SUMMARY
Adipose tissue contains a heterogeneous population of stromal vascular fraction (SVF) cells that work synergistically with resident cell types to enhance tissue healing. Ease of access and processing paired with therapeutic promise make SVF cells an attractive option for autologous applications in regenerative medicine. However, inherent variability in SVF cell therapeutic potential from one patient to another hinders prognosis determination for any one person. This study investigated the regenerative properties and inflammation responses of thirteen, medically diverse human donors. Using non-expanded primary lipoaspirate samples, SVF cells were assessed for robustness of several parameters integral to tissue regeneration, including yield, viability, self-renewal capacity, proliferation, differentiation potential, and immunomodulatory cytokine secretion. Each parameter was selected either for its role in regenerative potential, defined here as the ability to heal tissues through stem cell repopulation and subsequent multipotent differentiation, or for its potential role in wound healing through trophic immunomodulatory activity. These data were then analyzed for consistent and predictable patterns between and across measurements, while also investigating the influence of the donors’ relevant medical histories, particularly if the donor was in remission following breast cancer treatment. Analyses identified positive correlations among the expression of three cytokines: interleukin (IL)-6, IL-8, and monocyte chemoattractant protein (MCP)-1. The expression of these cytokines also positively related to self-renewal capacity. These results are potentially relevant for establishing expectations in both preclinical experiments and targeted clinical treatment strategies that use stem cells from patients with diverse medical histories.
Keywords: adipose-derived stromal cell, stem cell immunomodulation, breast cancer, inflammatory cytokines, heterogeneity, regenerative medicine, autologous cell therapy
INTRODUCTION
A practical challenge in the research, development, and application of adipose-derived stem/stromal cell (ASC) treatment is the inherent variability in therapeutic potential due to donor/patient health and medical history. Current knowledge and understanding of cell therapy outcomes is limited by each patient having a unique medical profile, due in part to the human body’s adjustments to trauma, disease, and drug treatments, which can have long-term and lasting effects. The effects that chronic pathologies and medical conditions exert on progenitor cells has been a well-explored area of regenerative medicine, and conditions such as arthritis, diabetes, and obesity have been shown to influence stem cell functions (1–4). With regard to adipose tissue specifically, the ways in which cancer and chemotherapy affect the functional efficacy of ASC therapy is of interest because of the prevalence of adipose grafting as a reconstructive procedure following cancer treatments (5, 6). Such treatments may influence the regenerative capacity of the patient’s cells in differential and unpredictable ways, in turn confounding associated data. Clinical trials are often limited by small sample sizes and significant variability in patient response, which can influence the resulting data and yield conclusions that inaccurately represent findings seen in broader applications (5, 7).
The stromal vascular fraction (SVF), a heterogeneous cell population that includes ASCs, is a therapeutically relevant cell source that requires relatively little processing and holds promise for rapid, point-of-care treatment with similar outcomes to ASC-exclusive treatment (6, 8). SVF cells have been shown to repair injuries through similar mechanisms as ASCs, with the potential to further enhance therapeutic outcomes due to synergistic activity between ASCs and the other resident cell types (8, 9). Additionally, comparisons of SVF cell and ASC therapeutic potential in models of several medical conditions have illustrated that SVF cell fractions and ASC-exclusive isolates have comparable clinical safety and efficacy (8, 10, 11). For these reasons, the therapeutic value of SVF cells was explored in this study.
Given the accelerating rate at which regenerative medicine is advancing, it is imperative to diligently investigate cellular regenerative properties that are clinically relevant to the implementation of safe and efficacious treatments. While the regenerative and therapeutic potentials of SVF cells and passaged ASCs have been well characterized, there is little information that compares the relationship of different measures of potential, in a patient-specific manner, in non-expanded SVF cells (12–14). To provide broader insight into the potential efficacy of non-expanded SVF cells in various applications, we quantitatively and semi-quantitatively measured the viability, self-renewal capacity, proliferative activity, differentiation potential, and immunomodulatory cytokine secretion of primary SVF cells isolated from thirteen, medically diverse, human donor samples. Further, we examined how medical history might influence these therapeutic parameters when establishing prognoses of SVF cell treatments. This work aimed to identify and characterize the therapeutic potential of SVF cells while also detecting trends or patterns that exist in regenerative properties, irrespective of confounding factors such as prior illness.
MATERIALS AND METHODS
Isolation of SVF from human adipose tissue
Lipoaspirate waste tissue from thirteen female human donors (ages 34–68, mean age 51.5) was obtained with consenting procedures approved by the Institutional Review Board at Rhode Island Hospital. Tissue isolates were derived from either thigh, abdominal, or axillary fat pads, with an average of 340 mL of tissue processed per donor (median: 300 mL, range 80–1200 mL). Donors represented medical histories of breast cancer (donors 1–6, at least one year in remission), lipodystrophy (donors 7–10), or macromastia (donors 11–13). SVF cells were isolated using established protocols with minor modifications (15). Lipoaspirate was washed 5–7 times in warm, sterile phosphate buffered saline (PBS) to remove blood and tumescent fluid. Resulting tissue was digested for one hour in a matching volume of 0.1% wt/vol collagenase (Worthington Biomedical Corporation), 1% vol/vol bovine serum albumin Fraction V (BSA, Invitrogen), and 2 mM calcium chloride in PBS while shaking at 37°C. Following digestion, released cells were centrifuged at 300 g to form a pellet, and the supernatant lipids and buoyant, mature adipocytes were removed. Pelleted cells were resuspended in stromal medium consisting of Dulbecco’s Modified Eagle Medium-Nutrient Mixture F-12 (DMEM-F/12, HyClone, GE Healthcare), 10% fetal bovine serum (FBS, Zen-Bio), and 1% antibiotic/antimycotic (A/A, HyClone, GE Healthcare) to neutralize residual collagenase. Following two additional washes in stromal medium, samples were incubated at room temperature in an erythrocyte lysis buffer (115 mM ammonium chloride, 10 mM potassium carbonate, and 0.1 mM ethylenediamine tetraacetic acid). Samples were centrifuged and cells were resuspended in stromal medium, then filtered sequentially through 100 μm and 70 μm strainers before counting cell numbers using a hemocytometer. Total cell yield and viability were determined through Trypan Blue live/dead staining. SVF cells were centrifuged and resuspended in 1 or 1.5 mL freezing medium consisting of 80% FBS, 10% dimethyl sulfoxide, and 10% DMEM F/12. The average number of cells frozen in each vial was 5.3×106 (median: 5.1×106, range 3.0×106 – 9.6×106). Samples were stored cryogenically until use. Cells were thawed by incubating vials retrieved from cryogenic storage in a 37°C water bath for 2–4 minutes. Following resuspension in 3–5 mL stromal medium and a 5-minute centrifugation (400 g) to remove residual DMSO, post-thaw viability of non-expanded SVF cells was recorded before specified cell numbers were plated directly into the culturing conditions described below for analysis of therapeutic parameters.
Clonogenicity
SVF cell capacity for self-renewal was measured using a colony forming unit-fibroblast (CFU-F) assay. Samples from each donor were seeded in 100 mm dishes (n = 3) at a density of 20 cells/cm2. After two weeks, plates were fixed and stained with 0.5% crystal violet in 100% methanol for 15 minutes. Samples were imaged using a Nikon D500 digital camera, and colonies containing > 50 cells were quantified by processing the images using ImageJ software (National Institutes of Health). Clonogenicity was reported as self-renewal capacity, calculated by dividing the number of colonies formed by the initial seeding density (16).
Population Doubling Time (PDT)
Proliferation rates for SVF cells from each donor were calculated by seeding 6-well plates with 2,500 cells, growing them in stromal medium for ten days, and recording cell numbers for Days 4–10 (n = 3 wells for each time point and donor). Medium was changed every 2–3 days. For counting, cells were fixed with 10% formalin, and nuclei stained with 4′,6-diamino-2-phenylindole (DAPI). Cell numbers were quantified using a Cytation cell imaging plate reader (BioTek) across six random fields of view for each well, and PDTs were calculated from the log phase of the growth curve formed by plotting cell counts for each time point (16, 17).
Multilineage Differentiation
The multipotent differentiation capacity of donor SVF cells was determined by chemical induction along osteogenic, adipogenic, and chondrogenic lineages. Assessment of differentiation potential was determined using quantitative or semi-quantitative bioassays for the production of lineage-specific metabolites.
Osteogenesis
Osteogenic differentiation potential was assessed by alkaline phosphate (ALP) activity and calcified matrix deposition. SVF cells from each donor were thawed and seeded into 96-well plates at a density of 8×103 cells/well and cultured for 4–6 days in expansion medium comprised of DMEM/F-12, 10% FBS, 1% A/A, 0.25 ng/mL transforming growth factor (TGF)-β1, 5 ng/mL epidermal growth factor, and 1 ng/mL fibroblast growth factor (R&D systems). Following expansion, confluent monolayers were cultured in either stromal medium or osteogenic medium, containing DMEM High Glucose (DMEM/HG), 10% FBS, 2.16 mg/mL β-glycerophosphate, 50 μg/mL mM ascorbate-2-phosphate, 20 nM dexamethasone, 3.85 ng/mL vitamin-D3, and 1% A/A (18). Media were changed every 2–3 days. After seven days, cells for each condition were lysed and stored at −80°C before being thawed and assessed for ALP activity (n = 4 per medium condition, per donor), which was quantified using a fluorometric assay (BioVision). After twenty-one days in either control or osteogenic media, the remaining samples were fixed with 10% formalin (n = 4 per medium condition, per donor), and calcified matrix deposition was visualized using pH-adjusted (4.1–4.3) alizarin red S stain (ARS, Sigma-Aldrich). Following imaging, ARS was eluted from wells using 10% cetylpyridinium chloride in 10 mM sodium phosphate at room temperature overnight. The eluent’s optical density (OD) was measured at 540nm using a Cytation spectrophotometer (Biotek) (19).
Adipogenesis
Adipogenic differentiation potential was assessed by intracellular lipid size, abundance, and staining. Cells were plated and expanded as described for osteogenesis. Confluent monolayers were cultured for twenty-one days in either stromal or adipogenic medium, containing DMEM/F-12, 10% FBS, 10 μM insulin, 1 μM dexamethasone, 0.25 mM isobutyl-methylxanthine, 200 μM indomethacin (Sigma-Aldrich), and 1% A/A (20, 21). Media were changed every 2–3 days. Following the induction period, adipogenic-induced and control wells were fixed with 10% formalin (Thermo Fisher Scientific). Oil red O (ORO, Sigma-Aldrich) staining was used to visualize intracellular lipid droplets in control and adipogenic cultures using a Cytation cell imaging system at 628ex/685em (n = 4 per medium condition, per donor). A custom MATLAB program was employed to quantify mean lipid areas and large lipid counts (> 2 μm diameter), as previously described (22). Following imaging, ORO stain was eluted using 100% isopropanol, and the eluent’s OD was measured at 500nm using a Cytation spectrophotometer (19).
Chondrogenesis
Chondrogenic differentiation potential was assessed by sulfated glycosaminoglycan (sGAG) production. SVF cells were plated in V-bottom 96-well plates at a density of 5×104 cells/well (n = 4 per medium condition, per donor). Plates were centrifuged at 400 g for 5 minutes to form cell pellets, and after a 24-hour equilibration period, pellets were incubated in either stromal medium or chondrogenic medium, containing DMEM/HG, 10% FBS, 2 ng/mL TGF-β1, 0.15 mM ascorbate-2-phosphate, 100 nM dexamethasone, 1% ITS premix (BD Biosciences), and 1% A/A (23). 80% of media was changed every 2–3 days. After twenty-one days, pellets were digested in 125 μg/mL papain (Sigma-Aldrich) at 65°C and pH 6.5 for 24 hours. The resultant solution was assayed for sGAGs using a dimethyl-methylene blue (DMMB) assay, as described previously (24). DMMB dye was dissolved in 1 mL of 100% ethanol and 10 mL of 0.3 M HCl containing 304 mg glycine and 237 mg sodium chloride. The dye was pH-adjusted to 1.5, and volume adjusted to 100 mL using distilled water. 200 μl of dye was added to 50 μl of digest solution, and the absorbance of the resulting mixture was measured at 525 nm using a Cytation spectrophotometer. Concentrations of sGAG were quantified using a standard curve generated using known quantities of chondroitin sulfate.
Analysis of Cytokine Secretion in Response to Inflammation
The biochemical response of SVF cells to an inflammatory stimulus was assessed by chemically stimulating donor-specific samples with pro-inflammatory molecules and then analyzing the supernatant for secreted pro- and anti-inflammatory cytokines. This analysis aimed to elucidate the donor-specific responses to an inflamed, injury-induced microenvironment and was performed using a fluorescent, multiplex enzyme-linked immunosorbent assay (ELISA) (RayBiotech, Norcross, GA). Culturing conditions were adapted from previously established protocols to meet suggested specifications provided by the multiplex ELISA manufacturer (25, 26). Confluent SVF cells in 24-well plates were cultured in serum-free base media containing Minimum Essential Medium (MEM, Gibco), 10% ITS premix solution, and 1% A/A with the addition of 3 ng/mL tumor-necrosis factor (TNF)-α and 10 ng/mL interferon (IFN)-γ for 24 hours. Control samples were cultured in the same serum-free base media without any addition of cytokines. Following treatment, control and immune-stimulated samples were washed twice with warm, sterile PBS and cultured for an additional 24 hours in base medium, which was collected for analysis. Multiplex ELISAs were performed following manufacturer’s instructions, and cytokine secretion levels were quantified using a Genepix 4000B scanner using average sample fluorescence at 532 nm measured against a standard curve (n = 4). Cytokines assessed included interleukin (IL)-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-13, monocyte chemoattractant protein (MCP)-1, IFN-γ, and TNF-α.
Statistical Analysis
Correlations of parameters across the entire donor population were calculated from raw data using a nonparametric Spearman rank analysis (27). Differentiation potential and inflammation-induced cytokine secretion of donor-matched pairs of experimental and control conditions were assessed using a two-tailed Student’s t-test. Diagnosis-dependent differences in donor clonogenicity were also assessed using a two-tailed Student’s t test. Assessment of non-normal, diagnosis-dependent variation in the differentiation potential and immunomodulatory cytokine secretion of donor SVF cells was performed using a nonparametric ANOVA on ranks followed by Dunn’s correction for multiple comparisons. All analyses were performed using SigmaPlot 12.5 software. For all tests, p-values < 0.05 indicated statistical significance.
RESULTS
SVF cell yields, viabilities, proliferation rates, and clonogenicity
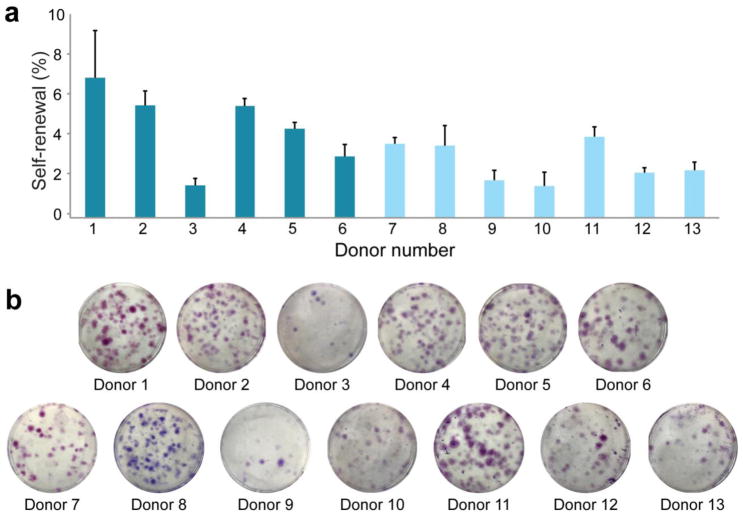
Cell yield varied across donors but in general was observed to be 1–6×105 cells/mL lipoaspirate. Average post-thaw cell viability was 79% (median: 78%, range: 70–90%). Average PDT was 32 hrs (median: 27 hrs, range: 15–48 hrs). One donor (Donor 10) had a significantly higher PDT of 84 hours, which was treated as an outlier and excluded from the average calculation. No correlative relationships or patterns were identified between these measures and the measurements of other parameters mentioned below. The average self-renewal capacity of donor SVF cells was 3.6% (median: 3.6%, range: 1.6–7.0%, Fig. 1).
Figure 1. Self-renewal capacity of SVF cells by donor.
(A) The self-renewal capacities of donor SVF cells were assessed using a CFU-F assay. (B) Individual colonies were stained with crystal violet and counted to determine clonogenicity. Data shown as mean ± standard deviation, with representative images for each donor. (Donors 1–6: breast cancer remission, Donors 7–13: no cancer diagnosis)
SVF cell differentiation potential
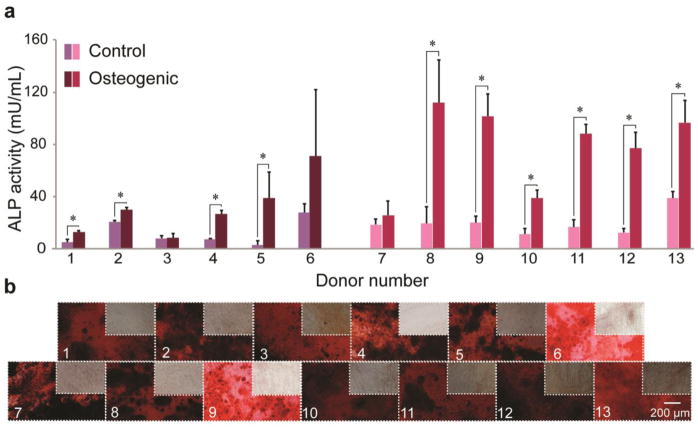
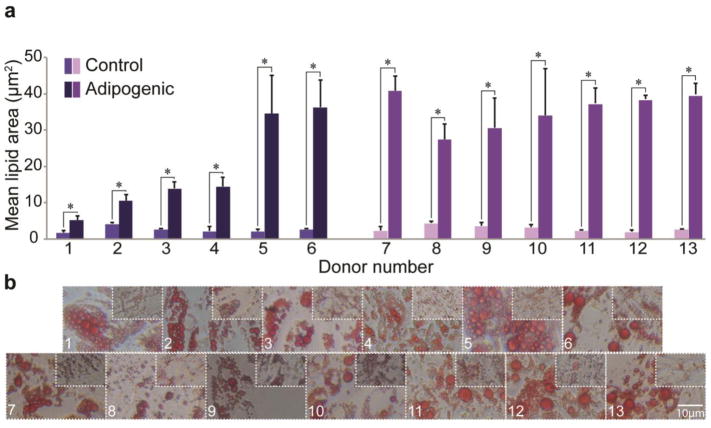
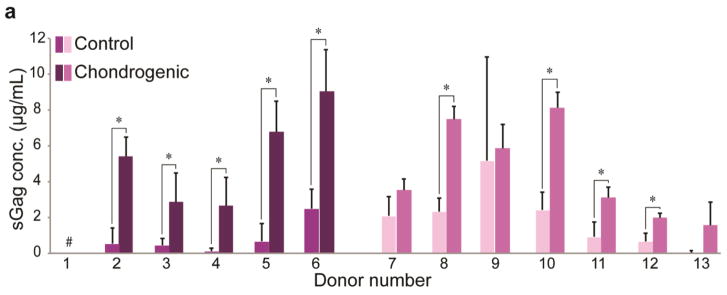
Multilineage differentiation potential of SVF cells was determined for each donor by quantifying lineage-specific metabolite production. Successful differentiation along osteogenic, adipogenic, and chondrogenic lineages was defined as having significantly higher metabolite production than matched stromal medium controls (Supplemental Table 1). For osteogenesis, 10/13 donors showed positive differentiation potential when assessing ALP activity, an early stage marker for this lineage (range: 1.4–13-fold higher than control, p < 0.05). Average values across all donors were 56 ± 14 mU/mL and 16 ± 4 mU/mL for osteogenic and control groups, respectively. However, 13/13 donors showed positive differentiation potential when assessing calcified matrix deposition via ARS eluent OD, a late stage marker for this lineage (range: 11–130-fold higher than control, Fig. 2). Average values across all donors were 9.3 ± 1.2 and 0.22 ± 0.03 for osteogenic and control groups, respectively. For adipogenesis, 13/13 donors exhibited robust differentiation compared to matched controls when assessing intracellular lipid size (range: 2.5–19-fold higher than control, p < 0.05). Values of mean lipid area in adipogenic-induced samples compared to spontaneously produced lipids in control samples were 28 ± 4.8 μm2 vs. 2.8 ± 0.6 μm2, respectively (Fig. 3). Using ORO OD as a measure of adipogenesis, only 8/13 donors exhibited statistically greater adipogenesis following induction. These increases ranged from 0.6–1.7-fold higher than controls. Interestingly, of the five donors that did not differentiate (Donors 2, 3, 8, 9, 10), four exhibited statistically higher ORO OD in control samples (Donors 2, 3, 8, 10). Average ORO ODs across all donors were 0.26 ± 0.02 and 0.19 ± 0.01 for adipogenic-induced and control conditions, respectively. For chondrogenesis, 9/13 donors showed positive differentiation potential when assessing sGAG production (range: 1.7–27-fold higher than control, p < 0.05). Mean sGAG concentrations across all donors were 4.51 ± 1.08 μg/mL and 1.39 ± 1.00 μg/mL for chondrogenic-induced and control samples, respectively (Fig. 4). The results from the four donors (Donors 1, 7, 9, 13) that did not produce significantly more sGAG when compared to their matched controls, possibly due to high variability among biological replicates for each donor. Donor 1 exhibited values of “0” for both control and chondrogenic-induced conditions, likely due to inadvertent loss of the cell pellet, and the donor was therefore removed from the chondrogenic differentiation analysis.
Figure 2. Osteogenic potential of SVF cells by donor.
(A) ALP activity for control and osteogenic-induced SVF cells for all donors. (B) Representative images of ARS-stained calcified matrix for osteogenic-induced and control samples. Data shown as mean ± standard deviation, with representative images for each donor. *represents p < 0.05. (Donors 1–6: breast cancer remission, Donors 7–13: no cancer diagnosis)
Figure 3. Adipogenic potential of SVF cells by donor.
(A) Mean lipid area for control and adipogenic-induced SVF cell samples for all donors. (B) Representative images of ORO-stained intracellular lipids for adipogenic-induced and control samples. Data shown as mean ± standard deviation, with representative images for each donor. *represents p<0.05. (Donors 1–6: breast cancer remission, Donors 7–13: no cancer diagnosis)
Figure 4. Chondrogenic potential of SVF cells by donor.
(A) sGAG production for control and chondrogenic-induced SVF cell samples for all donors. Data shown as mean ± standard deviation. # indicates no sample existed, due either to miniscule matrix production or inadvertent loss of pellet during processing. *represents p < 0.05. (Donors 1–6: breast cancer remission, Donors 7–13: no cancer diagnosis)
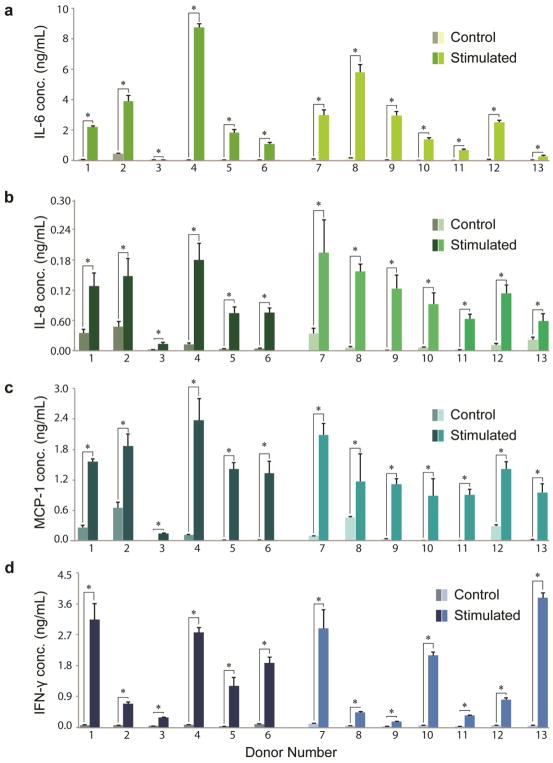
SVF cell immunomodulatory cytokine secretion
The supernatant medium of SVF cells exposed to pro-inflammatory stimuli was assessed using a multiplex ELISA array for the presence of cytokines that could potentially modulate an immune response in vivo. While most measures in unstimulated control conditions were zero or near-zero values, IL-6, IL-8, MCP-1 and IFN-γ expression was consistently robust and significantly up-regulated for all donors following stimulation (p < 0.05, Supplemental Table 2). Mean IL-6 expression was 2.64 ± 0.19 ng/mL and 0.070 ± 0.005 ng/mL for stimulated and control samples, respectively (p < 0.05, Fig. 5a). Mean IL-8 values were 0.109 ± 0.022 ng/mL and 0.014 ± 0.003 ng/mL for stimulated and control samples, respectively (p < 0.05, Fig. 5b). MCP-1 expression yielded mean values of 1.33 ± 0.21 ng/mL and 0.15 ± 0.02 ng/mL for stimulated and control samples, respectively (p < 0.05, Fig. 5c). IFN-γ values averaged 1.60 ± 0.16 ng/mL and 0.053 ± 0.005 ng/mL for stimulated and control samples, respectively (p < 0.05, Fig. 5d). The remaining six cytokines tested in the multiplex ELISA appeared in only trace amounts across all thirteen donors in control/induced conditions.
Figure 5. Immunomodulatory cytokine secretion of SVF cells by donor.
Cytokine secretion concentrations of (A) IL-6, (B) IL-8, (C) MCP-1, and (D) IFN-γ for control and pro-inflammatory cytokine-stimulated SVF cells. Data shown as mean ± standard deviation. *represents p < 0.05. (Donors 1–6: breast cancer remission, Donors 7–13: no cancer diagnosis)
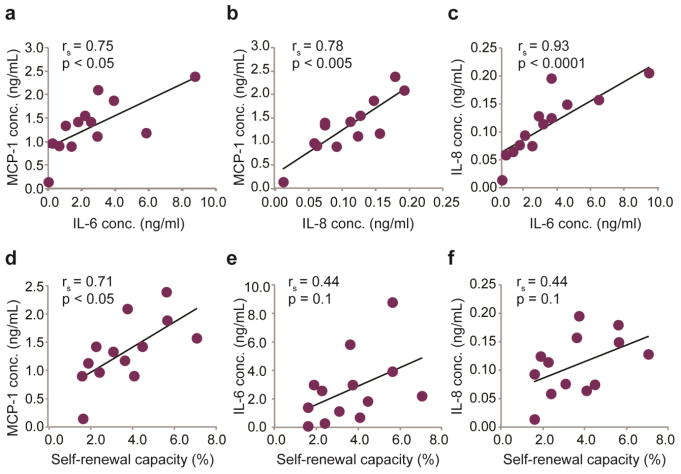
Correlations of SVF cell regenerative characteristics
To determine whether statistically significant correlations existed among the properties reported above, regression analyses were conducted using the thirteen donor samples as independent data points. Significant relationships were observed between sets of secreted immunomodulatory cytokine levels. There was a strong, statistically significant positive correlation observed between the pro-inflammatory cytokines IL-6 and MCP-1 (Fig. 6a, rs = 0.75, p < 0.05), IL-8 and MCP-1 (Fig. 6b rs = 0.78, p < 0.005), and IL-6 and IL-8 (Fig. 6c, rs = 0.93, p < 0.0001). Additionally, positive correlations were observed between donor self-renewal capacity and cytokine expression. The relationship between self-renewal and MCP-1 expression was statistically significant (Fig. 6d, rs = 0.71, p < 0.05), whereas self-renewal vs. IL-6 expression (Fig. 6e, rs = 0.44, p = 0.1) and IL-8 expression (Fig. 6f, rs = 0.44, p = 0.1) showed similar trends but failed to reach statistical significance. Correlations between lineage-specific differentiation potentials showed modest positive relationships, although none were statistically significant. The strongest of these was found between measures of osteogenesis and adipogenesis (rs = 0.36, p = 0.2), followed by osteogenesis and chondrogenesis (rs = 0.32, p = 0.3). No relationship was observed between adipogenesis and chondrogenesis (rs = −0.21, p = 0.5). A minor negative trend was observed between donor self-renewal capacity and osteogenic and adipogenic differentiation potentials (rs = 0.27 – 0.32, p = 0.3 – 0.4). Interestingly, sGAG production exhibited a strong negative correlation with post-thaw SVF cell viability (rs = −0.76, p < 0.005). Relatively little effect was observed due to donor age in relation to other measured characteristics, with IL-8 having the strongest correlation (rs = −0.51, p = 0.07). No significant relationship was identified between donor cell yields, viabilities, population doubling times, and any of the other measured parameters. No patterns, trends, or correlations were determined to be dependent on the anatomical source of lipoaspirate, although these groupings had limited sample sizes.
Figure 6. Correlation analyses of SVF cell therapeutic potential.
Nonparametric Spearman rank analyses were performed to identify potential relationships among SVF cell inflammatory cytokine secretion and regenerative properties. Statistically significant positive relationships were found between secreted immunomodulatory factor production for (A) IL-6 and MCP-1, (B) IL-8 and MCP-1, and (C) IL-6 and IL-8. The positive relationship between (D) self-renewal capacity and MCP-1 expression across donors was also determined to be statistically significant. A similar trend was observed for self-renewal compared to (E) IL-6 and (F) IL-8 expression, although statistical significance was not achieved.
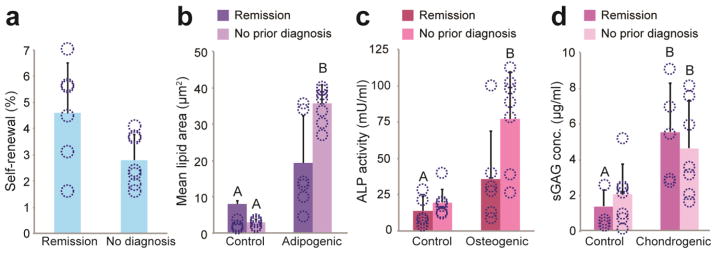
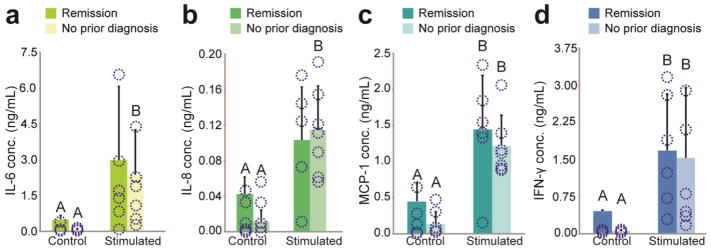
Medical history influence on SVF cell regenerative potential
Non-identifying medical history associated with SVF samples indicated that 6/13 donors had a previous diagnosis of breast cancer. Because breast reconstruction following mastectomy is a common clinical application of fat grafting with autologous SVF supplementation, we performed analyses to compare the regenerative characteristics of donor samples with a history of breast cancer to samples with alternative, non-cancer diagnoses. Results showed a strong trend towards increased clonogenic potential in breast cancer donors (p = 0.06, Fig. 7a). Additionally, differentiation potential for the osteogenic and adipogenic lineages in these samples were diminished, although not with statistical significance (p > 0.05, Fig. 7b and 7c). No such effects were observed for chondrogenesis (Fig. 7d). Prior history of breast cancer did not appear to have an effect on the cytokine secretion of donor SVF cells stimulated by pro-inflammatory conditions (Fig. 8, p > 0.05), However, breast cancer remission donors under control conditions had a consistent trend of higher baseline expressions of IL-6, IL-8, MCP-1, and IFN-γ, though it did not reach statistical significance (p > 0.05).
Figure 7. Medical history and regenerative potential.
(A) SVF cell self-renewal capacity for breast cancer remission donors was higher when compared to donors without a prior breast cancer diagnosis, nearing statistical significance. An opposing trend was observed for differentiation potential, suggesting breast cancer remission donors might have less robust (B) adipogenic and (C) osteogenic potential based on production of corresponding lineage-specific metabolites. (D) No differences were observed in chondrogenic potential. Data shown as mean ± standard deviation; * and unlike letters indicate statistical significance (p < 0.05). Overlaid circles indicate individual donor data points.
Figure 8. Medical history and immunomodulatory cytokine secretion.
Secretion of pro-inflammatory factors (A) IL-6, (B) IL-8, (C) MCP-1, and (D) IFN-γ were compared between breast cancer remission donors and donors without a history of breast cancer. Although no statistically significant differences were observed in cytokine secretion upon exposure to pro-inflammatory conditions, a consistent trend towards higher cytokine levels in remission samples was observed for control conditions. Data shown as mean ± standard deviation; unlike letters indicate statistical significance (p < 0.05). Overlaid circles indicate individual donor data points.
DISCUSSION
The results of this study indicate that SVF cells from individual donors vary dramatically in their regenerative characteristics and secretion profiles in response to an inflammatory stimulus and are therefore likely to have distinctly different therapeutic performance. Multilineage differentiation assays indicated that chemical induction of donor SVF cells along a given lineage yields variable responses, with some exhibiting high lineage-specific metabolite production and others showing limited ability. When investigated in more depth, significant relationships were observed among subsets of measured parameters. For example, production of three inflammatory cytokines positively correlated to one another. One possible explanation for donor-to-donor variability is diversity in medical histories. In the current work, a subset of samples was derived from patients with a past diagnosis of breast cancer. Interpreting findings within this context suggested that persistent changes associated with the disease could potentially alter stem cell clonogenicity and inflammatory cytokine expression, while having minimal effect on differentiation.
The pronounced effect of donor variability on ASC differentiation potential has been established since the cells were first identified and characterized in 2001 (28). Even with standardized techniques, the prognosis and efficacy of treatment can be vastly different from one patient to another. Attempts to characterize which qualities influence donor variability have shown that age and sex are two important factors affecting SVF cell therapeutic performance (12–14, 29). Across the thirteen female donor samples tested, age did not influence any of our measures of therapeutic potential. This discrepancy with previously published literature may be due to the relatively small, uniformly distributed range of ages across our donor samples (34–68), which prevented comparisons of performance between distinctly “young” and “old” subgroups. Our results also showed no differences due to the anatomical location of isolated SVF cells, although this factor has been previously noted to influence therapeutic potential (30). It is possible that additional confounding differences between our thirteen donors masked any tissue location-specific influences.
There were diverse responses to chemical induction for lineage-specific differentiation across the thirteen SVF cell samples. While there were slight, statistically non-significant positive trends between donor osteogenic differentiation potential and the two other lineages assessed, our results indicated that robust differentiation along one lineage is not a reliable predictor of the potential for differentiation along other lineages. This opposes a widely accepted paradigm that such relationships exist, e.g., an inverse relationship between osteogenic and adipogenic differentiation potentials (31, 32). It is possible that the trends we observed are due to the overall heterogeneity of SVF cells. Many studies that demonstrate the inverse quality of osteogenic and adipogenic potentials use stem cell populations enriched using expansion/passaging. Cellular expansion yields effectively clonal groups of highly proliferative cells that may exhibit the established seesaw effect of multipotent differentiation potential. However, this relationship has been shown to be absent in non-expanded or early passage stromal vascular cell populations (33–35).
ASCs derived from adipose tissue have been shown to act as both immune-enhancing and immunosuppressive mediators of inflammation, with most therapeutic applications focused primarily on the latter (36). In the current study, SVF cells for thirteen donors differentially expressed four pro-inflammatory cytokines following stimulation with TNF-α and IFN-γ. Surprisingly, no anti-inflammatory cytokines were expressed at significant levels. Non-expanded SVF cells may be predisposed to pro-inflammatory activity more so than extensively expanded, enriched ASC populations. Studies have shown that early passage ASCs express markers such as major histocompatibility complex (MHC) II, as well as CD45, CD80, and CD86, all of which are associated with the stimulation of antigen-presenting cells (36). Moreover, in mixed lymphocyte reaction cultures, non-expanded/P0 ASCs have been shown to promote the proliferation of allogeneic responder T cells, but this proliferative activity is attenuated in T cell co-cultures with ASCs beyond P1 (36). Although the exclusive up-regulation of pro-inflammatory cytokines was unexpected, the substantial variability in expression level across donors emphasized how therapeutic response could be difficult to predict, with certain donors expressing cytokines in concentrations orders of magnitude higher than others. Despite the variation, strong positive correlations among expression of IL-6, IL-8, and MCP-1 across donors are consistent with previously published studies (37–39), and they suggest that the mechanism through which SVF cells elicit a response may be consistent from donor to donor, even if the degree of the response is not.
The up-regulated cytokines in this study are implicated in many processes that promote wound healing. IL-6, IL-8, and MCP-1 are traditionally seen as pro-inflammatory markers because they stimulate neutrophil, monocyte, and macrophage infiltration at injury sites, but there is ample evidence to suggest these cytokines have anti-inflammatory capabilities as well. MCP-1, for example, can promote cardiac repair and angiogenesis following ischemic injury (40–43). IL-6 plays a role in angiogenesis and epidermal cell proliferation and has been shown to have pleiotropic qualities that can suppress inflammatory immune responses (44–48). Furthermore, localized ASC secretion of IL-6 potently modulates of the differentiation of dendritic cells (DC), limiting their pro-inflammatory ability and instead enhancing their secretion of IL-10 (36, 49–52). In mouse models of cutaneous wound healing, ASC conditioned medium containing high concentrations of IL-6 and IL-8 also promoted wound closure and healing, and inhibition of IL-6 and IL-8 activity attenuated this effect (53). Taken together, these findings indicate that ASCs found within SVF could exert therapeutic effects by depleting the number of activated antigen-presenting cells through secretion of traditionally pro-inflammatory factors, suppressing immune responses through secondary paracrine interactions with effector cells. Future work should examine how donor expression of IL-6, IL-8, MCP-1, and IFN-γ correlates to donor efficiency at depleting the functional activity of immune effector cells in an in vivo environment.
Patient medical history, specifically a previous diagnosis of breast cancer, was found to be a possible influence on the functional capacity and regenerative potential of SVF cells. The observed effects may be due to biological and/or physiological changes caused by the disease itself, or they may be residual, long-term responses to chemotherapeutic treatment(s) (19, 54, 55). Because samples were collected from donors who had been in remission for at least one year, any observed trends or relationships should not be due to existing disease or treatment effects, but rather, the result of lasting influences. The enhanced self-renewal capacity of SVF cells observed for these donor samples could be due a lingering systemic regenerative response to surgical and chemotherapeutic cancer treatments. However, our results also suggest that there may be a decrease in differentiation potential for both osteogenic and adipogenic lineages in cells derived from breast cancer remission patients, although this trend was variable and not statistically significant. Our previous work and others have indicated that ASCs can resist short-term changes in differentiation potential due to chemotherapeutic treatment (12, 19, 56); however, the current results suggest that long-term differentiation potential of these cells may not be similarly protected. Interestingly, results also showed elevated baseline levels of cytokine secretion in breast cancer remission samples in the absences of an inflammatory stimulus. This is to be expected given the well-established links between chronic, mild inflammation and the development and recurrence of cancer (57). However, it remains unclear whether the increase in cytokine production is causal or in reaction to breast cancer and its treatment. Many pathways implicated in mesenchymal stem cell immunomodulation also play important roles in the invasive and metastatic qualities of tumors. Increased PGE2 expression, for example, has been associated with increased metastatic capacity, along with increased expression of cytokines and growth factors including IL-6 and IL-8 (58). Since tumors and ASCs utilize similar molecular pathways for both metastasis and immunomodulation, care should be taken in assigning only a single explanation for cytokine secretion. A logical next step is to use an in vivo model to determine how the different measured parameters in this study influence long-term graft survival. The in vivo microenvironment provides a range of biologically relevant factors that might better predict therapeutic outcomes, including intercellular interactions, biomolecules, and mechanical cues from surrounding tissues and extracellular matrix.
The assaying of a broad set of therapeutically relevant stem cell characteristics across a modestly sized group of human donors yielded findings regarding the potential for SVF cells to exhibit therapeutic effects, but further categorization could be investigated based on medical history, donor age, harvest location, or any of many other considerations. The substantial variability observed in the therapeutic parameters of SVF cells highlights the current challenges facing scientists conducting experiments with these cells and clinicians using them in diverse patient populations. Additional work is necessary to determine the most influential, donor-specific characteristics that can accurately predict outcomes. Larger sample sizes strengthen statistical conclusions and encompass population variability more realistically; however, the specifics of each donor will still play a dominant role in how the associated stem cells behave. It should also be noted that several past studies have observed that long-term graft survival of lipofilling procedures using SVF was inferior to more homogenous populations of expanded adipose-derived stem/stromal cells (59–61). While this is relevant on a biological and physiological level, long-term, multi-passage culturing would be necessary to yield sufficient cell numbers for enhanced therapies. This consumption of time and resources may not be sensible for widespread clinical application, especially compared to the practical simplicity that point-of-care SVF cell treatment provides. This expansion also eliminates the potential synergistic regenerative effects that might be observed when using heterogeneous, autologous cell populations that include progenitor cells (8, 9).
The current work identified substantial, donor-specific variation in the regenerative potential and immunomodulatory secretion profiles of ASCs found in human SVF by evaluating the robustness of several contributing parameters to therapeutic qualities. Findings also suggest a possible enduring influence of breast cancer for therapeutic applications using autologous stem cells from fat tissue. By identifying and examining these relationships, this study can inform the prognoses of future clinical work featuring stromal vascular fraction cell therapy.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01 AR063642) and the National Science Foundation (EAGER CBET 1547819). The authors would like to thank Lisa White, Pa-C for aiding in the collection of donor samples and medical history data. Additionally, Christoph Schorl, PhD, of the Brown University genomics facility, provided assistance with microarray imaging and analysis software. Nicholas Labriola, PhD, produced the custom MATLAB program used to measure lineage-specific metabolite production. Vikram Mookerjee aided in proofreading and editing the manuscript.
Footnotes
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
AMP conducted experiments and analyses. DMC, PL, and EMD designed study. PL provided tissue samples. AMP, DMC, PL, and EMD contributed to writing and editing the manuscript.
References
- 1.Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis and rheumatism. 2002;46(3):704–13. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 2.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58(7):1550–7. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roldan M, Macias-Gonzalez M, Garcia R, Tinahones FJ, Martin M. Obesity short-circuits stemness gene network in human adipose multipotent stem cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25(12):4111–26. doi: 10.1096/fj.10-171439. [DOI] [PubMed] [Google Scholar]
- 4.van Tienen FH, van der Kallen CJ, Lindsey PJ, Wanders RJ, van Greevenbroek MM, Smeets HJ. Preadipocytes of type 2 diabetes subjects display an intrinsic gene expression profile of decreased differentiation capacity. International journal of obesity (2005) 2011;35(9):1154–64. doi: 10.1038/ijo.2010.275. [DOI] [PubMed] [Google Scholar]
- 5.Gimble JM, Bunnell BA, Guilak F. Human adipose-derived cells: an update on the transition to clinical translation. Regen Med. 2012;7(2):225–35. doi: 10.2217/rme.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu VM, Stransky CA, Bucky LP, Percec I. Fat grafting’s past, present, and future: why adipose tissue is emerging as a critical link to the advancement of regenerative medicine. Aesthetic surgery journal. 2012;32(7):892–9. doi: 10.1177/1090820X12455658. [DOI] [PubMed] [Google Scholar]
- 7.Stacey GN. The Challenge of Standardization in Stem Cell Research and Development. In: Ilic D, editor. Stem Cell Banking. New York, NY: Springer New York; 2014. pp. 11–8. [Google Scholar]
- 8.Nguyen A, Guo J, Banyard DA, Fadavi D, Toranto JD, Wirth GA, et al. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J Plast Reconstr Aesthet Surg. 2016;69(2):170–9. doi: 10.1016/j.bjps.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Kanthilal M. Characterization of mechanical and regenerative properties of human, adipose stromal cells. 2014;7(4):585–97. doi: 10.1007/s12195-014-0350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riordan NH, Ichim TE, Min WP, Wang H, Solano F, Lara F, et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med. 2009;7:29. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez JP, Murphy MP, Hong S, Madrigal M, March KL, Minev B, et al. Autologous stromal vascular fraction therapy for rheumatoid arthritis: rationale and clinical safety. International archives of medicine. 2012;5:5. doi: 10.1186/1755-7682-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varghese J, Griffin M, Mosahebi A, Butler P. Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem cell research & therapy. 2017;8(1):45. doi: 10.1186/s13287-017-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. doi: 10.1186/1479-5876-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aksu AE, Rubin JP, Dudas JR, Marra KG. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Annals of plastic surgery. 2008;60(3):306–22. doi: 10.1097/SAP.0b013e3180621ff0. [DOI] [PubMed] [Google Scholar]
- 15.Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5(7):1294–311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE. 2014;9(12):e115963. doi: 10.1371/journal.pone.0115963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng LW, Yip SK, Wong HK, Yam GH, Liu YM, Lui WT, et al. Adipose-derived stem cells from pregnant women show higher proliferation rate unrelated to estrogen. Hum Reprod. 2009;24(5):1164–70. doi: 10.1093/humrep/dep001. [DOI] [PubMed] [Google Scholar]
- 18.Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206(1):229–37. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 19.Beane OS, Fonseca VC, Darling EM. Adipose-derived stem cells retain their regenerative potential after methotrexate treatment. Exp Cell Res. 2014;327(2):222–33. doi: 10.1016/j.yexcr.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estes BT, Diekman BO, Guilak F. Monolayer cell expansion conditions affect the chondrogenic potential of adipose-derived stem cells. Biotechnol Bioeng. 2008;99(4):986–95. doi: 10.1002/bit.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng B, Cao B, Li G, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue engineering. 2006;12(7):1891–901. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- 22.Labriola NR, Darling EM. Temporal heterogeneity in single-cell gene expression and mechanical properties during adipogenic differentiation. J Biomech. 2015;48(6):1058–66. doi: 10.1016/j.jbiomech.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Cruz RD, Fonseca VC, Darling EM. Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. Proc Natl Acad Sci U S A. 2012;109(24):E1523–9. doi: 10.1073/pnas.1120349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marble HD, Sutermaster BA, Kanthilal M, Fonseca VC, Darling EM. Gene expression-based enrichment of live cells from adipose tissue produces subpopulations with improved osteogenic potential. Stem cell research & therapy. 2014;5(5):145. doi: 10.1186/scrt502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhukareva V, Obrocka M, Houle JD, Fischer I, Neuhuber B. Secretion profile of human bone marrow stromal cells: donor variability and response to inflammatory stimuli. Cytokine. 2010;50(3):317–21. doi: 10.1016/j.cyto.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187–95. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 27.Bigoni M, Sacerdote P, Turati M, Franchi S, Gandolla M, Gaddi D, et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J Orthop Res. 2013;31(2):315–21. doi: 10.1002/jor.22208. [DOI] [PubMed] [Google Scholar]
- 28.Sen A, Lea-Currie YR, Sujkowska D, Franklin DM, Wilkison WO, Halvorsen YD, et al. Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. Journal of cellular biochemistry. 2001;81(2):312–9. doi: 10.1002/1097-4644(20010501)81:2<312::aid-jcb1046>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Faustini M, Bucco M, Chlapanidas T, Lucconi G, Marazzi M, Tosca MC, et al. Nonexpanded mesenchymal stem cells for regenerative medicine: yield in stromal vascular fraction from adipose tissues. Tissue Eng Part C Methods. 2010;16(6):1515–21. doi: 10.1089/ten.TEC.2010.0214. [DOI] [PubMed] [Google Scholar]
- 30.Russo V, Yu C, Belliveau P, Hamilton A, Flynn LE. Comparison of Human Adipose-Derived Stem Cells Isolated from Subcutaneous, Omental, and Intrathoracic Adipose Tissue Depots for Regenerative Applications. Stem Cells Translational Medicine. 2014;3(2):206–17. doi: 10.5966/sctm.2013-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102(Pt 2):341–51. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 32.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol. 2004;4(3):290–4. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Zhu M, Kohan E, Bradley J, Hedrick M, Benhaim P, Zuk P. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J Tissue Eng Regen Med. 2009;3(4):290–301. doi: 10.1002/term.165. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez J, Pratta AS, Abbassi N, Fabre H, Rodriguez F, Debard C, et al. Evaluation of Three Devices for the Isolation of the Stromal Vascular Fraction from Adipose Tissue and for ASC Culture: A Comparative Study. Stem Cells Int. 2017;2017:9289213. doi: 10.1155/2017/9289213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prunet-Marcassus B, Cousin B, Caton D, Andre M, Penicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp Cell Res. 2006;312(6):727–36. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Leto Barone AA, Khalifian S, Lee WPA, Brandacher G. Immunomodulatory Effects of Adipose-Derived Stem Cells: Fact or Fiction? BioMed Research International. 2013;2013 doi: 10.1155/2013/383685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weitkamp JH, Reinsberg J, Bartmann P. Interleukin-8 (IL-8) preferable to IL-6 as a marker for clinical infection. Clin Diagn Lab Immunol. 2002;9(6):1401. doi: 10.1128/CDLI.9.6.1401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Georganas C, Liu H, Perlman H, Hoffmann A, Thimmapaya B, Pope RM. Regulation of IL-6 and IL-8 expression in rheumatoid arthritis synovial fibroblasts: the dominant role for NF-kappa B but not C/EBP beta or c-Jun. J Immunol. 2000;165(12):7199–206. doi: 10.4049/jimmunol.165.12.7199. [DOI] [PubMed] [Google Scholar]
- 39.Bertazzolo N, Punzi L, Stefani MP, Cesaro G, Pianon M, Finco B, et al. Interrelationships between interleukin (IL)-1, IL-6 and IL-8 in synovial fluid of various arthropathies. Agents Actions. 1994;41(1–2):90–2. doi: 10.1007/BF01986402. [DOI] [PubMed] [Google Scholar]
- 40.Gerhardt CC, Romero IA, Cancello R, Camoin L, Strosberg AD. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol. 2001;175(1–2):81–92. doi: 10.1016/s0303-7207(01)00394-x. [DOI] [PubMed] [Google Scholar]
- 41.Dietze-Schroeder D, Sell H, Uhlig M, Koenen M, Eckel J. Autocrine action of adiponectin on human fat cells prevents the release of insulin resistance-inducing factors. Diabetes. 2005;54(7):2003–11. doi: 10.2337/diabetes.54.7.2003. [DOI] [PubMed] [Google Scholar]
- 42.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niu J, Kolattukudy PE. Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci (Lond) 2009;117(3):95–109. doi: 10.1042/CS20080581. [DOI] [PubMed] [Google Scholar]
- 44.Behm B, Babilas P, Landthaler M, Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol. 2012;26(7):812–20. doi: 10.1111/j.1468-3083.2011.04415.x. [DOI] [PubMed] [Google Scholar]
- 45.Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996;4(4):411–20. doi: 10.1046/j.1524-475X.1996.40404.x. [DOI] [PubMed] [Google Scholar]
- 46.McFarland-Mancini MM, Funk HM, Paluch AM, Zhou M, Giridhar PV, Mercer CA, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184(12):7219–28. doi: 10.4049/jimmunol.0901929. [DOI] [PubMed] [Google Scholar]
- 47.Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73(6):713–21. doi: 10.1189/jlb.0802397. [DOI] [PubMed] [Google Scholar]
- 48.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 49.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem cells (Dayton, Ohio) 2007;25(8):2025–32. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 50.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem cells and development. 2012;21(14):2724–52. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 51.Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunology letters. 2009;126(1–2):37–42. doi: 10.1016/j.imlet.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Yanez R, Oviedo A, Aldea M, Bueren JA, Lamana ML. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010;316(19):3109–23. doi: 10.1016/j.yexcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Heo SC, Jeon ES, Lee IH, Kim HS, Kim MB, Kim JH. Tumor necrosis factor-alpha-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol. 2011;131(7):1559–67. doi: 10.1038/jid.2011.64. [DOI] [PubMed] [Google Scholar]
- 54.Beane OS, Darling LE, Fonseca VC, Darling EM. Disparate Response to Methotrexate in Stem Versus Non-Stem Cells. Stem Cell Rev. 2016;12(3):340–51. doi: 10.1007/s12015-016-9645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.(EBCTCG) EBCTCG. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet (London, England) 2012;379(9814):432–44. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pike S, Zhang P, Wei Z, Wu N, Klinger A, Chang S, et al. In vitro effects of tamoxifen on adipose-derived stem cells. Wound Repair Regen. 2015;23(5):728–36. doi: 10.1111/wrr.12322. [DOI] [PubMed] [Google Scholar]
- 57.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121(10):3804–9. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 59.Kolle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382(9898):1113–20. doi: 10.1016/S0140-6736(13)61410-5. [DOI] [PubMed] [Google Scholar]
- 60.Domergue S, Bony C, Maumus M, Toupet K, Frouin E, Rigau V, et al. Comparison between Stromal Vascular Fraction and Adipose Mesenchymal Stem Cells in Remodeling Hypertrophic Scars. PLoS One. 2016;11(5):e0156161. doi: 10.1371/journal.pone.0156161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Olmo D, Herreros D, Pascual M, Pascual I, De-La-Quintana P, Trebol J, et al. Treatment of enterocutaneous fistula in Crohn’s Disease with adipose-derived stem cells: a comparison of protocols with and without cell expansion. Int J Colorectal Dis. 2009;24(1):27–30. doi: 10.1007/s00384-008-0559-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.