Abstract
BACKGROUND
Chronic life stress, such as the stress of caregiving, can promote pathophysiology, but the underlying cellular mechanisms are not well understood. Chronic stress may induce recalibrations in mitochondria leading to changes either in mitochondrial content per cell, or in mitochondrial functional capacity (i.e., quality).
METHODS
Here we present a functional index of mitochondrial health (MHI) for human leukocytes that can distinguish between these two possibilities. The MHI integrates nuclear and mitochondrial DNA–encoded respiratory chain enzymatic activities and mitochondrial DNA copy number. We then use the MHI to test the hypothesis that daily emotional states and caregiving stress influence mitochondrial function by comparing healthy mothers of a child with an autism spectrum disorder (high-stress caregivers, n = 46) with mothers of a neurotypical child (control group, n = 45).
RESULTS
The MHI outperformed individual mitochondrial function measures. Elevated positive mood at night was associated with higher MHI, and nightly positive mood was also a mediator of the association between caregiving and MHI. Moreover, MHI was correlated to positive mood on the days preceding, but not following the blood draw, suggesting for the first time in humans that mitochondria may respond to proximate emotional states within days. Correspondingly, the caregiver group, which had higher perceived stress and lower positive and greater negative daily affect, exhibited lower MHI. This effect was not explained by a mismatch between nuclear and mitochondrial genomes.
CONCLUSIONS
Daily mood and chronic caregiving stress are associated with mitochondrial functional capacity. Mitochondrial health may represent a nexus between psychological stress and health.
Keywords: Chronic stress, Daily affect, Mind-body, Mitochondria, mtDNA copy number, Respiratory chain activity
Chronic psychological stress is a common human experience that has harmful effects on health and well-being. Years of chronic adversity cause affective, cognitive, behavioral, and metabolic changes that accelerate aging and predispose to disease (1–4). Caring for a family member with a chronic condition is an example of a stressful life situation and in some contexts is an independent risk factor for adverse cardiovascular events (5), dementia (6), and earlier mortality (7). However, the cellular mechanisms that underlie these adverse effects remain unclear (2), and there are likely multiple mechanisms underlying stress-induced pathophysiology and cellular aging (8,9). One potential mechanism mediating these effects is mitochondrial dysfunction (10), because it is known that mitochondria play a role in adaptation to stress (11) and aging (12) in animal models. However, mitochondrial health has not been well examined in relation to psychological stress in humans (13), owing in part to the lack of adequate methods to functionally assess mitochondria and the need for comprehensive life stress assessments. To bridge this gap, we developed a novel index of mitochondrial health for blood immune cells and applied it to a maternal caregiving model of chronic stress where we tested its sensitivity to daily mood.
Mitochondrial health is emerging as a major determinant of healthy physiological regulation and resilience (14). Mitochondria are multifaceted organelles, and there are hundreds in each cell of the body. They are the cellular powerhouse, providing energy for most cellular functions and generating signals of adaptation that influence the cell’s physiological response to stressors (11,15). Research in animal models has shown that mitochondrial defects cause profound alterations of stress response axes including the hypothalamic-pituitary-adrenal axis and sympathetic activation, which contribute to allostatic load and stress adaptation (11). In relation to disease, mitochondria also play a determinant role in cellular life and death (16), and they regulate disease-related processes that overlap with those of chronic stress pathophysiology (10), including inflammation, metabolic regulation, and cellular gene expression (17,18). Despite the important role of mitochondria in human health and potential association to stress and psychopathology (19–24), currently, methods available have not allowed assessments of mitochondrial health, or quality, in sizeable human cohorts.
There are different aspects of mitochondrial biology that must be taken into account to obtain an overall measure of mitochondrial health. Mitochondria contain multiple copies of their own genome known as the mitochondrial DNA (mtDNA). The mtDNA is critical for normal mitochondrial function because it encodes components of the respiratory chain complexes, where energy from food substrates and oxygen are transformed into adenosine triphosphate to power cellular processes (25). Two main causes of mitochondrial dysfunction are known: 1) reduction in the total amount of mitochondria— or mitochondrial content within cells; and 2) reduction in specific mitochondrial energy production capacity— or quality of each mitochondrion. For instance, individuals who inherit mitochondrial disease have a primary reduction in mitochondrial quality, but they can exhibit elevated mitochondrial content in blood leukocytes (26). As a result, mtDNA copy number (mtDNAcn) per cell can be increased as a compensatory mechanism for poor mitochondrial quality (26,27), and by itself mtDNAcn is not a reliable marker of mitochondrial content or quality. Empirically distinguishing between differences in mitochondrial content and quality is therefore essential to assess mitochondrial health and evaluate its relationship to aging, disease, and life stress exposure.
In this study, we tested the hypothesis that psychological states are associated with mitochondrial health. We first designed a mitochondrial health index (MHI) that mathematically integrates biochemical enzymatic activities and mtDNAcn into a single score, thus representing mitochondrial functional capacity on a per-mitochondrion basis. Next, we assessed the MHI in isolated peripheral blood mononuclear cells (PBMCs) in chronically stressed (caregiver) and control women and combined these data with psychosocial assessment of trait and daily emotional states, measured both prior to and after the collection of mitochondria (28). This enabled us to address two primary questions: 1) whether mitochondrial health was associated with perceived stress, negative affect, and daily mood; and 2) whether mitochondrial health was lower in caregivers. A mediation analysis demonstrated that the effect of caregiver status on mitochondrial health is partially mediated by lower daily positive mood. Overall, our results demonstrate that daily mood influences mitochondrial function, which may contribute to lower leukocyte mitochondrial health in chronically stressed caregivers.
METHODS AND MATERIALS
Study Cohort
Data for the present study were derived from a larger longitudinal study focused on the effects of caregiving stress on immunological aging. Individuals were recruited from the San Francisco Bay Area and deemed eligible to participate if they were nonsmokers between the ages of 20 and 50 years and had to be the mother of at least one child between the ages of 2 and 16 years. To be characterized as a high-stress maternal caregiver, the participant had to care for a child diagnosed with autism spectrum disorder and report a score of ≥13 on the Perceived Stress Scale (PSS) (29). In contrast, low-stress maternal control subjects were characterized as caring for a neurologically typical child and a reported PSS score of ≤19. The PSS eligibility criteria were based on prior national norms (29,30). Structured Clinical Interviews for Diagnostic and Statistical Manual for Mental Disorders for Axis I Disorders were carried out during the eligibility period, and individuals with current psychiatric conditions, including bipolar disorder, posttraumatic stress disorder, and eating disorders, were excluded. Substance use disorders were also exclusionary. Depression was exclusionary among our control participants; however, this was not exclusionary for high-stress caregivers. At the time point at which measures of mitochondrial health were assessed, only 2 participants, both of whom were high-stress caregivers, met diagnostic criteria for depression. Anxiety disorders were not assessed by structured interview and were not exclusionary. Structured Clinical Interviews were readministered at subsequent study time points. This study was approved by the Institutional Review Board at the University of California, San Francisco, and written informed consent was obtained for each study participant.
MHI Measurements
Platelet-free PBMCs were obtained by differential centrifugation and stored at −80°C until measurements were taken. All assays were performed on the same biological samples for each subject. Mitochondrial enzymes were selected based on four criteria: 1) they represent a known biological function (energy production capacity or mitochondrial content); 2) they are detectable in a microplate format for high throughput; 3) they have been shown to respond acutely to metabolic and biological stress; and 4) they are encoded by either the mitochondrial or nuclear genomes (see the Supplement for detailed information about each component). Thus, enzymatic activities were quantified spectrophotometrically for citrate synthase (CS), cytochrome c oxidase (COX, complex IV), and succinate dehydrogenase (SDH, complex II), and expressed per million cells. In parallel, mtDNA and nuclear DNA counts were measured by multiplex quantitative real-time polymerase chain reaction (qPCR) to normalize for cell number and calculate mtDNAcn. Finally, a composite measure, the MHI was calculated by mathematically integrating enzymatic activities and mtDNAcn, as described in Supplemental Figure S1 and in the Supplement, which includes a template MHI calculation file.
Stress and Mood Measures
Psychological stress was measured using the 10-item PSS (29), depressive symptoms were assessed using the Inventory of Depressive Symptomatology (31), and symptoms of anxiety were measured using the State-Trait Anxiety Inventory (32). Daily mood was assessed over 7 consecutive days using a morning and nightly diary, which encompassed the biological sample collection on day 4. The nightly diary assessed the intensity of positive (e.g., joy, inspired, feeling in control) and negative (e.g., feeling stressed, sad, tired) affective states, which were derived from the modified Differential Emotions Scale (33). Participants also completed a morning diary on awakening that assessed positive and negative affective states as well as expectations on the day to come. Additional details regarding nightly and morning diary measure are provided in the Supplemental Methods and Materials.
We examined mood on average over the week, and then, to take advantage of the daily data and explore the directionality of effect, examined mood either before or after the blood draw. Daily mood data were extracted and aggregated as pre–blood draw mood, averaging days 1 to 3, and as post–blood draw, averaging days 5 to 7. Thus, we performed analyses on a dual set of experience data to explore the temporal relationship between mood and MHI.
Data Analysis
Analyses were carried out in four stages. First, we analyzed individual mitochondrial measures and their mathematical integration as MHI. Second, we employed linear regressions to examine across groups whether trait measures of distress or daily mood measured before or after blood draw were related to MHI. Third, analyses of covariance were performed to evaluate group differences between caregiver status (caregiver vs. control) on individual measures of mitochondrial activity (COX, SDH, and CS), mtDNAcn, and MHI. Finally, we performed mediation analysis to examine whether the caregiver difference in MHI was explained in part by differences in trait measures of distress or acute experience in daily affect. Correlation coefficients are computed as Pearson’s r, unless noted otherwise. For partial least square discriminant analysis of MHI components, singular value decomposition was used to impute missing values (34) in Metaboanalyst 3.0 (http://www.metaboanalyst.ca/faces/docs/About.xhtml) (35), and data were mean centered and divided by the standard deviation of each variable to scale all variables. All other analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
For additional information about the study cohort, inclusion and exclusion criteria, questionnaires and daily mood measures, and complete experimental procedures for mitochondrial enzymatic assays, mtDNAcn, mitochondrial protein content, and data analysis, see the Supplement.
RESULTS
Demographics
The sample (n = 91) was 43.4 ± 5.4 (mean ± SD) years old on average, with a body mass index of 25.9 ± 5.3, and 17.0 ± 1.8 years of education. There were no significant differences between the caregivers and control subjects in any demographic factors (Supplemental Table S1). Caregiver duration was on average 5.1 ± 3.0 years (range 1.1–13.9). Two caregivers met diagnostic criteria for major depression, and 9 caregivers and 1 control subject were taking antidepressants for mood. This study was not powered to test the association between depression and mitochondrial health, so no conclusion about this potential association can be drawn here. Nevertheless, a sensitivity analysis of the primary analysis excluding those taking antidepressants was conducted, showing no significant effect.
Mitochondrial Measurements
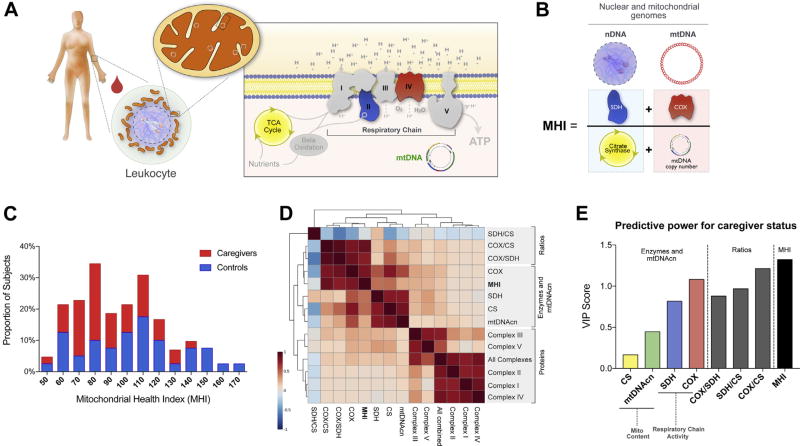
For each participant, PBMCs were homogenized and the activities of SDH (complex II), COX (complex IV), and CS were measured (Figure 1A). The same lysate for each sample was subsequently used to measure mtDNAcn and to quantify cell number by qPCR (Supplemental Figure S1).
Figure 1.
Mitochondrial health index (MHI) and mitochondrial profiling in human leukocytes. (A) Schematic of a human peripheral blood leukocyte and its mitochondria. The internal components of the mitochondrial respiratory chain (complexes I–V), the tricarboxilic acid ([TCA], also Krebs) cycle, and the mitochondrial genome (mtDNA) are shown in the inset with the inner mitochondrial membrane. (B) Mathematical integration of two nuclear DNA (nDNA)-encoded components (left), and mtDNA-related components (right) into the MHI. (C) Frequency distribution of MHI in the study sample. See Table 3 for statistics. (D) Correlation matrix generated by unsupervised clustering between enzymatic activities, calculated ratios, mtDNA copy number (mtDNAcn), and respiratory chain complexes protein levels. Three major clusters of correlated variables are highlighted. (E) Results from partial least square discriminant analysis model showing rank-ordered variables based on their variable importance in projection (VIP) scores for the first component of the full model; n = 89 to 91 for all. VIP values >1 are considered significant. ATP, adenosine triphosphate; COX, cytochrome c oxidase; CS, citrate synthase; Mito, mitochondrial; SDH, succinate dehydrogenase.
mtDNAcn and Mitochondrial Content
We first compared across the full study sample two markers of mitochondrial content—or the number of mitochondria per cell: CS enzymatic activity and mtDNAcn. Traditionally, mitochondrial enzymatic activity is normalized per total protein content (36), a procedure meant to correct for the total number of cells present in each sample. However, protein normalization does not take into account potential differences in cellular protein density or cell size. When applying this standard procedure, CS and mtDNAcn were not significantly correlated (Supplemental Figure S2A). We therefore applied qPCR to precisely adjust cell count in each cell lysate and used this metric to normalize enzymatic activity. This method uses a single copy nuclear gene B2M (β2-microglobulin) to determine relative cell number between samples and generate a correction factor that adjusts cell number per homogenate volume. When CS was normalized on a per-cell basis using this novel approach, the markers of mitochondrial content were significantly correlated and shared 69% of their variance (Supplemental Figure S2B), as expected.
We then repeated this analysis for enzymes of the respiratory chain, SDH and COX, two mitochondrial respiratory chain components that contribute to energy production capacity. Again, when normalized to protein, mtDNAcn was not correlated with SDH nor COX activities but was significantly correlated when expressed per cell using our novel approach (Supplemental Figure S2C–F). These results establish that normalization on a per-cell basis is a superior approach to assess mitochondrial functional capacity in human PBMCs.
Mitochondrial Content Versus Specific Mitochondrial Function
Mitochondrial content indexed by CS (37) was then compared with respiratory chain activity for SDH and COX. As expected, interindividual differences in mitochondrial content accounted for a substantial portion of the variance in total SDH (59%) and COX (64%) activities (Supplemental Figure S3A, B). Comparatively, SDH and COX activities were more weakly correlated to each other and showed only 33% overlap in variance (Supplemental Figure S3C). This indicated that the activities of both enzymes are likely regulated through largely independent mechanisms, consistent with the fact that SDH is entirely encoded by the nucleus, and that COX is partially encoded by mtDNA. Thus, SDH and COX must contribute mostly independent information about overall mitochondrial health. Similar findings were observed with measurements of mitochondrial respiratory chain proteins (Supplemental Figure S4).
Development of the MHI
To develop a composite measure of mitochondrial health, we integrated four functional parameters in a simple equation with two numerators and two denominators, which equally represent the nuclear and mitochondrial genomes. Respiratory chain activity (SDH and COX) are mean-centered and added as the numerator, and markers of mitochondrial content (CS and mtDNAcn) are also mean-centered and added as the denominator. The quotient of both terms therefore yields a scalar index, the MHI, which reflects respiratory chain capacity per unit of mitochondrial content (see Figure 1B).
In our cohort, the calculated MHI was normally distributed (D’Agostino and Pearson normality test, K2 = 4.25, p > .10), with a slight positive skew (skewness = 0.52, kurtosis = −0.34) (Figure 1C). A correlation matrix among all measured and calculated parameters of mitochondrial function showed that all enzyme activities, MHI, and mtDNAcn were most highly related (central cluster), whereas respiratory chain protein levels mostly behave independently from MHI (Figure 1D). Intercorrelations among the four mitochondrial parameters measured and the MHI index are shown in Supplemental Table S2, and the nonsignificant correlations with body mass index and age are presented in the Supplemental Results.
Comparison of Individual Measures Versus MHI
To further assess how well the MHI can discriminate between caregivers and control subjects relative to each of its individual components, we performed a partial least square discriminant analysis with caregiver status as the independent variable. We extracted the variable importance in projection scores on the first and most discriminant component of the final model, where a higher score indicates greater power to discriminate between groups (Figure 1E). Scores showed that mitochondrial content (CS and mtDNAcn) were weakest, followed by SDH (nuclear encoded) and COX (mtDNA encoded). Calculated ratios, in which enzymatic activities are normalized to CS (i.e., mitochondrial content), had higher scores than individual components, corroborating the presence of a specific reduction of mitochondrial energy production machinery, rather than a lower mitochondrial content. Of all parameters, the MHI had the highest variable importance in projection score on the first component, confirming that the mathematical integration of individual MHI components results in a composite index with superior predictive potential in relation to caregiver status.
Daily Mood and Mitochondrial Health
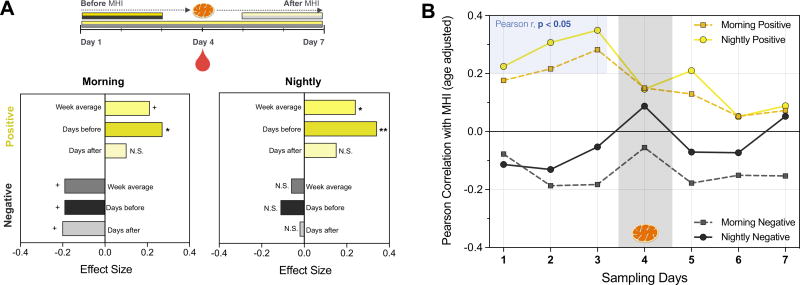
We then examined the relationship between daily mood and MHI. First, we averaged the mean of positive and negative mood measures across the full week to obtain a trait-like measure. In the evening, higher positive affect was significantly related to higher MHI (r = .24, p < .05) (Figure 2A). In the morning, there was a trend (p < .10) for MHI to be related to greater positive affect (r = .21) and lower negative affect (r = −.19). Then, to understand whether mood had a greater effect on mitochondrial function when it was more proximate to the MHI measurement, we conducted two exploratory analyses examining the association between MHI and daily mood both before and after biological sampling.
Figure 2.
Exploratory analysis showing the strength of the association between daily emotional states and peripheral blood mononuclear cells’ mitochondrial health index (MHI). (A) Combined effect size for the association between MHI and emotional states measured 1) across the week: Week average; 2) over 3 days preceding peripheral blood mononuclear cell collection: Days before; or 3) over 3 days after peripheral blood mononuclear cell collection: Days after. Note that effect sizes are larger for time points preceding blood draw, suggesting a directional relationship from mood to mitochondria. Mood was assessed in the morning and at night as described in the Supplemental Methods and Materials. +p < .10; *p < .05, **p < .01; n = 86 to 89. N.S., not significant. (B) Individual Pearson’s r correlation coefficients between daily measures of positive or negative mood and MHI measured from blood drawn at day 4; n = 86 to 89.
Mood was averaged for each individual over the 3 days before MHI was measured and was compared with the average of the 3 days after. For mood measures assessed before blood was collected, the cells were present in the body and thus exposed to any biological effects of emotional states. To the extent that mood varies daily within an individual, we expected to see stronger correlations with MHI in the “pre” blood draw mood than the “post” blood draw mood measures. Indeed, for mood measures before the biological sample was taken, we found that positive, but not negative, mood in the morning (r = .27, p < .05) and evening was significantly associated with higher MHI (r = .31, p < .01) (Figure 2A). In contrast, mood measured in the 3 days after sampling was not significantly associated with MHI (p > .1), suggesting a directional effect of mood on mitochondrial health.
This time-dependent association is further illustrated by plotting correlation coefficients between daily mood and MHI (measured at day 4) (Figure 2B). The strength of the association between positive mood and MHI appears to become stronger from day 1 to day 3 (only reaching significance for morning mood, on days 1, 2, and 3), reaching highest values (morning: r = .28, night: r = .35) the day immediately preceding blood draw. Thus, nightly mood on day 3 alone accounts for approximately 12.3% of the variance of MHI on day 4. None of the mood scores measured after the collection of PBMCs were correlated with MHI, and correlation coefficients appeared to regress toward zero for these time points (Figure 2B). The effect of negative mood was not significant when examined as separate days. This temporal association between positive mood and MHI suggests that the cells that were circulating during the experience of positive mood tended to have higher mitochondrial energy production capacity.
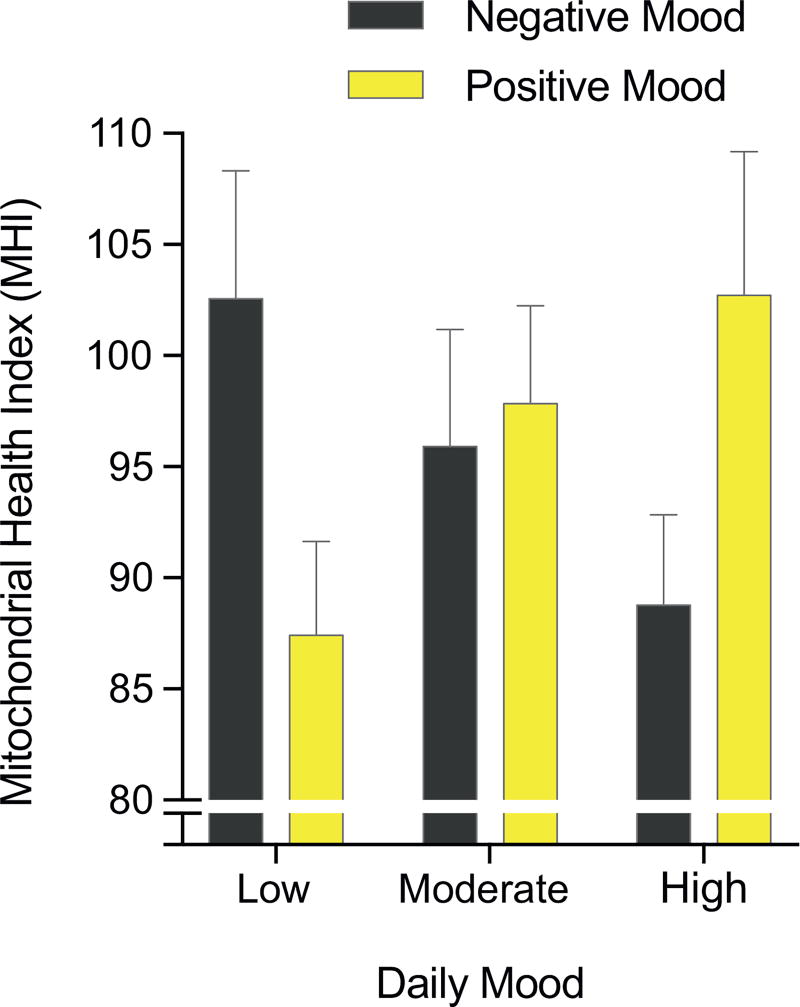
Lastly, we examined MHI in tertiles of positive and negative nightly mood across the whole sample. As shown in Figure 3, there was evidence for a dose response relationship, in the expected direction, between positive mood and higher MHI. Negative mood at night was also related to lower MHI. Individuals with the highest positive mood and/or lowest levels of negative mood had the highest MHI levels. The lowest and highest mood tertiles had MHI values that differed by 16% to 18%, which represent biologically meaningful differences in respiratory chain function.
Figure 3.
Cross-sectional association between mood and mitochondrial health index (MHI). Average MHI for tertiles of nightly negative and positive mood across the 3 days preceding mitochondrial measurements. Data are mean ± SEM; n = 27 to 29 per tertile.
Caregiver and Control Group Comparison
Caregivers reported significantly higher levels of perceived stress, depressive symptoms, and trait anxiety than control subjects (Table 1). Caregivers also showed lower levels of daily positive affect and higher negative affect over the week, both upon waking and at night (Table 2). Across the study sample, there were no significant associations between MHI and measures of distress (perceived stress, depressive symptoms, and anxiety) (Supplemental Table S3). There was only a trend suggesting higher levels of depressive symptoms with lower MHI, in the expected direction (p = .10). But there are relatively few individuals with high depression scores, and only two caregivers met criteria for major depression (none in the severe range), making this study not adequately powered to assess the association between depression and MHI.
Table 1.
Comparison of Weekly Averages of Daily Mood in Caregivers and Control Subjects
| Group | t | df | Effect Size |
||
|---|---|---|---|---|---|
| Caregivers | Control Subjects |
||||
| Morning Mood | |||||
| Positive | 1.98 ± 0.53 | 2.43 ± 0.45 | 4.33a,c | 86 | 0.92 |
| Negative | 1.60 ± 0.56 | 1.19 ± 0.58 | 3.37a,b | 86 | 0.73 |
| Nightly Mood | |||||
| Positive | 1.72 ± 0.61 | 2.26 ± 0.50 | 4.60a,c | 89 | 0.97 |
| Negative | 0.61 ± 0.47 | 0.46 ± 0.31 | 1.89 | 89 | 0.37 |
Data are mean ± SD. Effect size was calculated as Cohen’s d from t test.
Statistically significant value.
p < .01, two-tailed t test.
p ≤ .001, two-tailed t test.
Table 2.
Comparison of Mean Distress Scores in Caregivers and Control Subjects
| Group | t | df | Effect Size |
||
|---|---|---|---|---|---|
| Caregivers | Control Subjects |
||||
| Depression | 18.32 ± 8.32 | 10.83 ± 6.09 | 4.76a,b | 84 | 1.03 |
| Perceived Stress | 20.41 ± 5.74 | 14.61 ± 5.25 | 4.94a,b | 86 | 1.05 |
| Anxiety | 45.59 ± 10.83 | 37.16 ± 9.18 | 3.91a,b | 85 | 0.84 |
Values are mean ± SD. Effect size was calculated as Cohen’s d from t tests.
Statistically significant value.
p < .001, two-tailed t tests.
To test the hypothesis that caregivers have lower mitochondrial health, we used standard analysis of covariance models to examine caregiver versus control group differences in enzymatic activities, mtDNAcn, and MHI. Table 3 displays age-adjusted group means across key mitochondrial outcomes. Individual components of the MHI yielded no statistically significant group differences on their own. However, the MHI was significantly lower in caregivers compared with control subjects, indicating lower mitochondrial respiratory chain activity per unit of mitochondria. This is also apparent in Figure 1C, where a greater proportion of caregivers have lower MHI, and only control subjects have high MHIs >150. This result illustrates the sensitivity of the composite MHI over individual components of the index in relation to caregiver stress and indicates lower mitochondrial quality, but not content, in caregivers.
Table 3.
Mitochondrial Enzymatic Activities, mtDNAcn, and MHI by Group
| Group | t | df | Effect Size |
||
|---|---|---|---|---|---|
| Caregivers | Control Subjects |
||||
| COX | 4.35 ± 0.13 | 4.57 ± 0.14 | 1.11 | 83 | 0.24 |
| SDH | 5.40 ± 0.04 | 5.50 ± 0.04 | 1.66 | 86 | 0.36 |
| CS | 6.84 ± 0.05 | 6.88 ± 0.05 | 0.51 | 86 | 0.11 |
| mtDNAcn | 5.94 ± 0.03 | 5.97 ± 0.03 | 0.75 | 85 | 0.16 |
| MHI | 88.06 ± 3.95 | 104.57 ± 4.20 | 2.84a,b | 82 | 0.63 |
Mean estimates ± SEM are age adjusted. Effect size was calculated as Cohen’s d from t tests. Values are log transformed for CS, COX, SDH, and mtDNAcn. Mitochondrial health index (MHI) values are not transformed. Enzyme activities are expressed per million cells corrected by quantitative real-time polymerase chain reaction, and mtDNAcn as copies of mtDNA per cell.
COX, cytochrome c oxidase; CS, citrate synthase; mtDNA, mitochondrial DNA; mtDNAcn, mtDNA copy number; SDH, succinatedehydrogenase.
Statistically significant value.
p < .01, two-tailed t test.
Does Daily Mood Mediate MHI in Caregivers?
Given the temporal association between mood and mitochondrial function, and the differences in mood between groups, we reasoned that the differences in MHI between caregivers and control subjects could partially be explained by daily mood. To test this possibility, we ran a series of multiple regression models to test the indirect effects of caregiver status on MHI, via mood from days preceding the blood draw.
As shown in Table 4, in the total effect model (38) caregiver group status is a significant predictor of MHI for both morning and nightly positive mood. This showed an indirect effect of nightly positive mood (β = −5.12, p < .05) (Supplemental Figure S6), suggesting that low positive nightly mood, before the blood draw, may be partly responsible for the lower MHI measured in caregivers relative to control subjects.
Table 4.
Regression Models Testing Direct and Indirect Effects of Caregiver Group and Positive Mood on MHI
| Morning Positive Mood (n = 81) |
Nightly Positive Mood (n = 85) |
|
|---|---|---|
| Total Effect | ||
| Caregiver group | −17.62a,b ± 6.02 | −16.51a,b ± 5.82 |
| Full Effects | ||
| Intercept | 52.37 ± 27.10 | 31.56 ± 26.4 |
| Caregiver group | −13.62a,b ± 6.53 | −11.39 ± 6.03 |
| Mood | 8.16 ± 5.39 | 10.67a,b ± 4.38 |
| Model R2 | 0.13 | 0.16 |
| Indirect Effect (Bootstrapped, 95% CI) | ||
| Mood | −3.99 (−11.27, 0.82) | −5.12a,b (−11.61, −0.94) |
Values are mean ± SEM unstandardized B effects, representing units of mitochondrial health index (MHI), unless otherwise indicated. Daily mood measures were assessed before blood draw. Significance assessed using bias-corrected bootstrap confidence interval (CI). Age is a covariate in each model, not shown here.
Statistically significant value.
p < .05.
Exploration of Reasons for the Group Differences
One possible biological cause of lower MHI in caregivers is that they have an imbalance or mismatch between the amount of proteins synthesized by the mitochondrial and nuclear genomes, which is known to result from abnormal intracellular communication and influence the aging process (39,40). Comparing the balance of proteins levels for mtDNA-encoded COX II and nuclear-encoded complexes I and II subunits in control subjects and caregivers separately indicated that differences in MHI between caregivers and control subjects were unlikely due to altered communication between mitochondria and the cell nucleus (Supplemental Figure S5). The number of days since last menstrual period was also unrelated to MHI (r = −.01, p = .96).
As enzymatic activities could be affected by freezer storage time, we also evaluated duration in the freezer between sampling andmeasurements as a potential confounding variable. There was a significant correlation between storage time and MHI (r = −.56, p < .001), as well as with positive mood, perceived stress, anxiety, and depression (see the Supplement for analyses and discussion). This made it impossible to selectively covary out storage time across the whole sample without removing the effect of the group variable. But in the caregiver group, the correlation between positive mood and MHI remained significant and of similar effect size when adjusted for storage time compared with the unadjusted correlation that did not account for freezer time, indicating that the association between daily mood and MHI is invariant to freezer time. However, we cannot rule out the possibility that storage time may nevertheless have contributed to the group difference.
DISCUSSION
There are multiple pathways by which chronic stress can impact health. Here we developed a new way to quantify this relationship by combining biochemical and molecular mitochondrial measures in human blood leukocytes. The MHI approach integrates measures of both mitochondrial content and functional capacity assessed from the same samples, with a new procedure to normalize enzymatic activities on a per-cell basis. We first confirmed the sensitivity of this method and tested the hypothesis that mood is associated with leukocyte mitochondrial health. Collectively, our findings demonstrate three main points: 1) the integrative MHI is an indicator of mitochondrial functional capacity with superior sensitivity to mood and chronic stress than individual mitochondrial measures; 2) chronic caregiving stress may be associated with lower mitochondrial respiratory chain capacity, but not mitochondrial content; and 3) daily mood may partially mediate the effects of caregiving stress on mitochondria.
Despite growing evidence that aging- and stress-related diseases involve mitochondrial dysfunction (18,41,42), this hypothesis has remained difficult to test in sizeable human cohorts. Recent studies have found that life history of depression and early life adversity are associated with greater mtDNAcn measured in whole blood (19,20). However, other studies have found a smaller, rather than greater, mtDNAcn with posttraumatic stress disorder (21), with depression (22), and in the elderly (43). In addition, early life trauma has also been linked to changes in cellular baseline oxygen consumption measured in cryopreserved whole cells (23). But such measurements could reflect differences in cellular energy demand rather than intrinsic differences in the actual functional capacity of mitochondria. Maximal mitochondrial energy production capacity is more directly quantified in fresh permeabilized cells (44) or, as in the current study, by the maximal rate of key enzymatic activities such as COX and SDH (45).
A limitation of studies measuring only mtDNAcn—not the activity or functional outputs from mitochondria—is that while potentially indicating mitochondrial recalibrations, the number of mtDNA molecules per cell is biologically relevant only to the extent that it enables respiratory chain function. It is therefore difficult to interpret when measured alone. Furthermore, measures in whole blood are confounded by cellular composition. Differences in platelet number substantially alter apparent mtDNAcn without affecting leukocyte mitochondrial function (46,47). Thus, the biological significance of variation in mtDNAcn alone is unclear, particularly when measured in whole blood. In general, laboratory methods currently available to assess the functionality of mitochondria typically require a substantial amount of fresh cells or tissues, involve specialized equipment, have low throughput, and do not readily allow researchers to distinguish between differences in mitochondrial content versus functional capacity.
The MHI addresses these limitations and represents specific mitochondrial energy production capacity on a per-mitochondrion basis—or mitochondrial quality. Here, parallel measurements of the MHI with daily mood provide evidence that mitochondria may respond to psychological stress and mood states. These results are consistent with animal studies on the effects of acute and chronic stress on mitochondrial structure and function (13). However, more research is required to replicate and extend the current findings in humans and to identify the interacting psychobiological mechanisms that may transduce chronic psychosocial stress into changes in mitochondrial health.
Some limitations of the current study must be noted. Though using mothers caring for a child with autism spectrum disorder is a well-established stress model, it could be confounded by an underlying predisposition to mitochondrial dysfunction. This limitation applies to the finding on group differences, but not to the findings relying on within-person differences in mood across the week, which demonstrates a temporal relationship whereby positive mood predicts 10% to 15% of the variance in next day MHI, a meaningful biological effect. Furthermore, although the four-component MHI is a substantial advance over single mitochondrial measurements, it paints only a partial picture of the entire spectrum of possible stress-induced mitochondrial recalibrations. The accumulation of stress-induced mitochondrial structural and functional recalibrations, or mitochondrial allostatic load (10), could manifest at multiple different levels. Furthermore, although mood was measured daily over 7 days, MHI was only measured at one time point, which limits our ability to infer causality. Another technical limitation of this study is the use of mixed leukocyte populations in PBMCs, as opposed to sorted leukocytes lineages, which may contain different mitochondrial phenotypes (48). Furthermore, the potential confound of freezer storage time in relation to the caregiver-control group difference underscores the need to replicate these findings and the importance for future studies of leukocyte enzymatic activities and mitochondrial health to explicitly analyze storage time by clinical/experimental groups. Future studies should strive to comprehensively assess multiple facets of mitochondrial biology from homogenous leukocyte populations, in cohorts comprising both women and men.
In summary, we present a novel scalable approach to quantify mitochondrial health and establish the extent of interindividual variability in human leukocyte mitochondrial function. This approach provides the first evidence of a directional effect of mood on mitochondrial function, which mediated in part the effect of caregiver status on MHI. Chronic caregiving stress was associated with lower mitochondrial energy production capacity, but not content. These results are consistent with the adverse effects of psychological stress on multiple physiological systems, with mitochondria representing a potential site for the biological embedding of chronic stress exposure.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant Nos. 1R01AG030424-01A2, 5R24AG048024-02 and 5R21HL117727-02 to EE, MFE274188 and Wharton Fund contributions to MP, MOP136999 and RGPIN-2016-03932 (to YB).
The authors are grateful to Mary Elizabeth Sutherland for valuable comments and edits on this manuscript.
Footnotes
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2018.01.012.
References
- 1.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Brain on stress: How the social environment gets under the skin. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9:360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 4.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Colditz G, Berkman L, Kawachi I. Caregiving to children and grandchildren and risk of coronary heart disease in women. Am J Public Health. 2003;93:1939–1944. doi: 10.2105/ajph.93.11.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norton MC, Smith KR, Ostbye T, Tschanz JT, Corcoran C, Schwartz S, et al. Greater risk of dementia when spouse has dementia? The Cache County study. J Am Geriatr Soc. 2010;58:895–900. doi: 10.1111/j.1532-5415.2010.02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz R, Beach SR. Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: Linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackburn EH, Epel ES. Telomeres and adversity: Too toxic to ignore. Nature. 2012;490:169–171. doi: 10.1038/490169a. [DOI] [PubMed] [Google Scholar]
- 10.Picard M, McEwen BS. Psychological stress and mitochondria: A conceptual framework. Psychosom Med. 2018;80:126–140. doi: 10.1097/PSY.0000000000000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK, et al. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc Natl Acad Sci U S A. 2015;112:E6614–E6623. doi: 10.1073/pnas.1515733112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kauppila TE, Kauppila JH, Larsson NG. Mammalian mitochondria and aging: An update. Cell Metab. 2017;25:57–71. doi: 10.1016/j.cmet.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Picard M, McEwen BS. Psychological stress and mitochondria: A systematic review. Psychosom Med. 2018;80:141–153. doi: 10.1097/PSY.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juster RP, Seeman T, McEwen BS, Picard M, Mahar I, Mechawar N, et al. Social inequalities and the road to allostatic load: From vulnerability to resilience. In: Cicchetti D, editor. Developmental Psychopathology Handbook. 3. Cambridge, UK: Cambridge Press; 2016. [Google Scholar]
- 15.Chandel NS. Evolution of mitochondria as signaling organelles. Cell Metab. 2015;22:204–206. doi: 10.1016/j.cmet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Kasahara A, Scorrano L. Mitochondria: From cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014;24:761–770. doi: 10.1016/j.tcb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Picard M, Wallace DC, Burelle Y. The rise of mitochondria in medicine. Mitochondrion. 2016;30:105–116. doi: 10.1016/j.mito.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai N, Chang S, Li Y, Li Q, Hu J, Liang J, et al. Molecular signatures of major depression. Curr Biol. 2015;25:1146–1156. doi: 10.1016/j.cub.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS, et al. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiatry. 2016;79:78–86. doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bersani FS, Morley C, Lindqvist D, Epel ES, Picard M, Yehuda R, et al. Mitochondrial DNA copy number is reduced in male combat veterans with PTSD. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:10–17. doi: 10.1016/j.pnpbp.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Kim MY, Lee JW, Kang HC, Kim E, Lee DC. Leukocyte mitochondrial DNA (mtDNA) content is associated with depression in old women. Arch Gerontol Geriatr. 2011;53:e218–e221. doi: 10.1016/j.archger.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Boeck C, Koenig AM, Schury K, Geiger ML, Karabatsiakis A, Wilker S, et al. Inflammation in adult women with a history of child maltreatment: The involvement of mitochondrial alterations and oxidative stress. Mitochondrion. 2016;30:197–207. doi: 10.1016/j.mito.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Lindqvist D, Fernstrom J, Grudet C, Ljunggren L, Traskman-Bendz L, Ohlsson L, Westrin A. Increased plasma levels of circulating cell-free mitochondrial DNA in suicide attempters: associations with HPA-axis hyperactivity. Transl Psychiatry. 2016;6:e971. doi: 10.1038/tp.2016.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholls DG, Fergusson SJ. Bioenergetics. 4. Waltham, MA: Academic Press; 2013. [Google Scholar]
- 26.Giordano C, Iommarini L, Giordano L, Maresca A, Pisano A, Valentino ML, et al. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber’s hereditary optic neuropathy. Brain. 2014;137:335–353. doi: 10.1093/brain/awt343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu-Wai-Man P, Sitarz KS, Samuels DC, Griffiths PG, Reeve AK, Bindoff LA, et al. OPA1 mutations cause cytochrome c oxidase deficiency due to loss of wild-type mtDNA molecules. Hum Mol Genet. 2010;19:3043–3052. doi: 10.1093/hmg/ddq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith LE, Hong J, Seltzer MM, Greenberg JS, Almeida DM, Bishop SL. Daily experiences among mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord. 2010;40:167–178. doi: 10.1007/s10803-009-0844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 30.Cohen S, Williamson G. Psychological stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: SAGE; 1988. [Google Scholar]
- 31.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 32.Spielberger CD. State-Trait Anxiety Inventory: A Comprehensive Bibliography. Palo Alto, CA: Consulting Psychologists Press; 1984. [Google Scholar]
- 33.Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. J Pers Soc Psychol. 2003;84:365–376. doi: 10.1037//0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stacklies W, Redestig H, Scholz M, Walther D, Selbig J. pcaMethods—a bioconductor package providing PCA methods for incomplete data. Bioinformatics. 2007;23:1164–1167. doi: 10.1093/bioinformatics/btm069. [DOI] [PubMed] [Google Scholar]
- 35.Xia J, Wishart DS. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr Protoc Bioinformatics. 2016;55:14.10.11–14.10.91. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 36.Barrientos A, Fontanesi F, Diaz F. Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr Protoc Hum Genet. 2009;Chapter 19(Unit19.13) doi: 10.1002/0471142905.hg1903s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen S, Nielsen J, Neigaard Nielsen C, Nielsen LB, Wibrand F, Stride N, et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol. 2012;590:3349–3360. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A regression-Based Approach. New York, NY: Guilford; 2013. [Google Scholar]
- 39.Quiros PM, Mottis A, Auwerx J. Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol. 2016;17:213–226. doi: 10.1038/nrm.2016.23. [DOI] [PubMed] [Google Scholar]
- 40.Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nunnari J, Suomalainen A. Mitochondria: In sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Archer SL. Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369:2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 43.Mengel-From J, Thinggaard M, Dalgard C, Kyvik KO, Christensen K, Christiansen L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum Genet. 2014;133:1149–1159. doi: 10.1007/s00439-014-1458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 45.Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc. 2012;7:1235–1246. doi: 10.1038/nprot.2012.058. [DOI] [PubMed] [Google Scholar]
- 46.Urata M, Koga-Wada Y, Kayamori Y, Kang D. Platelet contamination causes large variation as well as overestimation of mitochondrial DNA content of peripheral blood mononuclear cells. Ann Clin Biochem. 2008;45:513–514. doi: 10.1258/acb.2008.008008. [DOI] [PubMed] [Google Scholar]
- 47.Hurtado-Roca Y, Ledesma M, Gonzalez-Lazaro M, Moreno-Loshuertos R, Fernandez-Silva P, Enriquez JA, Laclaustra M. Adjusting mtDNA quantification in whole blood for peripheral blood platelet and leukocyte counts. PLoS One. 2016;11:e0163770. doi: 10.1371/journal.pone.0163770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chacko BK, Kramer PA, Ravi S, Johnson MS, Hardy RW, Ballinger SW, Darley-Usmar VM. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab Invest. 2013;93:690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.