Abstract
Classic nucleophilic substitution reactions (SN1 and SN2) are not generally amenable to the enantioselective variants that use simple and racemic alkyl halide electrophiles. The merging of transition metal catalysis and radical chemistry with organometallic nucleophiles is a versatile method for addressing this limitation. Here, we report that visible light-driven catalytic asymmetric photoredox radical coupling can act as a complementary and generic strategy for the enantioconvergent formal substitution of alkyl haldies with readily available and bench-stable organic molecules. Single-electron reductive debrominations of racemic α-bromoketones generate achiral alkyl radicals that can participate in asymmetric Csp3–Csp3 bonds forming cross-coupling reactions with α-amino radicals derived from N-aryl amino acids. A wide range of valuable enantiomerically pure β2- and β2,2-amino ketones were obtained in satisfactory yields with good-to-excellent enantioselectivities by using chiral phosphoric acid catalysts to control the stereochemistry and chemoselectivity. Fluoro-hetero-quaternary and full-carbon quaternary stereocenters that are challenging to prepare were successfully constructed.
Enantioselective substitution reactions of racemic alkyl halides are inherently limited by the specificity of their stereochemical course (SN1 or SN2). Here, the authors report a radical coupling strategy for the enantioselective aminoalkylation of α-bromoketones for modular, highly enantioselective Csp3−Csp3 bond formation.
Introduction
The nucleophilic substitution of alkyl halides is a fundamental chemical transformation for precisely delivering molecular fragments to sp3-hybridized carbon atoms using halide (X) as a directing group1,2. SN1 and SN2 reactions are two classic pathways, and they are ubiquitous in organic synthesis. However, the enantioselective version of the SN1 approach for forming a stereogenic center at the carbon undergoing substitution by a simple alkyl halide, for which no special structural requirement for the removal of X is designated3–5, remains as an unsolved problem due to the reaction mechanism. Meanwhile, the SN2 reaction is stereospecific for which the generation of chiral products typically requires the utilization of the enantioenriched secondary alkyl halides. In this regard, Fu et al. made a major breakthrough by merging transition metal-catalyzed cross-coupling techniques with radical chemistry (Fig. 1a)6. In a bimetallic protocol such as this7, achiral alkyl radicals are generated from both racemic alkyl halide reactants through inner-sphere single-electron transfer (SET) with a chiral transition metal catalyst (Mcat*). After transmetalation of the generated Mcat*X species with organometallic nucleophiles (NuM) and oxidative addition of the alkyl radical, the alkylmetal–Nu complex, which is produced as a single stereoisomer, undergoes reductive elimination to afford enantioconvergent substitution products. Thus far, this catalytic system has inspired the development of numerous chemical transformations with various NuM species8–10. Recently, Fu extended this protocol to nonmetallic secondary amine nucleophiles, where SET between a photosensitive metal–nucleophile complex and an alkyl halide under visible light irradiation is responsible for the production of the alkyl radicals11. In this context, the capacity of the radical approach to C–X bond fragmentation has garnered substantial recognition for generating reactive species that can participate in asymmetric substitution reactions of simple alkyl halides.
Fig. 1.
Strategies for catalytic asymmetric radical-based substitution reactions of simple alkyl halides. a Bimetallic protocol (known). b Radical coupling under visible light-induced photoredox catalysis for enantioconvergent substitution of alkyl halides (design profile). c A dual-catalysis approach to enantioselective radical coupling of N-aryl amino acids to α-bromoketones irradiated by visible light (this work). X halide. PC, photoredox catalyst. SPINOL-CPAs = 1,1ʹ-spirobiindane-7,7ʹ-diol (SPINOL)-based spirocyclic chiral phosphoric acids. With the recent rapid development of the field of visible light-mediated photoredox catalysis, this strategy has been seen as a powerful tool for achieving radical-based transformations36,37. This catalysis platform has found considerable success in outer-sphere SET oxidations of a wide array of readily available and bench-stable organic molecules as it provides diverse radical species and shows high functional group tolerance. Meanwhile, reductive dehalogenations of alkyl halides via electron transfer-fragmentation processes allows the generation of alkyl radicals33,34. In conjunction with our studies19,20 on the development of enantioselective, dual-catalysis photoredox reactions38–41, we speculated that radical coupling reactions42,43 for connecting two distinct odd-electron partners using an extrinsic stereocontrol factor44–46 would provide a complementary and structurally versatile approach for accessing the enantioconvergent products (b). This method would avoid the limitation of the narrow substrate scope faced by direct SH2-based radical transformations47, where stereoselectivity is also underdeveloped. Notably, the precise and absolute stereocontrol in a reaction with such a low-energy barrier and rapid bond-forming process is a formidable challenge, especially when building an uncommon full-carbon quaternary stereocenter. Here, we demonstrate a generic radical recombination strategy for the enantioselective aminoalkylation of α-bromoketones for modular Csp3–Csp3 bond formation (c). SPINOL-based spirocyclic chiral phosphoric acid (SPINOL-CPA) catalysts were used to control the chemoselectivity and stereoselectivity of the redox-neutral radical coupling between α-bromoketones and N-aryl amino acids. Both secondary α-bromoketones and tertiary (α-fluoro)-α-bromoketones were compatible with this α-aminoalkyl-substitution reaction, leading to valuable chiral β2- and β2,2-amino ketones with high enantioselectivities
Results
Reaction optimization
The use of the non-fossil fuel-based α-amino acids as starting substrates always represents an attractive method in organic synthesis because of their environmentally benign features. In recent years, the groups of Das12, Tan13, MacMillan14–16, and Rueping17 successively reported the viability of generating the α-amino radicals from N-protected α-amino acids via facile visible light-driven single-electron oxidative decarboxylation. Inspired by these contributions, we began our study by exploring the model reaction between α-bromoketone 1a and N-phenyl glycine (2a) with our developed metal-free18 dicyanopyrazine-derived chromophore (DPZ)19,20 as the photoredox catalyst (Table 1). Initial investigations using Stern–Volmer experiments revealed that the excited DPZ (*DPZ) species can facilitate single-electron oxidation of 1a. Upon examining a range of reaction parameters, we observed that the reaction performed in 1,2-dimethoxyethane at 0 °C for 60 h in the presence of 0.5 mol% DPZ, 10 mol% chiral SPINOL-CPA21 C1, and 3 Å molecular sieves affords a desired α-aminoalkyl-substitution product 3a in 79% yield with 95% ee (enantiomeric excess) (entry 1). The substituents at the 6,6ʹ-positions of SPINOL affected the enantioselectivity, and as an evidence, catalysts C2–C4 showed different enantioselectivities for the formation of 3a (entries 2–4). Catalyst BINOL-CPA22–24 C5 gave 3a with 45% ee (entry 5), which confirmed the significant effect of the chiral backbone of the catalyst on the enantioselectivity. The reaction was also examined using l-amino acid-based urea-tertiary amine bifunctional catalyst20 C6, but the yield deteriorated and the reaction was not enantioselective (entry 6). In the absence of the chiral catalyst C1, 3a was formed as an achiral mixture in only 30% of yield (>95% conversion of 1a, entry 7). In both reactions (entries 6–7), the reductive debrominative protonation of 1a became a primary transformation, given that ethyl phenyl ketone (4) was obtained as the major product. The reaction conducted in the absence of DPZ provided 3a in only 20% of yield with 94% ee (23% conversion of 1a, entry 8). These results suggested that the chiral catalyst is crucial for both the chemoselectivity and enantioselectivity, and the photocatalyst is necessary for the reactivity25. Control experiments confirmed that visible light is necessary for the transformation (entry 9).
Table 1.
Optimization of reaction conditions

|
The reaction was performed on a 0.05 mmol scale. Entries 1–7, the chemical conversion of 1a was >95% determined by crude 1H NMR. Entry 8, the chemical conversion of 1a was 23%
aYields were determined from the isolated compound following chromatographic purification
bEnantiomeric excesses were determined by HPLC analysis on a chiral stationary phase
Substrate scope of secondary α-bromoketones
To examine the scope of this radical-based enantioconvergent substitution reaction, optimal conditions were evaluated with a wide range of N-aryl amino acids and racemic secondary α-bromoketones for the synthesis of β2-amino ketones (Fig. 2). For the couplings of 1a with N-aryl glycines 2a–f, which feature electron-withdrawing or electron-donating substituents on the aryl ring, products 3a–f were obtained in 69–80% yields with 94–95% ees. As for the scope of electrophiles, various secondary α-bromoketones with a variety of combinations of substituents (i.e., aryl, alkyl, and benzyl) at the 1- and 2-positions were compatible with the reaction conditions and provided the corresponding β2-amino ketones (3g–w) in 55–86% yields with 86–95% ees. Notably, the free hydroxyl group on the aromatic ring did not interfere with the reaction (product 3m). To improve the enantioselectivity, catalyst C2 was used to generate products 3s, t and 3v. The reaction to access 3a was attempted on a 1.0 mmol scale, but under an irradiation by two 3 W blue LEDs, and the similar yield (75%) and enantioselectivity (94% ee) were observed. Reactions of 1 with N-MeOPh (2g) and N-Ph (2h) alanines were also carried out, and adducts 3x−z containing two adjacent tertiary carbon stereocenters were obtained in 46–51% yields with 96–97% ees and >20:1 drs. A slightly decreased yield can be attributed to the decreased chemoselectivity of the coupling reactions. When examining other α-substituted N-aryl amino acids, this undesirable side reaction became dominant, and the reductive debrominative protonation product of 1 was observed almost exclusively.
Fig. 2.
Substrate scope of N-aryl amino acids and secondary α-bromoketones. Reactions were performed with 1 (0.1 mmol), 2 (0.2 mmol), DPZ (0.5 × 10−3 mmol), C1 (0.01 mmol), NaHCO3 (0.3 mmol), and 3 Å MS (50 mg) in 1,2-dimethoxyethane (1.5 mL) at 0 °C. Yields were determined from the isolated material after chromatographic purification. Enantiomeric excesses were determined by HPLC analysis on a chiral stationary phase. aOn a 1.0 mmol scale and 60 h, yield of 3a = 75% and ee = 94% (2 × 3 W blue LED). bCatalyst C2 was used instead of C1
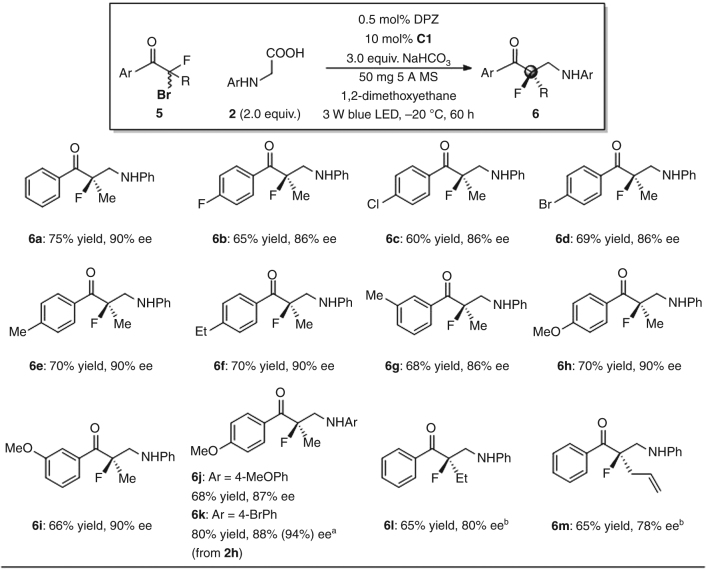
Substrate scope of α-bromo-α-fluoroketones
Motivated by the potential applications of organofluorine compounds in a variety of scientific disciplines26,27, the scope of α-bromo-α-fluoroketones 5 was examined next (Fig. 3). Under the established reaction conditions, but using 5 Å MS as an additive and at −20 °C, chiral β-fluoro-β-methyl amino ketones 6a–k were prepared in 60–80% yields with 84–94% ees. When the α-substituent of 5 was ethyl and allyl, the enantioselectivities were lower. However, switching from catalyst C1 to C3 (20 mol%) and performing the reaction at a lower temperature (−45 °C) allowed products 6l–m to be generated with good enantioselectivity.
Fig. 3.
Substrate scope of tertiary α-bromo-α-fluoroketones with N-aryl glycines. Reactions were performed with 5 (0.1 mmol), 2 (0.2 mmol), DPZ (0.5 × 10−3 mmol), C1 (0.01 mmol), NaHCO3 (0.3 mmol), and 5 Å MS (50 mg) in 1,2-dimethoxyethane (1.5 mL) at −20 °C. Yields were determined from the isolated material after chromatographic purification. Enantiomeric excesses were determined by HPLC analysis on a chiral stationary phase. aThe ee value in parenthesis was obtained after recrystallization. bCatalyst C3 (20 mol%) was used instead of C1 and T = −45 °C
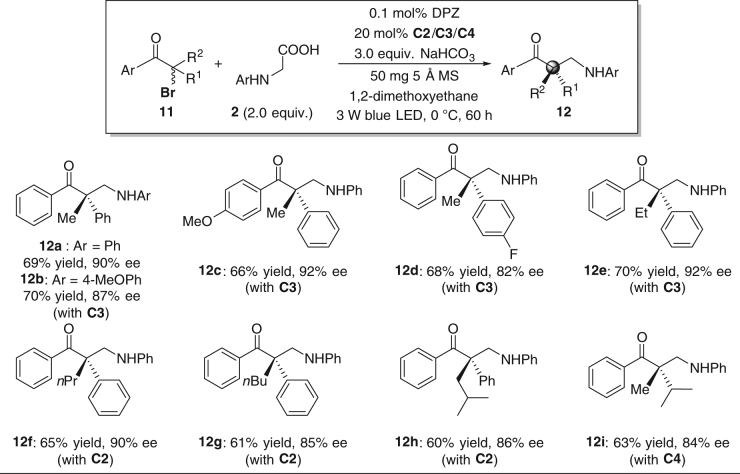
Substrate scope of tertiary α-bromoketones
Encouraged by these results, we extended the generality of the reaction with regard to racemic tertiary α-bromoketones 7 to address the challenge of forming full-carbon quaternary stereocenters (Fig. 4). Although the tertiary carbon radicals feature higher steric hindrance, the reactions proceeded smoothly with similar reactivity and chemoselectivity, and afforded β2,2-amino ketones 8a–i in 60–70% yields. To achieve the best enantioselectivity, catalyst C3 was used for the preparation of 8a–e (82–92% ee), C2 was used for 8f–h (84–90% ee), and C4 was used for 8i (84% ee). Other chiral β2,2-amino ketones could be obtained with high enantioselectivity by tuning the 6,6′-substituents of the catalyst.
Fig. 4.
Substrate scope of tertiary α-bromoketones. Reactions were performed with 7 (0.1 mmol), 2 (0.2 mmol), DPZ (0.1 × 10−3 mmol), C2/C3/C4 (0.02 mmol), NaHCO3 (0.3 mmol), and 5 Å MS (50 mg) in 1,2-dimethoxyethane (1.5 mL) at 0 °C. Yields were determined based on the isolated material after chromatographic purification. Enantiomeric excesses were determined by HPLC analysis on a chiral stationary phase
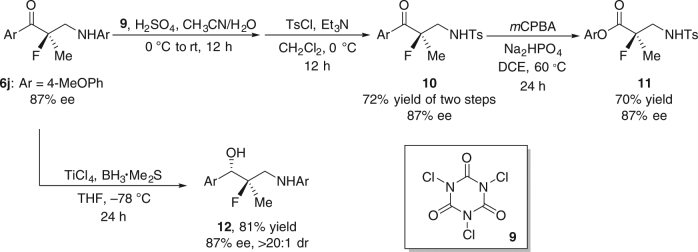
Synthetic applications
Enantiomerically pure β2- and β2,2-amino ketones are prevalent in biologically active compounds28 and are direct precursors29–32 to a number of important chiral structural motifs such as β2- and β2,2-amino acids, derivatives for peptide synthesis30,31, and fluorohydrins32. For example, the treatment of β2,2-amino ketone 6j with N,Nʹ,Nʺ-trichloroisocyanuric acid (9), followed by p-toluenesulfonyl chloride could replace the para-methoxyphenyl (PMB) N-protective group with a tosyl (Ts) group (Fig. 5). The resulting β2,2-amino ketone 10 was readily converted to fluorine-containing chiral ester 11 by a Baeyer–Villiger rearrangement with mCPBA. Meanwhile, the reduction of 6j using TiCl4 and BH3•Me2S in THF at −78 °C furnished fluorohydrin 12 with perfect diastereoselectivity and without erosion of the ee.
Fig. 5.
Synthetic utilities. The synthesis of β2-fluoro-β2-substituted amino ester 11 and fluorohydrin 12 from chiral β-fluoro-β-methyl amino ketone 6j
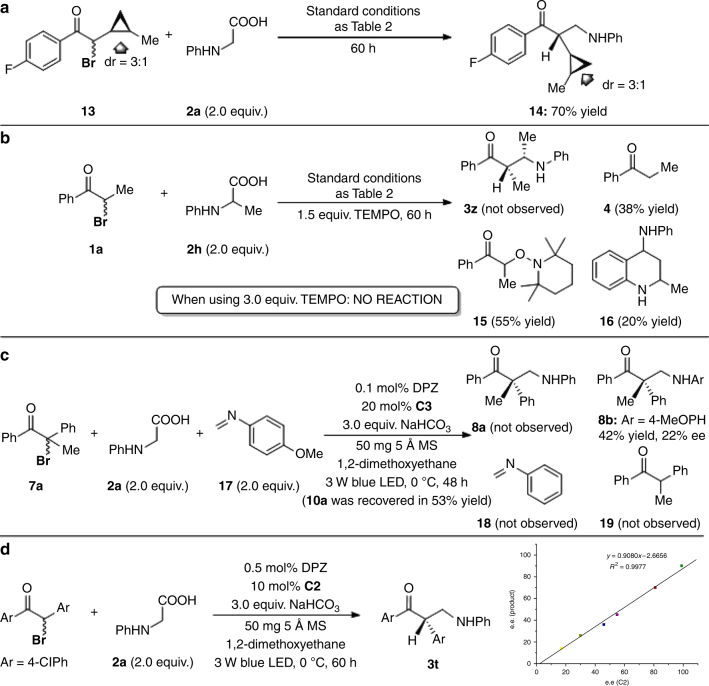
Mechanistic studies
Although the above results and previous reports18,33,34 are consistent with a redox-neutral radical process triggered by the reductive quenching of DPZ*, as depicted in Fig. 1c, control experiments were performed to further probe the reaction mechanism (Fig. 6). A radical-clock study using cis-cyclopropyl-substituted α-bromoketones 13 and 2a was conducted first (Fig. 6a). β2-amino ketone 14 was obtained in 70% yield, and no ring-opened product was observed due to the maintained configuration of the cyclopropyl moiety. This result confirmed that the radical intermediates generated from the SET reductive debromination of α-bromoketones are very active and quickly couple with the α-amino radicals. The high reactivity of these alkyl radicals is consistent with the result of the reaction between 1a and N-phenyl alanine (2h) in the presence of the radical scavenger TEMPO (1.5 equiv.), where product 15 was obtained in 55% yield via coupling with TEMPO (Fig. 6b). Notably, this reaction also generated products 4 and 4-amino tetrahydroquinoline 16 in 38% and 20% yields, respectively, but β2-amino ketone 3z was not observed. Product 16 is likely generated from the Povarov reaction between the imine formed by two SET oxidations of 2i and an enamine (the tautomer of the imine). Hence, the second reductive protonation of the alkyl radical must be fast and the substitution products should be formed by a radical cross-coupling reaction as the major operative process and not by a Mannich-type reaction of the α-ketone anion with an imine. Such an ionic pathway and a possible radical addition process could be further excluded by the control experiment shown in Fig. 6c, in which the reaction of α-bromoketone 7a with N-phenyl glycine 2a and N-MeOPh imine 17 derived from formaldehyde worked slightly sluggish, but very clean, only affording 8b in 42% yield with 22% ee after 48 h and without 8a being detected. Of note, the transformation of 7a with N-MeOPh glycine 2f under the reaction conditions furnished 8b with 87% ee (Fig. 4). The different enantioselective results suggest two distinct approaches to access 8b in both reactions. Furthermore, while 8a was not obtained, imine 18 through two single-electron oxidations of 2a was not observed yet, which also eliminated the possibility of β2- and β2,2-amino ketones obtained from the transformations with imines in our reaction system. For higher concentration of product 8b from imine 17, it should be obtained from a Mannich-type radical addition than the α-amino radical generated from 2a. In this process, the excellent reactivity that has been described by Knowles and co-workers28 could be demonstrated by the result that no ketone 19 as the debrominative protonation product was detected. It is also worth mentioning that no reaction was detected when three kinds of the parent ketones with imine 17 were under the corresponding standard reaction conditions.
Fig. 6.
Experimental studies to elucidate the mechanism. a Radical-clock examination. b Investigation on the effect of TEMPO. c The reaction of bromoketone 7a with N-phenyl glycine 2a and imine 17. d The study on the relationship between ee of the CPA C2 and ee of the product 3t
Chiral phosphoric acid catalysts were shown to provide higher selectivity for the radical coupling reaction over the reductive debrominative protonation (entries 1 and 7, Table 1). According to the persistent radical effect35, it is plausible that phosphoric acid would act as a bifunctional H-bonding catalyst29 to stabilize the electrophilic alkyl radical species and activate the nucleophilic α-amino radical variant, thus facilitating the selective C–C bond formation, but not the SET-redox reaction. The decreased yield with N-phenyl-N-methyl glycine as a starting material (45% yield, see Supplementary Table 3) and the 13C NMR analysis between substrate and catalyst (Supplementary Note 2) could be recognized as circumstantial evidences to demonstrate this hypothesized activation approach. This H-bonding effect offers another method of controlling the enantioselectivity of the formation of chiral substitution products. Additionally, a linear relationship was found between the ee of product 3t and the ee of acid catalyst C2 (Fig. 6d). This result suggests only a single molecule of chiral phosphate was involved in the crucial C–C bond-forming step. More investigations were continuously being conducted in our laboratory to make an accurate understanding of the mechanism.
Discussion
In summary, we have developed a catalytic asymmetric photoredox radical-coupling method as a versatile strategy for the enantioconvergent substitution of alkyl halides. Alkyl radicals generated from the visible light-driven photoreductive dehalogenation of simple alkyl halides undergoing diverse radical-based transformations have been well-studied33,34,36,37. The high enantioselectivity in conjunction with broad substrate scope for Csp3–Csp3 bond formation, especially the success in the construction of challenging fluoro-hetero-quaternary and full-carbon quaternary stereocenters, provides a crucial foundation for the pursuit of other valuable enantioconvergent substitution transformations of alkyl halides with readily available and bench-stable organic molecules.
Methods
General information
For the NMR spectra of compounds in this manuscript, see Supplementary Figs. 1–80. For the HPLC spectra of compounds in this manuscript, see Supplementary Figs. 81–141. For details of optimization of reaction conditions, see Supplementary Tables 1–3. For the synthesis of substrates 13, 17, and 22, see Supplementary Note 1. For details of mechanistic studies, see Supplementary Note 2. For details of synthetic applications, see Supplementary Note 3. For the determination of absolute configuration of products, sees Supplementary Note 4. For general information, general experimental procedure, and analytic data of compounds synthesized, see Supplementary Methods.
Preparation of 3
A total of 35.4 μL (0.0005 mmol, 0.005 equiv.) of DPZ solution (1.0 mg of DPZ in 200 μL of toluene) was added into a 10-mL Schlenk tube and then solvent was removed in vacuo. Subsequently, 1 (0.1 mmol, 1.0 equiv.), 2 (0.2 mmol, 2.0 equiv.), C1 (0.01 mmol, 0.1 equiv.) for 3a–r, u, w, x, y and z or C2 (0.01 mmol, 0.1 equiv.) for 3s, t, and v, 3 Å molecular sieves (50.0 mg), and NaHCO3 (25.2 mg, 3.0 equiv.) in 1,2-dimethoxyethane (1.5 mL) were sequentially added and then degassed three times by the freeze-pump-thaw method. The reaction mixture was stirred under an argon atmosphere at 0 °C (the temperature was maintained in an incubator) for 30 min without light, and then irradiated by a 3 W blue LED (λ = 450–455 nm) from a 3.0 cm distance for another 60 h. The reaction mixture was directly loaded onto a short silica gel column, followed by gradient elution with petroleum ether/ethyl acetate (200/1‒20/1 ratio). Removing the solvent in vacuo afforded products 3a–z.
Preparation of 6
A total of 35.4 μL (0.0005 mmol, 0.005 equiv.) of DPZ solution (1.0 mg of DPZ in 200 μL of toluene) was added into a 10-mL Schlenk tube and then the solvent was removed in vacuo. Subsequently, 5 (0.1 mmol, 1.0 equiv.), 2 (0.2 mmol, 2.0 equiv.), C1 (0.01 mmol, 0.1 equiv.) for 6a–k or C3 (0.02 mmol, 0.2 equiv.) for 6l and m, 5 Å molecular sieves (50.0 mg), NaHCO3 (25.2 mg, 3.0 equiv.) in 1,2-dimethoxyethane (1.5 mL) were sequentially added and then degassed three times by the freeze-pump-thaw method. The reaction mixture was stirred under an argon atmosphere at −20 °C for 6a–k or at −45 °C for 6l and m (the temperature was maintained in an incubator) for 30 min without light, and then irradiated by a 3 W blue LED (λ = 450–455 nm) from a 3.0 cm distance for another 60 h. The reaction mixture was directly loaded onto a short silica gel column, followed by gradient elution with petroleum ether/ethyl acetate (200/1‒20/1 ratio). Removing the solvent in vacuo afforded products 6a–m.
Preparation of 8
A total of 7.1 μL (0.0001 mmol, 0.001 equiv.) of DPZ solution (1.0 mg of DPZ in 200 μL of toluene) was added into a 10-mL Schlenk tube and then solvent was removed in vacuo. Subsequently, 7 (0.1 mmol, 1.0 equiv.), 2 (0.2 mmol, 2.0 equiv.), C2 (0.02 mmol, 0.2 equiv.) for 8f–h, C3 (0.02 mmol, 0.2 equiv.) for 8a–e, or C4 (0.02 mmol, 0.2 equiv.) for 8i, 5 Å molecular sieves (50.0 mg), NaHCO3 (25.2 mg, 3.0 equiv.) in 1,2-dimethoxyethane (1.5 mL) were sequentially added and then degassed three times by the freeze-pump-thaw method. The reaction mixture was stirred under an argon atmosphere at 0 °C (the temperature was maintained in an incubator) for 30 min without light, and then irradiated by a 3 W blue LED (λ = 450–455 nm) from a 3.0 cm distance for another 60 h. The reaction mixture was directly loaded onto a short silica gel column, followed by gradient elution with petroleum ether/ethyl acetate (200/1‒20/1 ratio). Removing the solvent in vacuo afforded products 8a–i.
Data availability
The X-ray crystallographic coordinates for structures that support the findings of this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the accession code CCDC 1587114 (12), 1589705 (25), and 1814679 (26). The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.
Electronic supplementary material
Acknowledgements
Grants from NSFC (Nos. 21072044 and 21672052), Innovation Scientists and Technicians Troop Construction Projects of Henan Province, and Henan University are gratefully acknowledged. We also appreciate Miss Xinyi Ye and Prof. Choon-Hong Tan (NTU) for their generous help in the analysis of HRMS data and Mr. Yangyang Shen (ICIQ) for constructive discussions.
Author contributions
Z.J. conceived and designed the experiments. J.L., M.K., and B.Q. performed the experiments and prepared the Supplementary Information. R.L. and X.Z. helped with isolating the new compounds and analyzing the data. Z.J. wrote the paper. J.L., M.K., and B.Q. contributed equally to this work. All authors discussed the results and commented on the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Jiangtao Li, Manman Kong, Baokun Qiao.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-04885-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hartshorn SR. Aliphatic Nucleophilic Substitution. London: Cambridge University Press; 1973. [Google Scholar]
- 2.Anslyn, E. V. & Dougherty, D. A. (eds) in Modern Physical Organic Chemistry Ch 11 (University Science Books, Sausalito, CA, 2006).
- 3.Reisman SE, Doyle AG, Jacobsen EN. Enantioselective thiourea-catalyzed additions to oxocarbenium ions. J. Am. Chem. Soc. 2008;130:7198–7199. doi: 10.1021/ja801514m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma S, Han X, Krishnan S, Virgil SC, Stoltz BM. Catalytic enantioselective stereoablative alkylation of 3-halooxindoles: facile access to oxindoles with C3 all-carbon quaternary stereocenters. Angew. Chem. Int. Ed. 2009;48:8037–8041. doi: 10.1002/anie.200902943. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy CR, et al. Mechanism-guided development of a highly active bis-thiourea catalyst for anion-abstraction catalysis. J. Am. Chem. Soc. 2016;138:13525–13528. doi: 10.1021/jacs.6b09205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer C, Fu GC. Asymmetric nickel-catalyzed Negishi cross-couplings of secondary α-bromo amides with organozinc reagents. J. Am. Chem. Soc. 2005;127:4594–4595. doi: 10.1021/ja0506509. [DOI] [PubMed] [Google Scholar]
- 7.Schley ND, Fu GC. Nickel-catalyzed Negishi arylations of propargylic bromides: a mechanistic investigation. J. Am. Chem. Soc. 2014;136:16588–16593. doi: 10.1021/ja508718m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherney AH, Kadunce NT, Reisman SE. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C-C bonds. Chem. Rev. 2015;115:9587–9652. doi: 10.1021/acs.chemrev.5b00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu GC. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes. ACS Cent. Sci. 2017;3:692–700. doi: 10.1021/acscentsci.7b00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi J, Fu GC. Transition metal-catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling chemistry. Science. 2017;356:eaaf7230. doi: 10.1126/science.aaf7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kainz QM, et al. Asymmetric copper-catalyzed C-N cross-couplings induced by visible light. Science. 2016;351:681–684. doi: 10.1126/science.aad8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajesh CS, Thanulingam TL, Das S. Photoelectron transfer induced decarboxylation of substituted carboxylic acids across a liquid/liquid interface. Tetrahedron. 1997;53:16817–16834. doi: 10.1016/S0040-4020(97)10084-9. [DOI] [Google Scholar]
- 13.Chen L, et al. Amphiphilic methyleneamino synthon through organic dye catalyzed-decarboxylative aminoalkylation. Org. Biomol. Chem. 2013;11:5922–5925. doi: 10.1039/c3ob41091a. [DOI] [PubMed] [Google Scholar]
- 14.Zuo Z, MacMillan DWC. Decarboxylative arylation of α-amino acids via photoredox catalysis: a one-step conversion of biomass to drug pharmacophore. J. Am. Chem. Soc. 2014;136:5257–5260. doi: 10.1021/ja501621q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuo Z, et al. Enantioselective decarboxylative arylation of α-amino acids via the Merger of photoredox and nickel catalysis. J. Am. Chem. Soc. 2016;138:1832–1835. doi: 10.1021/jacs.5b13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston CP, Smith RT, Allmendinger S, MacMillan DWC. Metallaphotoredox-catalysed sp3-sp3 cross-coupling of carboxylic acids with alkyl halides. Nature. 2016;536:322–325. doi: 10.1038/nature19056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millet A, Lefebvre Q, Rueping M. Visible-Light photoredox-catalyzed Giese reaction: decarboxylative addition of amino acid derived α-amino radicals to electron-deficient olefins. Chem. Eur. J. 2016;22:13464–13468. doi: 10.1002/chem.201602257. [DOI] [PubMed] [Google Scholar]
- 18.Romero NA, Nicewicz DA. Organic photoredox catalysis. Chem. Rev. 2016;116:10075–10166. doi: 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- 19.Wei G, et al. Enantioselective aerobic oxidative C(sp3)-H olefination of amines via cooperative photoredox and asymmetric catalysis. ACS Catal. 2016;6:3708–3712. doi: 10.1021/acscatal.6b00846. [DOI] [Google Scholar]
- 20.Lin L, et al. Organocatalytic enantioselective protonation for photoreduction of activated ketones and ketimines induced by visible light. Angew. Chem. Int. Ed. 2017;56:13842–13846. doi: 10.1002/anie.201707899. [DOI] [PubMed] [Google Scholar]
- 21.Čorić I, Müller S, List B. Kinetic resolution of homoaldols via catalytic asymmetric transacetalization. J. Am. Chem. Soc. 2010;132:17370–17373. doi: 10.1021/ja108642s. [DOI] [PubMed] [Google Scholar]
- 22.Akiyama T. Stronger Brønsted acids. Chem. Rev. 2007;107:5744–5758. doi: 10.1021/cr068374j. [DOI] [PubMed] [Google Scholar]
- 23.Rono LJ, Yayla HG, Wang DY, Armstrong MF, Knowles RR. Enantioselective photoredox catalysis enabled by proton-coupled electron transfer: development of an asymmetric Aza-pinacol cyclization. J. Am. Chem. Soc. 2013;135:17735–17738. doi: 10.1021/ja4100595. [DOI] [PubMed] [Google Scholar]
- 24.Proctor, R. S. J., Davis, H. J. & Phipps, R. J. Catalytic enantioselective Minisci-type addition to heteroarenes. Science 360, 419–422 (2018). Science360, 419–422 (2018). [DOI] [PubMed]
- 25.Arceo E, Jurberg ID, Álvarez-Fernández A, Melchiorre P. Photochemical activity of a key donor-acceptor complex can drive stereoselective catalytic α-alkylation of aldehydes. Nat. Chem. 2013;5:750–756. doi: 10.1038/nchem.1727. [DOI] [PubMed] [Google Scholar]
- 26.Liang Y, Fu GC. Catalytic asymmetric synthesis of tertiary alkyl fluorides: Negishi cross-couplings of racemic α,α-dihaloketones. J. Am. Chem. Soc. 2014;136:5520–5524. doi: 10.1021/ja501815p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuadros S, Dell’Amico L, Melchiorre P. Forging fluorine-containing quaternary stereocenters by a light-driven organocatalytic aldol desymmetrization process. Angew. Chem. Int. Ed. 2017;56:11875–11879. doi: 10.1002/anie.201706763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moffett RB, Hester JB. Central nervous system agents. 4. Analogs of 3-amino-2-phenylpropiophenone. J. Med. Chem. 1972;15:1243–1247. doi: 10.1021/jm00282a010. [DOI] [PubMed] [Google Scholar]
- 29.Carlier PR, et al. Synthesis of a potent wide-spectrum Serotonin-, Norepinephrine-, Dopamine-Reuptake Inhibitor (SNDRI) and a species-selective dopamine-reuptake inhibitor based on the gamma-amino alcohol functional group. Bioorg. Med. Chem. Lett. 1998;8:487–492. doi: 10.1016/S0960-894X(98)00062-6. [DOI] [PubMed] [Google Scholar]
- 30.Peddie, V. et al. Fluorinated β2- and β3-amino acids: synthesis and inhibition of α-chymotrypsin. Synthesis 2010, 1845–1859 (2010).
- 31.Yu JS, Noda H, Shibasaki M. Quaternary β2,2-amino acids: catalytic asymmetric synthesis and incorporation into peptides by Fmoc-based solid-phase peptide synthesis. Angew. Chem. Int. Ed. 2018;57:818–822. doi: 10.1002/anie.201711143. [DOI] [PubMed] [Google Scholar]
- 32.Haufe G. Regio- and stereoselective synthesis of vicinal fluorohydrins. J. Fluor. Chem. 2004;125:875–894. doi: 10.1016/j.jfluchem.2004.01.023. [DOI] [Google Scholar]
- 33.Narayanam JMR, Tucker JW, Stephenson CRJ. Electron-transfer photoredox catalysis: development of a tin-free reductive dehalogenation reaction. J. Am. Chem. Soc. 2009;131:8756–8757. doi: 10.1021/ja9033582. [DOI] [PubMed] [Google Scholar]
- 34.Staveness D, Bosque R, Stephenson CRJ. Free radical chemistry enabled by visible light-induced electron transfer. Acc. Chem. Res. 2016;49:2295–2306. doi: 10.1021/acs.accounts.6b00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Studer A. The persistent radical effect in organic synthesis. Chem. Eur. J. 2001;7:1159–1164. doi: 10.1002/1521-3765(20010316)7:6<1159::AID-CHEM1159>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 36.Prier CK, Rankic DA, MacMillan DWC. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw MH, Twilton J, MacMillan DWC. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016;81:6898–6926. doi: 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicewicz DA, MacMillan DWC. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science. 2008;322:77–80. doi: 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen AE, MacMillan DWC. Synergistic catalysis: a powerful synthetic strategy for new reaction development. Chem. Sci. 2012;3:633–658. doi: 10.1039/c2sc00907b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brimioulle R, Lenhart D, Maturi MM, Bach T. Enantioselective catalysis of photochemical reactions. Angew. Chem. Int. Ed. 2015;54:3872–3890. doi: 10.1002/anie.201411409. [DOI] [PubMed] [Google Scholar]
- 41.Skubi KL, Blum TR, Yoon TP. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 2016;116:10035–10074. doi: 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie J, Jin H, Hashimi ASK. The recent achievements of redox-neutral radical C-C cross-coupling enabled by visible-light. Chem. Soc. Rev. 2017;46:5193–5203. doi: 10.1039/C7CS00339K. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, et al. Building congested ketone: substituted hantzsch ester and nitrile as alkylation reagents in photoredox catalysis. J. Am. Chem. Soc. 2016;138:12312–12315. doi: 10.1021/jacs.6b06379. [DOI] [PubMed] [Google Scholar]
- 44.Uraguchi D, Kinoshita N, Kizu T, Ooi T. Synergistic catalysis of ionic Brønsted acid and photosensitizer for a redox neutral asymmetric α-coupling of N-arylaminomethanes with aldimines. J. Am. Chem. Soc. 2015;137:13768–13771. doi: 10.1021/jacs.5b09329. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, et al. Asymmetric radcial-radical cross-coupling through visible-light-activated iridium catalysis. Angew. Chem. Int. Ed. 2016;55:685–688. doi: 10.1002/anie.201509524. [DOI] [PubMed] [Google Scholar]
- 46.Ma J, et al. Visible-light-activated asymmetric β-C-H functionalization of acceptor-substituted ketones with 1,2-dicarbonyl compounds. J. Am. Chem. Soc. 2017;139:17245–17248. doi: 10.1021/jacs.7b09152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renaud P, Gerster M. Use of lewis acids in free radical reactions. Angew. Chem. Int. Ed. 1998;37:2562–2579. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2562::AID-ANIE2562>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures that support the findings of this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the accession code CCDC 1587114 (12), 1589705 (25), and 1814679 (26). The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.