Abstract
Polycomb repressive complex 2 (PRC2) is a chief epigenetic regulator. Chen et al. describe the crystal structure of the heterotetrameric PRC2 holo complex that provides new mechanistic insights into subunits organization and PRC2 association with chromatin.
PRC2 orchestrates a broad range of epigenetic-driven programs, ranging from gene silencing and DNA damage response to cellular differentiation and development. This major lysine methyltransferase complex modifies chromatin by producing H3K27me3, an epigenetic mark coupled to the initiation, maintenance, and spreading of transcriptional repression [1]. The catalytic subunit of the complex, EZH2, and the scaffold subunits SUZ12 and EED form a minimal assembly that retains methyltransferase activity and along with the fourth subunit RBBP7/4 comprise the PRC2 core (Fig. 1A). In addition, several non-core accessory proteins, including JARID2, AEBP2 and PHF19, associate with PRC2 and modulate its chromatin localization and catalytic activity. Owing to its vital roles in normal biological processes and in disease, atomic-resolution level architecture of PRC2 has been of immense interest for many years, however this information was challenging to obtain due to high complexity and large size of the complex.
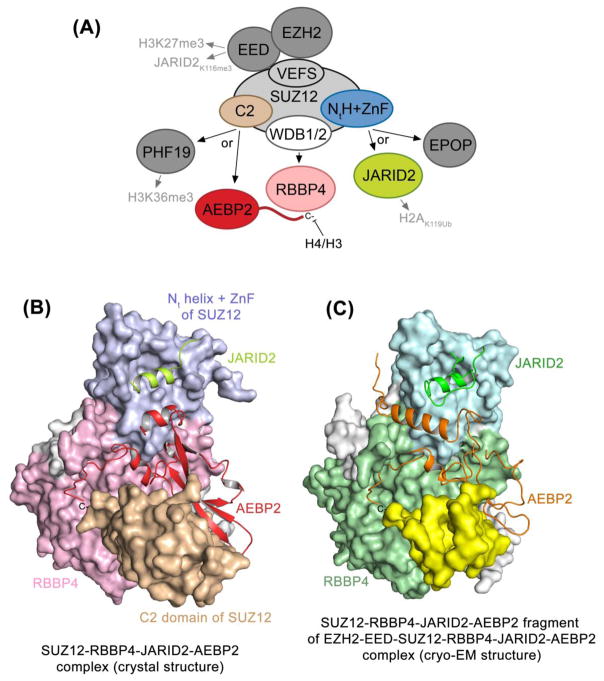
Figure 1. PRC2 architecture and subunits interactions.
(A) A schematic of the PRC2 complex with the interacting regions and/or subunits described by Chen et al. colored. (B) The crystal structure of the SUZ12-RBBP4-AEBP2-JARID2 complex determined by Chen et al. (PDB ID 5WAI) [4]. (C) The SUZ12-RBBP4-AEBP2-JARID2 fragment of the cryo-EM structure of the EZH2, EED, SUZ12, RBBP4, AEBP2 and JARID2 complex obtained by Kasinath et al. is shown (PDB ID 6C23) [9].
An exciting breakthrough in our understanding of the PRC2 catalytic mechanism and allosteric regulation came from the crystal structures of core subunits of fungal and human PRC2 complexes [2, 3]. EZH2, EED and the VEFS domain of SUZ12 were shown to form a globular assembly that exists in either a basal state or in the stimulated state induced by trimethylated peptides. While binding of the stimulatory peptides such as histone H3K27me3 or JARID2K116me3 to EED allosterically promotes the catalytic activity, trapping an oncogenic mutant peptide H3K27M in the active site of EZH2 results in PRC2 inhibition. These groundbreaking studies of the EZH2-EED-SUZ12(VEFS) assembly provided seminal insights into the structural basis underlying the core subunit interactions and methyltransferase function of the PRC2 complex.
In a more recent study, published in Molecular Cell, Chen et al. continue building momentum on these impressive achievements and now describe the crystal structure of the missing part of the PRC2 complex [4]. The authors report on the heterotetrameric assembly formed by SUZ12, RBBP4, the C-terminal region of AEBP2, and the trans-repression (TR) region of JARID2 that comprise a minimal combination of PRC2 subunits essential for nucleosome binding. The SUZ12 subunit functions as a platform that bridges all four subunits, two core (SUZ12 and RBBP4) and two accessory (AEBP2 and JARID2), together. Specifically, Chen et al. show that a pair of separate WD40-binding (WDB) domains of SUZ12 associate with RBBP4, whereas the N-terminal helix and a zinc finger (NtH+ZnF) of SUZ12 create a binding site for the α-helical TR region of JARID2, and AEBP2 binds to and stabilizes a previously unidentified C2 domain of SUZ12 (Fig. 1B).
The discovery of the C2 domain in SUZ12 is exciting and unexpected. Typically, C2 domains bind phospholipids in a Ca2+-dependent manner and are implicated in transporting their host proteins to cell membranes. Here, the C2 domain is in direct contact with AEBP2, or, in the absence of AEBP2, with RBBP4 as it inserts an arginine residue into an acidic patch of RBBP4 and impedes binding of histone H3K4 tail to RBBP4 [4]. Intriguingly, the putative Ca2+/phospholipid-binding loops in the SUZ12 C2 domain are positioned away from the complex and could be available for binding of ligands. Whether this domain is capable of sensing nuclear phospholipids and whether calcium levels can mediate accessory subunit selectivity and PRC2 activity remain open questions.
The AEBP2 and JARID2 subunits are found in one of the classes of PRC2 holo complexes, and a separate class is defined by the presence of the polycomblike protein PHF19, which co-localizes with a PRC2 associated factor EPOP. Using biochemical binding and cell co-immunoprecipitation assays Chen et al. demonstrate that not only PHF19 and EPOP coexist in the same holo PRC2 complex, but they also compete with AEBP2 and JARID2, respectively, for the same binding sites in SUZ12 [4]. These data reinforce the idea that EPOP represents yet another accessory subunit of the PRC2 complex and unambiguously show that binding of AEBP2/JARID2 and PHF19/EPOP to SUZ12 is stoichiometric and mutually exclusive.
Recruitment of PRC2 to specific genomic loci and its dismissal are fine-tuned processes that require multiple cooperative and competitive contacts with DNA and histones, often involving several subunits of the complex. Although RBBP4 and its homologs have been shown to recognize unmodified histone H3 and H4 [5, 6], the new data from Chen et al. demonstrate that SUZ12-RBBP4 assembly exhibits poor nucleosome binding activity [4]. This finding is consistent with the structure of the dimeric SUZ12-RBBP4 complex that reveals partial occlusion of the histone binding site of RBBP4 by the SUZ12 C2 and WD40-binding domains. Interestingly, AEBP2 and JARID2 substantially enhance the nucleosome binding, but only when both are present in the complex. The basis of such an enhancement remains unclear because in the structure of the tetrameric SUZ12-RBBP4-AEBP2-JARID2 complex, the H3-binding site of RBBP4 remains blocked – now by the C-terminal tail of AEBP2. Furthermore, the AEPB2 tail is engaged with a similar set of RBBP4 residues that engages histone H3 tail, and EMSA experiments show that the H3 tail is dispensable for the interaction of the tetrameric complex with reconstituted nucleosomes as the complex binds equally well to the nucleosomes with and without H3 tails. JARID2 on the other hand contains a cluster of positively charged residues in the TR region, mutation of which indeed eliminates binding of the tetrameric complex to the nucleosome. Together, these new exciting results call for further evaluation of the role of RBBP4 and JARID2 in chromatin targeting of PRC2, where alterations in subunit organization, global and local conformational changes, and allosteric effects may affect the histone or DNA binding abilities of individual subunits. In addition, it will be important to extend structural analysis of PRC2 onto the characterization of other direct contacts with histones. These include the recognition of H2AK119Ub by the ubiquitin-binding domain of JARID2 that promotes H3K27 methylation and the recognition of H3K36me3 by the Tudor domain of PHF19 (or PHF1 that inhibits the PRC2 catalytic activity) [7, 8].
The structural architecture of the SUZ12-RBBP4-AEBP2-JARID2 assembly has been corroborated independently by the no less than spectacular cryo-electron microscopy structure of the entire PRC2 complex containing EZH2, EED, SUZ12, RBBP4, AEBP2 and JARID2 determined by the Nogales group [9]. Particularly, the cryo-EM structure uncovers the molecular mechanisms by which AEBP2 and JARID2 mimic histone H3 tails and shows how SUZ12 interacts with all other subunits to add to the complex stability (Fig. 1C). Furthermore, the cryo-EM structure of the PRC2 complex bound to bifunctional dinucleosomes offers a long-sought after model helping to explain the mechanism for H3K27me3 propagation and spreading of repressive chromatin. The data of Poespsel et al. reveal that DNA binding of the CXC domain of EZH2 positions the EED subunit to interact with the stimulatory H3K27me3 tail of one nucleosome while orienting the catalytic SET domain of EZH2 to simultaneously engage the substrate – an unmodified H3 tail of the adjacent nucleosome [10]. Collectively, the latest structural details reported by the Liu and Nogales groups provide a comprehensive view of how the PRC2 complex is activated and inhibited and acts in a varying epigenetic landscape.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343–9. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiao L, Liu X. Structural basis of histone H3K27 trimethylation by an active polycomb repressive complex 2. Science. 2015;350(6258):aac4383. doi: 10.1126/science.aac4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Justin N, et al. Structural basis of oncogenic histone H3K27M inhibition of human polycomb repressive complex 2. Nat Commun. 2016;7:11316. doi: 10.1038/ncomms11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, et al. Unique Structural Platforms of Suz12 Dictate Distinct Classes of PRC2 for Chromatin Binding. Mol Cell. 2018;69(5):840–852. e5. doi: 10.1016/j.molcel.2018.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitges FW, et al. Histone methylation by PRC2 is inhibited by active chromatin marks. Molecular Cell. 2011;42(3):330–41. doi: 10.1016/j.molcel.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Murzina NV, et al. Structural basis for the recognition of histone H4 by the histone-chaperone RbAp46. Structure. 2008;16(7):1077–85. doi: 10.1016/j.str.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper S, et al. Jarid2 binds mono-ubiquitylated H2A lysine 119 to mediate crosstalk between Polycomb complexes PRC1 and PRC2. Nat Commun. 2016;7:13661. doi: 10.1038/ncomms13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musselman CA, et al. Molecular basis for H3K36me3 recognition by the Tudor domain of PHF1. Nat Struct Mol Biol. 2012;19(12):1266–72. doi: 10.1038/nsmb.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasinath V, et al. Structures of human PRC2 with its cofactors AEBP2 and JARID2. Science. 2018;359(6378):940–944. doi: 10.1126/science.aar5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poepsel S, et al. Cryo-EM structures of PRC2 simultaneously engaged with two functionally distinct nucleosomes. Nat Struct Mol Biol. 2018;25(2):154–162. doi: 10.1038/s41594-018-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]