Abstract
Background
Major depressive disorder (MDD) affects women approximately twice as often as men. Women are three times as likely to have atypical depression, with hypersomnia and weight gain. This suggests that the molecular mechanisms of MDD may differ by sex.
Methods
To test this hypothesis, we performed a large-scale gene expression meta-analysis across three corticolimbic brain regions, the dorsolateral prefrontal cortex, subgenual anterior cingulate cortex, and basolateral amygdala (N=26 men, 24 women with MDD and sex-matched controls). Results were further analyzed using a threshold-free approach, gene ontology, and cell type-specific analyses. A separate dataset was used for independent validation [N=13 MDD subjects/sex; 22 controls (13 males, 9 females)].
Results
Of the 706 genes differentially expressed in men with MDD and 882 genes differentially expressed in women with MDD, only 21 were changed in the same direction in both sexes. Notably, 52 genes displayed expression changes in opposite directions between men and women with MDD. Similar results were obtained using a threshold-free approach, where the overall transcriptional profile of MDD was opposite in men and women. Gene ontology indicated that men with MDD had decreases in synapse-related genes, whereas women with MDD exhibited transcriptional increases in this pathway. Cell type-specific analysis indicated that men with MDD exhibited increases in oligodendrocyte- and microglia-related genes, while women with MDD had decreases in markers of these cell types.
Conclusions
The brain transcriptional profile of MDD differs greatly by sex, with multiple transcriptional changes in opposite directions between men and women with MDD.
Keywords: Sex difference, Depression, Mood disorders, Genetics, Meta-analysis, Corticolimbic
Introduction
Major depressive disorder (MDD) is a leading cause of disability worldwide (1), but its impact differs substantially between sexes. Women are twice as likely to be diagnosed with a single MDD episode, and four times more likely to be diagnosed with recurrent MDD (e.g., (2–7). Women with MDD also report greater illness severity, more symptoms (3, 8–10), and different symptomatology than men. For instance, women are three times more likely to have atypical depression, characterized by hypersomnia and weight gain (11–15). Comorbidity of MDD with other disorders also differs between sexes. For instance, women are more likely to have comorbid anxiety disorders, whereas men are more likely to have comorbid substance use disorders (e.g., (16–19)). Some studies suggest that women have more positive treatment outcomes with selective serotonin reuptake inhibitors and monoamine oxidase inhibitors (20, 21), whereas men seem to respond better to tricyclic antidepressants.
Research suggests dysfunction of the corticolimbic network of mood regulation in MDD. We consider three network nodes, the dorsolateral prefrontal cortex (DLPFC; Brodmann area 9 (BA9)), subgenual anterior cingulate cortex (ACC; BA25), and amygdala (AMY). Structural and functional neuroimaging implicates these regions in MDD [e.g. (22–30)]. Since some studies were performed in only women (24, 31), it is unclear whether results are generalizable to both sexes. Additionally, studies that included both sexes often lacked statistical power to stratify by sex. The idea that these brain regions are differentially affected in men and women with MDD is supported by sex differences in activation during normal emotional states. fMRI studies of non-depressed subjects suggest differential regional during emotion-related tasks, with women having more AMY activation and men more cortical activation [e.g., (32–34)].
Postmortem brain studies report reduced density and number of glial cells in MDD in the DLPFC (35), ACC (36, 37), and AMY (38, 39). Additionally, there is reduced neuron size in DLPFC (36) and ACC (37) in MDD. However, these analyses were not stratified by sex. Gene expression studies on tissue homogenate from postmortem brains have identified sex differences in MDD. In the ACC, we reported brain derived neurotrophic factor (BDNF)/TrkB expression changes with greater effect in men compared to women with MDD (40). We also reported a more robust reduction in the GABA neuron marker, somatostatin, in the DLPFC, ACC, and AMY of women compared to men with MDD (41). The AMY of women with MDD exhibited a GABA-/BDNF-related dysfunction not seen in men with MDD (41–43). We also found sex differences in cholinergic signaling changes in the AMY of MDD subjects (44). Sex differences in glutamate-related genes were reported in the DLPFC of MDD subjects, with increased expression of glutamate-related genes in women with MDD and decreases in these same genes in men with MDD (45). Finally, Labonte et al recently reported sex-specific transcriptional signatures of depression (46).
Here, we use large-scale gene expression studies, meta-analysis across corticolimbic brain regions, and meta-regression for sex to examine the brain molecular pathology in MDD. Given the sex differences in MDD incidence, symptomatology, and neuroimaging, we hypothesize that the molecular signature of MDD is distinct in men and women.
Materials and Methods
Detailed methods are available in the supplements.
Human subjects and microarray studies
Brain samples were obtained during autopsies conducted at the Allegheny County Medical Examiner’s Office (Pittsburgh, USA) after next-of-kin consent using procedures approved by University of Pittsburgh’s Institutional Review Board and Committee for Oversight of Research Involving the Dead. Consensus DSM-IV diagnoses were made by an independent committee of experienced clinical research scientists using information from clinical records, toxicology results, and a standardized psychological autopsy. Unaffected comparison subjects were assessed with identical procedures.
50 MDD subjects (26 men, 24 women) and 50 sex-matched unaffected comparison subjects were included. We combined 8 microarray datasets from three brain regions, with half the studies performed in men, half in women (43, 47, 48). Four studies were in ACC (2/sex), two in DLPFC (1/sex), and two in AMY (1/sex). Tables S1 and S2 contain details on subjects and areas investigated. Group means for age, postmortem interval (PMI), RNA integrity number (RIN), and brain pH were nearly identical and not statistically different.
For replication, we used recently published publically available RNA-seq data (BA11, BA25) generated using brains from a different brain bank (GEO GSE102556; (46)). The effect of MDD was analyzed separately in men and women. We then assessed overlap in DE genes identified in men and women and the percent of overlapping genes that were changed in opposite directions in men and women with MDD.
Meta-analysis of gene expression in MDD
Datasets and meta-analysis methods and results were described previously (49–51). Briefly, we adopted linear models to account for potential confounding covariates and applied a meta-analysis pipeline to combine studies for identification of MDD-associated genes. A random effects model (REM) was used to detect changes in gene expression by combining effects across studies. We adopted REM separately for the 4 female and 4 male studies. We used q<0.05 as the cutoff for differential expression (DE). We then combined all eight studies by REM and used meta-regression to probe for genes that were changed differently in men and women with MDD (q<0.05).
Overlap of gene expression profiles in MDD in men and women
Rank-rank hypergeometric overlap test (RRHO)
We used RRHO (52) to compare MDD DE genes between men and women. RRHO is a threshold-free algorithm that identifies trends of overlap between two ranked lists of DE genes. The genes are ranked by the −log10 of DE p-value multiplied by the effect size direction. Up, down, and unchanged genes are at the bottom, top, and middle of the list, respectively. A one-sided p-value for the overlap of gene lists from two datasets is calculated according to the hypergeometric distribution.
Spearman’s Correlation
We used Spearman’s rank-order correlation as a complementary threshold-free method. We compared ranked effect sizes between men and women for all genes.
Gene ontology enrichment analysis
The area under the receiver operating curve (AUROC) statistic was used to measure enrichment of Gene Ontology (GO) groups in a specific gene ranking. This value is equal to the probability that a gene in a GO group will rank higher than a gene not in the group. DE results were ranked from the most significant gene in the negative direction to the most significant gene in the positive direction (signed −log(pvalues)). Mann-Whitney U test p-values were calculated. GO groups with 10–200 genes were used (5081 groups).
Cell type-specific analysis
From single-cell transcriptome analysis of healthy human adult cortex, we obtained six lists of the top 21 most enriched genes in transcriptomic-determined cell types (astrocytes, neurons, oligodendrocytes, oligodendrocyte precursors, microglia, and endothelial cells; Table S3 in (53)). The number of genes tested varies because not all 21 genes were assayed in our meta-analysis. We calculated AUROC statistics and Mann-Whitney U p-values for each cell-type list with Bonferroni correction.
Results
Divergent molecular signatures of MDD in men and women
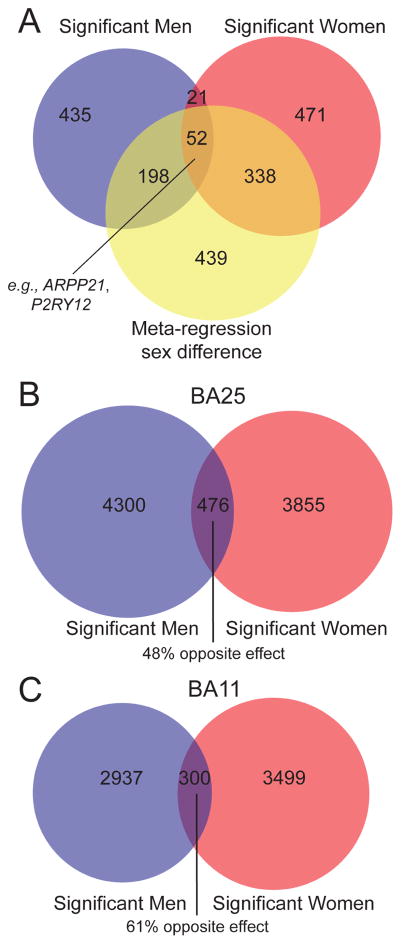
We used large-scale gene expression meta-analysis to probe for sex differences in the brains of men and women with MDD (See strategy in Figure S1). We first performed the meta-analysis in each sex separately. There were 706 DE transcripts (252 upregulated, 454 downregulated) in men and 882 DE transcripts (524 upregulated, 358 downregulated) in women. When comparing DE genes in men and women with MDD, 633 of 706 transcripts were found in men only and 809 of 882 transcripts in women only. Interestingly, only 73 genes were DE in both MDD men and women, and 52 of these 73 genes were changed in opposite directions between sexes. Therefore, only 21 DE genes were affected in the same direction in men and women with MDD. Results are summarized in Figure 1A. Results are not driven by differences in sex chromosomes, as only 2.5% of genes identified in men and 3.2% of genes identified in women are found on sex chromosomes.
Figure 1. Distinct transcriptional changes in men and women with MDD.
(A) Venn diagram displaying overlap in differentially expressed genes in men with MDD, in women with MDD, and in genes identified via meta-regression for sex (q < 0.05). We confirmed these results using an independent replication dataset (p < 0.05); there was very little overlap in DE genes identified in men and women with MDD in BA25 (B) and BA11 (C).
Next, we performed meta-regression on all studies to directly test for expression differences between men and women with MDD. This approach is more stringent than assessing the overlap of DE gene lists identified in men and women separately and is better powered since it includes all eight studies. We identified 1027 genes that were significantly differentially altered in men and women with MDD (Figure 1A). A comparison of the meta-regression gene list (1027 genes) indicates that these genes are changed in opposite directions in men and women with MDD (Figure 2A). Of these meta-regression genes, 198 and 338 were significant in men or women only, respectively. 52 of the 1027 meta-regression genes were significant for both men and women with MDD, but in opposite directions. These same 52 genes were identified in the previous men/women separate analysis (Table S3; Figure 2B). The remaining genes with meta-regression main effect (439) did not reach significance (q<0.05) in either sex. Notably, less than 1% of the meta-regression genes were sexually dimorphic in control subjects, indicating that the differential effects in MDD are not driven by baseline sex differences (Table S4).
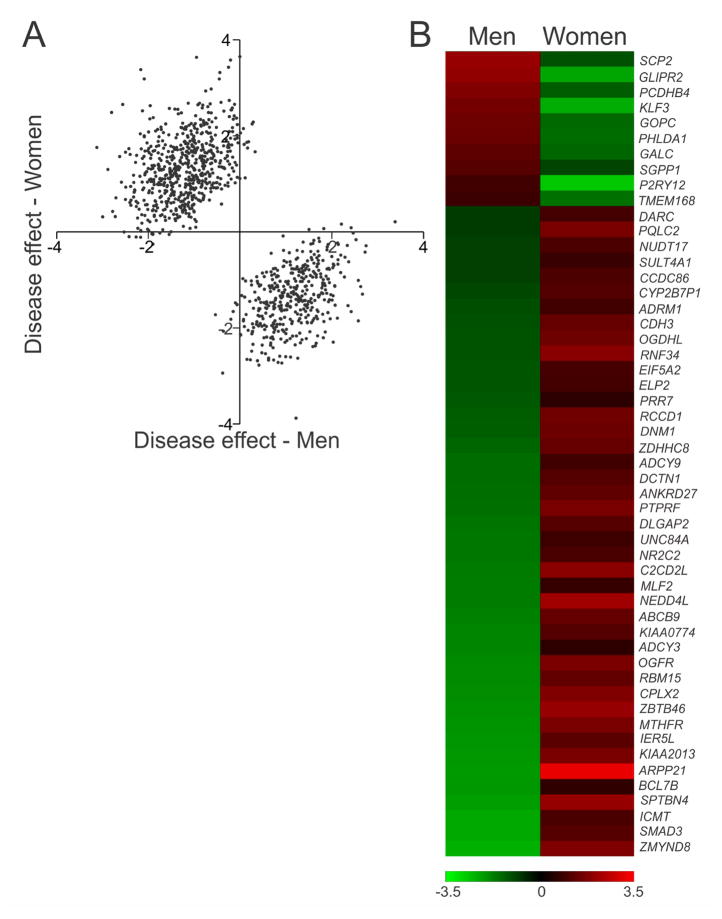
Figure 2. Genes affected in opposite directions in men and women with MDD.
(A) Scatterplot indicating the overall pattern of opposite effect size directions for the full meta-regression by sex gene list (1027 genes). (B) Heatmap indicating opposite effect sizes of the 52 genes significantly (q < 0.05) changed in opposite directions in men and women with MDD. These genes were identified in both the meta-regression dataset as well as in the sex-specific meta-analysis datasets.
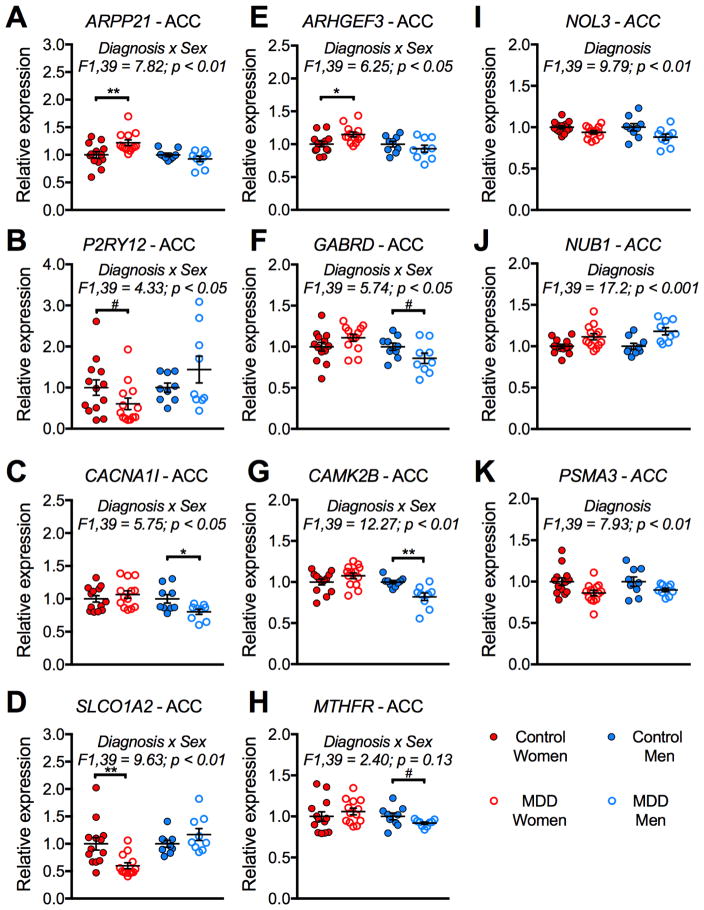
Although most of our male and female MDD studies were performed in separate experiments, one ACC study was performed at the same time in men and women; we directly compared individual gene expression results in this study. We selected three meta-regression genes that were significantly changed in opposite directions in males and females with MDD (ARPP21, P2RY12, MTHFR). We selected an additional 5 genes identified by meta-regression that were significant in only one sex (CACNA1I, ARHGEF3, SLCO1A2, GABRD, CAMK2B). We confirmed significant interactions of sex and diagnosis for 7/8 genes (Figure 3A–H). We also confirmed main effects of diagnosis on expression of NOL3, NUB1, and PSMA3; these genes were changed in the same direction in men and women with MDD (Figure 3I–K). To confirm that these changes were consistent across brain regions, we performed an independent qPCR experiment in the AMY for ARPP21, P2RY12, and MTHFR, and found significant interactions of sex and diagnosis for ARPP21 and P2RY12 (Figure S3). In the AMY, there was a sex difference in MTHFR, but no interaction of sex and diagnosis, suggesting that the meta-regression result is driven primarily by the ACC and DLPFC for MTHFR.
Figure 3. Verification of meta-regression results using arrays in the ACC.
The MD2 ACC microarray experiments were performed at the same time in men and women with MDD, allowing us to directly compare expression changes from the microarray studies. There were significant sex x diagnosis interactions for ARPP21 (A), P2RY12 (B), CACNA1I (C), SLCO1A2 (D), ARHGEF3 (E), GABRD (F), and CAMK2B (G). There was a significant main effect of diagnosis on expression of NOL3 (I), NUB1 (J), and PSMA3 (K). (A) There was a significant increase in ARPP21 expression in only women with MDD. (B) There was a trend for a decrease in P2RY12 expression in only women with MDD. (C) There was a significant decrease in CACNA1I expression in only men with MDD. (D) There was a decrease in SLC01A2 expression in only women with MDD. (E) There was an increase in ARHGEF3 expression in only women with MDD. (F) There was a trend for a decrease in GABRD expression in only men with MDD. (G) There was a decrease in CAMK2B expression in only men with MDD. (H) There was a trend for a decrease in MTHFR expression in only men with MDD. (I) There was a significant decrease in NOL3 expression in both men and women with MDD. (J) There was a significant increase in NUB1 expression in both men and women with MDD. (K) There was a significant decrease in PSMA3 expression in men and women with MDD. *, p < 0.05; **, p < 0.01; #, p < 0.1.
We confirmed this opposite direction effect in male and female MDD using a separate RNA-seq dataset generated using subjects from a different brain bank (46). We again found very little overlap in DE genes in men and women with MDD (~8%). Notably, ~55% of these overlapping genes changed in opposite directions in men and women with MDD (Figure 1B–C; Tables S5–S6).
Opposite transcriptional profiles in men and women with MDD
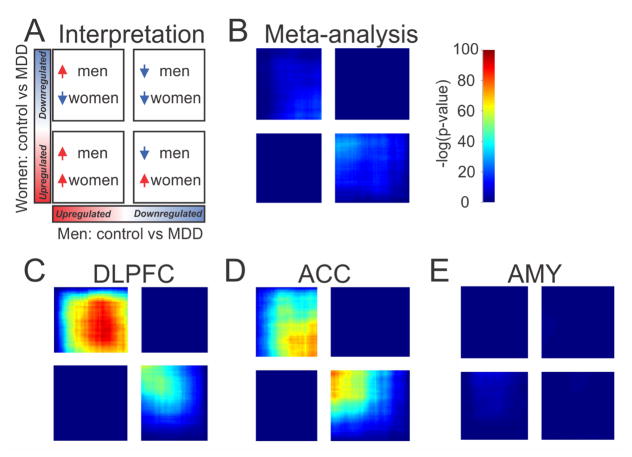
We used a threshold-free approach to validate our divergent gene expression findings in men and women with MDD. Typical DE studies use somewhat arbitrary DE and effect size thresholds to identify relevant genes, which might miss small but reproducible changes. To complement this approach, we used RRHO as an exploratory, threshold-free method to assess patterns of overlap between two DE datasets. For each of the two datasets, RRHO ranks the entire gene list by DE p-value and effect size direction, with one dataset represented on the X-axis and one on the Y-axis. We first performed RRHO using results from the cross-brain region meta-analysis. We compared the rank ordered gene list generated in depressed men compared to controls (X-axis Figure 4) to the rank ordered gene list generated in depressed women compared to controls (Y-axis Figure 4). Figure 4A indicates interpretation of RRHO plots. Consistent with the lack of overlap in DE genes reported above, there was no statistically significant overlap in genes that were upregulated in both men and women with MDD or downregulated in both sexes (Figure 4B). However, there was a statistically significant overlap in genes affected in opposite directions in men and women with MDD (Figure 4B; Figure S4A). We confirmed this result using Spearman correlation. There was a significant negative correlation in effect sizes for genes in the male-specific dataset to the effect sizes of genes in the female-specific dataset (ρ=−0.130; slope=−0.127; p=4.39×10−41), indicating that genes were changed in opposite directions in men and women with MDD.
Figure 4. Threshold-free differential expression patterns reveal that men and women with MDD have opposite molecular signatures.
(A) Schematic indicating interpretation of RRHO plots. A hot spot in the bottom left corner indicates overlap in genes up in both men and women with MDD. A hot spot in the top right corner indicates overlap in genes down in both men and women with MDD. A hot spot in the top left indicates overlap in genes up in men and down in women with MDD. A hot spot in the bottom right indicates overlap in genes down in men and up in women with MDD. Note that in the RRHO plots, the quadrants are not always of equal size; this is due to the fact that there is typically not an even split in the number of genes that are up and down regulated. (B) There was no significant overlap in genes that were up in both men and women with MDD or down in both men and women with MDD. However, there was a weak overlap in genes that were changed in opposite directions in men and women with MDD. There was a strong overlap in genes that were affected in opposite directions in the DLPFC (C) and ACC (D) of men and women with MDD. (E) There was no overlap in gene expression profiles in the AMY.
We also performed RRHO and Spearman correlations separately for each brain region. There was no statistically significant overlap in genes upregulated in both men and women with MDD or downregulated in both sexes for any brain region (Figure 4C, D, E). Instead, we observed in the DLPFC and ACC a statistically significant overlap in genes affected in opposite directions in men and women with MDD (Figure 4C and 3D; Figure S4B and S4C). We confirmed this negative correlation in effect size direction using Spearman correlation in the DLPFC (ρ=−0.204; slope=−0.197; p=2.20×10−100) and ACC (ρ=−0.224; slope=−0.149; p=4.94×10−122). In the AMY, there was no statistically significant overlap in genes changed in opposite directions in men and women with MDD (Figure 4E). Importantly, RRHO analysis in the replication cohort confirmed these opposite transcriptional profile in male and female depression (Figure S5).
Pathway analysis of molecular signatures of MDD in men and women
In men, the DE genes were enriched for synapse-related pathways, inner mitochondrial membrane protein complex, and G-protein coupled amine receptor activity (Table 1; top three pathways are synapse-related, with overlapping genes (Figure S6)). Results indicated that genes in these pathways were downregulated in men with MDD.
Table 1.
List of top 5 gene ontology pathways identified in men with MDD.a
| Pathway | Men | Women | ||
|---|---|---|---|---|
|
|
|
|||
| p-value | AUROC | p-value | AUROC | |
| Regulation of synapse structure or activityb | < 10−9 | 0.373 ↓ | < 0.01 | 0.559 ↑ |
| Regulation of synaptic plasticityb | < 10−6 | 0.378 ↓ | < 0.15 | 0.544 ↑ |
| Positive regulation of synapse assemblyb | < 10−5 | 0.312 ↓ | < 0.01 | 0.618 ↑ |
| Inner mitochondrial membrane protein complex | < 10−5 | 0.375 ↓ | < 10−6 | 0.347 ↓ |
| G-protein coupled amine receptor activity | < 10−5 | 0.169 ↓ | < 0.15 | 0.622 ↑ |
Abbreviation: AUC, area under the curve.
AUC < 0.5 indicates a pathway is enriched in genes that were downregulated in MDD in that sex. AUC > 0.5 indicates a pathway is enriched in genes that were upregulated in MDD in that sex.
The synapse-related pathways have highly overlapping gene lists (see Figure S7). Bold indicates pathways affected in opposite directions in men and women with MDD.
In women, the DE genes were enriched for pathways related to antigens and mitochondrial function (Table 2; top four pathways are antigen-related, with overlapping genes (Figure S7)). Genes in these top pathways were downregulated in women with MDD.
Table 2.
List of top 5 gene ontology pathways identified in women with MDD.a
| Pathway | Women | Men | ||
|---|---|---|---|---|
|
|
|
|||
| p-value | AUROC | p-value | AUROC | |
| Antigen processing & presentationb | < 10−10 | 0.353 ↓ | NS | 0.522 |
| Antigen processing & presentation of exogenous peptide antigenb | < 10−10 | 0.343 ↓ | NS | 0.515 |
| Antigen processing & presentation of exogenous antigenb | < 10−9 | 0.346 ↓ | NS | 0.511 |
| Antigen processing & presentation of peptide antigenb | < 10−9 | 0.354 ↓ | NS | 0.516 |
| Mitochondrial translational termination | < 10−97 | 0.337 ↓ | <0.05 | 0.428 ↓ |
Abbreviation: AUC, area under the curve.
AUC < 0.5 indicates a pathway is enriched in genes that were downregulated in MDD in that sex. AUC > 0.5 indicates a pathway is enriched in genes that were upregulated in MDD in that sex.
The antigen-related pathways have highly overlapping gene lists (see Figure S8).
Given that some pathways might still be enriched in both men and women with MDD, but to varying degrees, we examined the top pathways identified in each sex in the opposite sex. In other words, a pathway might be enriched in both sexes, but might only be a top pathway in one sex. Interestingly, all five pathways identified in men were also enriched in women (Table 1). However, while genes in 4 of the 5 top male pathways were downregulated in men with MDD these same pathways had genes that were upregulated in women with MDD. When we examined the top female identified pathways in men, most were not enriched in men with MDD (Table 2).
We next performed GO pathway analysis using genes identified by meta-regression. These genes enriched for regulation of synapse-related pathways, antigen-related pathways, and MHC protein complex (Table 3; overlap of genes in the top pathways in Figure S8).
Table 3.
List of top 5 gene ontology pathways identified by metaR dataset.
| Pathway | p-value | Men Effect size | Women Effect size |
|---|---|---|---|
| Regulation of synapse structure or activitya | < 10−8 | − 0.50 | 0.20 |
| Antigen processing & presentationb | < 10−7 | − 0.003 | − 0.50 |
| MHC protein complexb | < 10−7 | 0.50 | − 1.20 |
| Regulation of synapse organizationa | < 10−7 | − 0.46 | 0.51 |
| Antigen processing & presentation of exogenous peptide antigenb | < 10−7 | − 0.04 | − 0.56 |
The synapse-related pathways have highly overlapping gene lists.
The antigen-related pathways pathways have highly overlapping gene lists (see Figure S9).
Cell type enrichment analysis
We next asked whether sex-specific DE genes were enriched for markers of particular cell types. The goal is to identify candidate cell populations that are likely disrupted in MDD. Results are summarized in Table 4. Genes specifically expressed in oligodendrocytes and microglia were upregulated in men with MDD, but downregulated in women with MDD. Genes specifically expressed in astrocytes were upregulated in men with MDD, but unchanged in women with MDD. Neuronal genes were downregulated in men with MDD, but unchanged in women with MDD. We confirmed these cell-type specific results using a single cell dataset generated in mouse cortex (Table S7). Together, this cell type enrichment analysis suggests that oligodendrocytes and microglia are oppositely affected in men and women with MDD.
Table 4.
Sex-specific associations of transcriptomic cell-type enriched gene sets.a
| Cell type | Men | Women | ||
|---|---|---|---|---|
|
|
|
|||
| q-value | AUC | q-value | AUC | |
| Oligodendrocytes | < 0.005 | 0.763 ↑ | < 0.1 | 0.319 ↓ |
| Astrocytes | < 0.005 | 0.734 ↑ | NS | 0.434 |
| Microglia | < 0.05 | 0.710 ↑ | < 10−4 | 0.134 ↓ |
| Neurons | < 0.05 | 0.330 ↓ | NS | 0.525 |
| Oligodendrocyte precursor cells | < 0.12 | 0.672 ↑ | < 0.19 | 0.682 ↑ |
| Endothelial cells | NS | 0.559 | NS | 0.542 |
Abbreviation: AUC, area under the curve.
AUC > 0.5 indicates a cell type is enriched in genes that were downregulated in MDD in that sex. AUC < 0.5 indicates a cell type is enriched in genes that were upregulated in MDD in that sex. Bold indicates cell-types affected in opposite directions in men and women with MDD.
We previously reported reduced expression of oligodendrocyte-specific genes in the AMY of men with MDD (42). Thus, we were surprised that when all three corticolimbic brain regions were combined, there was an increase in expression of oligodendrocyte-specific genes in men with MDD. A closer look at the cell type-specific findings for each brain region in fact confirms our previous AMY finding. While oligodendrocyte-specific genes increase in expression in the DLPFC and ACC of men with MDD, these same genes are decreased in the AMY (Figure S9A). Interestingly, the oligodendrocyte-specific genes were upregulated in the AMY, but downregulated in DLPFC and ACC in women with MDD. Together, the cortical patterns for oligodendrocyte-specific genes drives the cross-brain region findings. A closer look at microglia- and neuronal-specific genes showed consistent findings across all three brain regions (Figure S9B, S9C).
Discussion
We report almost no overlap in transcriptional changes across corticolimbic brain regions in men and women with MDD, but instead opposite transcriptional changes. Our results suggest that men with MDD have decreases, but women with MDD have increases in synapse-related genes. Immune-related reductions characterized female MDD. Cell type-specific analysis suggests increases in oligodendrocyte- and microglia-specific genes in men with MDD, but decreases in markers of these cell types in women with MDD. Together, these findings point towards distinct, and even opposite molecular changes in MDD in men and women.
Our results are partially consistent with results from a recent publication reporting sex-specific changes in MDD (46). While we also found very little overlap in DE genes in men and women with MDD, our results indicate a high level of transcriptional overlap in genes changed in opposite directions. In fact, we used our statistical methods on the data generated by Labonte et al. (46) and found very similar, but unreported opposite transcriptional results. Brains used in the previous publication were from a different brain bank, supporting the generalizability of our findings. Here, we include results from the AMY, which is not included in Labonte et al. (46). Although consistent with our hypothesis, it is somewhat surprising that these sex-specific molecular changes in MDD were not reported previously. One reason might be because many previous postmortem brain analyses in MDD were performed in mostly (or only) men. Studies that included both sexes mostly did not have sufficient statistical power to stratify by sex, although a few prior reports have hinted at sex differences in MDD (see examples in the Introduction) We believe that our meta-analysis/regression approach gave us the statistical power to investigate larger-scale profiles of molecular changes occurring in the brains of men and women with MDD.
Previous studies reported reduced expression of neuron-specific genes in the AMY of men with MDD and reduced neuronal density in DLPFC (35, 42). Our findings in men with MDD are consistent with those reports. However, we did not find a significant change in neuronal genes in women with MDD. In fact, our results, while not significant after correction for multiple testing, suggest upregulation of neuron-specific genes in women with MDD. Hence future studies should directly compare neuron and synapse density in the brains of men and women with MDD.
Our findings in men with MDD are consistent with previous reports (that included mostly men) showing reduced markers of synapses, increased markers of inflammation, and reduced spine synapses in the DLPFC (54, 55). Specifically, we report reduced expression of genes related to synapse function and increased expression of microglia-specific genes in men with MDD. Our current findings suggest opposite synapse and inflammation-related changes in women with MDD.
Prior studies demonstrated reduced glial cell densities in DLPFC, ACC, and AMY in MDD (35–37, 39). These studies included both men and women, but did not stratify by sex. Thus, it is unclear whether the findings are sex-specific. Here, we report increases in markers of glia in men, but decreases in women with MDD. Making comparisons between density of glia and changes in expression of glia-specific cells might not be appropriate, as reduced glia density does not necessarily translate into reduced expression of glia-specific genes. Future studies will examine the glial deficits in both sexes, with attention to different glia cell types.
The region-specific findings for oligodendrocyte-specific genes are interesting. In men with MDD, we report increases in oligodendrocyte-specific genes in the DLPFC and ACC, but decreases in expression of these same genes in the AMY. Additionally, in women with MDD, these genes showed decreased expression in the DLPFC and ACC, but increased expression in the AMY (Figure 4). Thus, the oligodendrocyte changes in MDD are not only sex-specific, but brain region-specific as well. The cell type-specific findings for microglia-, astrocyte-, and neuronal-specific genes were consistent across brain regions.
The sex differences in MDD that we report might be driven by developmental processes. Developmental exposure to testosterone around the time of birth and through puberty permanently masculinizes the structure of several brain regions (termed organizational effects of hormones). Notably, adolescence is also a sensitive developmental time-period in which there is extensive neuroanatomical, functional, and chemical brain maturation. Events during adolescence that interact with these developmental processes can increase risk for adult psychopathology. We and others have used various rodent models to manipulate gonadal hormone exposure during critical periods of brain development (perinatal through puberty). For instance, we showed that giving newborn female mice a single dose of testosterone partially masculinizes adult mood-related behavior (i.e., these females had lower anxiety-/depressive-like behaviors in adulthood) (56). Differences in gonadal hormone exposure during development might also influence how the brain responds to a challenge (e.g., chronic stress) in adulthood. Sex differences due to developmental processes might be more relevant to the developmental origins of MDD compared to sex differences emerging in adulthood (e.g., reflecting environmental effects) (57). Since our study includes only adults, we are unable to determine whether the observed sex differences emerge during development or in adulthood.
Our cell type specific and pathway analyses suggest divergent changes in the brains of men and women with MDD. It is quite interesting that in both men and women, the neuronal- and microglial-related changes occur in opposite directions. Women with MDD have decreased markers of immune function and microglia, with increased markers of synaptic function and neurons. On the other hand, men with MDD have increased markers of microglia, with decreased markers of synaptic function and neurons. This opposite direction of effect on microglia and synapses is consistent with a growing literature suggesting that activated microglia have more frequent and prolonged contacts with, and may have increased phagocytosis of, dendritic spines (58). Since our work here is performed in the human postmortem brain, it is unclear whether the synaptic changes observed in MDD (decreased in men, increased in women) are driven by microglia changes, or vice versa. Additionally, it is unclear whether the opposite molecular signatures of MDD in men and women might drive sex differences in MDD symptomatology. To glean more definitive links, follow-up studies in rodent models would perturb immune function in both directions (in both sexes) and assess MDD-associated behavioral domains (e.g., anxiety-, anhedonia-, despair-related behavior).
Limitations of these results are inherent to studies involving human postmortem brains and the heterogeneity of psychiatric cohorts. Although many covariates could affect gene expression independently of psychiatric diagnosis, our statistical method only included the top two relevant covariates for each gene. This increased our statistical power, but might have ignored additional relevant covariates. We were not sufficiently powered for some cofactors, including recurrent/single episode MDD and comorbid drug abuse. Our meta-analysis/regression approach gave us statistical power to identify consistent molecular changes across brain regions in MDD. However, we note that this method might miss brain region-specific changes important for disease progression. Future studies will use large cohorts of MDD subjects and matched controls, with sufficient statistical power to detect potential sex-specific MDD changes. Although men and women with MDD tend to have differential responses to antidepressants, the medications taken by our subjects were largely similar between men and women, and antidepressant usage was used as a potential cofactor; thus, our results were not driven by different medications between the sexes.
To conclude, our study reveals divergent corticolimbic molecular changes in men and women with MDD. Thus, it follows that potential novel treatments should target sex-specific pathology. For instance, our results suggest that treatments to suppress immune function might be more appropriate for men with MDD, while treatments which boost immune function might be more appropriate for women with MDD. Alternatively, future treatments might aim to target the limited shared pathology present in both men and women with MDD. The implications of MDD cell-specific changes between men and women remain to be further investigated.
Supplementary Material
Figure S1. Overview of experimental design for meta-analysis, meta-regression, and downstream analyses.
Figure S2. Correlation of gene expression across studies used in meta-analysis. (A) In ACC studies performed in males on the same Affymetrix platform, there was a significant correlation of gene expression (Pearson correlation = 0.518). Results are shown by scatterplot. (B) In ACC studies performed in females on different platforms (Affymetrix and Illumina), there was a significant correlation of gene expression (Pearson correlation = 0.376). Results are shown by scatterplot.
Figure S3. Verification of meta-regression results using qPCR in AMY. There were sex x diagnosis interactions for ARPP21 (A) and P2RY12 (B), but not for MTHFR (C). For ARPP21 (A), there was a significant increase in expression in only women with MDD. For P2RY12 (B), there was a significant decrease in expression in only women with MDD. *, p < 0.05.
Figure S4. Overlap in opposite molecular profiles in men and women with MDD. (A) Venn diagrams indicating overlap in RRHO-identified genes from the full meta-analysis. (B) Venn diagrams indicating overlap in RRHO-identified genes from the DLPFC. (C) Venn diagrams indicating overlap in RRHO-identified genes from the ACC.
Figure S5. RRHO analysis of replication dataset from Labonte et al. (6) confirmed the opposite transcriptional profile of male and female depression in BA25 (left) and BA11 (right).
Figure S6. Overlap of top 10 biological pathways identified in men with MDD. Note the high level of overlap in the synapse-related pathways.
Figure S7. Overlap of top 10 biological pathways identified in women with MDD. Note the high level of overlap in the antigen-related pathways. Additionally, the mitochondrial translation-related pathways overlapped with each other, but not with the mitochondrial membrane-related pathways.
Figure S8. Overlap of top 10 biological pathways identified in the meta-regression dataset. Note the high level of overlap in the antigen-related and MHC pathways. Additionally, the synapse-related pathways are also highly overlapping.
Figure S9. Cell type-specific changes in MDD. (A) There were sex-specific and brain region-specific changes in oligodendrocyte genes. The overall cell type-specific signal when all three brain regions were combined indicated upregulation of oligodendrocyte-specific genes in men with MDD and downregulation of these same genes in women with MDD. This finding was driven by the DLPFC and ACC, with opposite direction of effects in AMY. (B) Across all three brain regions, there were increases in microglia-specific genes in men with MDD, but decreases in these same genes in women with MDD. (C) Across brain regions, there were consistent decreases in neuron-specific genes in men with MDD. There were nonsignificant increases in these same neuron-specific genes in women with MDD.
Table S1. Description of eight MDD microarray studies, including data pre-processing and number of genes investigated. See also previous reports on the cohorts and datasets (11–14).
Table S2. Demographic and technical details on individual subjects included in each microarray study.
Table S3. Genes identified via meta-regression which are changed in opposite directions in men and women with MDD.
Table S4. Overlap in DE genes from male MDD and female MDD with genes that are DE between male and female healthy controls.a
Table S5. Sex-specific depression changes confirmed using a different brain bank cohort.a
Table S6. Replication cohort: top 10 transcripts significantly changed in opposite directions in men and women with MDD.
Table S7. Sex-specific associations of transcriptomic cell-type enriched gene sets using mouse reference dataset.a
Table S8. Primers used in qPCR studies.
Acknowledgments
This work was supported by National Institute of Mental Health (NIMH) MH084060 (ES), MH085111 (ES), and MH103473 (MS). Drs. Sibille and Seney are supported by a NARSAD Distinguished Scientist and Young Investigator Award, respectively, from the Brain and Behavior Research Foundation. The funding agencies had no role in the study design, data collection and analysis, decision to publish and preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health.
Footnotes
Financial Disclosures
David A. Lewis currently receives investigator-initiated research support from Pfizer. In 2015–2017, he served as a consultant in the areas of target identification and validation and new compound development to Sunovion. Seney, Huo, Cahill, French, Puralewski, Zhang, Logan, Tseng, and Sibille report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. The global burden of disease, 2004 update. 2008:1–146. [Google Scholar]
- 2.Perugi G, Musetti L, Simonini E, Piagentini F, Cassano GB, Akiskal HS. Gender-mediated clinical features of depressive illness. The importance of temperamental differences. Br J Psychiatry. 1990;157:835–841. doi: 10.1192/bjp.157.6.835. [DOI] [PubMed] [Google Scholar]
- 3.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in chronic major and double depression. J Affect Disord. 2000;60:1–11. doi: 10.1016/s0165-0327(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J Affect Disord. 1994;30:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 6.Weissman MM, Klerman GL. Sex differences and the epidemiology of depression. Arch Gen Psychiatry. 1977;34:98–111. doi: 10.1001/archpsyc.1977.01770130100011. [DOI] [PubMed] [Google Scholar]
- 7.Preisig M, Merikangas KR, Angst J. Clinical significance and comorbidity of subthreshold depression and anxiety in the community. Acta Psychiatr Scand. 2001;104:96–103. doi: 10.1034/j.1600-0447.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 8.Angst J, Dobler-Mikola A. Do the diagnostic criteria determine the sex ratio in depression? J Affect Disord. 1984;7:189–198. doi: 10.1016/0165-0327(84)90040-5. [DOI] [PubMed] [Google Scholar]
- 9.Young MA, Fogg LF, Scheftner WA, Keller MB, Fawcett JA. Sex differences in the lifetime prevalence of depression: does varying the diagnostic criteria reduce the female/male ratio? J Affect Disord. 1990;18:187–192. doi: 10.1016/0165-0327(90)90035-7. [DOI] [PubMed] [Google Scholar]
- 10.Scheibe S, Preuschhof C, Cristi C, Bagby RM. Are there gender differences in major depression and its response to antidepressants? J Affect Disord. 2003;75:223–235. doi: 10.1016/s0165-0327(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 11.Angst J, Gamma A, Sellaro R, Zhang H, Merikangas K. Toward validation of atypical depression in the community: results of the Zurich cohort study. J Affect Disord. 2002;72:125–138. doi: 10.1016/s0165-0327(02)00169-6. [DOI] [PubMed] [Google Scholar]
- 12.Benazzi F. Prevalence and clinical features of atypical depression in depressed outpatients: a 467-case study. Psychiatry Res. 1999;86:259–265. doi: 10.1016/s0165-1781(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 13.Posternak MA, Zimmerman M. The prevalence of atypical features across mood, anxiety, and personality disorders. Compr Psychiatry. 2002;43:253–262. doi: 10.1053/comp.2002.33498. [DOI] [PubMed] [Google Scholar]
- 14.Matza LS, Revicki DA, Davidson JR, Stewart JW. Depression with atypical features in the National Comorbidity Survey: classification, description, and consequences. Arch Gen Psychiatry. 2003;60:817–826. doi: 10.1001/archpsyc.60.8.817. [DOI] [PubMed] [Google Scholar]
- 15.Frank E, Carpenter LL, Kupfer DJ. Sex differences in recurrent depression: are there any that are significant? Am J Psychiatry. 1988;145:41–45. doi: 10.1176/ajp.145.1.41. [DOI] [PubMed] [Google Scholar]
- 16.Angst J, Vollrath M. The natural history of anxiety disorders. Acta Psychiatr Scand. 1991;84:446–452. doi: 10.1111/j.1600-0447.1991.tb03176.x. [DOI] [PubMed] [Google Scholar]
- 17.Breslau N, Schultz L, Peterson E. Sex differences in depression: a role for preexisting anxiety. Psychiatry Res. 1995;58:1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- 18.Kornstein SG, Schatzberg AF, Yonkers KA, Thase ME, Keitner GI, Ryan CE, et al. Gender differences in presentation of chronic major depression. Psychopharmacol Bull. 1995;31:711–718. [PubMed] [Google Scholar]
- 19.Najt P, Fusar-Poli P, Brambilla P. Co-occurring mental and substance abuse disorders: a review on the potential predictors and clinical outcomes. Psychiatry Res. 2011;186:159–164. doi: 10.1016/j.psychres.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 20.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 21.Davidson J, Pelton S. Forms of atypical depression and their response to antidepressant drugs. Psychiatry Res. 1986;17:87–95. doi: 10.1016/0165-1781(86)90063-6. [DOI] [PubMed] [Google Scholar]
- 22.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 23.Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, et al. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry. 1999;156:1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 25.Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- 26.Baxter LR, Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 27.Bench CJ, Friston KJ, Brown RG, Frackowiak RS, Dolan RJ. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol Med. 1993;23:579–590. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- 28.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 30.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 31.Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- 32.Koch K, Pauly K, Kellermann T, Seiferth NY, Reske M, Backes V, et al. Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia. 2007;45:2744–2754. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Lebron-Milad K, Abbs B, Milad MR, Linnman C, Rougemount-Bucking A, Zeidan MA, et al. Sex differences in the neurobiology of fear conditioning and extinction: a preliminary fMRI study of shared sex differences with stress-arousal circuitry. Biol Mood Anxiety Disord. 2012;2:7. doi: 10.1186/2045-5380-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mak AK, Hu ZG, Zhang JX, Xiao Z, Lee TM. Sex-related differences in neural activity during emotion regulation. Neuropsychologia. 2009;47:2900–2908. doi: 10.1016/j.neuropsychologia.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 36.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 37.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 39.Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:1194–1202. doi: 10.1176/appi.ajp.2012.12020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seney ML, Chang LC, Oh H, Wang X, Tseng GC, Lewis DA, et al. The Role of Genetic Sex in Affect Regulation and Expression of GABA-Related Genes Across Species. Front Psychiatry. 2013;4:104. doi: 10.3389/fpsyt.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, et al. A molecular signature of depression in the amygdala. Am J Psychiatry. 2009;166:1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bassi S, Seney ML, Argibay P, Sibille E. Elevated Hippocampal Cholinergic Neurostimulating Peptide precursor protein (HCNP-pp) mRNA in the amygdala in major depression. J Psychiatr Res. 2015;63:105–116. doi: 10.1016/j.jpsychires.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry. 2015;20:1057–1068. doi: 10.1038/mp.2015.91. [DOI] [PubMed] [Google Scholar]
- 46.Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23:1102–1111. doi: 10.1038/nm.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tripp A, Kota RS, Lewis DA, Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42:116–124. doi: 10.1016/j.nbd.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Lin Y, Song C, Sibille E, Tseng GC. Detecting disease-associated genes with confounding variable adjustment and the impact on genomic meta-analysis: with application to major depressive disorder. BMC Bioinformatics. 2012;13:52. doi: 10.1186/1471-2105-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Kang DD, Shen K, Song C, Lu S, Chang LC, et al. An R package suite for microarray meta-analysis in quality control, differentially expressed gene analysis and pathway enrichment detection. Bioinformatics. 2012;28:2534–2536. doi: 10.1093/bioinformatics/bts485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding Y, Chang LC, Wang X, Guilloux JP, Parrish J, Oh H, et al. Molecular and Genetic Characterization of Depression: Overlap with other Psychiatric Disorders and Aging. Mol Neuropsychiatry. 2015;1:1–12. doi: 10.1159/000369974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plaisier SB, Taschereau R, Wong JA, Graeber TG. Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 2010;38:e169. doi: 10.1093/nar/gkq636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darmanis S, Sloan SA, Zhang Y, Enge M, Caneda C, Shuer LM, et al. A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci U S A. 2015;112:7285–7290. doi: 10.1073/pnas.1507125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang HJ, Adams DH, Simen A, Simen BB, Rajkowska G, Stockmeier CA, et al. Gene expression profiling in postmortem prefrontal cortex of major depressive disorder. J Neurosci. 2007;27:13329–13340. doi: 10.1523/JNEUROSCI.4083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seney ML, Walsh C, Stolakis R, Sibille E. Neonatal testosterone partially organizes sex differences in stress-induced emotionality in mice. Neurobiol Dis. 2012;46:486–496. doi: 10.1016/j.nbd.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi L, Zhang Z, Su B. Sex Biased Gene Expression Profiling of Human Brains at Major Developmental Stages. Sci Rep. 2016;6:21181. doi: 10.1038/srep21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Overview of experimental design for meta-analysis, meta-regression, and downstream analyses.
Figure S2. Correlation of gene expression across studies used in meta-analysis. (A) In ACC studies performed in males on the same Affymetrix platform, there was a significant correlation of gene expression (Pearson correlation = 0.518). Results are shown by scatterplot. (B) In ACC studies performed in females on different platforms (Affymetrix and Illumina), there was a significant correlation of gene expression (Pearson correlation = 0.376). Results are shown by scatterplot.
Figure S3. Verification of meta-regression results using qPCR in AMY. There were sex x diagnosis interactions for ARPP21 (A) and P2RY12 (B), but not for MTHFR (C). For ARPP21 (A), there was a significant increase in expression in only women with MDD. For P2RY12 (B), there was a significant decrease in expression in only women with MDD. *, p < 0.05.
Figure S4. Overlap in opposite molecular profiles in men and women with MDD. (A) Venn diagrams indicating overlap in RRHO-identified genes from the full meta-analysis. (B) Venn diagrams indicating overlap in RRHO-identified genes from the DLPFC. (C) Venn diagrams indicating overlap in RRHO-identified genes from the ACC.
Figure S5. RRHO analysis of replication dataset from Labonte et al. (6) confirmed the opposite transcriptional profile of male and female depression in BA25 (left) and BA11 (right).
Figure S6. Overlap of top 10 biological pathways identified in men with MDD. Note the high level of overlap in the synapse-related pathways.
Figure S7. Overlap of top 10 biological pathways identified in women with MDD. Note the high level of overlap in the antigen-related pathways. Additionally, the mitochondrial translation-related pathways overlapped with each other, but not with the mitochondrial membrane-related pathways.
Figure S8. Overlap of top 10 biological pathways identified in the meta-regression dataset. Note the high level of overlap in the antigen-related and MHC pathways. Additionally, the synapse-related pathways are also highly overlapping.
Figure S9. Cell type-specific changes in MDD. (A) There were sex-specific and brain region-specific changes in oligodendrocyte genes. The overall cell type-specific signal when all three brain regions were combined indicated upregulation of oligodendrocyte-specific genes in men with MDD and downregulation of these same genes in women with MDD. This finding was driven by the DLPFC and ACC, with opposite direction of effects in AMY. (B) Across all three brain regions, there were increases in microglia-specific genes in men with MDD, but decreases in these same genes in women with MDD. (C) Across brain regions, there were consistent decreases in neuron-specific genes in men with MDD. There were nonsignificant increases in these same neuron-specific genes in women with MDD.
Table S1. Description of eight MDD microarray studies, including data pre-processing and number of genes investigated. See also previous reports on the cohorts and datasets (11–14).
Table S2. Demographic and technical details on individual subjects included in each microarray study.
Table S3. Genes identified via meta-regression which are changed in opposite directions in men and women with MDD.
Table S4. Overlap in DE genes from male MDD and female MDD with genes that are DE between male and female healthy controls.a
Table S5. Sex-specific depression changes confirmed using a different brain bank cohort.a
Table S6. Replication cohort: top 10 transcripts significantly changed in opposite directions in men and women with MDD.
Table S7. Sex-specific associations of transcriptomic cell-type enriched gene sets using mouse reference dataset.a
Table S8. Primers used in qPCR studies.