal of providing study drug to CATT patients with no out-of-pocket expense was achieved. The Revised Medicare Clinical Trial Policy supported ranibizumab use in the trial and bevacizumab was covered by NEI funds. The NEI agreed to pay all co-pays not covered by supplemental insurance policies. The Congressional amendment insured no further coverage and payment issues would occur and should facilitate future comparative efficacy trials conducted with public funds. Masking of the drug identity occurred at the local level with masked visual acuity examiners and masking of the treating physician. Patients were unmasked, but that proved to be immaterial as most patients were unaware of their treatment assignment when they were queried at the end of the study. The political process, Congressional amendment, and policy changes required to launch CATT consumed more than 2 years.78
The Comparison of AMD Treatments Trials (CATT)
Between February 2008 and December 2009, 1185 patients with newly diagnosed, untreated neovascular AMD were enrolled in CATT. Patients were randomly assigned to one of four treatment groups: ranibizumab monthly, bevacizumab monthly, ranibizumab PRN or bevacizumab PRN. In the PRN arms, a single injection was given to initiate treatment and subsequent injections given only if there was fluid on OCT or other signs of active CNV. The primary outcome was mean change in visual acuity at one year. A second randomization occurred at the end of Year 1 in the two monthly treatment arms with half of patients randomly assigned to continued monthly treatment or to PRN treatment during the second year. The final study visit was at two years.
In 2011, the one year results were reported.79 First, ranibizumab and bevacizumab were found to be equivalent for visual acuity. Second, there was a greater reduction in retinal thickness and fluid on OCT in ranibizumab-treated patients, but the absolute difference in thickness and fluid between drugs was small and did not translate into a difference in visual acuity. Third, there was no difference between drugs in the rate of death, myocardial infarction, stroke or vascular death. Finally, there were more serious adverse events (SAEs) in patients assigned to bevacizumab. However, after extensive review, there seemed to be no biological plausibility and this finding was not replicated in subsequent clinical trials. In 2012, the two-year findings were published confirming the one year results (Figure 8).80
Figure 8.
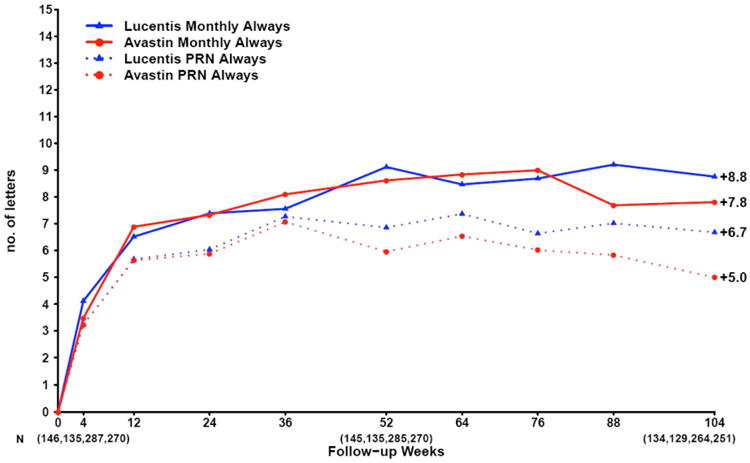
Mean change in visual acuity over time in patients treated with the same dosing regimen for 2 years in CATT.
Other Bevacizumab-Ranibizumab Trials
The equivalence of ranibizumab and bevacizumab for visual acuity was subsequently confirmed in five other international randomized clinical trials (Figure 9). The IVAN (Alternative Treatments to Inhibit VEGF in Age-related Choroidal Neovascularisation) study randomized 610 patients in the United Kingdom to one of four treatment arms: monthly bevacizumab, monthly ranibizumab, discontinuous (PRN) bevacizumab or discontinuous (PRN) ranibizumab.81,82 At one and two years, there was less than a 2 letter difference in visual acuity between drugs, but the findings were statistically inconclusive. When the IVAN data was pooled with CATT, however, the meta-analysis confirmed equivalence between drugs. The MANTA (Multicenter Anti-VEGF Trial in Austria) trial enrolled 320 patients in Austria who were randomly assigned to ranibizumab PRN or bevacizumab PRN.83 At one year, bevacizumab and ranibizumab were equivalent for visual acuity. The GEFAL (Groupe d'Etude Français Avastin versus Lucentis dans la DMLA néovasculaire) study enrolled 500 patients in France who were randomly assigned to ranibizumab PRN or bevacizumab PRN.84 At one year, ranibizumab and bevacizumab were equivalent for visual acuity. The BRAMD (Bevacizumab and Ranibizumab in Age-Related Macular Degeneration) trial enrolled 327 patients in the Netherlands who were randlomly assigned to ranibizumab monthly or bevacizumab monthly.85 At one year, there was no significant difference between drugs for visual acuity. The LUCAS (Lucentis Compared with Avastin Study) trial enrolled 432 patients in Norway who were randomly assigned to bevacizumab or ranibizumab with all patients treated using a treat and extend schedule.86 At one and two years, bevacizumab was equivalent to ranibizumab for visual acuity. Several subsequent meta-analyses have been performed using data from most of these trials and have concluded that ranibizumab and bevacizumab are equivalent for visual acuity for the treatment of neovascular AMD and that there are no appreciable differences in safety outcomes between drugs.87-89 Subsequent trials comparing ranibizumab and bevacizumab for the treatment of diabetic macular edema90 and macular edema associated with retinal vein occlusion91,92 have also shown no difference in visual acuity outcomes after one year of treatment.
Figure 9.
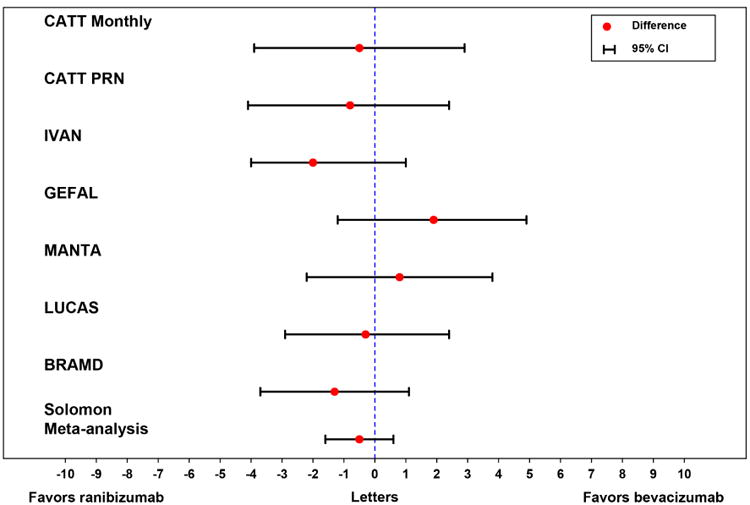
Difference between bevacizumab and ranibizumab in mean visual acuity at one year in the CATT, IVAN, MANTA, GEFAL, BRAMD, and LUCAS trials.
Other CATT Findings (Selected)
Since the publication of the one and two year results, the CATT Study Group has published, as of this writing, 50 manuscripts, editorials and letters. Many of these publications have importantly informed the management of AMD and continue to shape the use of intravitreal therapy.
Dosing
At one year, eyes assigned to PRN dosing had two letters less gain in visual acuity than monthly treated eyes. Although this was not significant at one year, it became statistically significant at two years.80 IVAN and HARBOR (pHase III, double-masked, multicenter, randomized, Active treatment-controlled study of the efficacy and safety of 0.5 mg and 2.0 mg Ranibizumab administered monthly or on an as-needed Basis (PRN) in patients with subfoveal neOvasculaRage-related macular degeneration), a 1098 patient clinical trial that compared monthly vs PRN dosing of ranibizumab at two different doses, also showed two letters less gain in visual acuity with PRN dosing when compared with monthly treatment,93 increasing the likelihood that the observed 2 letter difference was not due to chance. Overall visual acuity results were still excellent with PRN dosing and it required 10 fewer injections over a two year period than monthly treatment.80 Today, monthly treatment is infreqently employed and the most common treatment algorithm in use at present is “treat and extend”.94 However, there is still a powerful rationale to start with PRN treatment. A review of the distribution of the number of injections given during Year 1 of CATT reveals that 14% of patients needed only three or fewer injections to resolve all fluid during the first year of treatment (Figure 10A&B). Committing to any fixed dosing regimen at the initiation of treatment, including the modified fixed regimen of treat and extend, will result in substantial overtreatment in 1 in 7 patients.
Figure 10.
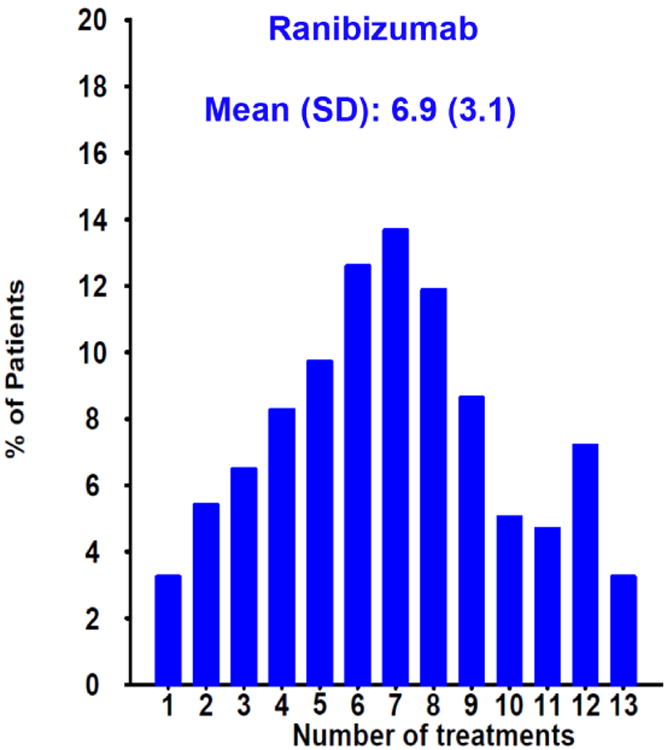
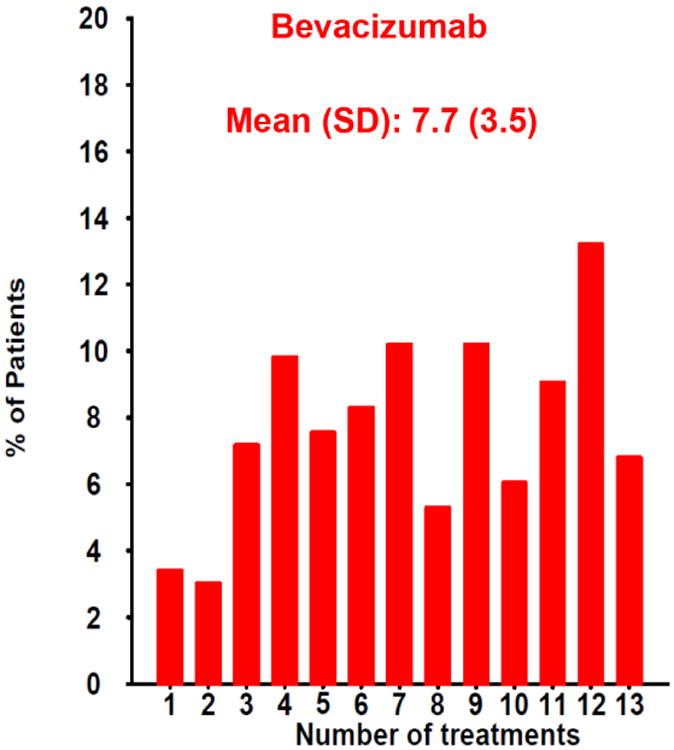
Distribution of the number of injections given in the PRN arms of CATT during the first year of the study for patients treated with (A) ranibizumab or (B) bevacizumab. One in seven (14%) of patients in both groups needed only three or fewer injections to resolve all fluid during the first year of treatment as determined by the treating ophthalmologist.
Predictors of the Numbers of Injections
The number of injections delivered in the PRN arms of CATT over two years was highly variable (Figure 11A&B). There were several baseline variables that predicted a small decrease in the mean number of injections over a two-year period. These include the presence of a retinal angiomatous proliferation (RAP) lesion, the presence of subretinal or sub-RPE hemorrhage, and the absence of subretinal fluid. However, there were no baseline characteristics that were associated with large differences in the number of injections.95,96 Investigations of the major AMD risk alleles and several VEGF and VEGF receptor single nucleotide polymorphisms (SNPs) failed to identify a genetic explanation for the wide heterogeneity in the number of injections given.97-99
Figure 11.
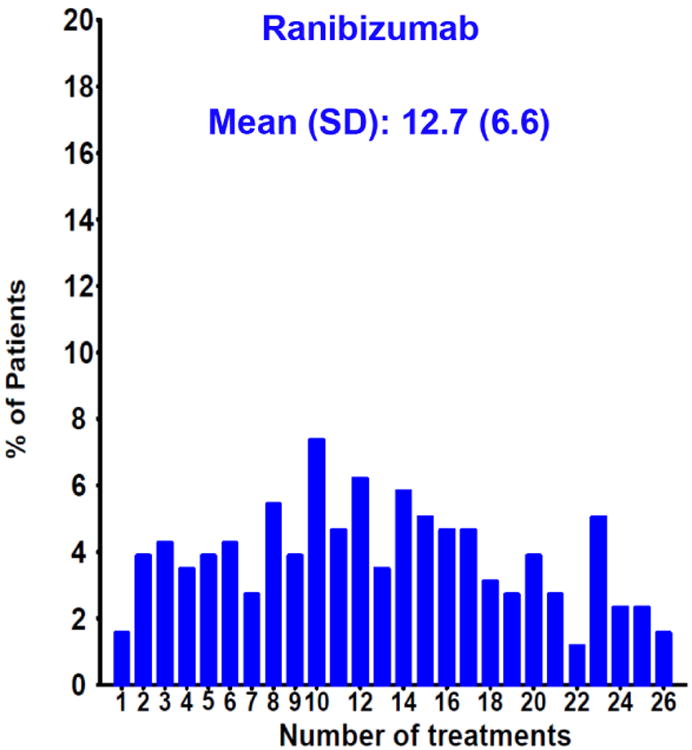
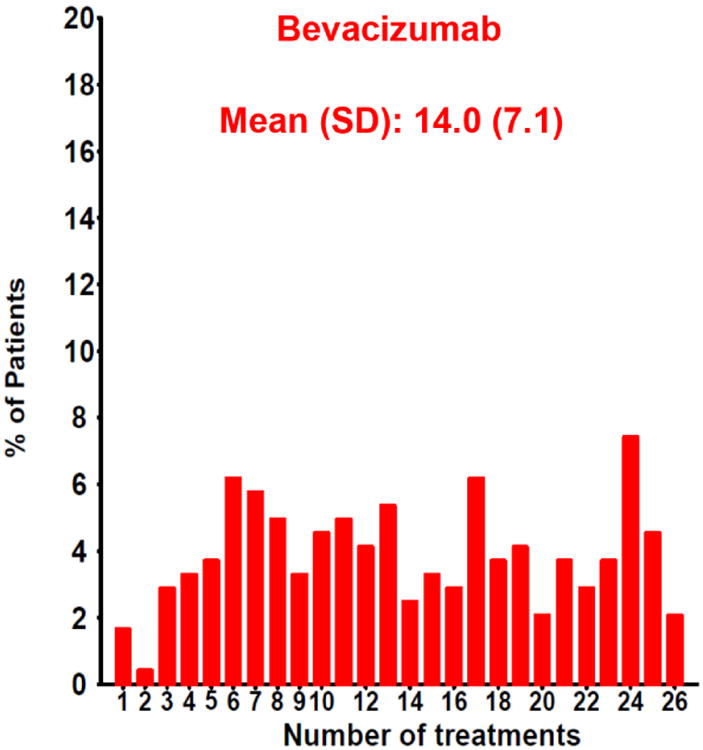
Distribution of the number of injections given in the PRN arms of CATT over two years for patients treated with (A) ranibizumab or (B) bevacizumab.
Predictors of Visual Acuity Outcomes
There are a number of baseline variables that predict longterm visual acuity outcomes, such as baseline visual acuity, age, and size of CNV lesion.100 However, their cumulative predictive value is less than 10%. The most powerful predictor by far of one and two year visual acuity outcomes is visual acuity response to treatment at 12 weeks.101 A patient with a one-line or greater gain at 12 weeks has an 85% chance of maintaining that gain at one year and 79% at two years (Figure 12).
Figure 12.
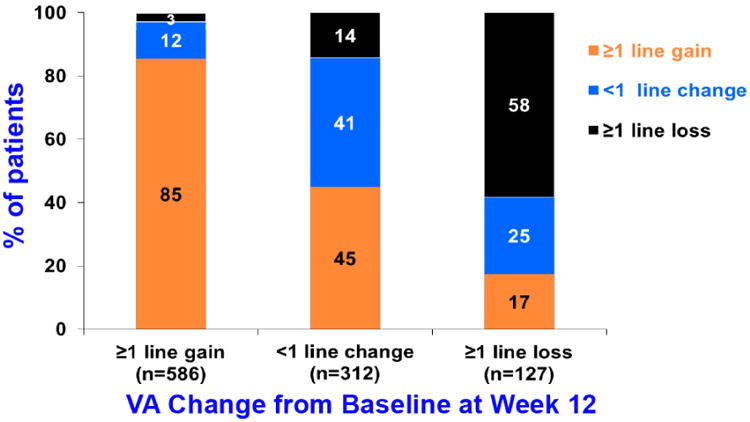
Bar graph showing visual acuity results at 1 year by early VA response to treatment at 12 weeks in CATT.
Impact of Fluid on Vision
Despite the fact that the vast majority of patients achieve substantial reduction of retinal thickness and fluid on OCT with anti-VEGF treatment, a small amount of fluid persists in most patients.79,80 Persistence of intraretinal fluid, especially in the center of the fovea, adversely affected vision at one and two years.102,103 Paradoxically, the persistence of subretinal fluid in the center of the fovea was associated with better visual acuity at all times points (Figure 13A&B).
Figure 13.
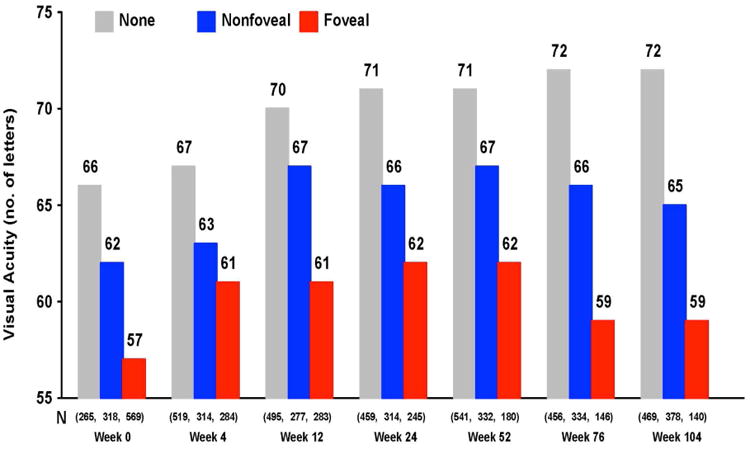
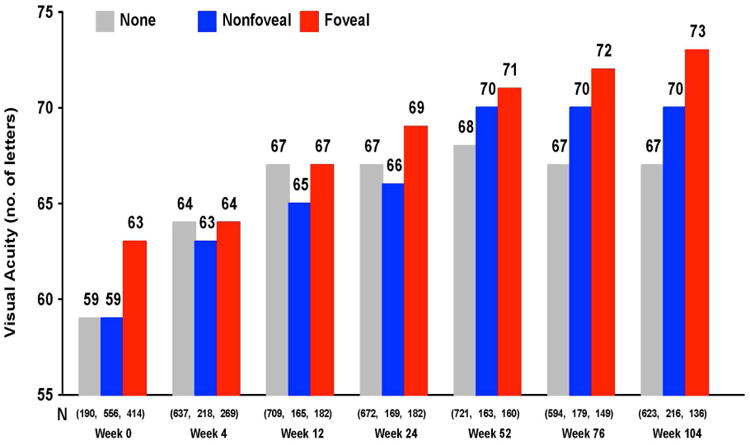
Mean visual acuity by status of fluid over two years in CATT for (A) intraretinal fluid and (B) subretinal fluid.
Geographic (Macular) Atrophy
At two years, monthly dosing of both ranibizumab and bevacizumab was associated with a higher rate of geographic atrophy than PRN dosing (p=0.003).80, 104-106 Most of the atrophy occurred in the bed of the original CNV lesion. Findings in IVAN were similar with a higher rate of atrophy in patients treated with continuous versus discontinuous dosing (p=0.03).82 A third randomized clinical trial, HARBOR, also showed an increased rate of atrophy in patients treated with monthly dosing versus PRN p<0.05).93 The reason for increased atrophy in monthly treated eyes is unknown, but the fact that this was observed in three large independent clinical trials makes this association unlikely to be due to chance alone.
Subretinal Hemorrhage
Unlike previous AMD treatment trials, CATT included eyes that had greater than 50% of their lesion composed of blood at baseline.107 These 84 patients (7.1% of the 1,185 patients in CATT) did remarkably well with a mean visual acuity improvement that was similar to those who never had a hemorrhage (Figure 14), although final mean visual acuity was slightly lower.
Figure 14.
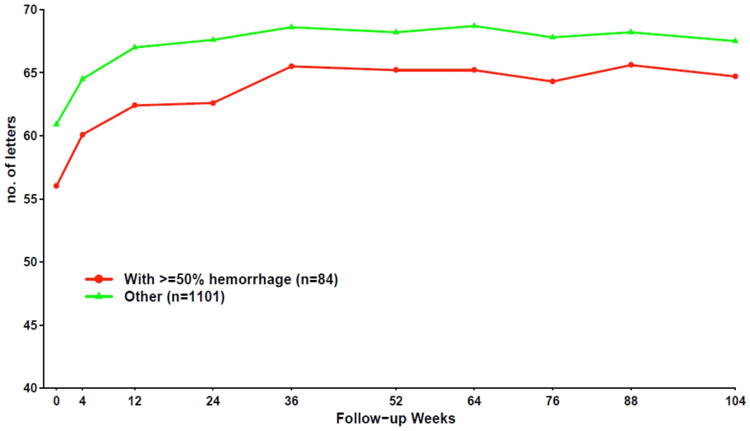
Mean visual acuity over time by presence of ≥ 50% hemorrhage at baseline.
Long-Term Follow-up
Five years after initiation of anti-VEGF treatment in CATT, 50% of patients had visual acuity of 20/40 or better (Figure 15A).108 However, there was a disappointing 11-letter decline in vision on average between Year 2 and Year 5 (Figure 15B). There were fewer injections given between Years 2 and 5 when compared to the number of injections given in the first 2 years of CATT in the PRN arms. However the reasons for fewer injections were multifactorial, and unavoidable in some cases, and likely do not alone explain the decline in vision. Between Year 2 and Year 5, there was considerable increase in atrophy of the retina and in atrophy of the RPE/choriocapillaris that likely contributed to VA decline. Giving more injections over this time would not have altered the evolution of atrophy and in fact may have increased it given the increased atrophy observed in 3 studies when more frequent injections were given. This increase in atrophy represents one the greatest challenges for improving long term outcomes in patients with neovascular AMD.
Figure 15.
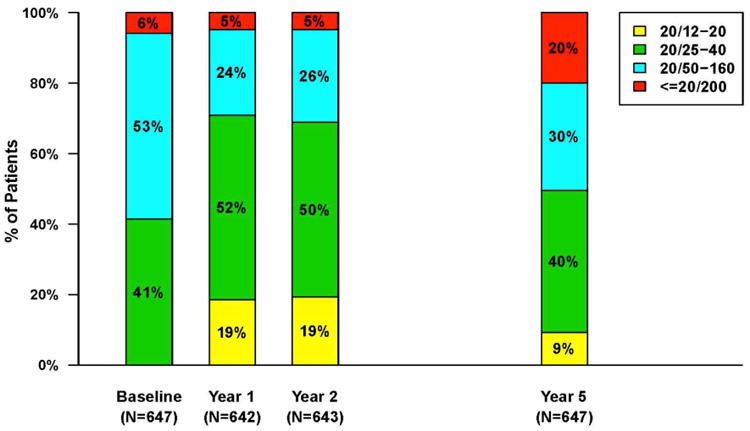
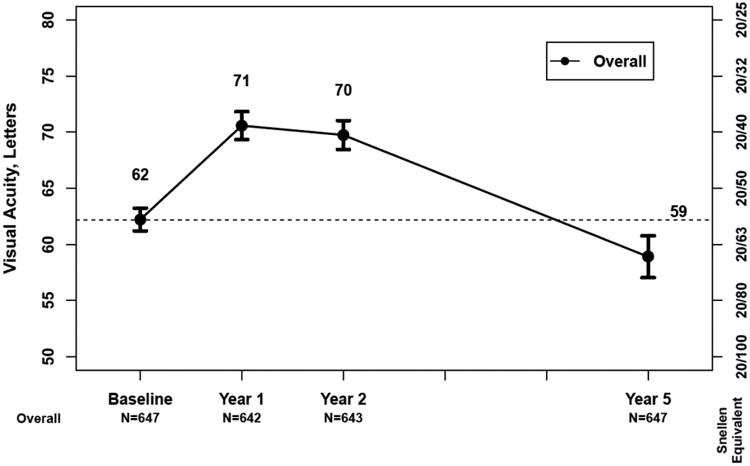
A) Bar graph showing the distribution of visual acuity over time for 647 patients in the CATT Follow-up Study. B) Mean visual acuity over time for patients in the CATT Follow-up Study
Other Intravitreal Drugs
A number of important additions to the retina specialist's intravitreal arsenal have been FDA approved in recent years. These include aflibercept and two sustained-release steroid formulations. Aflibercept is a soluble decoy receptor that binds vascular endothelial growth factor-A (VEGF-A) and placental growth factor (PIGF). Clinical trials showed aflibercept to be equivalent to ranibizumab for the treatment of neovascular AMD and also demonstrated that aflibercept dosed every 8 weeks was as effective as both ranibizumab and aflibercept dosed every 4 weeks.109 In other trials, aflibercept has been shown to be superior to ranibizumab and bevacizumab for the treatment of eyes with center-involved diabetic macular edema (DME) and visual acuity of 20/50 or worse,90 but equivalent to bevacizumab for the treatment of central retinal vein occlusion associated macular edema when each drug was dosed monthly for 6 months.110 The dexamethasone intravitreal implant (Ozurdex) is a sustained-release bioerodable formulation of dexamethasone that when injected in the vitreous cavity releases steroid continuously over a 3-6 month period. It has been shown to be effective for the treatment of diabetic macular edema,111 noninfectious uveitis,112 and macular edema associated with retinal vein occlusion.113 The fluocinolone acetonide intravitreal implant (Iluvien) is a non-erodible sustained release formulation of fluocinolone that when injected into the vitreous cavity releases steroid continuously for two to three years. It has been shown to be effective for the treatment of diabetic macular edema.114
Summary
Pharmacotherapeutic advances in retinal disease have been remarkable over the last 25 years. Almost all of the new drugs developed have required intravitreal adminstration to be highly effective, leading to an exponential increase in the annual number of intravitreal injections given. Ganciclovir was the first widely available, relatively inexpensive, compounded drug that was used for repeat intravitreal injection to treat a chronic retinal disease, followed by triamcinolone for DME and bevacizumab and ranibizumab for neovascular AMD. Ganciclovir was able to be formulated for sustained release drug delivery to avoid frequent intravitreal injection, a goal that has been more elusive for anti-VEGF drugs due in part to their molecular size and the challenges of protein stability at body temperature over time. The political obstacles encountered while conducting some of the trials to evaluate these treatments were substantial. Addressing the issues they raised led to important national policy changes that will impact the conduct of future clinical trials in ophthalmology as well as in all areas of medicine. The first comparative efficacy trial of intravitreal therapies was the Comparison of AMD Treatments Trials (CATT). The primary results from CATT and the many publications that followed continue to shape the use of intravitreal therapy today. Finally, the importance of the roles of pharmaceutical company sponsored trials, NIH sponsored studies and networks, and partnership between the two in the development of future intravitreal treatments cannot be overstated. Recent collaborations between the pharmaceutical industry and the DRCR Network underscore how successful these public-private partnerships can be. Given the number of intravitreal drugs already approved and in development, the expanding clinical indications for the use of these drugs, and the considerable efforts to reduce treatment burden by developing drugs with longer intraocular residence times or the development novel drug delivery mechanisms, it appears certain that intravitreal therapy will continue to evolve and push current therapeutic boundaries to improve visual acuity outcomes for retinal diseases.
Acknowledgments
This work was supported in part by cooperative agreements (U10EY017828 and U10EY023530) from the National Eye Institute, National Institutes of Health, Department of Health and Human Services. The author would like to thank Maureen G. Maguire PhD, Stuart L. Fine MD and Frederick L. Ferris MD for their advice and critical review of the manuscript.
Footnotes
Conflict of interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Williams G. Intravitreal injections: health policy implications. Review of Ophthalmology. 2014;(June) https://www.reviewofophthalmology.com/article/ivt-injections-health-policy-implications.
- 2.RBRVS DataManager Online. Copyright American Medical Association [Google Scholar]
- 3.Machemer R, Aaberg TM. Vitrectomy. New York: Grune & Stratton; 1979. [Google Scholar]
- 4.Peyman GA, Herbst R. Bacterial endophthalmitis Treatment with intraocular injection of gentamicin and dexamethasone. Arch Ophthalmol. 1974;91:416–418. doi: 10.1001/archopht.1974.03900060428017. [DOI] [PubMed] [Google Scholar]
- 5.May DR, Ericson ES, Peyman GA, Axelrod AJ. Intraocular injection of gentamicin. Single injection therapy of experimental bacterial endophthalmitis. Arch Ophthalmol. 1974;91:487–489. doi: 10.1001/archopht.1974.03900060501015. [DOI] [PubMed] [Google Scholar]
- 6.Peyman GA, Greenberg D, Fishman GA, Fiscella R, Thomas A. Evaluation of toxicity of intravitreal antineoplastic drugs. Ophthalmic Surg. 1984;15:411–413. [PubMed] [Google Scholar]
- 7.Peyman GA, Lad EM, Moshfeghi DM. Intravitreal injection of therapeutic agents. Retina. 2009;29:875–912. doi: 10.1097/IAE.0b013e3181a94f01. [DOI] [PubMed] [Google Scholar]
- 8.Martin DF, Ficker LA, Aguilar HE, Gardner SK, Wilson LA, Meredith TA. Vitreous cefazolin levels after intravenous injection: Effects of inflammation, repeated antibiotic doses, and surgery. Arch Ophthalmol. 1990;108:411–414. doi: 10.1001/archopht.1990.01070050109043. [DOI] [PubMed] [Google Scholar]
- 9.Axelrod JL, Klein RM, Bergen RL, Sheikh MZ. Human vitreous levels of selected antistaphylococcal antibiotics. Am J Ophthalmol. 1985;100:570–575. doi: 10.1016/0002-9394(85)90683-x. [DOI] [PubMed] [Google Scholar]
- 10.Kuppermann BD, Quiceno JI, Flores-Aguilar M, et al. Intravitreal ganciclovir concentration after intravenous administration in AIDS patients with cytomegalovirus retinitis: implications for therapy. J Infect Dis. 1993;168:1506–1509. doi: 10.1093/infdis/168.6.1506. [DOI] [PubMed] [Google Scholar]
- 11.Arevalo JF, Gonzalez C, Capparelli EV, et al. Intravitreous and plasma concentrations of ganciclovir and foscarnet after intravenous therapy in patients with AIDS and cytomegalovirus retinitis. J Infect Dis. 1995;172:951–956. doi: 10.1093/infdis/172.4.951. [DOI] [PubMed] [Google Scholar]
- 12.Baum J, Payman GA, Barza M. Intravitreal administration of antibiotic in the treatment of bacterial endophthalmitis, III: consensus. Surv Ophthalmol. 1982;26:204–206. doi: 10.1016/0039-6257(82)90080-7. [DOI] [PubMed] [Google Scholar]
- 13.The Endophthalmitis Vitrectomy Study Group. Results of the endophthalmitis vitrectomy study. Arch Ophthalmol. 1995;113:1479–1496. [PubMed] [Google Scholar]
- 14.Jabs DA. Cytomegalovirus retinitis and acquired immunodeficiency syndrome – bench to bedside: LXVII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2011;151:198–216. doi: 10.1016/j.ajo.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo IC, Kempen JH, Dunn JP, Vogelsang G, Jabs DA. Clinical characterisics and outcomes of cytomegalovirus retinitis in persons without human immunodeficiency virus infection. Am J Ophthalmol. 2004;138:338–346. doi: 10.1016/j.ajo.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Kuppermann BD, Petty JG, Richman DD, et al. Correlation between CD4+ counts and prevalence of cytomegalovirus retinitis and human immunodeficiency virus-related noninfectious retinal vasculopathy in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993;115:575–582. doi: 10.1016/s0002-9394(14)71453-9. [DOI] [PubMed] [Google Scholar]
- 17.Gallant JE, Moore RD, Richman DD, Keruly J, Chaisson RE the Zidovudine Epidemiology Study Group. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. J Infect Dis. 1992;166:1223–1227. doi: 10.1093/infdis/166.6.1223. [DOI] [PubMed] [Google Scholar]
- 18.Hoover DR, Saah AJ, Bacellar H, et al. Clinical manifestations of AIDS in the era of pneumocystis prophylaxis. N Engl J Med. 1993;329:1922–1926. doi: 10.1056/NEJM199312233292604. [DOI] [PubMed] [Google Scholar]
- 19.Holland GN, Pepose JS, Pettit TH, Gottlieb MS, Yee RD, Roos RY. Acquired immune deficiency syndrome: ocular manifestations. Ophthalmology. 1983;90:859–873. doi: 10.1016/s0161-6420(83)80009-8. [DOI] [PubMed] [Google Scholar]
- 20.Studies of the Ocular Complications of AIDS Research Group, in collaboration with the AIDS Clinical Trials Group. Mortality in patients with the acquired immunodeficiency syndrome treated with either foscarnet or ganciclovir for cytomegalovirus retinitis. N Engl J Med. 1992;326:213–220. doi: 10.1056/NEJM199201233260401. [DOI] [PubMed] [Google Scholar]
- 21.Drew WL, Ives D, Lalezari JP, et al. Oral ganciclovir as maintenance treatment for cytomegalovirus retinitis in patients with AIDS. N Engl J Med. 1995;333:615–620. doi: 10.1056/NEJM199509073331002. [DOI] [PubMed] [Google Scholar]
- 22.Delta Coordinating Committee. Delta: a randomised double-blind controlled trial comparing combinations of zidovudine plus didanosine or zalcitabine with zidovudine alone in HIV-infected individuals. Lancet. 1996 Aug 3;348(9023):283–291. [PubMed] [Google Scholar]
- 23.Hammer SM, Katzenstein DA, Hughes MD, et al. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. AIDS Clinical Trials Group Study 175 Study Team. N Engl J Med. 1996 Oct 10;335(15):1081–1090. doi: 10.1056/NEJM199610103351501. [DOI] [PubMed] [Google Scholar]
- 24.Fischl MA, Richman DD, Grieco MH, et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex: a double-blind, placebo-controlled trial. N Engl J Med. 1987;317:185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- 25.Collaborative DHPG Treatment Study Group. Treatment of serious cytomegalovirus infections with 9-(1,3-dihydroxy-2-propoxymethyl) guanine in patients with AIDS and other immunodeficiencies. N Engl J Med. 1986;314:801–805. doi: 10.1056/NEJM198603273141301. [DOI] [PubMed] [Google Scholar]
- 26.Studies of the Ocular Complications of AIDS Research Group, in collaboration with the AIDS Clinical Trials Group. Foscarnet-ganciclovir cytomegalovirus retinitis trial. 4. Visual outcomes. Ophthalmology. 1994;101:1250–1261. [PubMed] [Google Scholar]
- 27.Henry K, Cantrill HL, Fletcher C, et al. Use of intravitreal ganciclovir (dihydroxypropoxymethyl guanine) for cytomegalovirus retinitis in a patient with AIDS. Am J Ophthalmol. 1987;103:17–23. doi: 10.1016/s0002-9394(14)74163-7. [DOI] [PubMed] [Google Scholar]
- 28.Ussery FM, III, Gibson SR, Conklin RH, et al. Intravitreal ganciclovir in the treatment of AIDS-associated cytomegalovirus retinitis. Ophthalmology. 1988;95:640–648. doi: 10.1016/s0161-6420(88)33147-7. [DOI] [PubMed] [Google Scholar]
- 29.Cantrill HL, Henry K, Melroe NH, et al. Treatment of cytomegalovirus retinitis with intravitreal ganciclovir: long-term results. Ophthalmology. 1989;96:367–374. doi: 10.1016/s0161-6420(89)32900-9. [DOI] [PubMed] [Google Scholar]
- 30.Cochereau-Massin I, Le Hoang P, Lautier-Frau M, et al. Efficacy and tolerance of intravitreal ganciclovir in cytomegalovirus retinitis in acquired immune deficiency syndrome. Ophthalmology. 1991;98:1348–1353. doi: 10.1016/s0161-6420(91)32135-3. [DOI] [PubMed] [Google Scholar]
- 31.Young S, Morlet N, Besen G, et al. High dose (2000 ug) intravitreous ganciclovir in the treatment of cytomegalovirus retinitis. Ophthalmology. 1998;105:1404–1410. doi: 10.1016/s0161-6420(98)98020-4. [DOI] [PubMed] [Google Scholar]
- 32.Smith TJ, Pearson PA, Blandford DL, et al. Intravitreal sustained-release ganciclovir. Arch Ophthalmol. 1992;110:255–258. doi: 10.1001/archopht.1992.01080140111037. [DOI] [PubMed] [Google Scholar]
- 33.Sanborn GE, Anand R, Torti RE, et al. Sustained-release ganciclovir therapy for treatment of cytomegalovirus retinitis: use of an intravitreal device. Arch Ophthalmol. 1992;110:188–195. doi: 10.1001/archopht.1992.01080140044023. [DOI] [PubMed] [Google Scholar]
- 34.Anand R, Nightingale SD, Fish RH, Smith TJ, Ashton P. Control of cytomegalovirus retinitis using sustained release of intraocular ganciclovir. Arch Ophthalmol. 1993;111:223–227. doi: 10.1001/archopht.1993.01090020077027. [DOI] [PubMed] [Google Scholar]
- 35.Martin DF, Parks DJ, Mellow SD, et al. Treatment of cytomegalovirus retinitis with an intraocular sustained-release ganciclovir implant: A randomized, controlled clinical trial. Arch Ophthalmol. 1994;112:1531–1539. doi: 10.1001/archopht.1994.01090240037023. [DOI] [PubMed] [Google Scholar]
- 36.Musch DC, Martin DF, Gordon JF, Davis MD, Kuppermann BD the Ganciclovir Implant Study Group. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant: a multicenter, randomized, controlled trial. N Engl J Med. 1997;337:83–90. doi: 10.1056/NEJM199707103370203. [DOI] [PubMed] [Google Scholar]
- 37.Martin DF, Kuppermann BD, Wolitz RA, Palestine AG, Li H, Robinson CA Roche Ganciclovir Study Group. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. N Engl J Med. 1999;340:1063–1070. doi: 10.1056/NEJM199904083401402. [DOI] [PubMed] [Google Scholar]
- 38.Jabs DA, Enger C, Dunn JP, Forman M or the Cytomegalovirus Retinitis and Viral Resistance Research Group. Cytomegalovirus retinitis and viral resistance: ganciclovor resistance. J Infect Dis. 1998;177:770–773. doi: 10.1086/514249. [DOI] [PubMed] [Google Scholar]
- 39.Collier AC, Coombs RW, Schoenfeld DA, et al. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. N Engl J Med. 1996;334:1011–1018. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 40.Cameron DW, Heath-Chiozzi M, Danner S, et al. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. Lancet. 1998;351:543–549. doi: 10.1016/s0140-6736(97)04161-5. [DOI] [PubMed] [Google Scholar]
- 41.Hammer SM, Squires KE, Hughes MD the AIDS Clinical Trials Group 320 Study Team. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cells of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 42.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 43.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 44.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 45.Martin DF, Dunn JP, Davis JL, et al. Use of the ganciclovir implant for the treatment of cytomegalovirus retinitis in the era of potent antiretroviral therapy: recommendations of the International AIDS Society-USA panel. Am J Ophthalmol. 1999;127:329–339. doi: 10.1016/s0002-9394(98)00441-3. [DOI] [PubMed] [Google Scholar]
- 46.Martin DF, Sierra-Madero J, Walmsley S, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002;346:1119–1126. doi: 10.1056/NEJMoa011759. [DOI] [PubMed] [Google Scholar]
- 47.Jaffe GJ, Ben-Nun J, Guo H, Dunn JP, Ashton P. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology. 2000 Nov;107(11):2024–2033. doi: 10.1016/s0161-6420(00)00466-8. [DOI] [PubMed] [Google Scholar]
- 48.Jaffe GJ, Yang CH, Guo H, et al. Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Invest Ophthalmol Vis Sci. 2000;41:3569–3575. [PubMed] [Google Scholar]
- 49.Jaffe GJ, Martin DF, Callanan DG, Pearson PA, Levy B, Comstock T Fluocinolone Acetonide Uveitis Study Group. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four week results of a multicenter randomized clinical study. Ophthalmology. 2006;113:1020–1027. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 50.Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Levy B, Comstock T. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126:1191–1201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 51.Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group. Kempen JH, Altaweel MM, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118:1916–1926. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearson PA, Abou-Jaoude ES, Smith TJ, Ashton P. American Academy of Ophthalmology. Dallas, TX: Oct, 2000. A phase I evaluation of sustained delivery fluocinolone in the treatment of diabetic macular edema (DME) [Google Scholar]
- 53.Jonas JB, Sofker A. Intraocular injection of crystalline cortisone as adjunctive treatment of diabetic macular edema. Am J Ophthalmol. 2001;132:425–427. doi: 10.1016/s0002-9394(01)01010-8. [DOI] [PubMed] [Google Scholar]
- 54.Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920–927. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 55.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–1449. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Augustin AJ, Schmidt-Erfurth U. Verteporfin therapy combined with intravitreal triamcinolone in all types of choroidal neovascularization due to age-related macular degeneration. Ophthalmology. 2006;113:14–22. doi: 10.1016/j.ophtha.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Spaide RF, Sorenson J, Maranan L. Photodynamic therapy with verteporfin combined with intravitreal injection of triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 2005;112:301–304. doi: 10.1016/j.ophtha.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Ip MS, Scott IU, Van Veldhuisen PC, et al. SCORE Study Research Group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101–1114. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antcliff RJ, Spalton DJ, Stanford MR, et al. Intravitreal triamcinolone for uveitic cystoid macular edema (an optical coherence tomography study) Ophthalmology. 2001;108:765–772. doi: 10.1016/s0161-6420(00)00658-8. [DOI] [PubMed] [Google Scholar]
- 61.Conway MD, Canakis C, Livir-Rallatos C, Peyman GA. Intravitreal triamcinolone acetonide for refractory chronic pseudophakic cystoid macular edema. J Cataract Refract Surg. 2003;29:27–33. doi: 10.1016/s0886-3350(02)01441-4. [DOI] [PubMed] [Google Scholar]
- 62.Miller JW, Adamis AP, Shima DT, D'Amore PA, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994;145:574–584. [PMC free article] [PubMed] [Google Scholar]
- 63.Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;36:855–868. [PubMed] [Google Scholar]
- 64.Frank RN, Amin RH, Eliott D, Puklin JE, Abrams GW. Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol. 1996;122:393–403. doi: 10.1016/s0002-9394(14)72066-5. [DOI] [PubMed] [Google Scholar]
- 65.The EyeTech Study Group. Preclinical and phase 1A clinical evaluation of an anti-VEGF pegylated aptamer (EYE001) for the treatment of exudative age-related macular degeneration. Retina. 2002;22:143–152. doi: 10.1097/00006982-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 66.Mordenti J, Cuthbertson RA, Ferrara N, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 1251-labeled full length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999;27:536–44. doi: 10.1177/019262339902700507. [DOI] [PubMed] [Google Scholar]
- 67.Mordenti J, Thomsen K, Licko V, Berleau L, et al. Intraocular pharmacokinetics and safety of a humanized monoclonal antibody in rabbits after intravitreal administration of a solution or a PLGA microsphere formulation. Toxicol Sci. 1999;52:101–6. doi: 10.1093/toxsci/52.1.101. [DOI] [PubMed] [Google Scholar]
- 68.Gragoudas ES, Adamis AP, Cunningham ET, Jr, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 69.Chakravarthy U, Adamis AP, Cunningham ET, Jr, et al. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology. 2006;113:1508 e1–25. doi: 10.1016/j.ophtha.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 70.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 71.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 72.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–335. [PubMed] [Google Scholar]
- 73.Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age- related macular degeneration: twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–1047. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 74.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–372. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 75.Bashshur ZF, Haddad ZA, Schakal A, Jaafar RF, Saab M, Noureddin BN. Intravitreal bevacizumab for treatment of neovascular age-related macular degeneration: a one-year prospective study. Am J Ophthalmol. 2008;145:249–256. doi: 10.1016/j.ajo.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 76.Arevalo JF, Sánchez JG, Wu L, et al. Pan-American Collaborative Retina Study Group. Intravitreal bevacizumab for subfoveal choroidal neovascularization in age-related macular degeneration at twenty-four months: the Pan-American Collaborative Retina Study. Ophthalmology. 2010;117:1974–1981. doi: 10.1016/j.ophtha.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 77.Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable-dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 78.Martin DF, Maguire MG, Fine SL. Identifying and eliminating the roadblocks to comparative effectiveness research. N Engl J Med. 2010;363:105–107. doi: 10.1056/NEJMp1001201. [DOI] [PubMed] [Google Scholar]
- 79.Martin DF, Maguire MG, Ying GS, et al. Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin DF, Maguire MG, Fine SL, et al. Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: 2-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakravarthy U, Harding SP, Rogers CA, et al. IVAN Study Investigators. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 82.Chakravarthy U, Harding SP, Rogers CA, et al. IVAN Study Investigators. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258–1267. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 83.Krebs I, Schmetterer L, Boltz A, et al. MANTA Research Group. A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2013;97:266–271. doi: 10.1136/bjophthalmol-2012-302391. [DOI] [PubMed] [Google Scholar]
- 84.Kodjikian L, Souied EH, Mimoun G, et al. GEFAL Study Group. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL Noninferiority Randomized Trial. Ophthalmology. 2013;120:2300–2309. doi: 10.1016/j.ophtha.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 85.Schauwvlieghe AM, Dijkman G, Hooymans JM, et al. Comparing the effectiveness of bevacizumab to ranibizumab in patients with exudative age-related macular degeneration. The BRAMD study PLoS One. 2016;11:e0153052. doi: 10.1371/journal.pone.0153052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berg K, Pederson TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122:146–152. doi: 10.1016/j.ophtha.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 87.Moja L, Lucenteforte E, Kwag K, et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014;(9):CD011230. doi: 10.1002/14651858.CD011230.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Solomon SD, Lindsley KB, Krzystolik MG, et al. Intravitreal bevacizumab versus ranibizumab for treatment of neovascular age-related macular degeneration: findings from a Cochrane Systematic Review. Ophthalmology. 2016;123:70–77. doi: 10.1016/j.ophtha.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maguire MG, Shaffer J, Ying GS, et al. the Bevacizumab-Ranibizumab International Trials Group. Serious adverse events with bevacizumab or ranibizumab for age-related macular degeneration: Meta-analysis of individual patient data. Ophthalmology Retina. 2017;1:375–381. doi: 10.1016/j.oret.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.The Diabetic Retinopathy Clinical Research Network. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. N Engl J Med. 2015;372:1193–1203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rajagopal R, Shah GK, Blinder KJ, et al. Bevacizumab versus ranibizumab in the treatment of macular edema due to retinal vein occlusion: 6-month results of the CRAVE study. Ophthalmic Surg Lasers Imaging Retina. 2015;46:844–850. doi: 10.3928/23258160-20150909-09. [DOI] [PubMed] [Google Scholar]
- 92.Narayanan R, Panchal B, Das T, Chhablani J, Jalali S, Ali MH MARVEL study group. A randomised, double-masked, controlled study of the efficacy and safety of intravitreal bevacizumab versus ranibizumab in the treatment of macular oedema due to branch retinal vein occlusion: MARVEL Report No. 1 Br J Ophthalmol. 2015;99:954–959. doi: 10.1136/bjophthalmol-2014-306543. [DOI] [PubMed] [Google Scholar]
- 93.Busbee BG, Ho AC, Brown DM, et al. HARBOR Study Group. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120:1046–1056. doi: 10.1016/j.ophtha.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 94.Stone TW, editor. ASRS 2017 Preferences and Trends Membership Survey. Chicago, IL: American Society of Retina Specialists; 2017. [Google Scholar]
- 95.Martin DF, Ying GS, Huang J, Maguire MG the CATT Research Group. Predictors of the number of injections among patients treated PRN with ranibizumab or bevacizumab in the Comparison of AMD Treatments Trials (CATT) Invest Ophthalmol Vis Sci. 2014;55 ARVO E-Abstract 859. [Google Scholar]
- 96.Daniel E, Shaffer J, Ying GS, et al. Comparison of AMD Treatments Trials (CATT) Research Group. Outcomes in eyes with retinal angiomatous proliferation in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Ophthalmology. 2016;123:609–616. doi: 10.1016/j.ophtha.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hagstrom SA, Ying GS, Pauer GJT, et al. Comparison of AMD Treatments Trials (CATT) Research Group. Pharmacogenetics for genes associated with age-related macular degeneration in the Comparison of AMD Treatments Trials (CATT) Ophthalmology. 2013;120:593–599. doi: 10.1016/j.ophtha.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hagstrom SA, Ying GS, Pauer GJT, et al. VEGFA and VEGFR2 gene polymorphisms and response to anti-vascular endothelial growth factor therapy in the Comparison of AMD Treatments Trials (CATT) JAMA Ophthalmology. 2014;132:521–527. doi: 10.1001/jamaophthalmol.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hagstrom SA, Ying GS, Maguire MG, et al. CATT Research Group and IVAN Study Investigators. VEGFR2 gene polymorphisms and response to anti-vascular endothelial growth factor therapy in age-related macular degeneration. Ophthalmology. 2015;122:1563–1568. doi: 10.1016/j.ophtha.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ying GS, Huang J, Maguire MG, et al. CATT Research Group. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120:122–129. doi: 10.1016/j.ophtha.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ying GS, Maguire MG, Daniel E, et al. CATT Research Group. Association of baseline characteristics and early vision response with two-year outcomes in the Comparison of AMD Treatments Trials. Ophthalmology. 2015;122:2523–2531. doi: 10.1016/j.ophtha.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jaffe GJ, Martin DF, Toth CA, et al. CATT Research Group. Macular morphology and visual acuity in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Ophthalmology. 2013;120:1860–1870. doi: 10.1016/j.ophtha.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharma S, Toth CA, Daniel E, et al. CATT Research Group. Macular morphology and visual acuity in the second year of the Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Ophthalmology. 2016;123:865–875. doi: 10.1016/j.ophtha.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grunwald JE, Daniel E, Huang J, et al. CATT Research Group. Risk of geographic atrophy in Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2014;121:150–161. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grunwald JE, Pistilli M, Ying GS, Maguire MG, Daniel E, Martin DF the CATT Research Group. Growth of geographic atrophy in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Ophthalmology. 2015;122:809–816. doi: 10.1016/j.ophtha.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grunwald JE, Pistilli M, Daniel E, et al. the CATT Research Group. Incidence and growth of geographic atrophy during 5 years of Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Ophthalmology. 2017;124:97–104. doi: 10.1016/j.ophtha.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Altaweel MM, Daniel E, Martin DF, et al. CATT Research Group. Outcomes of eyes with lesions composed of >50% blood in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Ophthalmology. 2015;122:391–398. doi: 10.1016/j.ophtha.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2016;123:1751–1761. doi: 10.1016/j.ophtha.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 110.Scott IU, VanVeldhuisen PC, Ip MS, et al. SCORE2 Investigator Group. Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion. JAMA. 2017;317:2072–2087. doi: 10.1001/jama.2017.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boyer DS, Yoon HY, Belfort R, Jr, et al. Ozurdex MEAD Study Group. Three-Year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 112.Lowder C, Belfort R, Jr, Lightman S, et al. Ozurdex HURON Study Group. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545–553. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- 113.Haller JA, Bandello F, Belfort R, Jr, et al. Ozurdex GENEVA Study Group. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1446. doi: 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 114.Campochiaro PA, Brown DM, Pearson A et al. FAME Study Group. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626–635. doi: 10.1016/j.ophtha.2010.12.028. [DOI] [PubMed] [Google Scholar]


