Abstract
Objective
We examined the associations of muscle area and radiodensity with adiponectin and leptin.
Methods
1944 participants enrolled in the Multi-Ethnic Study of Atherosclerosis underwent computed tomography to quantify body composition and measurements of adiponectin, leptin, interleukin-6, C-reactive protein, and resistin.
Results
The mean age and body mass index of participants was 64.7 years and 28.1 kg/m2, respectively, and 49% were female. With adjustment for age, gender, race/ethnicity, traditional cardiovascular disease risk factors, inflammatory biomarkers, physical activity, and sedentary behavior, a 1-standard deviation (SD) increment in total abdominal, stability, and locomotor muscle area was associated with a 19%, 17%, and 12% lower adiponectin level, respectively (p<0.01 for all) but not leptin (p>0.05). Muscle radiodensity was more robustly associated with adiponectin and leptin in the multivariable linear regression models. That is, with full adjustment for all covariates, a 1-SD increment in total abdominal, stability, and locomotor muscle radiodensity was associated with a 31%, 31%, and 18% lower adiponectin level (p<0.01 for all) and 6.7%, 4.6%, and 8.1% higher leptin level (p<0.05 for all), respectively.
Conclusions
Our data suggest that increases in muscle area and radiodensity may have positive impacts on chronic inflammation, and in turn, reduce the risk of cardiometabolic disease.
Keywords: Sarcopenia, inflammation, adipokines, body composition
Introduction
Obesity is a growing global public health problem and a major risk factor for both type 2 diabetes and cardiovascular disease. It is well established that obesity is characterized by low-grade inflammation including elevated levels of pro-inflammatory cytokines and reduced levels of anti-inflammatory cytokines (1). Among these cytokines, adiponectin and leptin have emerged as important regulators of glucose and lipid metabolism in skeletal muscle, contributing to insulin sensitivity and reduced risk of metabolic and cardiovascular disease (1,2).
Adiponectin is an abundant protein derived primarily from adipose tissue and acts directly on skeletal muscle to increase fatty acid oxidation and glucose uptake, and decrease triglyceride content in skeletal muscle (2,3). Higher concentrations of adiponectin have been associated with both a reduced and higher risk of metabolic and cardiovascular disease (4,5). More recently, adiponectin has been positively associated with cachexia (4) and inversely associated with muscle fiber size (6) and strength (4,7,8) in healthy adults and heart failure patients.
Leptin is secreted by adipose and muscle tissue and plays an important role in energy balance and glucose homeostasis (9). In this regard, leptin increases fatty acid oxidation and stimulates intramuscular triglyceride hydrolysis in skeletal muscle (1,2). In addition, leptin has anabolic effects on skeletal muscle (9). For example, leptin treatment in mice increases skeletal muscle mass and decreases the expression of factors associated with atrophy (9,10).
While adiponectin and leptin have been studied extensively in relation to adiposity, little is known about the role of skeletal muscle mass and density on adiponectin and leptin. Notably, skeletal muscle radiodensity has emerged as an important marker of the quality of muscle, with a lower radiodensity indicating greater fat infiltration of the muscle (11,12). Therefore, the aim of this study was to examine the associations of skeletal muscle area and radiodensity with adiponectin and leptin in a multi-ethnic cohort, and to determine if the associations were independent of relevant covariates including abdominal visceral and subcutaneous fat. We hypothesized that skeletal muscle area and radiodensity would be negatively associated with adiponectin and positively associated with leptin, independent of relevant covariates.
Methods
Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal cohort study of the characteristics of subclinical cardiovascular disease and the risk factors that predict progression to clinically overt cardiovascular disease or progression of the subclinical disease in adults from six regions across the US. The overall design of the MESA study has been published (13). In brief, the cohort included a total of 6814 men and women aged 45–84 years who were free from clinically apparent cardiovascular disease at the time of enrollment (July 2000 to August 2002). The racial/ethnic groups of participants included African American, Chinese American, Hispanic and non-Hispanic white. Participants who were enrolled in the study returned for follow-up clinic visits approximately 2, 4, 6, and 10 years after the baseline clinic visit.
At clinic visits 2 and 3 (from 2002 to 2005), a random subset of 1970 participants were enrolled in an ancillary study where abdominal computed tomography (CT) scans were obtained and subsequently used to quantify abdominal muscle area, muscle radiodensity, visceral adipose tissue and subcutaneous adipose tissue. Approximately half of the participants had their abdominal CT scan at visit 2 and the other half at visit 3. Participants who had complete data on muscle area, muscle radiodensity, adiponectin and leptin were included in the study. To make measurements contemporaneous, we used visit-matched data for each participant for all analyses. The MESA studies were approved by the Institutional Review Board of each study site and all participants provided written informed consent.
Data collection
At all study visits, standard questionnaires were used to obtain information on participant sociodemographics, ethnicity and health history. Cigarette smoking was defined as current, former, or never smoker. Height and weight were measured to the nearest 0.1 cm and 0.5 kg, respectively, with participants wearing minimal clothing and no shoes. Body mass index (BMI) was calculated as weight in kg divided by height in meters squared. Waist, at the level of the umbilicus, and hip circumferences were measured using a standard flexible, tension-regulated tape measure. Blood pressure was measured with an automated monitor after 5 minutes of seated rest, with the last two of three readings averaged and recorded (Dinamap Monitor Pro 100, GE Healthcare, Waukesha, WI). Each participant’s arm circumference was measured and selection of a proper cuff size was based on the guideline that the length of the inflatable bladder in the cuff should be at least 40% of the arm circumference. Hypertension was defined as systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥80 mmHg or taking antihypertensive medication (14).
Sedentary Behavior and Physical Activity Assessment
At each clinic visit, participants self-reported their time spent engaged in sedentary behavior and physical activity using the Typical Week Physical Activity Survey (TWPAS). This survey was adapted from the Cross-Cultural Activity Participation Study (15) and designed to identify the frequency of and time spent in sedentary behavior and in various physical activities during a typical week in the previous month. Survey responses were quantified into minutes per week of sedentary behavior (sedentary behavior) and MET-minutes per week of moderate-to-vigorous physical activity defined as moderate and vigorous activities from all categories.
Laboratory
At each clinic visit, venous blood was collected after a 12-hour fast and shipped to the MESA central laboratory for analysis of total and HDL cholesterol, triglycerides, and glucose. Stored fasting blood samples from visits 2 or 3 were analyzed for insulin, C-reactive protein (CRP), adiponectin, leptin, tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and resistin. All biomarkers were measured using Bio-Rad Luminex flow cytometry (Millipore, Billerica, MA) at the laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). Average analytic coefficients of variation across several control samples ranged from 6.0% to 13.0%.
Dyslipidemia was defined as a total cholesterol/HDL-cholesterol ratio >5.0 or if the participant was taking medication to reduce cholesterol (16). Diabetes was defined as fasting glucose ≥ 126 mg·dL−1 or use of diabetes medication (17). Estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease-Epi equation (18).
Computed Tomography (CT) Measurements
To determine abdominal body composition by computed tomography, electron-beam scanners (Imatron C-150, Imatron Inc., South San Francisco, CA) were used at Northwestern University and University of California, Los Angeles, with the following settings: collimation 3 mm, slice thickness 6 mm, reconstruction using 25 6-mm slices with 35-cm field of view and normal kernel. Multidetector CT scanners (Sensation 64 [Siemens, Malvern, PA] and GE Lightspeed [GE Healthcare, Waukesha, WI], Siemens S4 Volume Zoom, and Siemens Sensation 16 [Siemens, Malvern, PA]) were used at Columbia University, Wake Forest University, and University of Minnesota, respectively. The specific scanning methodologies employed in the MESA study have been reported elsewhere (19). Abdominal slices from these scans were processed using MIPAV 4.1.2 software (National Institutes of Health, Bethesda, MD) that measured adipose tissue, skeletal muscle, and total tissue using a semi-automated method. Adipose tissue was identified as being between −190 and −30 Hounsfield units (HU), whereas skeletal muscle tissue was identified as being between 0 and 100 HU (20). Radiodensities between 0 and −30 HU were labeled as undefined tissue type (21). Six transverse cross sectional slices were analyzed at the following spine levels: 2 at L2/L3, 2 at L3/L4 and 2 at L4/L5.
Using the pixel radiodensities, and the HU criteria provided above, adipose tissue and skeletal muscle areas were calculated for subcutaneous and visceral adipose tissue, as well as abdominal muscle groups using a single slice obtained at L4/L5. Bilateral oblique, rectus abdominus, paraspinus and psoas muscles were defined within their unique fascial planes. These muscles were grouped into muscles of stabilization (oblique, rectus abdominus, paraspinus muscles), muscles of locomotion (psoas muscle), and total abdominal muscle (oblique, rectus abdominus, paraspinus muscles, and psoas). For each muscle, area was determined by summing the number of pixels of 0 to 100 HU within that muscle’s corresponding fascial plane. Muscle radiodensity was the average HU measurement within the muscle’s distinct fascial plane for those with an HU value within the appropriate range (20). Subcutaneous adipose tissue was defined as the adipose tissue outside of the visceral cavity, not including the adipose tissue located within the muscular fascia. Visceral adipose tissue area was computed as the sum of the pixels of the appropriate HU range (−30 to −190 HU) and within the visceral cavity. CT imaging was interpreted by staff who were blinded to participants’ clinical information. Inter and intra-rater reliability for total abdominal, subcutaneous, and visceral cavity areas were 0.99 for all measurements. Inter and intra-rater reliability for all muscle groups ranged from 0.93 to 0.98.
Statistics
Analyses for this report were completed in 2017. Among the 1970 participants, 1944 had complete data on muscle area, muscle radiodensity and adiponectin or leptin. There were 153 individuals who were missing values for adiponectin and the covariates, resulting in a final analytic sample of 1791 participants when adiponectin was the outcome in regression analyses. Similarly, there were 160 individuals who were missing values for leptin and the covariates, leaving a sample size of 1784 for these analyses. Characteristics of the population were summarized with mean and standard deviation (SD) for continuous variables and frequency and percentage of the study population for categorical variables. Skewed variables are presented as median with interquartile range. ANCOVA was used to determine the means of muscle area and radiodensity by quartiles of adiponectin and leptin, after adjusting for age, sex, and race/ethnicity. The ANCOVA was the first step in the analyses to determine if muscle area and radiodensity were different across the quartiles of adiponectin and leptin, while controlling for non-modifiable significant confounders.
To normalize the distribution, adiponectin and leptin were log-transformed and then linear regression analysis was used to determine the association between these variables and both muscle area and radiodensity in continuous and (per 1 SD increment) and categorical (i.e., quartiles) forms. The initial model (model 1) adjusted for age, sex, race/ethnicity, smoking, dyslipidemia, hypertension, coronary artery calcium (CAC), physical activity and sedentary behavior. Model 2 included model 1 plus C-reactive protein, interleukin-6 and adiponectin (in leptin analysis) or leptin (in adiponectin analysis). Model 3 included model 2 plus subcutaneous and visceral adiposity. All statistical analyses were conducted using Stata (Version 13; StataCorp) and a p-value <0.05 was used to determine statistical significance.
Results
Table 1 presents the characteristics of the study sample. The mean age was 64.7 years and 49% were female. Forty percent of participants were non-Hispanic white, 21% were African American, 26% were Hispanic/Latino, and 13% were Chinese American. On average, participants were overweight, with a mean BMI and waist circumference of 28.1 kg·m−2 and 98.3 cm, respectively. Thirty-one percent of participants had a BMI greater than 30 kg·m−2. Almost half (46%) of participants were never smokers, 40% were dyslipidemic, 61% were hypertensive, and 14% had diabetes mellitus. Over half (58%) of participants had CAC with a mean score of 178.5. Mean adiponectin and leptin levels were 20.7 μg/mL and 20.7 ng/mL, respectively.
Table 1.
Characteristics of the study cohort: The Multi-Ethnic Study of Atherosclerosis.
| Characteristic | Men (n=983) | Women (n=961) | Total (n=1944) |
|---|---|---|---|
| Age (years, M [SD]) | 64.3 (9.2) | 65.1 (9.4) | 64.7 (9.7) |
| Ethnicity (% [Freq]) | |||
| White | 42.2 (415) | 38.5 (370) | 40.3 (785) |
| Chinese American | 13.5 (133) | 12.3 (118) | 12.9 (251) |
| African American | 18.4 (181) | 23.5 (226) | 20.9 (407) |
| Hispanic | 25.8 (254) | 25.7 (247) | 25.8 (501) |
| Ever Smoker (% [Freq]) | 43.5 (428) | 33.5 (322) | 54.2 (1051) |
| BMI (M [SD]) | 27.9 (4.4) | 28.4 (5.0) | 28.1 (5.2) |
| Dyslipidemia (% [Freq]) | 43.5 (428) | 33.5 (322) | 39.6 (750) |
| Diabetes (% [Freq]) | 16.1 (158) | 12.6 (121) | 14.4 (279) |
| Hypertension (% [Freq]) | 60.6 (596) | 61.1 (585) | 60.9% (1181) |
| Coronary Artery Calcium (% [Freq]) | 66.2 (651) | 45.3 (435) | 57.6 (1086) |
| Waist circumference (cm, M [SD]) | 99.2 (12.0) | 97.3 (15.8) | 98.3 (14.1) |
| Subcutaneous fat area (cm2, M [SD]) | 211.6 (95.0) | 296.6 (122.9) | 253.7 (117.7) |
| Visceral fat area (cm2, M [SD]) | 164.1 (72.5) | 131.8 (61.6) | 148.0 (69.2) |
| Abdominal muscle area (cm2, M [SD]) | 116.4 (23.9) | 80.3 (17.4) | 98.3 (27.6) |
| Stability muscle area (cm2, M [SD]) | 87.3 (20.1) | 62.0 (15.1) | 74.6 (21.8) |
| Locomotor muscle area (cm2, M [SD]) | 29.0 (6.1) | 18.3 (3.8) | 23.7 (7.4) |
| Abdominal muscle radiodensity (HU, M [SD]) | 44.4 (4.9) | 40.1 (5.2) | 42.2 (5.5) |
| Stability muscle radiodensity (HU, M [SD]) | 42.1 (5.4) | 37.0 (5.7) | 39.5 (6.1) |
| Locomotor muscle radiodensity (HU, M [SD]) | 51.1 (5.0) | 49.3 (5.3) | 50.2 (5.2) |
| Sedentary Behavior (min·wk−1, Mdn [IQR]) | 1421 (1260) | 1560 (1511) | 1470 (1451) |
| MVPA (MET-min·wk−1, Mdn [IQR]) | 3983 (5320) | 3229 (4025) | 3555 (4538) |
| Systolic blood pressure (mmHg, M [SD]) | 123 (9) | 126 (23) | 124 (21) |
| Diastolic blood pressure (mmHg, M [SD]) | 73 (9) | 67 (10) | 70 (10) |
| Coronary artery calcium score (Mdn [IQR]) | 33.7 (230.6) | 0.0 (59.7) | 8.9 (135.5) |
| Insulin (pmol·L−1) | 294.3 (438.4) | 285.8 (214.6) | 290.1 (346.4) |
| Glucose (mg·dL−1, M [SD]) | 100.0 (27.6) | 96.9 (27.7) | 98.3 (27.7) |
| Triglycerides (mg·dL−1, M [SD]) | 135.0 (110.3) | 132.2 (76.3) | 133.6 (95.0) |
| High-density lipoprotein (mg·dL−1, M [SD]) | 46.3 (12.4) | 56.8 (15.9) | 51.5 (15.1) |
| Low-density lipoprotein (mg·dL−1, M [SD]) | 109.6 (31.1) | 114.5 (31.5) | 112.1 (31.4) |
| eGFR (mL·min−1, M [SD]) | 79.7 (16.8) | 78.6 (18.1) | 79.2 (17.4) |
| C-reactive protein (mg·L−1, Mdn [IQR] | 1.2 (1.8) | 1.9 (3.4) | 1.5 (2.6) |
| Adiponectin (μg·mL−1, Mdn [IQR]) | 14.5 (10.9) | 21.5 (16.6) | 17.4 (14.5) |
| Leptin (ng·mL−1, Mdn [IQR]) | 7.1 (10.8) | 25.0 (29.8) | 13.4 (22.7) |
| Resistin (ng·mL−1, Mdn [IQR]) | 14.8 (6.8) | 15.2 (7.6) | 15.0 (7.2) |
| Interleukin-6 (pg·mL−1, Mdn [IQR]) | 1.8 (1.6) | 1.9 (1.7) | 1.9 (1.7) |
| TNF-α (pg·mL−1, Mdn [IQR]) | 4.8 (2.7) | 4.5 (1.9) | 4.6 (2.9) |
Note. M, mean; SD, standard deviation; %, percent; Freq, frequency; Mdn, median; IQR, interquartile range; BMI, body mass index; HU, Hounsfield units; MVPA, moderate-to-vigorous physical activity; MET, metabolic equivalents; eGFR, estimated glomular filtration rate; TNF-α, tumor necrosis factor alpha; waist circumference – at umbilicus level.
Muscle area and radiodensity by quartiles of leptin and adiponectin
The mean muscle area and radiodensity by quartile of adiponectin and leptin are provided in Table 2. With adjustment for age, sex, and race/ethnicity, the mean levels of total abdominal and stability muscle radiodensity decreased across increasing quartiles of adiponectin (p<0.01), whereas the decrease in locomotor muscle radiodensity was not significant (p=0.17). Somewhat similarly, total abdominal, stability and locomotor muscle radiodensity also decreased across increasing quartiles of leptin. Moreover, total abdominal, stabilization, and locomotor muscle area also decreased across increasing quartiles of adiponectin (p<0.01). There were no differences in muscle area across quartiles of leptin.
Table 2.
Mean muscle areas and radiodensities by adiponectin and leptin quartiles
| Adiponectin Quartile | |||||
|---|---|---|---|---|---|
| Q1 (n=465) | Q2 (n=476) | Q3 (n=473) | Q4 (n=481) | P value | |
| Muscle Radiodensity | |||||
| Total abdominal | 44.4 | 42.9 | 41.4 | 40.2 | 0.007 |
| Stability | 42.0 | 40.4 | 38.6 | 37.2 | 0.005 |
| Locomotor | 51.5 | 50.6 | 49.7 | 49.1 | 0.174 |
| Muscle Area | |||||
| Total abdominal | 111.8 | 103.7 | 94.2 | 84.6 | <0.001 |
| Stability | 85.2 | 78.8 | 71.4 | 63.8 | <0.001 |
| Locomotor | 26.6 | 24.9 | 22.8 | 20.6 | 0.004 |
| Leptin Quartile | |||||
|---|---|---|---|---|---|
| Q1 (n=480) | Q2 (n=475) | Q3 (n=468) | Q4 (n=465) | P value | |
| Muscle Radiodensity | |||||
| Total abdominal | 44.7 | 43.1 | 41.4 | 39.7 | <0.001 |
| Stability | 42.3 | 40.5 | 38.6 | 36.7 | <0.001 |
| Locomotor | 51.7 | 50.7 | 49.6 | 48.7 | <0.001 |
| Muscle Area | |||||
| Total abdominal | 108.7 | 102.4 | 95.8 | 87.2 | 0.168 |
| Stability | 81.6 | 77.4 | 73.0 | 67.2 | 0.074 |
| Locomotor | 27.1 | 25.0 | 22.7 | 19.9 | 0.246 |
Note. Adiponectin quartiles (μg·mL−1): Q1 <11.80; Q2=11.80–17.35; Q3=17.36–26.27; Q4≥26.28; Leptin quartiles (ng·mL−1): Q1 <5.64; Q2=5.64–13.46; Q3=13.47–28.30; Q4≥28.31.
Adjusted for age, sex, race/ethnicity.
Multivariable associations of muscle area and radiodensity with adiponectin and leptin
Multivariable linear regression models were constructed to determine the independent associations between muscle radiodensity and area and the adipokines (Table 3). With adjustment for age, sex, race/ethnicity, smoking, dyslipidemia, hypertension, CAC, physical activity and sedentary behavior (model 1), a 1-SD increment in total abdominal, stability, and locomotor muscle area was associated with a 24%, 22%, and 11% lower adiponectin levels, respectively (p < 0.01 for all). These associations were slightly attenuated but remained significant with the addition of all covariates including abdominal subcutaneous and visceral adipose tissue (model 3, 19%, 17%, and 13%, respectively, p < 0.01 for all). Muscle radiodensity was more robustly related to adiponectin in the multivariable linear regression models. That is, with adjustment for all covariates (model 3), a 1-SD increment in total abdominal, stability, and locomotor muscle radiodensity was associated with a 31%, 31%, and 18% lower adiponectin level, respectively (p < 0.01 for all).
Table 3.
Multivariate linear regression models for the associations between muscle area and radiodensity with adiponectin (n=1791) and leptin (n=1784). Data are presented as standardized betas.
| Model | ||||||
|---|---|---|---|---|---|---|
| Total Abdominal muscle area | 1 | P-value | 2 | P-value | 3 | P-value |
| Adiponectin | −0.236 | <0.001 | −0.236 | <0.001 | −0.190 | <0.001 |
| Leptin | 0.025 | 0.391 | 0.005 | 0.860 | 0.020 | 0.413 |
| Stability muscle area | ||||||
| Adiponectin | −0.218 | <0.001 | −0.213 | <0.001 | −0.166 | <0.001 |
| Leptin | 0.032 | 0.215 | 0.010 | 0.703 | 0.018 | 0.415 |
| Locomotor muscle area | ||||||
| Adiponectin | −0.110 | 0.001 | −0.132 | <0.001 | −0.125 | 0.001 |
| Leptin | −0.039 | 0.202 | −0.035 | 0.260 | 0.011 | 0.672 |
| Total Abdominal muscle radiodensity | ||||||
| Adiponectin | −0.113 | <0.001 | −0.181 | <0.001 | −0.310 | <0.001 |
| Leptin | −0.211 | <0.001 | −0.208 | <0.001 | 0.067 | 0.003 |
| Stability muscle radiodensity | ||||||
| Adiponectin | −0.119 | <0.001 | −0.184 | <0.001 | −0.305 | <0.001 |
| Leptin | −0.219 | <0.001 | −0.213 | <0.001 | 0.046 | 0.041 |
| Locomotor muscle radiodensity | ||||||
| Adiponectin | −0.056 | 0.013 | −0.101 | <0.001 | −0.175 | <0.001 |
| Leptin | −0.128 | <0.001 | −0.122 | <0.001 | 0.081 | <0.001 |
Note. model 1: age, sex, race/ethnicity, smoking, dyslipidemia, hypertension, coronary artery calcium, physical activity and sedentary behavior; model 2: model 1 plus C-reactive protein, interleukin-6 and adiponectin (in leptin analysis) or leptin (in adiponectin analysis); model 3: model 2 plus subcutaneous and visceral adipose tissue.
With adjustment for age, sex, race/ethnicity, smoking, dyslipidemia, hypertension, CAC, physical activity and sedentary behavior (model 1), a 1-SD increment in total abdominal, stability, and locomotor muscle radiodensity was associated with a 21%, 22%, and 13% lower leptin level, respectively (p<0.001 for all). Additional adjustments for C-reactive protein, interleukin-6, and adiponectin did not materially change the magnitude or significance of the associations. Notably, with the addition of subcutaneous and visceral adipose tissue (model 3) a 1-SD increment in total abdominal, stability, and locomotor muscle radiodensity was associated with a 6.7%, 4.6%, and 8.1% higher leptin level, respectively (p < 0.05 for all). Muscle area was not independently associated with leptin in any of the models (p>0.05).
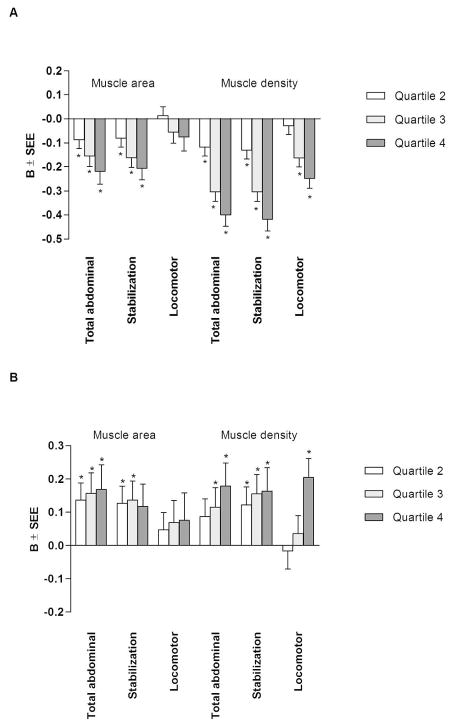
The results of multivariable linear regression when muscle radiodensity and area were considered as categorical variables and divided into quartiles are provided in Figure 1 (model 3 is presented). Compared with the lowest quartile, and after adjustment for age, sex, race/ethnicity, smoking, dyslipidemia, hypertension, CAC, physical activity, sedentary behavior and the other adipokines (model 2), there was a stepwise decrease in adiponectin with each higher quartile of total abdominal (9, 16, 29%, respectively) and stabilization (8, 18, 29%, respectively) muscle area, as well as total abdominal (9, 21, 24%, respectively) and stabilization (10, 21, 26%, respectively) muscle radiodensity, which was maintained in the fully adjusted model (p<0.05 for all). After full adjustment and compared with the lowest quartile, the third and fourth quartiles of locomotor muscle radiodensity were associated with a 16% and 25%, respectively, lower adiponectin level (p<0.001).
Figure 1.
Multivariable-adjusted associations between quartiles of muscle area and radiodensity with levels of adiponectin (A) and leptin (B) for model 3.
Referent category: Quartile 1. Muscle area quartile cutpoints (cm2): total abdominal: Q1<77.01, Q2= 77.02–94.66, Q3=94.67–116.84, Q4≥116.85; stabilization: Q1<58.19, Q2=58.20–72.12, Q3=72.13–89.13, Q4≥89.14; locomotor: Q1<17.88, Q2=17.88–22.75, Q3=22.76–28.83, Q4≥28.84; Muscle radiodensity quartile cutpoints (HU): total abdominal: Q1<38.17, Q2=38.17–42.69, Q3=42.70–46.34, Q4≥46.35; stabilization: Q1<35.24, Q2=35.24–39.86, Q3=39.87–44.06, Q4≥44.07; locomotor: Q1<47.14, Q2=47.15–50.84, Q3=50.85–53.89; Q4≥53.90. Model 3 adjusted for age, sex, race/ethnicity, dyslipidemia, hypertension, diabetes, smoking, glomerular filtration rate, physical activity, sedentary behavior, adipokines, and subcutaneous and visceral adipose tissue. B=slope; SEE, standard error of the estimate; *p<0.05.
Compared with the lowest quartile, and after adjustment for age, sex, race/ethnicity, smoking, dyslipidemia, hypertension, CAC, physical activity, sedentary behavior and the other adipokines (model 2) there was a stepwise decrease in leptin with each higher quartile of total abdominal (24, 40, 61%, respectively), stabilization (16, 34, 59%, respectively), and locomotor (26, 29, 28%, respectively) muscle radiodensity (p<0.001 for all). With additional adjustment for subcutaneous and visceral adipose tissue (model 3), and compared to the first quartile, the second, third and fourth quartiles of stabilization muscle radiodensity were associated with a 12, 15, and 16% higher leptin level (p<0.05), whereas the third (12%) and fourth (18%) quartiles of total abdominal muscle radiodensity and the fourth quartile (20%) of locomotor muscle radiodensity were associated with higher leptin levels (p<0.05 for all). After full adjustment, and compared to the first quartile, the second, third and fourth quartiles of total abdominal muscle area were associated with a 14, 16, and 17% higher leptin level (p<0.05), whereas the second and third quartiles of stabilization muscle area were associated with 13 and 14% higher leptin level (p<0.05). Locomotor muscle area was not independently associated with leptin in any of the models (p>0.05).
Discussion
In this multiethnic cross-sectional analysis of older adults, higher levels of abdominal muscle radiodensity were associated with significantly lower levels of adiponectin and higher levels of leptin. Notably, these associations were independent of relevant covariates including subcutaneous and visceral adiposity, cardiovascular disease risk factors, and other markers of inflammation. Further, abdominal muscle area was independently associated with levels of adiponectin but not leptin. Overall, the associations of abdominal muscle radiodensity were more robust for adiponectin than leptin. These results suggest that decreased skeletal muscle area and radiodensity are independent risk factors for chronic inflammation and may contribute to an increased risk of cardiometabolic disease.
Little is known about the precise role of “muscle quality” on inflammatory cytokines. The robust association between muscle area and radiodensity with adiponectin in the current study are consistent with previous literature and suggest that adiponectin may be an important clinical marker for sarcopenia. Bucci et al. (8) compared 412 participants from the European research network study MYOAGE and reported that total adiponectin was inversely associated with quadriceps and handgrip strength in older but not younger adults. Similarly, population-based cohort studies have reported inverse associations between adiponectin and muscular strength, physical function, and disability in community-dwelling older adults (7,22,23). Our study extends the literature by investigating muscle area and radiodensity by CT scan, which provides information on “muscle quality”, as well as area.
It is well recognized that aging is associated with higher levels of adiponectin (8,23) despite the increase in visceral adiposity and insulin resistance that typically occurs with age (24). Adiponectin affects several metabolic processes involved in the control of energy homeostasis, by stimulating fatty acid oxidation and glucose uptake in skeletal muscle through AMP-activated protein kinase signaling (2,4). A dysregulation in adiponectin with age and/or obesity may blunt this process, reducing fat metabolism and increasing fat deposition within skeletal muscle, thereby reducing muscle density. The inverse association between muscle radiodensity and adiponectin demonstrated in our study provides further support for this hypothesis.
To date, several lines of evidence suggest a role for adiponectin in muscle morphology, with data from both human and animal models. In animal models, adiponectin knockout mice demonstrate a greater type IIb muscle fiber area when compared to wild-type controls. Similarly, in in humans, inverse associations between adiponectin concentrations and muscle fiber size (6) and arm muscle mass as measured by dual-energy x-ray absorptiometry (25) have been reported. The inverse association between muscle area and adiponectin demonstrated in our study is consistent with these findings and provides additional evidence for a link between adiponectin and skeletal muscle morphology.
Importantly, our data show that the association between muscle radiodensity with leptin is positive when subcutaneous and visceral adiposity are accounted for in the model. Subcutaneous and visceral adipose tissue were negatively correlated with muscle radiodensity but positively correlated with leptin, indicating significant reverse confounding. Specifically, when subcutaneous and visceral adipose tissue were included in the model, the negative association between leptin and subcutaneous and visceral adipose tissue was accounted for (i.e., removed from the association between leptin and muscle radiodensity) and the association between leptin and muscle radiodensity changed from negative to positive. Further, the associations with leptin were stronger for muscle radiodensity than area. In mouse models leptin treatment is associated with increases in muscle mass and decreases the expression of factors associated with atrophy (10). One of the major physiological roles of leptin is suggested to be the prevention of lipid accumulation in peripheral tissues, including skeletal muscle (26). In this regard, higher leptin levels would limit the amount of lipid deposition in muscle, thereby maintaining a high radiodensity or quality of muscle.
Aging is associated with a progressive decline of muscle mass and quality, a condition termed sarcopenia. In addition, low muscle mass in aged individuals is associated with higher rates of disability and mortality (27). Therefore, understanding the factors associated with the age-related loss of skeletal muscle and function is of growing importance. Several proposed mechanisms for age-related muscle atrophy include physical inactivity, poor diet, oxidative stress, inflammation, and hormonal changes, with the mechanisms purported to be multifactorial (27). It is appreciated that both adiponectin and leptin affect several metabolic processes involved in the control of energy homeostasis, with increasing fatty acid oxidation in skeletal muscles one of the most important mechanisms by which insulin sensitivity is maintained. These findings also suggest that adiponectin and leptin may play an important role in skeletal muscle health. Skeletal muscle secretes myokines, including leptin (9) and adiponectin (4) that are thought to have endocrine, autocrine and paracrine effects. Thus, the amount and quality of skeletal muscle may play a protective role in inflammatory-related conditions, including obesity, insulin resistance, and cardiometabolic disease (28).
The strengths of the current study include a relatively large, well-characterized, multi-ethnic sample of men and women, the use of objective measures of abdominal muscle and adipose tissue via CT scan, careful assessment of many potentially confounding factors, and inflammatory markers that were analyzed at a central laboratory, with a high level of reproducibility. Nonetheless, our findings should be considered in the context of several limitations. First, measures of physical activity and sedentary behavior were self-reported and subject to recall bias. Second, this was a cross-sectional analyses and direction of associations cannot be determined. It is possible that adiponectin and leptin levels resulted in changes in muscle mass (i.e., reverse causation). Given this, prospective studies are needed to determine relationships of muscle area and radiodensity with adiponectin and leptin over time. Third, although abdominal subcutaneous and visceral adipose tissue were included in this study, we did not assess intramuscular adipose tissue. Finally, the use of multiple CT scanners from different manufacturers to measure body composition may introduce bias; however, research suggests that electron beam and multi-detector row CT scanners that were used in this study have equivalent reproducibility, particularly for CAC measurements (29–31). Mao et al. (31) reported a high degree of agreement (99%, 92%, and 92%) for presence of CAC, Agatston score and volume score, respectively, between electron beam and multidetector scanners. Further a study investigating between scanner variability for soft tissue reported a small mean difference and standard error in subcutaneous adipose tissue (−4.2 cm2 and 2.8%, respectively), visceral adipose tissue (−2.6 and 5.5%, respectively) and muscle tissue (1.8 cm2 and 1.9%, respectively) at L4 (32). Notably, all of the scans for our study were processed using the same software and inter and intra-rater reliabilities for total abdominal, subcutaneous, and visceral cavity areas were 0.99 for all measurements. Inter and intra-rater reliability for all muscle groups ranged from 0.93 to 0.98.
Conclusions
In summary, abdominal muscle radiodensity was inversely associated with adiponectin levels and positively associated with leptin levels, whereas abdominal muscle area was associated with levels of adiponectin but not leptin. These associations were independent of relevant covariates including physical activity, sedentary behavior, cardiovascular disease risk factors, central adiposity, and other markers of inflammation. Our data suggest that increasing total abdominal muscle area and radiodensity may have positive impacts on chronic inflammation, and in turn, reduce the risk of cardiometabolic disease.
Adiponectin and leptin have emerged as important regulators of metabolism in skeletal muscle, contributing to insulin sensitivity and muscle morphology.
Few population-based studies have investigated associations between skeletal muscle and adipokines.
We show muscle area and radiodensity are independently related to adiponectin and leptin.
These associations were independent of relevant covariates including subcutaneous and visceral adipose tissue, which are both important contributors to levels of these adipokines.
Acknowledgments
FUNDING: This research was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169 and R01HL088451) and National Center for Advancing Translational Sciences (UL1-TR-000040 and UL1-TR-001079).
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
DISCLOSURE: The authors report no conflicts of interest.
References
- 1.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Dyck DJ. Adipokines as regulators of muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab. 2009;34:396–402. doi: 10.1139/H09-037. [DOI] [PubMed] [Google Scholar]
- 3.Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab. 2006;9:282–289. doi: 10.1111/j.1463-1326.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 4.Sente T, Van Berendoncks AM, Hoymans VY, Vrints CJ. Adiponectin resistance in skeletal muscle: pathophysiological implications in chronic heart failure. J Cachexia Sarcopenia Muscle. 2016;7:261–274. doi: 10.1002/jcsm.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teoh H, Strauss MH, Szmitko PE, Verma S. Adiponectin and myocardial infarction: a paradox or a paradigm? Eur Heart J. 2006;27:2266–2268. doi: 10.1093/eurheartj/ehl248. [DOI] [PubMed] [Google Scholar]
- 6.Pisto P, Santaniemi M, Turpeinen JP, Ukkola O, Kesaniemi YA. Adiponectin concentration in plasma is associated with muscle fiber size in healthy middle-aged men. Scand J Clin Lab Invest. 2012;72:395–402. doi: 10.3109/00365513.2012.687759. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Tomata Y, Kakizaki M, et al. High circulating adiponectin levels predict decreased muscle strength among older adults aged 70 years and over: a prospective cohort study. Nutr Metab Cardiovasc Dis. 2015;25:594–601. doi: 10.1016/j.numecd.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Bucci L, Yani SL, Fabbri C, et al. Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology. 2013;14:261–272. doi: 10.1007/s10522-013-9428-5. [DOI] [PubMed] [Google Scholar]
- 9.Hamrick MW. Role of the cytokine-like hormone leptin in muscle-bone crosstalk with aging. J Bone Metab. 2017;24:1–8. doi: 10.11005/jbm.2017.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamrick MW, Herberg S, Arounleut P, et al. The adipokines leptin increases skeletal muscle mass and significantly alters skeletal muscle miRNA expression profile in aged mice. Biochem Biophys Res Commun. 2010;400:379–383. doi: 10.1016/j.bbrc.2010.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and bias of its biological variation. Acta Physiologica. 2014;210:489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 13.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 14.Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017 doi.org/10.1161/HYP.0000000000000065.
- 15.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Women’s Health Gend Based Med. 1999;8(6):805–13. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 16.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adutls. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 18.Levery AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–27. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, THaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann NY Acad Sci. 2000;904:18–24. doi: 10.1111/j.1749-6632.2000.tb06416.x. [DOI] [PubMed] [Google Scholar]
- 21.Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014;210:489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hozawa A, Sugawara Y, Tomata Y, et al. Relationship between serum adiponectin levels and disability-free survival among community-dwelling elderly individuals: The Tsurugaya project. J Gerontol A Biol Sci Med Sci. 2012;67A(5):530–536. doi: 10.1093/gerona/glr191. [DOI] [PubMed] [Google Scholar]
- 23.Kizer JR, Arnold AM, Strotmeyer ES, et al. Change in circulating adiponectin in advanced old age: determinants and impact on physical function and mortality. The Cardiovascular Health Study All Starts Study. J Gerontol A Biol Sci Med Sci. 2010;65A(11):1208–1214. doi: 10.1093/gerona/glq122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 25.Loncar G, Bozic B, von Haehling S, et al. Association of adiponectin with peripheral muscle status in elderly patients with heart failure. Eur J Int Med. 2013;24:818–823. doi: 10.1016/j.ejim.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Dyck DJ, Heigenhauser GJF, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf) 2006;186:5–16. doi: 10.1111/j.1748-1716.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- 27.Sakuma K, Yamaguchi A. Sarcopendic obesity and endocrinal adaptation with age. Int J Endocrinol. 2013 doi: 10.1155/2013/204164. Article ID 204164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 29.Deltrano RC, Anderson M, Nelson J, et al. Coronary calcium measuremetns: effect of CT scanner type and calcium measusre on rescan reproducibility- MESA study. Radiology. 2005;236:477–484. doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- 30.Ghadri JR, Goetti R, Fiechter M, et al. Inter-scan variability of coronary artery calcium scoring assessed on 64-multidetector computed tomography vs. dual-source computed tomography: a head to head comparison. Eur Heart J. 2011;32:1865–1874. doi: 10.1093/eurheartj/ehr157. [DOI] [PubMed] [Google Scholar]
- 31.Mao SS, Pal RS, McKay CR, et al. Comparison of coronary artery calcium scores between electron beam computed tomography and 64-multidetector computed tomographic scanner. J Comput Assist Tomogr. 2009;33:175–178. doi: 10.1097/RCT.0b013e31817579ee. [DOI] [PubMed] [Google Scholar]
- 32.Brandberg J. Methodological and applied studies. Intellecta DocuSys; Gothenburg, Sweden: 2009. Computed tomography and magnetic resonance imaging in determination of human body composition. [Google Scholar]