Abstract
The underlying genetic causes and altered signaling pathways of brain arteriovenous malformations remains unknown. A study published in The New England Journal of Medicine reported that KRAS somatic mutations (p.Gly12Val/Asp) were identified in brain arteriovenous malformations of human subjects and endothelial cell-enriched cultures, which might specifically activate the MAPK-ERK signaling pathway in brain endothelial cells.
Keywords: KRAS, KRAS4A, somatic mutation, endothelial cells, brain arteriovenous malformations
Brain arteriovenous malformations of the brain, a tangle of abnormal blood vessels connecting arteries and veins in the brain, are caused by direct connections between high-flow arterial vessels and low-resistance venous capacitance vessels ("steal phenomena") and further result from the direct and indirect effects of flow disturbances and rupture-associated haemorrhage [1] (Figure 1). Brain arteriovenous malformations have been reported to cause ischemic and hemorrhagic stroke in pediatric patients [2]. However, the underlying genetic causes and genomic factors contributing to the etiology of arteriovenous malformations of the brain remains unknown, especially for somatic mutations.
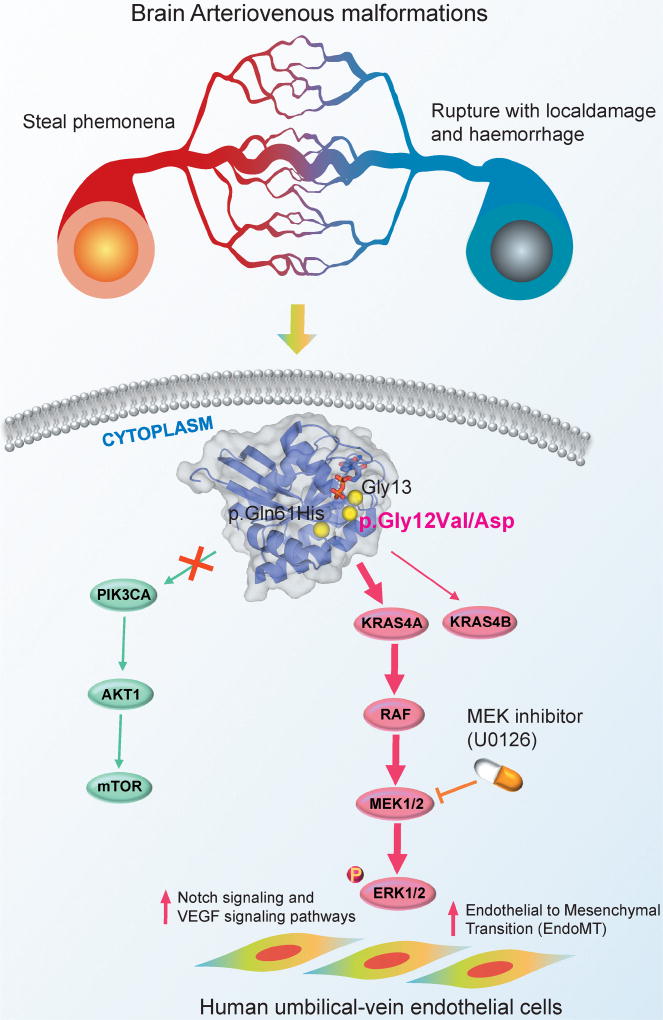
Figure 1. Diagram illustrating the proposed signaling pathways altered by KRAS activating somatic mutations in human brain arteriovenous malformations (AVMs).
(a) Activating somatic mutations (i.e., p.Gly12Val and p.Gly12Asp) were detected in brain AVMs of human subjects and endothelial cell-enriched cell cultures derived from patients with brain AVMs. (b) Human umbilical-vein endothelial cells expressing KRAS4A derived from p.Gly12Val on KRAS specifically activate ERK (extracellular signal-regulated kinase)-MAPK signaling pathway, not PI3K (phosphoinositide 3 kinase)-AKT signaling pathway; (c) Over-expression of KRAS4Ap.Gly12Val induces the expression of several AVMs-related pathways, including Endothelial to Mesenchymal Transition (EndoMT), Notch signaling, and VEGF signaling pathways, in human umbilical-vein endothelial cells. Furthermore, over-expression of KRAS4Ap.Gly12Val further enhances migratory behavior in human arterial endothelial cell lines, supporting mechanistically its activating roles triggering AVMs in human brain.
A recent study published in The New England Journal of Medicine reported that KRAS activating somatic mutations were observed in brain arteriovenous malformations [3]. Using the threshold of variants with sequence reads over 0.5% in the whole-exome sequencing (WES) analysis, Nikolaev et al., found that 12 of the 26 brain arteriovenous malformations patients were identified the KRAS activating mutations: 4 patients have the KRAS p.Gly12Val and 8 patients have the KRAS p.Gly12Asp. Interestingly, no KRAS mutations was observed in the 17 paired blood samples. They further performed droplet digital PRC analysis to identify the KRAS mutations with low variant allele frequency in heterogeneous samples. They re-identified the KRAS mutations in 12 samples that had been detected mutations in WES analysis and found KRAS mutations in 6 negative samples in WES analysis. In addition, they further identified that 11 human subjects among 13 additional tissue samples have been detected KRAS mutations. In total, 74% samples (29/39) had been detected activating mutations on KRAS: 19 samples with p.Gly12Asp, 9 samples with p.Gly12Val, and 1 sample with p.Gln61His. They showed that 48% samples (16/33) of brain arteriovenous malformations in an independent Finnish subject validation group had been detected the KRAS mutations using droplet digital PCR analysis. Moreover, none of the 21 paired blood samples was detected the KRAS mutations. These data suggested that activating somatic mutations on KRAS may trigger etiology of arteriovenous malformations in the human brain [3].
They further isolated endothelial cells from six fresh cell cultures derived from arteriovenous malformations of the human brain and from three control cultures of normal vascular cells derived from cortical vessels using magnetic-activated cell sorting [3]. Droplet digital PCR analysis reveals that five of six samples of endothelial cell-enriched cultures have been detected the KRAS activating mutations (p.Gly12Asp or p.Gly12Val). Moreover, control cell cultures derived from normal cortical vessels were not detected KRAS mutations by droplet digital PCR analysis. Collectively, these data suggested that endothelial cells derived from fresh cell cultures of brain arteriovenous malformations were positive for KRAS mutations.
They further inspected the down-stream signaling pathways activated by KRAS activating mutations, including MAPK (mitogen-activated protein kinase)-ERK (extracellular signal-regulated kinase) and PI3K (phosphoinositide 3 kinase)-AKT pathways [4]. They found the elevated levels of ERK1/2 phosphorylation in endothelial cell-enriched cultures derived from arteriovenous malformations of the brain with p.Gly12Val on KRAS compared to the primary endothelial cell-enriched cultures derived from normal brain vessels, while no elevated expression levels of AKT or p38 phosphorylation [3]. In addition, the elevated levels of ERK1/2 phosphorylation was further observed in human umbilical-vein endothelial cells expressing one of KRAS specific isoforms, KRAS4Ag.Gly12Val, suggesting that KRAS activating mutations specifically activates the MAPK-ERK signaling pathway in endothelial cells (see Figure 1). Subsequently, expression of KRAS4Ag.Gly12Val cause disassembly of vascular endothelial cadherin junctions in human umbilical-vein endothelial cells. In addition, over4 expression of KRAS4Ag.Gly12Val induces the expression of several arteriovenous malformations-related pathways, including Endothelial to Mesenchymal Transition (EndoMT), Notch signaling, and VEGF signaling pathways (see Figure 1), in human umbilical-vein endothelial cells. Furthermore, over-expression of KRAS4Ag.Gly12Val further enhances migratory behavior in human arterial endothelial cell lines, supporting mechanistically its activating roles triggering brain arteriovenous malformations. Finally, the inhibition of MAPK-ERK signaling by a MEK inhibitor (U0126) reduces lamellipodia formation by restoring localization of vascular endothelial cadherin to the junctions of endothelial cells in human umbilical-vein endothelial cells, suggesting a potential therapeutic strategy for arteriovenous malformations of human brain with KRAS mutations by MEK inhibitors (Figure 1).
Several specific open questions are not explored in current study. A previous study has revealed that activating mutations on PI3K-AKT pathway were detected in brain malformations [5]. Whether KRAS activating somatic mutations are exclusively with mutations on PI3K-AKT pathway in brain arteriovenous malformations is still unknown. Although the MEK inhibitor reduces lamellipodia formation by restoring localization of vascular endothelial cadherin to the junctions of endothelial cells, whether the clinical benefits of MEK inhibitor for brain arteriovenous malformations patients with KRAS mutations are not confirmed owing to low KRAS variant allele frequency (0.5 to 4%) [3] in heterogeneous samples and the unknown blood brain barrier profiles of MEK inhibitors. Thus, in vivo preclinical validation and prospective clinical trials should be conducted in the future. Finally, cancer and stroke have been shown the comorbidity by different mechanisms, such as hypercoaguability, direct tumor compression of blood vessels, or treatment-related effects which potentiate stroke [6]. However, the precise molecular mechanisms of KRAS mutations that specifically drive brain arteriovenous malformations, not brain tumors in human subjects, remain unclear.
Several recent studies have revealed that somatic mutations might contribute to atherosclerotic cardiovascular diseases [7] and neurological diseases [8]. Study how somatic mutations involving in cardiovascular diseases (i.e., stroke) and neurological diseases may offer novel therapeutic targets for treating those complex diseases. For example, repurposing the approved molecularly targeted agents that target specific signaling pathways altered by somatic mutations may offer new treatments for cardiovascular diseases and neurological diseases that are caused by somatic mutations. A vascular endothelial growth factor (VEGF) inhibitor (SU5416 or Semaxanib) in the treatment of cancer was shown to reduce post-stroke vascular damage [9]. Systematic identification of crosstalk pathways (i.e., disease module in the human protein-protein interactome [10]) driven by somatic mutations between cancer and cardiovascular diseases or neurological diseases may shed novel insights in etiologies of the diseases, offering novel targets and therapeutic strategies for drug discovery, such as drug repurposing.
Acknowledgments
This work was supported by K99HL138272 from NHLBI.
References
- 1.Lawton MT, et al. Brain arteriovenous malformations. Nat. Rev. Dis. Primers. 2015;1:15008. doi: 10.1038/nrdp.2015.8. [DOI] [PubMed] [Google Scholar]
- 2.Smith ER. Structural causes of ischemic and hemorrhagic stroke in children: moyamoya and arteriovenous malformations. Curr. Opin. Pediatr. 2015;27:706–711. doi: 10.1097/MOP.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 3.Nikolaev SI, et al. Somatic activating KRAS mutations in arteriovenous malformations of the Brain. N. Engl. J. Med. 2018;378:250–261. doi: 10.1056/NEJMoa1709449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nussinov R, et al. Calmodulin and PI3K signaling in KRAS cancers. Trends Cancer. 2017;3:214–224. doi: 10.1016/j.trecan.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen LA, et al. PI3K/AKT pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain. 2015;138:1613–1628. doi: 10.1093/brain/awv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dearborn JL, et al. Stroke and cancer- A complicated relationship. J. Neurol. Transl. Neurosci. 2014;2:1039. [PMC free article] [PubMed] [Google Scholar]
- 7.Jaiswal S, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poduri A, et al. Somatic mutation, genomic variation, and neurological disease. Science. 2013;341:1237758. doi: 10.1126/science.1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reeson P, et al. Delayed inhibition of VEGF signaling after stroke attenuates blood-brain barrier breakdown and improves functional recovery in a comorbidity-dependent manner. J. Neurosci. 2015;35:5128–5143. doi: 10.1523/JNEUROSCI.2810-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng F, Loscalzo J. Pulmonary comorbidity in lung cancer. Trends Mol. Med. 2018;24:239–241. doi: 10.1016/j.molmed.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]