Summary
Increased protein citrullination is linked to various diseases including rheumatoid arthritis (RA), Lupus, and cancer. Citrullinated autoantigens, a hallmark of RA, are recognized by anti-citrullinated protein antibodies (ACPA) which are used to diagnose RA. ACPA recognizing citrullinated enolase, vimentin, keratin and filaggrin are also pathogenic. Here, we used a chemoproteomic approach to define the RA-associated citrullinome. The identified proteins include numerous serine protease inhibitors (Serpins), proteases and metabolic enzymes. We demonstrate that citrullination of antiplasmin, antithrombin, t-PAI, and C1 inhibitor (P1-Arg-containing Serpins) abolishes their ability to inhibit their cognate proteases. Citrullination of nicotinamide N-methyl transferase (NNMT) also abolished its methyltransferase activity. Overall, these data advance our understanding of the roles of citrullination in RA and suggest that extracellular Protein Arginine Deiminase (PAD) activity can modulate protease activity with consequent effects on Serpin regulated pathways. Moreover, our data suggest that inhibition of extracellular PAD activity will be therapeutically relevant.
eTOC blurb
Tilvawala et al. demonstrated that protein citrullination is elevated in RA and defined the RA associated citrullinome. Tilvawala et al. further discovered that Serpin citrullination abolishes their ability to inhibit their cognate proteases. These studies open a new avenue to understand the links between protein citrullination and numerous diseases.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that affects 0.5–1.0% of the adult population (Cross et al., 2014). This disease is characterized by systemic inflammation resulting in damaged cartilage, bone and soft tissue, ultimately leading to restricted movement and disability. RA is triggered by a combination of genetic and environmental factors. Accumulating evidence indicates that aberrantly increased protein citrullination is also a disease trigger (Schellekens et al., 1998; Vossenaar and van Venrooij, 2004). This evidence includes the fact that up to 75% of RA patients produce anti-citrullinated protein antibodies (ACPA), which bind and recognize citrullinated epitopes present on numerous proteins including vimentin, fibrin and enolase (van Beers et al., 2010; Vossenaar et al., 2004a). Retrospective analyses have shown that ACPA begin to accumulate in patient sera 4–5 years before clinical onset of symptoms (van der Helm-van Mil et al., 2006; van Venrooij et al., 2006) and the detection of ACPA is the most specific diagnostic test for RA (Taylor et al., 2011). Importantly, higher ACPA titers correlate with a more severe disease course. As such, a positive anti-CCP test, which detects ACPA, is now part of the clinical criteria for diagnosing RA (Chandra et al., 2011).
Citrullination is the post-translational modification of an arginine residue to citrulline in proteins and peptides. This reaction is catalyzed by the calcium-dependent protein arginine deiminase (PAD) family of enzymes (Fuhrmann et al., 2015; Vossenaar et al., 2003). The conversion of a positively charged arginine to polar uncharged citrulline can drastically influence hydrogen bonding and ionic interactions with consequent effects on activity, protein-protein interactions and protein-nucleic acid interactions (Fuhrmann et al., 2015). Mammals have five PAD isozymes (PAD 1, 2, 3, 4 and 6) (Fuhrmann et al., 2015). However, only PADs 1–4 are catalytically active; PAD6 has a number of mutations that render it inactive (Raijmakers et al., 2007). With regards to RA, PAD2 and PAD4 are the most relevant because they are predominantly overexpressed in immune cells including macrophages and neutrophils (Vossenaar et al., 2004b). PADs are generally inactive under physiological conditions since relatively high calcium concentrations are required for activity (Fujisaki and Sugawara, 1981). However, once activated, these enzymes citrullinate numerous different proteins including vimentin, fibrin, filaggrin and keratin (Inagaki et al., 1989; Senshu et al., 1995). PAD1, 2 and 4 also citrullinate various histones (i.e., H1, H2A, H3 and H4) and histone citrullination contributes to the epigenetic control of gene transcription (Christophorou et al., 2014; Khan et al., 2016; Wang et al., 2004; Zhang et al., 2012). Although less is known about the functional consequences of vimentin, fibrin, and filaggrin citrullination, ACPA targeting these structural proteins are present in RA patients.
Apart from RA, dysregulated PAD activity is observed in other inflammatory diseases (Jones et al., 2009; Vossenaar et al., 2003) including type 1 diabetes (Rondas et al., 2015), Parkinson’s disease (Nicholas, 2011), Alzheimer’s disease (Ishigami et al., 2005), atherosclerosis (Kinloch et al., 2008), lupus (Knight et al., 2015), multiple sclerosis (Moscarello et al., 2007), psoriasis (Ishida-Yamamoto et al., 2000), chronic obstructive pulmonary disease (COPD) (Obermayer et al., 2014) and neuron injury (Lange et al., 2011), as well as several types of cancers (Chang and Han, 2006; Chang et al., 2009). Notably, PAD inhibitors show remarkable efficacy in many of the aforementioned diseases (Chumanevich et al., 2011; Knight et al., 2015; Knight et al., 2013; Lange et al., 2011; Willis et al., 2011). Since ACPA are generally restricted to RA, it is unclear how the PADs contribute to so many different pathologies. Potential explanations include the modulation of gene expression patterns via their histone citrullination activity (Shen and Casaccia-Bonnefil, 2008; Wang and Wang, 2013). Evidence supporting this possibility includes the recent demonstration that PAD4 modulates the expression of E2F (Ghari et al., 2016) and NF-kB target genes including IL-1β and TNFα in neutrophils (Chang et al., 2016). A second explanation is the role of the PADs in NET (Neutrophil Extracellular Trap) formation (Knight et al., 2015; Li et al., 2010; Rohrbach et al., 2012), a pro-inflammatory form of programmed cell death in which neutrophils decondense their chromatin to form web-like structures that can capture pathogens. NET formation also results in the release of numerous enzymes to the extracellular environment including myeloperoxidase, various proteases and the PADs (Damgaard et al., 2014). Previous reports demonstrated that PAD4 knock-out mice do not form NETs, suggesting that inhibition of PAD4 represents a viable approach for preventing this type of programmed cell death (Li et al., 2010). A recent report, however, suggests that PAD4 activity is not essential for human NET formation (Kenny et al., 2017). Nevertheless, histone citrullination represents an important event in NETosis as the PADs get activated once the neutrophil membrane is breached. Although NET formation is a part of the body’s innate immune system, abnormal NET formation is observed in many autoimmune disorders including RA (Khandpur et al., 2013), lupus (Yu and Su, 2013) and Alzheimer’s disease (Pietronigro et al., 2017).
Given this background, it is evident that abnormally elevated protein citrullination plays a key role in the onset and development of RA. Better knowledge of PAD substrates and the effect of citrullination on their functions would not only improve our understanding of disease pathology but will also help to develop better diagnostics and treatments. However, only a handful of PAD substrates are known due to the technical challenges associated with identifying citrullinated proteins; the mass change is only 0.98 Da. In previous reports, we described the development of two new reagents, rhodamine-PG (Rh-PG) and biotin-PG that can be used to visualize and isolate citrullinated proteins (Bicker et al., 2012; Lewallen et al., 2015). Herein, we describe their use to identify novel citrullinated proteins from RA patient serum, synovial fluid and synovial tissues. Remarkably, we identified an increase in many ACPA-target proteins in RA samples consistent with the hypothesis that ACPAs are disease causing. Moreover, we identified numerous metabolic enzymes as well as serine-protease inhibitors (Serpins). Importantly, for a subset of Serpins, we show that citrullination can abolish their inhibitory activity and thereby modulate Serpin-regulated pathways including the complement pathway, blood clotting, fibrinolysis, and cell motility. Increased protease activity would allow for enhanced complement activation and degradation of the extracellular matrix that would enable the influx of other immune cells and consequently potentiate the inflammatory response.
Results
Phenylglyoxal-based probes for the detection of protein citrullination in biological samples
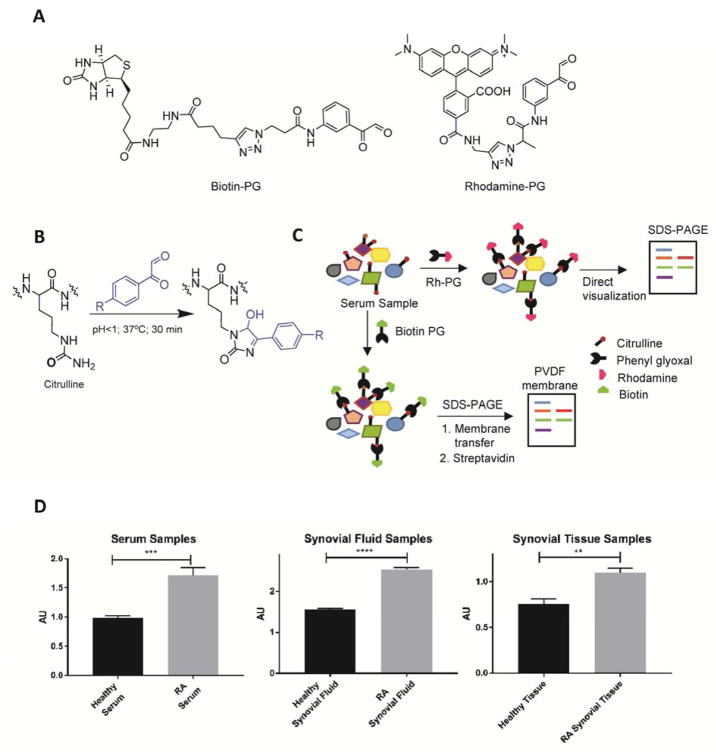
We previously developed a suite of phenylglyoxal (PG)-based probes that facilitate the isolation and/or visualization of citrullinated proteins in complex proteomes (Bicker et al., 2012; Lewallen et al., 2015). These probes, rhodamine-PG (Rh-PG) and biotin-PG (Figure 1A), covalently modify the urea group of citrulline under acidic conditions (Figure 1B). This covalent adduct can be visualized either directly by SDS-PAGE, in the case of Rh-PG, or by electroblotting in the case of biotin-PG (Figure 1C). Given their unique capabilities and the important role that aberrant protein citrullination plays in RA, we hypothesized that they could be used to identify the citrullinated proteins associated with RA. We further hypothesized that this information would provide novel insights into PAD biology as well as identify RA-specific biomarkers. To this end, we employed Rh-PG to quantify the relative amount of citrullinated proteins in RA-patient serum, synovial fluid and synovial tissue samples as compared to healthy individuals. The serum samples were obtained from healthy donors (n=16; 7 females and 9 males) and RA patients (n=16; 9 females and 7 males). The synovial fluid samples were collected from healthy donors (n=16; 6 females and 10 males) and RA patients (n=16; 6 females and 10 males). The synovial tissue samples were obtained from one healthy and two RA patients. The RA serum, synovial fluid and synovial tissue samples were obtained from confirmed RA patients but different individuals. The serum and synovial fluid samples were pooled to generate representative samples. Sample pooling enables higher throughput, minimizes sample heterogeneity, and enriches for proteins that are uniformly dysregulated across the entire sample group. Citrullinated proteins present in these samples were first labeled with Rh-PG, separated on SDS-PAGE gels and the fluorescence intensities of labeled proteins were quantified (Figure S1A). As depicted in Figure 1D, our data clearly demonstrate that protein citrullination levels are elevated in RA serum, synovial fluid and synovial tissue compared to healthy controls.
Figure 1. Phenylglyoxal based probes for the detection of protein citrullination in biological samples.
A) Structure of biotin-phenylglyoxal (biotin-PG) and rhodamine-phenylglyoxal (Rh-PG). B) Phenylglyoxal probes selectively label citrullinated proteins under acidic conditions. C) Schematic representation of methods used for labeling protein citrullination in healthy and RA patient samples using Rh-PG (top) and biotin-PG (bottom). D) Comparison of citrullinated protein levels detected from healthy and RA serum, synovial fluid and synovial tissue samples using Rh-PG. See also Figure S1A.
The RA-associated citrullinome
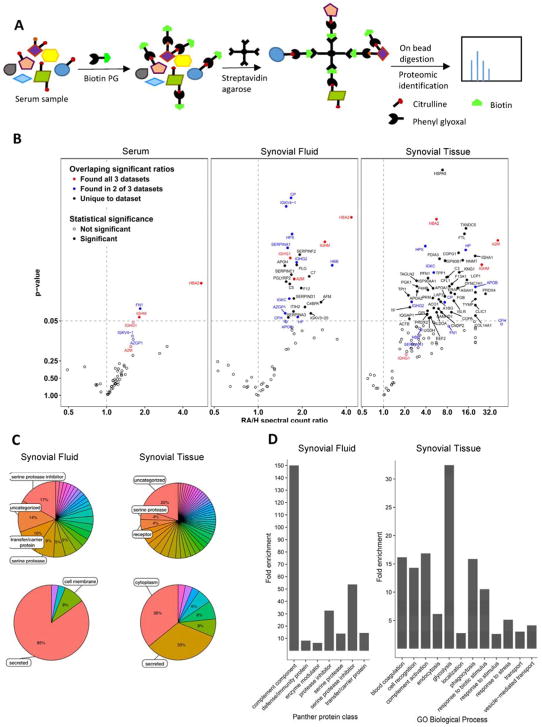
Next, we used biotin-PG to define the RA-associated citrullinome in serum, synovial fluid (the fluid surrounding joints), and synovial tissue samples (granulation tissue which surrounds joints). Briefly, RA and healthy controls were labeled with biotin-PG and citrullinated proteins enriched on streptavidin-agarose. After extensive washing, we performed an on-bead trypsin digest and subsequent LC/LC-MS/MS analysis (Figure 2A). Protein identities were determined by database searches using the SEQUEST algorithm. Relative quantitation of citrullinated protein abundance in RA and healthy patient samples was achieved by spectral counting. The relative abundance of a protein in the RA versus healthy datasets can be used to compare the relative citrullination level of the protein in human patients. Only proteins with an average of greater than 10 spectral counts in RA patients were used to calculate spectral count ratios.
Figure 2. Identification of the RA-associated citrullinome.
A) Schematic representation of experimental procedure used for the detection of protein citrullination by proteomic analysis. B) Volcano plot showing identities of citrullinated proteins that are elevated in RA serum (left), synovial fluid (middle) and synovial tissue (right) compared to healthy samples. The y-axis is p-value and the x-axis is the RA/Healthy ratio. C) Pie charts show distribution of elevated citrullinated proteins from synovial fluid and synovial tissue samples. Functional classification of citrullinated proteins from RA synovial fluid (top left) and synovial tissue (top right). Endogenous expression of citrullinated proteins from RA synovial fluid (bottom left) and synovial tissue (bottom right). D) Statistical over representation test for Panther protein classes citrullinated in synovial fluid (left) and biological processes citrullinated in tissue (right). The percentage of proteins which belong to a particular category in the input list (citrullinated proteins in RA) are compared to the percentage of proteins in the reference list (complete human genome) belonging to the same category. The categories which showed the highest degree of enrichment in RA synovial fluid and synovial tissue are shown.
Proteins with RA/healthy spectral count ratios >1 and p-values <0.05 are selectively citrullinated in the RA samples (Figure 2B). One readily apparent trend is that there is an expansion in number of citrullinated proteins as one moves from serum to synovial fluid and onto the synovial tissue. The RA/H ratios also increase significantly. These observations are consistent with enhanced citrullination occurring within the inflamed region. Notably, several proteins that show high relative ratios with significant p-values in synovial fluid, also appear in the serum data at lower levels and at lower confidence. This likely reflects the leaching of these proteins from synovial fluid into serum. Amongst the various other citrullinated proteins discovered, the majority were secreted (85% in synovial fluid and 33% in synovial tissue) and cytoplasmic (36% in synovial tissue) proteins (Figure 2C bottom), suggesting that both intra- and extra- cellular citrullination contributes to pathogenesis. Notably, we detected elevated levels of known citrullinated proteins including vimentin, fibrin, keratin and enolase from synovial tissues and synovial fluid. In addition to these known substrates, we identified a wide variety of novel citrullinated proteins covering various functional classes, amongst which serine protease inhibitors (17% in synovial fluid), serine proteases (8% in synovial fluid and 4% in synovial tissues), carriers/transporters (10% in synovial fluid) and complement system components (4% in synovial fluid) were the major classes (Figure 2C top). A PANTHER overrepresentation test (Mi et al., 2016) was used to identify protein classes and biological processes which were enriched in synovial fluid and synovial tissue. The complement pathway, serine protease inhibitors and serine proteases were the most elevated in synovial fluid while metabolic enzymes were highly enriched in synovial tissues (Figure 2D).
Quantification of citrullinated proteins detected through proteomic analysis
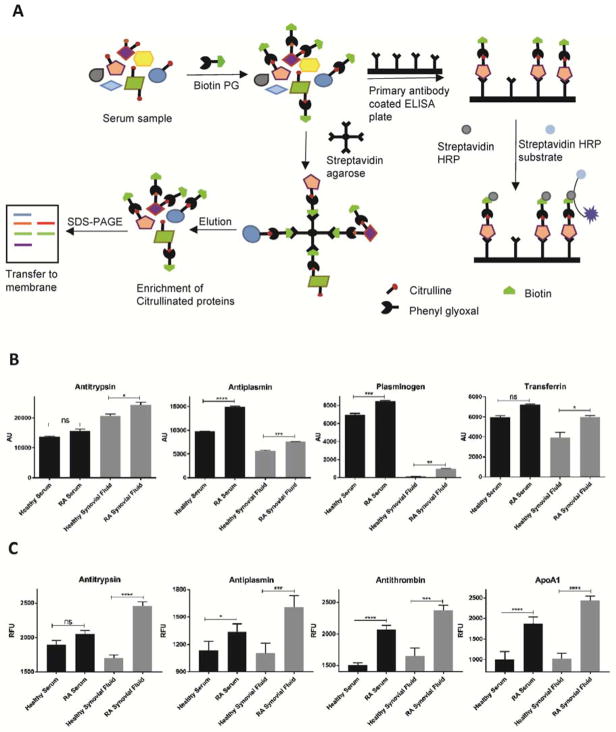
Having demonstrated that we could define the RA-associated citrullinome, we next quantitatively analyzed the citrullination levels of specific proteins in these samples using western blot and ELISA. In the western blot assay (Figure S1B), the red band represents the input level of the specific protein of interest while the green band shows the level of citrullination of that same protein (Figure S1B). We carried out western blot analysis of antitrypsin, antiplasmin, plasminogen and transferrin from RA patient serum and synovial fluid and compared the level of citrullination with healthy controls (Figure 3B). The results clearly demonstrate that these proteins were more heavily citrullinated in RA patients. We further confirmed our results using a biotin-PG based ELISA assay. In this assay, biotin-PG labeled proteins or protein mixtures were incubated with capture antibody coated 96-well plates in order to isolate the protein of interest (Figure S1C). The plates were next incubated with streptavidin-HRP followed by addition of fluorescent HRP substrate (Figure 3A, Figure S1C). Using this workflow, we quantified the citrullination levels of antitrypsin, antiplasmin, antithrombin III and ApoA1. As shown in Figure 3C, the ELISA data confirmed that antitrypsin, antiplasmin, antithrombin III and ApoA1 show increased citrullination in RA samples compared to the healthy controls.
Figure 3. Quantification of citrullinated proteins by Western blot and ELISA.
A) Schematic representation of experimental approaches used to quantitate citrullinated proteins by sandwich ELISA and Western blot. B) Quantification of citrullinated antiplasmin, antitrypsin, plasminogen and transferrin from RA and healthy serum and synovial fluid samples. Serum and synovial fluid samples were labeled with biotin-PG and citrullinated proteins isolated with streptavidin agarose. The inputs and eluents were probed by Western blotting using antibodies against the protein of interest. The blot was incubated with the appropriate secondary antibody and streptavidin. Experiments were carried out in triplicate and p-values calculated. C) Quantification of citrullinated antiplasmin, antithrombin, antitrypsin and ApoA1 from RA and healthy serum and synovial fluid samples. Serum and synovial fluid samples were labeled with biotin-PG and incubated with capture antibody coated 96-well plates to isolate the protein of interest. The plates were further incubated with streptavidin-HRP followed by addition of fluorescent HRP substrate. Experiments were carried out in triplicate and p-values calculated. See also Figure S1B and S1C.
Functional effects of citrullination
Although previous studies have confirmed higher levels of citrullinated enolase, vimentin, filaggrin in RA, the effect of citrullination on the activity of these and other proteins remain unknown. The RA citrullinome revealed that glycolytic enzymes are one of the major classes of highly enriched citrullinated proteins (Figure 2C and Figure 2D); nine out of eleven glycolytic enzymes are citrullinated. Citrullinated glycolytic enzymes discovered in RA synovial tissue are marked in yellow in the glycolytic pathway (Figure S2). The other interesting enzyme we discovered is nicotinamide N-methyl transferase (NNMT). NNMT is overexpressed in various cancers (Ulanovskaya et al., 2013) and contributes to diet induced obesity (Kraus et al., 2014). Given the importance of these metabolic pathways, we evaluated the effect of citrullination on the activity of NNMT as well as pyruvate kinase isoform M2 (PKM2) and α-enolase. We focused on these glycolytic enzymes because PKM2 activity represents a key control point for flux through the glycolytic pathway (Anastasiou et al., 2012) while α-enolase is a known antigen found in RA patients (Kinloch et al., 2005).
To test the effect of citrullination, we first treated NNMT, PKM2, aldolase and α-enolase with PAD1, PAD2, PAD3 and PAD4 and confirmed that they are citrullinated (Figure S3A). Next, we evaluated the effect of citrullination on enzyme activity. The NNMT activity assay was carried out using quinoline as an alternative substrate and SAM as the cofactor (van Haren et al., 2016). The activity of NNMT was measured in two different settings: 1) variable quinoline in the presence of a fixed SAM concentration; and 2) variable SAM in the presence of a fixed quinoline concentration. The rate of reaction was monitored by formation of 1-methylquinolinium. Notably, treatment of NNMT with PAD1 and PAD2 abolished its activity against both SAM and quinoline (Figure S3B and S3C and Table S1). PAD3 and PAD4 treated NNMT, however, only showed a moderate reduction in activity (Supplementary Figure 3B and 3C). The activity of PKM2 was measured in three different scenarios: 1) variable phosphoenol pyruvate (PEP) in the presence of a saturating concentration of ADP; 2) variable PEP in the presence of a saturating concentration of ADP and fructose 1, 6-bisphosphate (FBP); and 3) variable ADP in the presence of a saturating concentration of PEP (Figure S4A, S4B and S4C, Table S2). FBP is a known activator of PKM2 (Jurica et al., 1998). In all three scenarios, the activity of citrullinated PKM2 was, on an average, 2–3-fold higher compared to the uncitrullinated controls (Figure S4D). By contrast, citrullinated α-enolase showed a modest 2-fold decrease in catalytic efficiency (Figure S4E).
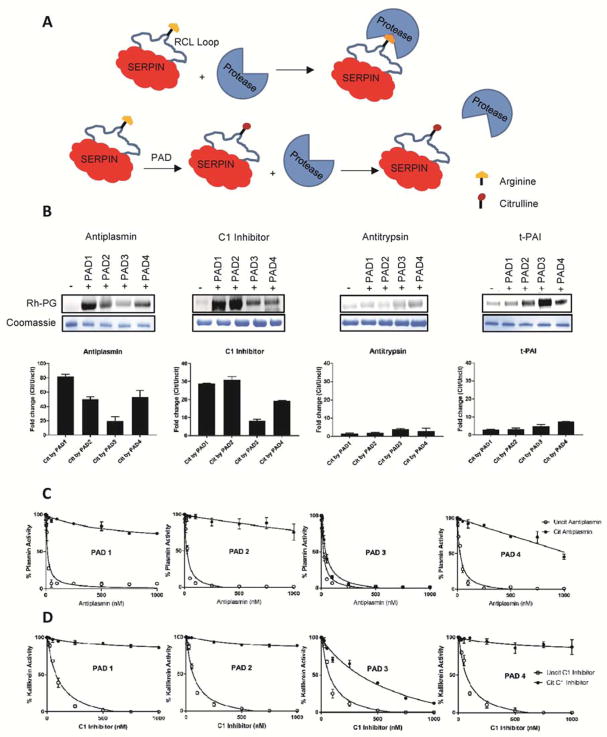
Given the preponderance of Serpins (Serine Protease Inhibitors) in our data sets (Figure 2B, 2C and 2D), we next investigated the effect of citrullination on Serpin activity. The Serpin superfamily contains well over 1500 members, including 36 human Serpins (Huntington, 2011). In humans, Serpins regulate protease cascades involved in blood clotting, fibrinolysis, complement activation, cell motility, inflammation and cell death (Silverman et al., 2001). Serpins react with serine proteases through a loop called the reactive center loop (RCL). The RCL is an extended, exposed sequence above the body of the Serpin scaffold. Upon RCL cleavage, the Serpin undergoes a conformational change that traps the protease in an inactive acyl-enzyme complex. In some Serpins, the P1 position in the RCL is occupied by an arginine residue which is responsible for docking the RCL into the protease active site (Khan et al., 2011). Given the important role of the P1-arginine in the Serpin-protease interaction, we hypothesized that citrullination of this arginine would abolish the inhibitory activity of Serpins containing a P1-arginine (Figure 4A). To this end, we initially focused on antiplasmin, C1 inhibitor, and tissue plasminogen activator inhibitor (t-PAI) as these Serpins play important roles in regulating fibrinolysis (Carpenter and Mathew, 2008), tissue repair (Sun and Yang, 2004), the complement pathway (Pappalardo et al., 2002) and blood coagulation (Sprengers and Kluft, 1987). Initially, we confirmed that these Serpins are PAD substrates by treating them with PAD1, PAD2, PAD3 and PAD4 followed by labeling with Rh-PG. As demonstrated in Figure 4B, PAD treated Serpins showed significantly higher band intensities compared to the untreated controls, confirming that Serpins are indeed PAD substrates.
Figure 4. Functional effect of citrullination on Serpins.
A) Schematic diagram showing the effect of citrullination on Serpin activity. Citrullination of the P1-arginine in Serpins abolishes their inhibitory activity against their cognate proteases. B) Citrullination of antiplasmin, C1 inhibitor, antitrypsin and t-PAI (10 μM each). Proteins citrullinated by PAD1, PAD2, PAD3 and PAD4 were labelled with Rh-PG. Proteins were separated by SDS-PAGE and rhodamine fluorescence imaged (top). Coomassie images (bottom) confirm equal loading. Band intensities were quantified, normalized against uncitrullinated proteins and plotted against PAD1, PAD2, PAD3 and PAD4. C) IC50 plots showing inhibition of plasmin by citrullinated and uncitrullinated antiplasmin. Plasmin (50 nM) was mixed with different concentrations of citrullinated or uncitrullinated antiplasmin and incubated for 30 min at room temperature. Hydrolysis of the plasmin substrate D-Val-Leu-Lys-pNA (1 mM) was monitored spectrophotometrically at 405 nm. D) IC50 plots showing inhibition of kallikrein by various concentrations of citrullinated and uncitrullinated C1 inhibitor. Kallikrein (100 nM) was mixed with different concentrations of citrullinated or uncitrullinated C1 inhibitor and incubated for 30 min at room temperature. Hydrolysis of the kallikrein substrate N-benzoyl-Pro-Phe-Arg-pNA (1 mM) was monitored spectrophotometrically at 405 nm. See also Figure S5 and Table S3.
We next measured the inhibitory activity of PAD treated Serpins and compared them with untreated controls. In this experiment, a fixed concentration of protease was mixed with various concentrations of citrullinated or uncitrullinated Serpin and incubated for 30 min at room temperature. The reaction mixtures were then placed in a 96-well plate followed by the rapid addition of the appropriate protease substrate. Hydrolysis of the latter was monitored spectrophotometrically to obtain IC50 values (Figure S5A). Citrullination of antiplasmin, C1 inhibitor and t-PAI, by PAD1, PAD2 and PAD4 virtually abolished their inhibitory activity against their corresponding proteases (Figure 4C, 4D and Figure S5B, Table S3). PAD3 treated Serpins showed a lesser effect. We performed the same experiments with antitrypsin. PAD treated antitrypsin did not show any significant change in its inhibition profile against neutrophil elastase, a known antitrypsin substrate (Figure S5C). This result is consistent with the fact that antitrypsin contains a P1-methionine whereas antiplasmin, t-PAI, and C1 inhibitor contain P1-arginines. To confirm that Serpin inactivation is due to citrullination of the P1-arginine, we generated an antiplasmin variant in which the P1-arginine was mutated to a lysine (i.e., R376K) and evaluated its ability to inhibit plasmin. In stark contrast to wild type antiplasmin, citrullination of the R376K mutant does not alter the inhibition profile of this mutant Serpin; the IC50 values for citrullinated and uncitrullinated mutant enzymes are almost identical (Figure S5D, Table S3). In total, these results demonstrate that citrullination inhibits a large subset of Serpins via citrullination of the P1-arginine.
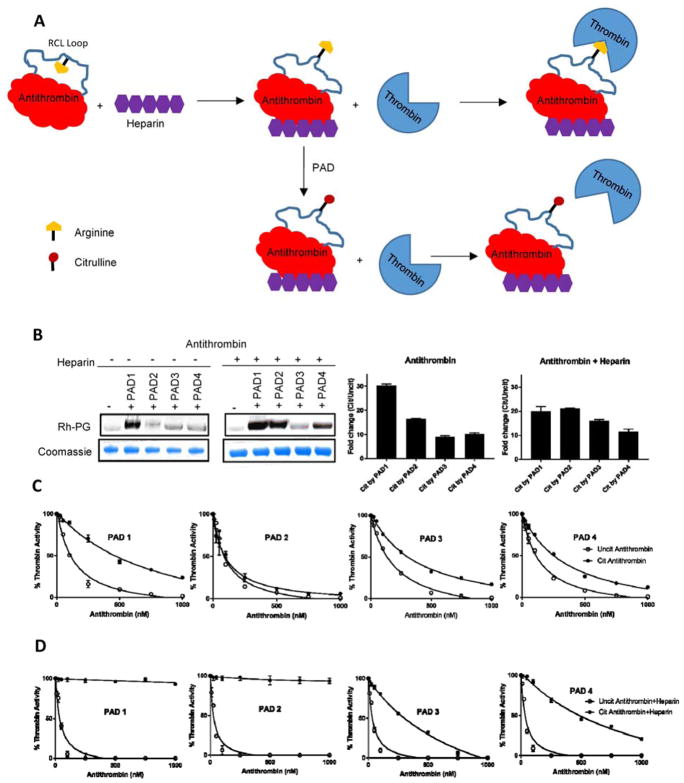
Our citrullinome data also identified elevated levels of citrullinated antithrombin. Rh-PG labeling of PAD treated antithrombin confirmed its citrullination (Figure 5B). Next, we measured the inhibitory activity of citrullinated antithrombin against thrombin. Given the fact that antithrombin also contains an arginine residue at the P1 position, we expected that its citrullination would abolish its inhibitory activity towards thrombin. Notably, citrullination of antithrombin only moderately affected its inhibition profile (Figure 5C, Table S4). Previous studies have shown that antithrombin in its native state is poorly reactive towards proteases, and only upon binding to heparin-like pentasaccharide molecules, does it become active (Huntington, 2011). The thrombin and heparin bound ternary complex of antithrombin revealed that in its native state the P1-arginine of the RCL is pointing inwards, facing the body of Serpin, making it relatively inaccessible to thrombin. Upon interaction with heparin, the P1 residue flips outwards into a protease-accessible conformation (Li et al., 2004). We therefore hypothesized that heparin would enhance citrullination of the P1-arginine in antithrombin (Figure 5A). To test this hypothesis, we incubated antithrombin in the presence and absence of heparin for 30 min before citrullinating it with PADs followed by labeling with Rh-PG. Notably, heparin treated antithrombin was more heavily citrullinated than that of the untreated control (Figure 5B). We further evaluated the effect of heparin on the inhibitory activity of the PAD treated antithrombin. As expected, the citrullination of the heparin treated antithrombin completely abolished its inhibitory activity against thrombin (Figure 5D, Table S4).
Figure 5. Functional effect of citrullination on antithrombin.
A) Schematic diagram showing the effect of citrullination on antithrombin activity. Upon interaction with heparin, the P1 residue flips into a protease-accessible conformation. Heparin also enhances citrullination of the P1-arginine. B) Citrullination of antithrombin in the absence and presence of heparin. Antithrombin (10 μM) was incubated in the presence and absence of heparin (1 mM) for 30 min at 37 °C before incubation with PADs followed by labeling with Rh-PG. Proteins were separated by SDS-PAGE and rhodamine fluorescence imaged (top). Coomassie images (bottom) confirm equal loading. Intensities were quantified, normalized against uncitrullinated proteins and plotted against PAD1, PAD2, PAD3 and PAD4. C) IC50 plots showing inhibition of thrombin (50 nM) by citrullinated and uncitrullinated antithrombin in the absence of heparin. Thrombin (50 nM) was mixed with citrullinated or uncitrullinated antithrombin and incubated for 30 min at room temperature. Hydrolysis of the thrombin substrate tosyl-Gly-Pro-Ala-pNA (1 mM) was monitored spectrophotometrically at 405 nm. IC50 plots showing inhibition of thrombin by various concentrations of citrullinated and uncitrullinated antithrombin in the presence of heparin. Antithrombin (10 μM) was incubated with heparin (1 mM) for 30 min at 37 °C before citrullinating it with PADs. Thrombin (50 nM) was mixed with citrullinated or uncitrullinated antithrombin and incubated for 30 min at room temperature. Hydrolysis of the thrombin substrate tosyl-Gly-Pro-Ala-pNA (1 mM) was monitored spectrophotometrically at 405 nm. See also Table S4.
Discussion
Aberrant citrullination is linked to many human diseases including RA, Lupus, atherosclerosis and various cancers (Chang and Han, 2006; Chang et al., 2009; Knight et al., 2015; Vossenaar and van Venrooij, 2004). To understand the role of citrullination in pathophysiology, there is a need to comprehensively identify the citrullinated proteins associated with these diseases. Towards this end, we used our suite of citrulline-reactive probes to define the citrullinated proteins elevated in RA serum, synovial fluid and synovial tissue samples. Along with known PAD substrates such as enolase, vimentin, keratin and fibrin, we identified more than 150 novel citrullinated proteins, thereby providing the most comprehensive list of citrullinated proteins in RA. Amongst the various proteins we identified are completely novel protein classes such as Serpins, glycolytic enzymes and serine proteases. Notably, an overlapping set of citrullinated proteins was identified in previous studies (Tutturen et al., 2013; van Beers et al., 2013; Wang et al., 2016). However, these studies lack the depth of coverage of the entire citrullinome that we achieved using our suite of citrulline-specific probes and were limited to synovial fluid samples. Moreover, Tutturen and coworkers (Tutturen et al., 2013) digested their samples with trypsin and then labelled with a phenylglyoxal based probe for MS analysis. The major limitation of this approach is that certain peptides containing other post-translation modifications or low abundance will not be detected; as a result, the number of proteins identified was much lower. By contrast in this study, we used an optimized work flow which includes enriching intact proteins to further enhance the peptide coverage, giving a higher confidence that the proteins isolated are truly citrullinated. Since the labeling reactions occur under denaturing condition (20% TCA), we also gain access to otherwise buried citrullines. One limitation of our study is the use of pooled samples. While sample pooling enables higher throughput and typically minimizes the possibility that the results are due to anomalous signals in a single sample, it will nonetheless be interesting to evaluate individual patient samples in future experiments.
In contrast to previous studies, we also validated our results and further evaluated the role of citrullination on the activity of numerous proteins identified in our study. Strikingly, citrullination showed moderate to drastic effects on the activity of metabolic enzymes and Serpins. Studies have shown that the dysregulation of metabolic enzymes plays an important role in cancer biology and is used by cells for survival and growth. We detected higher citrullination of many metabolic proteins in RA samples, including PKM2 and NNMT. NNMT catalyzes the N-methylation of nicotinamide, pyridines and other structural analogs, playing a pivotal role in the biotransformation and detoxification of many xenobiotics (Pissios, 2017). Recent studies have shown that overexpression of NNMT in a diverse set of cancers regulates protein methylation states in tumor cells through a distinct mechanism involving the alteration of the cellular SAM/SAH ratio which promotes a pro-growth phenotype (Ulanovskaya et al., 2013). The citrullination of NNMT by PAD1 and PAD2 abolished its activity. Thus, NNMT citrullination may represent a mechanism to regulate NNMT activity in cancer cells. Notably, PAD2 appears to act as a tumor suppressor in a subset of breast cancers (Horibata et al., 2017) and its ability to downregulate NNMT activity may play a role in this process.
PKM2 is a master regulator of glycolysis that has been demonstrated to balance the production of biomolecular building blocks and the generation of pyruvate and ATP in normal and cancer cells (Gupta and Bamezai, 2010). The activity of PKM2 in cells are allosterically regulated by switching between a high activity tetrameric form and a low activity dimeric form (Anastasiou et al., 2012). The catalytically active tetrameric form, expressed in normal cells, is associated with ATP synthesis and catabolic metabolism whereas the less active dimeric form is mostly expressed in cancer cells where it promotes the production of glycolytic intermediates that enter glycolysis branch pathways, including glycerol synthesis and the pentose phosphate pathway (Anastasiou et al., 2012). PKM2 citrullination results in a significant, albeit modest, increase in its activity towards both PEP and ADP. Since wild-type PKM2 is present as a dimer in solution, citrullination of PKM2 could shift its equilibrium towards the more active tetrameric form, however, preliminary size exclusion chromatography experiments do not support this possibility. Notably, increased PKM2 activity would be expected to inhibit cancer cell growth similarly to the expected response for NNMT citrullination. The activity of enolase, a known RA antigen, was moderately decreased upon citrullination.
The most striking results were obtained with the Serpins. In most cases, Serpin citrullination virtually abolished their inhibitory activity towards their cognate proteases. We further demonstrated that citrullination of the P1-arginine of the RCL is responsible for the loss of Serpin activity as antitrypsin which contains a P1-methionine does not lose activity when citrullinated. Moreover, heparin, which is known to expose the P1-arginine in antithrombin, enhanced its citrullination. Finally, when the P1-arginine in antiplasmin is changed to a lysine, citrullination no longer impacts its ability to inhibit plasmin. In total, all our evidence strongly indicates that citrullination plays an important role in regulating protease activity in the human body. The P1-arginine-containing Serpins are found in all branches of life and control proteolytic pathways related to human health and diseases (Khan et al., 2011). Dysregulation of Serpin activity in blood coagulation, complement and fibrinolysis pathways leads to increased extracellular proteolysis ultimately resulting in thrombosis, shock, and inflammation. Antiplasmin and t-PAI inhibit plasmin and t-PA, respectively. These two proteases degrade fibrin and cleave extracellular matrix (ECM)-associated molecules resulting in the liberation and/or activation of bioactive molecules such as growth factors and cytokines and activate matrix metalloproteases (MMPs) (Serrano and Munoz-Canoves, 2010). C1 inhibitor is a broad-spectrum Serpin, which inhibits the activated forms of several members of the complement pathway (C1r and C1s), the coagulation system (FXIIa, FXIa, and kallikrein) as well as fibrinolytic proteases (plasmin, tPA, and uPA) and thereby regulates the activation of the classical pathway, the lectin pathway as well as the fibrinolytic, coagulation, and kinin pathways (Pappalardo et al., 2002). Antithrombin acts as an anticoagulant by inhibiting thrombin, Factor IX, and Factor X, thereby regulating the coagulation pathway and dysregulation of antithrombin leads to a high risk of thrombosis (Quinsey et al., 2004). Various studies have demonstrated that the P1-arginine is key to Serpin activity. For example, the mutation of the P1-arginine in C1 inhibitor to any other amino acid except lysine abolishes its inhibitory activity against all cognate proteases whereas the P1-arginine mutation in antithrombin changes its specificity from thrombin to trypsin and chymotrypsin (Chuang et al., 2001; Eldering et al., 1992). Mutation of the P1-arginine to methionine and alanine in t-PAI and antiplasmin, respectively, result in loss of their inhibitory activity against their cognate proteases t-PA and plasmin (Holmes et al., 1987; Keijer et al., 1991). A rare mutation of P1-methionine to arginine in antitrypsin, commonly known as the Pittsburg variant, turns it into a thrombin inhibitor responsible for a life threatening disease state (Lewis et al., 1978). Thus, Serpin citrullination is likely to have profound effects on protease activity within the inflamed regions where the PADs are localized during inflammation.
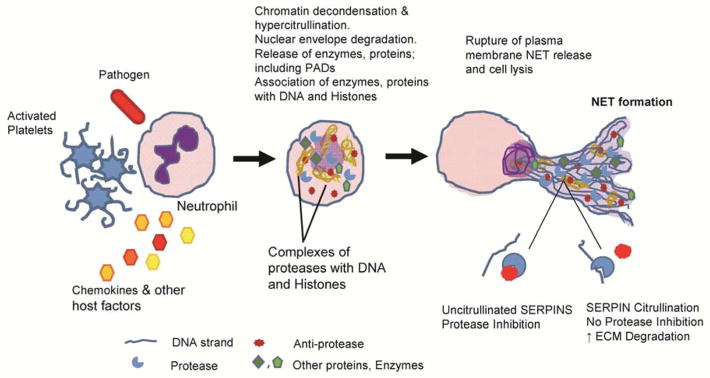
NETosis plays a key role in many autoimmune disorders including Alzheimer’s disease, lupus, and RA (Khandpur et al., 2013; Pietronigro et al., 2017; Yu and Su, 2013). NET formation is a part of our body’s natural defense system, where, in response to stimuli neutrophils decondense and extravate their chromatin (DNA and histones), enzymes and proteins to form NET like structures that capture pathogens (Figure 6). However, abnormal NET formation in chronic inflammatory states has serious consequences. Evidence suggests that dysregulated PAD activity is associated with aberrant NET formation (Knight et al., 2015; Li et al., 2010; Rohrbach et al., 2012). We further suggest that the PADs are released from the neutrophils in their active form thereby providing a mechanism for the presence of citrullinated proteins in serum and synovial fluid. Indeed, active PAD isozymes are found in RA synovial fluid (Spengler et al., 2015). Serpin citrullination abolishes their inhibitory activity, leaving proteases enzymatically active. Under these circumstances, the active proteases would be able to increase the degradation of extracellular matrix as well as modulate the activity of various cellular processes including blood coagulation, complement activation and fibrinolysis (Figure 6).
Figure 6. Schematic representation of NETosis.
In response to a stimulus, such as activated platelets, pathogens, or chemokines, neutrophils decondense and externalize their chromatin (DNA and histones), enzymes and proteins to form NET like structures that capture pathogens. The PADs are amongst the enzymes that are released during this process where they go on to citrullinate numerous Serpins, ultimately leading to their inactivation with a consequent increase in protease activity.
In summary, using our unique techniques, we have successfully defined the RA-associated citrullinome and discovered completely new classes of proteins that were previously unknown to play a role in RA pathophysiology. For the first time, we showed that citrullination modulates, both negatively and positively, the activity of Serpins and metabolic enzymes. This new information enhances our understanding of protein citrullination in RA, which we predict will profoundly influence future research into how the PADs modulate autoimmunity. Projecting forward, it will be interesting to investigate the possible presence of ACPA against these newly discovered proteins in RA patient serum and synovial fluid samples. Once discovered, these ACPA can be used as additional biomarkers to diagnose RA. Moreover, these results indicate that extracellular citrullination is an essential component of RA with profound effects on Serpin activity, suggesting that inhibition of extracellular PAD activity may be a viable therapeutic approach.
STAR METHODS
A detailed description of experimental procedures is available in the online version of this paper and include the following.
KEY RESOURCES TABLE
CONTACTS FOR RESOURCES AND RESOURCE SHARING
-
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human studies
-
METHOD DETAILS
Rhodamine-PG (Rh-PG) labeling of serum, synovial fluid and synovial tissue samples
Biotin-PG labeling and proteomic analysis
LC/LC-MS/MS and data processing
Streptavidin pull down and quantification by Western blot
Quantification by sandwich ELISA
Detection of citrullinated proteins using rhodamine-PG
Detection of citrullinated proteins using biotin-PG
Purification of recombinant human NNMT
Human NNMT activity assay
Purification of recombinant human pyruvate kinase M2 (PKM2)
Human PKM2 activity assay
Human enolase activity assay
Protease-Serpin activity assay
Purification of recombinant human wildtype and R376K a2-antiplasmin
Construction of R376K antiplasmin mutant
QUANTIFICATION AND STATISTICAL ANALYSIS
Supplementary Material
Table S5. Serum Proteomic Data, Related to Figure 2 (attached as an Excel file).
Table S6. Synovial Fluid Proteomic Data, Related to Figure 2 (attached as an Excel file).
Table S7. Synovial Tissue Proteomic Data, Related to Figure 2 (attached as an Excel file).
SIGNIFICANCE.
Herein, we used our suite of citrulline-specific probes to identify more than 150 novel citrullinated proteins from RA serum, synovial fluid and synovial tissue samples, including Serpins and metabolic enzymes. This is the most comprehensive survey of the RA-associated citrullinome carried out to date. Moreover, we demonstrate for the first time that citrullination has profound effects on the activity of a range of metabolic enzymes, including NNMT, and Serpins. Serpin citrullination abolishes their inhibitory activity, thus effectively activating their cognate proteases. Under these circumstances, the active proteases would be able to increase the degradation of the extracellular matrix as well as modulate the activity of various cellular processes including blood coagulation, complement activation and fibrinolysis. Since aberrant citrullination is a hallmark of numerous diseases, including lupus, atherosclerosis and various cancers, we predict that our findings will open new avenues of study into the role of citrullination in human disease.
Highlights.
Identified citrullinated proteins in RA serum, synovial fluid, and synovial tissue.
Citrullination of NNMT abolishes its methyltransferase activity.
Serpin citrullination abolishes their ability to inhibit their cognate proteases.
Serpin citrullination modulates Serpin-regulated pathways.
Acknowledgments
This work was supported in part by NIH grant GM109767 and Janssen Research, Spring House, PA. We thank Suzanne Cole and Xuefeng Yin for preparing healthy volunteer and RA samples for the study.
Abbreviations
- Biotin-PG
Biotin-Phenylglyoxal
- DTT
Dithiothreitol
- Rh-PG
Rhodamine-Phenylglyoxal
- TCA
Trichloroacetic acid
- EDTA
Ethylenediaminetetraacetic acid
- HRP
horseradish peroxidase
- BSA
Bovine serum albumin
- PBS
Phosphate buffered saline
- ACPA
Anti-citrullinated protein antibodies
- PAD
Protein Arginine Deiminase
- ELISA
Enzyme-linked immunosorbent assay
- NNMT
Nicotinamide N-Methyltransferase
Footnotes
AUTHOR CONTRIBUTION
R.T. designed experiments, performed the experiments, analyzed the data, and wrote the manuscript. S.H.N. designed experiments, and performed the experiments. A.M. performed and analyzed mass spectrometry experiments. V.V.N. designed and performed NNMT enzyme experiments. M.N. provided purified PAD2. S. N. designed experiments and analyzed data. A.J.S performed and analyzed mass spectrometry experiments. E.W. designed and analyzed mass spectrometry experiments. P.R.T. designed experiments, analyzed the data, and wrote the manuscript
Declaration of Interest
SN is an employee and shareholder of Johnson & Johnson. PRT was a founder, consultant, and chair of the scientific advisory board for Padlock Therapeutics, which was acquired by Bristol Myers Squibb in April 2016. PRT holds a patent that covers the probes, Rh-PG and biotin-PG, used in this study.
Supporting Information includes five figures, four tables, three Excel files and supplemental text.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A, et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8:839–847. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicker KL, Subramanian V, Chumanevich AA, Hofseth LJ, Thompson PR. Seeing citrulline: development of a phenylglyoxal-based probe to visualize protein citrullination. J Am Chem Soc. 2012;134:17015–17018. doi: 10.1021/ja308871v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter SL, Mathew P. Alpha2-antiplasmin and its deficiency: fibrinolysis out of balance. Haemophilia. 2008;14:1250–1254. doi: 10.1111/j.1365-2516.2008.01766.x. [DOI] [PubMed] [Google Scholar]
- Chandra PE, Sokolove J, Hipp BG, Lindstrom TM, Elder JT, Reveille JD, Eberl H, Klause U, Robinson WH. Novel multiplex technology for diagnostic characterization of rheumatoid arthritis. Arthritis Res Ther. 2011;13:R102. doi: 10.1186/ar3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, Liu GY, Dwivedi N, Sun B, Okamoto Y, Kinslow JD, Deane KD, Demoruelle MK, Norris JM, Thompson PR, et al. A molecular signature of preclinical rheumatoid arthritis triggered by dysregulated PTPN22. JCI Insight. 2016;1:e90045. doi: 10.1172/jci.insight.90045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Han J. Expression of peptidylarginine deiminase type 4 (PAD4) in various tumors. Mol Carcinog. 2006;45:183–196. doi: 10.1002/mc.20169. [DOI] [PubMed] [Google Scholar]
- Chang X, Han J, Pang L, Zhao Y, Yang Y, Shen Z. Increased PADI4 expression in blood and tissues of patients with malignant tumors. BMC Cancer. 2009;9:40. doi: 10.1186/1471-2407-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- Christophorou MA, Castelo-Branco G, Halley-Stott RP, Oliveira CS, Loos R, Radzisheuskaya A, Mowen KA, Bertone P, Silva JC, Zernicka-Goetz M, et al. Citrullination regulates pluripotency and histone H1 binding to chromatin. Nature. 2014;507:104–108. doi: 10.1038/nature12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YJ, Swanson R, Raja SM, Bock SC, Olson ST. The antithrombin P1 residue is important for target proteinase specificity but not for heparin activation of the serpin. Characterization of P1 antithrombin variants with altered proteinase specificity but normal heparin activation. Biochemistry. 2001;40:6670–6679. doi: 10.1021/bi002933d. [DOI] [PubMed] [Google Scholar]
- Chumanevich AA, Causey CP, Knuckley BA, Jones JE, Poudyal D, Chumanevich AP, Davis T, Matesic LE, Thompson PR, Hofseth LJ. Suppression of colitis in mice by Cl-amidine: a novel peptidylarginine deiminase inhibitor. Am J Physiol Gastrointest Liver Physiol. 2011;300:G929–938. doi: 10.1152/ajpgi.00435.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy KW, Weerapana E, Thompson PR. Detection and identification of protein citrullination in complex biological systems. Curr Opin Chem Biol. 2016;30:1–6. doi: 10.1016/j.cbpa.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, Williams B, Gabriel S, Lassere M, Johns N, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1316–1322. doi: 10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- Damgaard D, Senolt L, Nielsen MF, Pruijn GJ, Nielsen CH. Demonstration of extracellular peptidylarginine deiminase (PAD) activity in synovial fluid of patients with rheumatoid arthritis using a novel assay for citrullination of fibrinogen. Arthritis Res Ther. 2014;16:498. doi: 10.1186/s13075-014-0498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldering E, Huijbregts CC, Lubbers YT, Longstaff C, Hack CE. Characterization of recombinant C1 inhibitor P1 variants. J Biol Chem. 1992;267:7013–7020. [PubMed] [Google Scholar]
- Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- Fuhrmann J, Clancy KW, Thompson PR. Chemical biology of protein arginine modifications in epigenetic regulation. Chem Rev. 2015;115:5413–5461. doi: 10.1021/acs.chemrev.5b00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki M, Sugawara K. Properties of peptidylarginine deiminase from the epidermis of newborn rats. J Biochem. 1981;89:257–263. doi: 10.1093/oxfordjournals.jbchem.a133189. [DOI] [PubMed] [Google Scholar]
- Ghari F, Quirke AM, Munro S, Kawalkowska J, Picaud S, McGouran J, Subramanian V, Muth A, Williams R, Kessler B, et al. Citrullination-acetylation interplay guides E2F-1 activity during the inflammatory response. Sci Adv. 2016;2:e1501257. doi: 10.1126/sciadv.1501257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Bamezai RN. Human pyruvate kinase M2: a multifunctional protein. Protein Sci. 2010;19:2031–2044. doi: 10.1002/pro.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes WE, Lijnen HR, Collen D. Characterization of recombinant human alpha 2-antiplasmin and of mutants obtained by site-directed mutagenesis of the reactive site. Biochemistry. 1987;26:5133–5140. doi: 10.1021/bi00390a036. [DOI] [PubMed] [Google Scholar]
- Horibata S, Rogers KE, Sadegh D, Anguish LJ, McElwee JL, Shah P, Thompson PR, Coonrod SA. Role of peptidylarginine deiminase 2 (PAD2) in mammary carcinoma cell migration. BMC Cancer. 2017;17:378. doi: 10.1186/s12885-017-3354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington JA. Serpin structure, function and dysfunction. J Thromb Haemost. 2011;9(Suppl 1):26–34. doi: 10.1111/j.1538-7836.2011.04360.x. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Takahara H, Nishi Y, Sugawara K, Sato C. Ca2+-dependent deimination-induced disassembly of intermediate filaments involves specific modification of the amino-terminal head domain. J Biol Chem. 1989;264:18119–18127. [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Senshu T, Takahashi H, Akiyama K, Nomura K, Iizuka H. Decreased deiminated keratin K1 in psoriatic hyperproliferative epidermis. J Invest Dermatol. 2000;114:701–705. doi: 10.1046/j.1523-1747.2000.00936.x. [DOI] [PubMed] [Google Scholar]
- Ishigami A, Ohsawa T, Hiratsuka M, Taguchi H, Kobayashi S, Saito Y, Murayama S, Asaga H, Toda T, Kimura N, et al. Abnormal accumulation of citrullinated proteins catalyzed by peptidylarginine deiminase in hippocampal extracts from patients with Alzheimer’s disease. J Neurosci Res. 2005;80:120–128. doi: 10.1002/jnr.20431. [DOI] [PubMed] [Google Scholar]
- Jones JE, Causey CP, Knuckley B, Slack-Noyes JL, Thompson PR. Protein arginine deiminase 4 (PAD4): Current understanding and future therapeutic potential. Curr Opin Drug Discov Devel. 2009;12:616–627. [PMC free article] [PubMed] [Google Scholar]
- Jurica MS, Mesecar A, Heath PJ, Shi W, Nowak T, Stoddard BL. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure. 1998;6:195–210. doi: 10.1016/s0969-2126(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Keijer J, Ehrlich HJ, Linders M, Preissner KT, Pannekoek H. Vitronectin governs the interaction between plasminogen activator inhibitor 1 and tissue-type plasminogen activator. J Biol Chem. 1991;266:10700–10707. [PubMed] [Google Scholar]
- Kenny EF, Herzig A, Kruger R, Muth A, Mondal S, Thompson PR, Brinkmann V, Bernuth HV, Zychlinsky A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017:6. doi: 10.7554/eLife.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MS, Singh P, Azhar A, Naseem A, Rashid Q, Kabir MA, Jairajpuri MA. Serpin Inhibition Mechanism: A Delicate Balance between Native Metastable State and Polymerization. J Amino Acids. 2011;2011:606797. doi: 10.4061/2011/606797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Edwards BS, Muth A, Thompson PR, Cherrington BD, Navratil AM. GnRH Stimulates Peptidylarginine Deiminase Catalyzed Histone Citrullination in Gonadotrope Cells. Mol Endocrinol. 2016;30:1081–1091. doi: 10.1210/me.2016-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra140. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinloch A, Lundberg K, Wait R, Wegner N, Lim NH, Zendman AJ, Saxne T, Malmstrom V, Venables PJ. Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum. 2008;58:2287–2295. doi: 10.1002/art.23618. [DOI] [PubMed] [Google Scholar]
- Kinloch A, Tatzer V, Wait R, Peston D, Lundberg K, Donatien P, Moyes D, Taylor PC, Venables PJ. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1421–1429. doi: 10.1186/ar1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JS, Subramanian V, O’Dell AA, Yalavarthi S, Zhao W, Smith CK, Hodgin JB, Thompson PR, Kaplan MJ. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis. 2015;74:2199–2206. doi: 10.1136/annrheumdis-2014-205365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JS, Zhao W, Luo W, Subramanian V, O’Dell AA, Yalavarthi S, Hodgin JB, Eitzman DT, Thompson PR, Kaplan MJ. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013;123:2981–2993. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus D, Yang Q, Kong D, Banks AS, Zhang L, Rodgers JT, Pirinen E, Pulinilkunnil TC, Gong F, Wang YC, et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508:258–262. doi: 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Gogel S, Leung KY, Vernay B, Nicholas AP, Causey CP, Thompson PR, Greene ND, Ferretti P. Protein deiminases: new players in the developmentally regulated loss of neural regenerative ability. Dev Biol. 2011;355:205–214. doi: 10.1016/j.ydbio.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewallen DM, Bicker KL, Subramanian V, Clancy KW, Slade DJ, Martell J, Dreyton CJ, Sokolove J, Weerapana E, Thompson PR. Chemical Proteomic Platform To Identify Citrullinated Proteins. ACS Chem Biol. 2015;10:2520–2528. doi: 10.1021/acschembio.5b00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JH, Iammarino RM, Spero JA, Hasiba U. Antithrombin Pittsburgh: an alpha1-antitrypsin variant causing hemorrhagic disease. Blood. 1978;51:129–137. [PubMed] [Google Scholar]
- Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Johnson DJ, Esmon CT, Huntington JA. Structure of the antithrombin-thrombin-heparin ternary complex reveals the antithrombotic mechanism of heparin. Nat Struct Mol Biol. 2004;11:857–862. doi: 10.1038/nsmb811. [DOI] [PubMed] [Google Scholar]
- Lu BG, Sofian T, Law RH, Coughlin PB, Horvath AJ. Contribution of conserved lysine residues in the alpha2-antiplasmin C terminus to plasmin binding and inhibition. J Biol Chem. 2011;286:24544–24552. doi: 10.1074/jbc.M111.229013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Poudel S, Muruganujan A, Casagrande JT, Thomas PD. PANTHER version 10: expanded protein families and functions, and analysis tools. Nucleic Acids Res. 2016;44:D336–342. doi: 10.1093/nar/gkv1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan HP, O’Reilly FJ, Wear MA, O’Neill JR, Fothergill-Gilmore LA, Hupp T, Walkinshaw MD. M2 pyruvate kinase provides a mechanism for nutrient sensing and regulation of cell proliferation. Proc Natl Acad Sci U S A. 2013;110:5881–5886. doi: 10.1073/pnas.1217157110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscarello MA, Mastronardi FG, Wood DD. The role of citrullinated proteins suggests a novel mechanism in the pathogenesis of multiple sclerosis. Neurochem Res. 2007;32:251–256. doi: 10.1007/s11064-006-9144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas AP. Dual immunofluorescence study of citrullinated proteins in Parkinson diseased substantia nigra. Neurosci Lett. 2011;495:26–29. doi: 10.1016/j.neulet.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Obermayer A, Stoiber W, Krautgartner WD, Klappacher M, Kofler B, Steinbacher P, Vitkov L, Grabcanovic-Musija F, Studnicka M. New aspects on the structure of neutrophil extracellular traps from chronic obstructive pulmonary disease and in vitro generation. PLoS One. 2014;9:e97784. doi: 10.1371/journal.pone.0097784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo E, Zingale LC, Terlizzi A, Zanichelli A, Folcioni A, Cicardi M. Mechanisms of C1-inhibitor deficiency. Immunobiology. 2002;205:542–551. doi: 10.1078/0171-2985-00153. [DOI] [PubMed] [Google Scholar]
- Peng Y, Sartini D, Pozzi V, Wilk D, Emanuelli M, Yee VC. Structural basis of substrate recognition in human nicotinamide N-methyltransferase. Biochemistry. 2011;50:7800–7808. doi: 10.1021/bi2007614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietronigro EC, Della Bianca V, Zenaro E, Constantin G. NETosis in Alzheimer’s Disease. Front Immunol. 2017;8:211. doi: 10.3389/fimmu.2017.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissios P. Nicotinamide N-Methyltransferase: More Than a Vitamin B3 Clearance Enzyme. Trends Endocrinol Metab. 2017;28:340–353. doi: 10.1016/j.tem.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinsey NS, Greedy AL, Bottomley SP, Whisstock JC, Pike RN. Antithrombin: in control of coagulation. Int J Biochem Cell Biol. 2004;36:386–389. doi: 10.1016/s1357-2725(03)00244-9. [DOI] [PubMed] [Google Scholar]
- Raijmakers R, Zendman AJ, Egberts WV, Vossenaar ER, Raats J, Soede-Huijbregts C, Rutjes FP, van Veelen PA, Drijfhout JW, Pruijn GJ. Methylation of arginine residues interferes with citrullination by peptidylarginine deiminases in vitro. J Mol Biol. 2007;367:1118–1129. doi: 10.1016/j.jmb.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET formation. Front Immunol. 2012;3:360. doi: 10.3389/fimmu.2012.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondas D, Crevecoeur I, D’Hertog W, Ferreira GB, Staes A, Garg AD, Eizirik DL, Agostinis P, Gevaert K, Overbergh L, et al. Citrullinated glucose-regulated protein 78 is an autoantigen in type 1 diabetes. Diabetes. 2015;64:573–586. doi: 10.2337/db14-0621. [DOI] [PubMed] [Google Scholar]
- Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick C, Kamachi Y, Bartuski AJ, Cataltepe S, Schechter NM, Pemberton PA, Silverman GA. Squamous cell carcinoma antigen 2 is a novel serpin that inhibits the chymotrypsin-like proteinases cathepsin G and mast cell chymase. J Biol Chem. 1997;272:1849–1855. doi: 10.1074/jbc.272.3.1849. [DOI] [PubMed] [Google Scholar]
- Senshu T, Akiyama K, Kan S, Asaga H, Ishigami A, Manabe M. Detection of deiminated proteins in rat skin: probing with a monospecific antibody after modification of citrulline residues. J Invest Dermatol. 1995;105:163–169. doi: 10.1111/1523-1747.ep12317070. [DOI] [PubMed] [Google Scholar]
- Serrano AL, Munoz-Canoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res. 2010;316:3050–3058. doi: 10.1016/j.yexcr.2010.05.035. [DOI] [PubMed] [Google Scholar]
- Shen S, Casaccia-Bonnefil P. Post-translational modifications of nucleosomal histones in oligodendrocyte lineage cells in development and disease. J Mol Neurosci. 2008;35:13–22. doi: 10.1007/s12031-007-9014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- Spengler J, Lugonja B, Ytterberg AJ, Zubarev RA, Creese AJ, Pearson MJ, Grant MM, Milward M, Lundberg K, Buckley CD, et al. Release of Active Peptidyl Arginine Deiminases by Neutrophils Can Explain Production of Extracellular Citrullinated Autoantigens in Rheumatoid Arthritis Synovial Fluid. Arthritis Rheumatol. 2015;67:3135–3145. doi: 10.1002/art.39313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengers ED, Kluft C. Plasminogen activator inhibitors. Blood. 1987;69:381–387. [PubMed] [Google Scholar]
- Sun Z, Yang P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol. 2004;5:182–190. doi: 10.1016/S1470-2045(04)01414-7. [DOI] [PubMed] [Google Scholar]
- Tabb DL, McDonald WH, Yates JR., 3rd DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res. 2002;1:21–26. doi: 10.1021/pr015504q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P, Gartemann J, Hsieh J, Creeden J. A systematic review of serum biomarkers anti-cyclic citrullinated Peptide and rheumatoid factor as tests for rheumatoid arthritis. Autoimmune Dis. 2011;2011:815038. doi: 10.4061/2011/815038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutturen AE, Holm A, Fleckenstein B. Specific biotinylation and sensitive enrichment of citrullinated peptides. Anal Bioanal Chem. 2013;405:9321–9331. doi: 10.1007/s00216-013-7376-1. [DOI] [PubMed] [Google Scholar]
- Ulanovskaya OA, Zuhl AM, Cravatt BF. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat Chem Biol. 2013;9:300–306. doi: 10.1038/nchembio.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers JJ, Raijmakers R, Alexander LE, Stammen-Vogelzangs J, Lokate AM, Heck AJ, Schasfoort RB, Pruijn GJ. Mapping of citrullinated fibrinogen B-cell epitopes in rheumatoid arthritis by imaging surface plasmon resonance. Arthritis Res Ther. 2010;12:R219. doi: 10.1186/ar3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beers JJ, Schwarte CM, Stammen-Vogelzangs J, Oosterink E, Bozic B, Pruijn GJ. The rheumatoid arthritis synovial fluid citrullinome reveals novel citrullinated epitopes in apolipoprotein E, myeloid nuclear differentiation antigen, and beta-actin. Arthritis Rheum. 2013;65:69–80. doi: 10.1002/art.37720. [DOI] [PubMed] [Google Scholar]
- van der Helm-van Mil AH, Breedveld FC, Huizinga TW. Aspects of early arthritis. Definition of disease states in early arthritis: remission versus minimal disease activity. Arthritis Res Ther. 2006;8:216. doi: 10.1186/ar1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haren MJ, Sastre Torano J, Sartini D, Emanuelli M, Parsons RB, Martin NI. A Rapid and Efficient Assay for the Characterization of Substrates and Inhibitors of Nicotinamide N-Methyltransferase. Biochemistry. 2016;55:5307–5315. doi: 10.1021/acs.biochem.6b00733. [DOI] [PubMed] [Google Scholar]
- van Venrooij WJ, Zendman AJ, Pruijn GJ. Autoantibodies to citrullinated antigens in (early) rheumatoid arthritis. Autoimmun Rev. 2006;6:37–41. doi: 10.1016/j.autrev.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Vossenaar ER, Despres N, Lapointe E, van der Heijden A, Lora M, Senshu T, van Venrooij WJ, Menard HA. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004a;6:R142–150. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenaar ER, Radstake TR, van der Heijden A, van Mansum MA, Dieteren C, de Rooij DJ, Barrera P, Zendman AJ, van Venrooij WJ. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis. 2004b;63:373–381. doi: 10.1136/ard.2003.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenaar ER, van Venrooij WJ. Citrullinated proteins: sparks that may ignite the fire in rheumatoid arthritis. Arthritis Res Ther. 2004;6:107–111. doi: 10.1186/ar1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- Wang F, Chen FF, Gao WB, Wang HY, Zhao NW, Xu M, Gao DY, Yu W, Yan XL, Zhao JN, et al. Identification of citrullinated peptides in the synovial fluid of patients with rheumatoid arthritis using LC-MALDI-TOF/TOF. Clin Rheumatol. 2016;35:2185–2194. doi: 10.1007/s10067-016-3247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wang Y. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim Biophys Acta. 2013;1829:1126–1135. doi: 10.1016/j.bbagrm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, Sonbuchner LS, McDonald CH, Cook RG, Dou Y, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- Weerapana E, Speers AE, Cravatt BF. Tandem orthogonal proteolysis-activity-based protein profiling (TOP-ABPP)--a general method for mapping sites of probe modification in proteomes. Nat Protoc. 2007;2:1414–1425. doi: 10.1038/nprot.2007.194. [DOI] [PubMed] [Google Scholar]
- Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, Luo Y, Levitt B, Glogowska M, Chandra P, et al. N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186:4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Su K. Neutrophil Extracellular Traps and Systemic Lupus Erythematosus. J Clin Cell Immunol. 2013:4. doi: 10.4172/2155-9899.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bolt M, Guertin MJ, Chen W, Zhang S, Cherrington BD, Slade DJ, Dreyton CJ, Subramanian V, Bicker KL, et al. Peptidylarginine deiminase 2-catalyzed histone H3 arginine 26 citrullination facilitates estrogen receptor alpha target gene activation. Proc Natl Acad Sci U S A. 2012;109:13331–13336. doi: 10.1073/pnas.1203280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S5. Serum Proteomic Data, Related to Figure 2 (attached as an Excel file).
Table S6. Synovial Fluid Proteomic Data, Related to Figure 2 (attached as an Excel file).
Table S7. Synovial Tissue Proteomic Data, Related to Figure 2 (attached as an Excel file).