Abstract
The thermal inactivation kinetics of enzymes, including polyphenol oxidase (PPO) and peroxidase (POD), in chicory (Cichorium intybus L.) leaves were evaluated. In addition, the influences of different drying techniques (shade drying, hot air drying and freeze drying) on the phenolic profiles and antioxidant activities of chicory leaves were determined. The antioxidant activities of chicory leaves were evaluated on the basis of their 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, reducing power, and 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity. The results showed that the activation energy for PPO and POD inactivation were 123.00 kJ/mol and 78.99 kJ/mol, respectively. Preliminary treatment with hot water for 3 min at 90 °C was beneficial for preserving the phenolics present in fresh leaves. Hot air drying was better for the phenolics preservation. The hot air-dried and freeze-dried leaves possessed good antioxidant activities. The leaves with higher phenolics contents had better antioxidant activities, which indicated that the preservation of the phenolics was important for maintaining the antioxidant activity of chicory leaves.
Introduction
Chicory (Chicorium intybus L.), a major crop in northwestern Europe, has been used in indigenous medicine for hundreds of years1,2. In fact, chicory cultivation has multiple purposes; its leaves can be used as a vegetable and forage crop, and its roots can be used to produce both inulin and a coffee substitute3. Chicory has potent hepatoprotective, antioxidant, hypoglycemic, hydragogue and immunoregulatory activities4. It has been reported that chicory leaves contain a high content of phenolics (190 ± 2.03 mg/g of the dry matter)5. The content of phenolic compounds is strongly correlated with antioxidant capacity in plant samples, which suggests that chicory leaves are a good source of antioxidants6. However, the shelf-life of fresh chicory leaves is short because of enzymatic reactions and microbial growth. Thermal treatment, a preliminary processing method, and drying, a preservation technique, are deemed to be the most effective methods for preserving the quality of fresh materials7.
Polyphenol oxidase (PPO) is widespread in plant materials. The chemical conversion of phenolic compounds to quinones is catalyzed by PPO and leads to enzymatic browning and the loss of phenolics in fresh plant materials8. Peroxidase (POD) is also rich in plant materials. The chemical oxidation of phenolic compounds to phenoxy radicals is catalyzed by POD with hydrogen peroxide and causes the oxidation of chlorophyll9. Because of its thermostability and high content in most plants, POD is often used as an indicator in thermal food processing treatments10. Hot water blanching, an important thermal treatment method, is normally used for the inactivation of enzymes. The differences between the parameters used for heat-labile and heat-resistant isoenzyme fractions suggest the importance of the kinetics of POD and PPO inactivation in different raw materials7. However, no comparative studies have been reported on the enzyme inactivation kinetics of chicory leaves, which is important for the retention of their initial quality.
In addition, drying methods play an important role in food processing, and it has two vital functions: inhibiting microbial growth and facilitating storage11. To some extent, drying can affect the initial quality in terms of appearance and the preservation of unstable components. To date, several drying methods have been used to dehydrate plant materials7. Sun and shade drying are two common drying methods for fresh plant materials. They are operationally simple and are inexpensive. Shade drying is beneficial for the preservation of sun-unstable components. Hot air drying is applied to accelerate the drying process. However, oxidation or pyrolysis reactions during sun drying, shade drying or hot air drying may affect the chemical components of the plant materials12. Because of the low-temperature and low-pressure environment, freeze drying has advantages for preserving the quality of plant materials13. However, although freeze drying is beneficial for the preservation of sensory attributes, it might cause the loss of active ingredients14. Different drying methods affect the characteristic chemical compounds and the antioxidant activities of medicinal plants in different ways11. The effect of a particular drying method on the retention of raw quality is not predictable and depends on the compounds and the specific plant involved. Therefore, a significant amount of information for improving the qualities of products such as functional food ingredients or nutraceuticals can be revealed by the comparative evaluation of various drying technologies. However, information on the changes in the phenolic profiles and antioxidant activities of chicory leaves after drying with different methods is very limited.
Propper postharvest treatments (including preliminary processing and preservation technique) of chicory leaves possessing high levels of phenolics and antioxidant activities are essential for the retention of their initial quality. Therefore, the purposes of this research were: (1) establishing the mathematic relation between hot water blanching and enzyme (PPO and POD) inactivation by an enzymatic kinetic model and (2) evaluating the phenolic profiles and antioxidant activities of chicory leaves after drying by different techniques (shade, hot air and freeze drying).
Results and Discussion
Kinetic parameters of PPO and POD inactivation during thermal inactivation
The kinetic parameters for PPO and POD inactivation after water blanching are shown in Table 1. The changes in the residual PPO activity versus water blanching time at different temperatures (75, 80, 85, 90 and 95 °C) are shown in Fig. 1. The PPO activity in chicory leaves was significantly influenced by the blanching temperature and duration. Due to the high determination coefficients (R2) in the 0.9398 to 0.9974 range, the experimental results were well fit by a first-order kinetic model of enzymatic reactions under these experimental conditions. The k values of PPO inactivation in chicory leaves increased from 0.158 to 1.751 as the blanching temperature increased. The activation energy for PPO inactivation was 123.00 kJ/mol based on the calculation shown in Fig. 1.
Table 1.
Kinetic parameters of the first-order kinetic model for PPO and POD inactivation by water blanching.
| Temperature (°C) | PPO | POD | ||
|---|---|---|---|---|
| k/min | R 2 | k/min | R 2 | |
| 75 | 0.158 | 0.9886 | 0.273 | 0.9858 |
| 80 | 0.238 | 0.9974 | 0.468 | 0.9851 |
| 85 | 0.464 | 0.9756 | 0.663 | 0.9778 |
| 90 | 0.633 | 0.9905 | 1.097 | 0.9758 |
| 95 | 1.751 | 0.9398 | 1.133 | 0.9666 |
PPO, polyphenol oxidase; POD, peroxidase.
Figure 1.
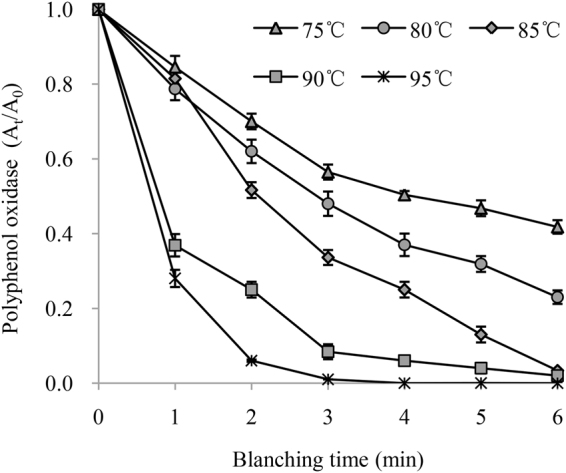
Residual polyphenol oxidase activity in chicory leaves during water blanching.
The plots of residual POD activity in the chicory leaves versus blanching time at five different temperatures (75, 80, 85, 90 and 95 °C) are shown in Fig. 2. Similar to PPO, the water blanching temperature and duration had significant influences on the POD activity in chicory leaves. According to the high determination coefficients (R2), which ranged from 0.9666 to 0.9858, the experimental results were well fit by a first-order kinetic model of enzymatic reactions under the tested temperatures. As the blanching temperature increased, the k values of POD inactivation in chicory leaves ranged from 0.273 to 1.133. The activation energy for POD inactivation was 78.99 kJ/mol based on the analysis shown in Fig. 2. The activation energy of PPO inactivation was higher than that of POD, which indicated PPO was more heat resistant than POD, and PPO was recommended as the enzymatic reference material for the heat treatment of chicory leaves.
Figure 2.
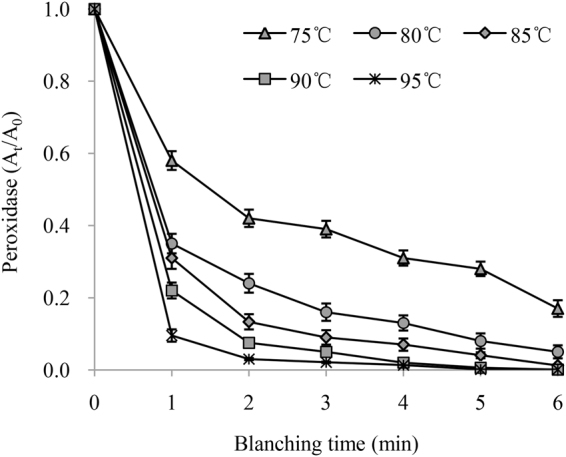
Residual peroxidase activity in chicory leaves during water blanching.
Amount of total phenolics preserved and DPPH radical scavenging activity after thermal inactivation
Hot water blanching is a common method of inactivating enzymes and preserving the initial quality of fresh materials. However, some thermally sensitive compounds including phenolics may lose their activities due to oxidization or diffusion into the water during hot water treatments. Nevertheless, the preservation of phenolics is important for maintaining the biological activities of the samples5. Therefore, the amount of phenolic compounds remaining after blanching can be used as an indicator for estimating the quality of chicory leaves.
The total phenolics extraction yields of chicory leaves after water blanching are shown in Fig. 3. At temperatures of 75, 80 and 85 °C, the extraction yields of total phenolics improved as the blanching time increased. These results may be due to the increased thermal inactivation of PPO and POD in chicory leaves as the treatment time increased at 75, 80 and 85 °C. The yield of total phenolics extracted from the leaves which were subjected a preliminary water blanching treatment for 3 min at 90 °C (5.58 ± 0.17%) was higher than those of other blanching temperatures. One possible reason was that treatment at higher temperatures may more effectively inactivate the PPO and POD in chicory leaves. Nevertheless, the total phenolics extraction yields decreased as the blanching time exceeding 2 min at 95 °C or 3 min at 90 °C (P < 0.05). This may be due to the serious thermal degradation of phenolics that occurs at 90 and 95 °C when blanching for an extended period. In addition, the phenolics extraction yields of leaves treated with hot water for 5–6 min at 95 °C were lower than those of leaves treated for the same time at 75, 80, 85 and 90 °C. Lin et al. found that the phenolics yields from Rabdosia serra (Maxim.) Hara leaf decreased as the blanching temperature increased from 70 to 100 °C, and the total phenolics yields decreased as the blanching time exceeding 4 min at 70 and 100 °C or 5 min at 90 °C7. As shown in Fig. 4, the DPPH radical scavenging activities of chicory leaves followed the same trends when blanched at different temperatures for 1 to 6 min, which indicated that the preservation of the phenolics is important for preserving the DPPH radical scavenging activity of chicory leaves.
Figure 3.
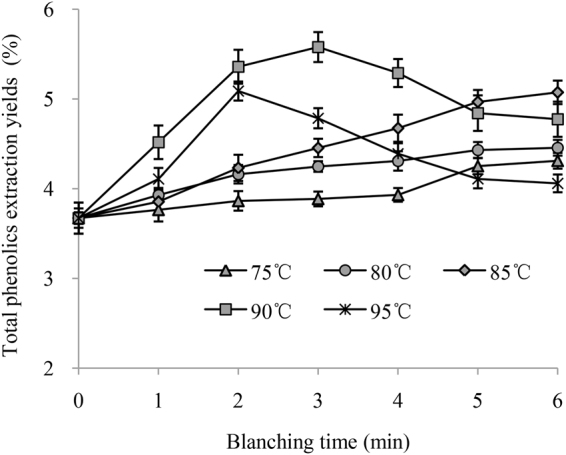
Total phenolics extraction yields of chicory leaves after water blanching.
Figure 4.
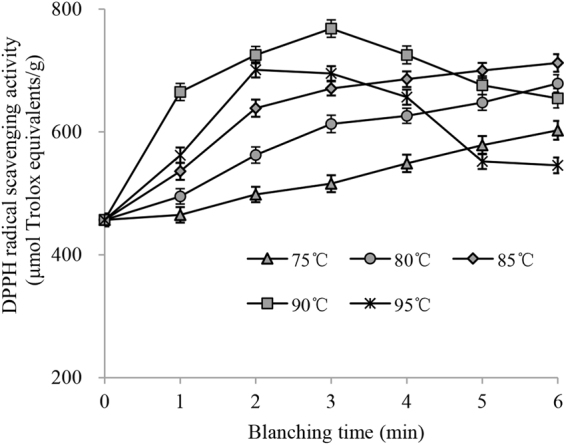
DPPH radical scavenging activity of chicory leaves after water blanching.
Therefore, considering the effectiveness of enzymatic inactivation and the preservation of total phenolics and the DPPH radical scavenging activity, the chicory leaves should be blanched with hot water for a relatively short time at a higher temperature. Water blanching for 3 min at 90 °C is recommended as the most effective thermal treatment for chicory leaves.
Assessment of different drying methods for chicory leaves
The moisture contents of leaves after dehydration using different methods decreased in the following order: shade-dried leaves (12.54 ± 0.18%) > freeze-dried leaves (12.07 ± 0.31%) > hot air-dried leaves (11.66 ± 0.12%) (P > 0.05). During freeze drying, the moisture in the plant materials was first frozen and then lost through sublimation. During shade drying and hot air drying, moisture is normally lost through evaporation. The key difference between these methods is that hot air could improve the water loss efficiency during hot air drying.
Inactivation kinetic parameters of PPO during drying processes
Based on the experiments on the thermal inactivation of enzymes conducted in this study, PPO is recommended as the enzyme reference material for the inactivation treatments of chicory leaves. Therefore, the PPO activity in chicory leaves was measured during different drying processes. The kinetic parameters and residual activities of PPO in chicory leaves during different drying processes are shown in Fig. 5. Due to the high determination coefficients (R2), which were in the 0.9299 to 0.9476 range, the experimental results were well fit by a first-order kinetic model of enzymatic reactions under the experimental conditions for these drying methods. The residual activities of PPO at the end of shade drying and freeze drying were 0.175 and 0.137, respectively. The residual activity of PPO was 0.062 after hot air drying. Therefore, hot air drying was more effective for PPO inactivation than shade drying and freeze drying.
Figure 5.
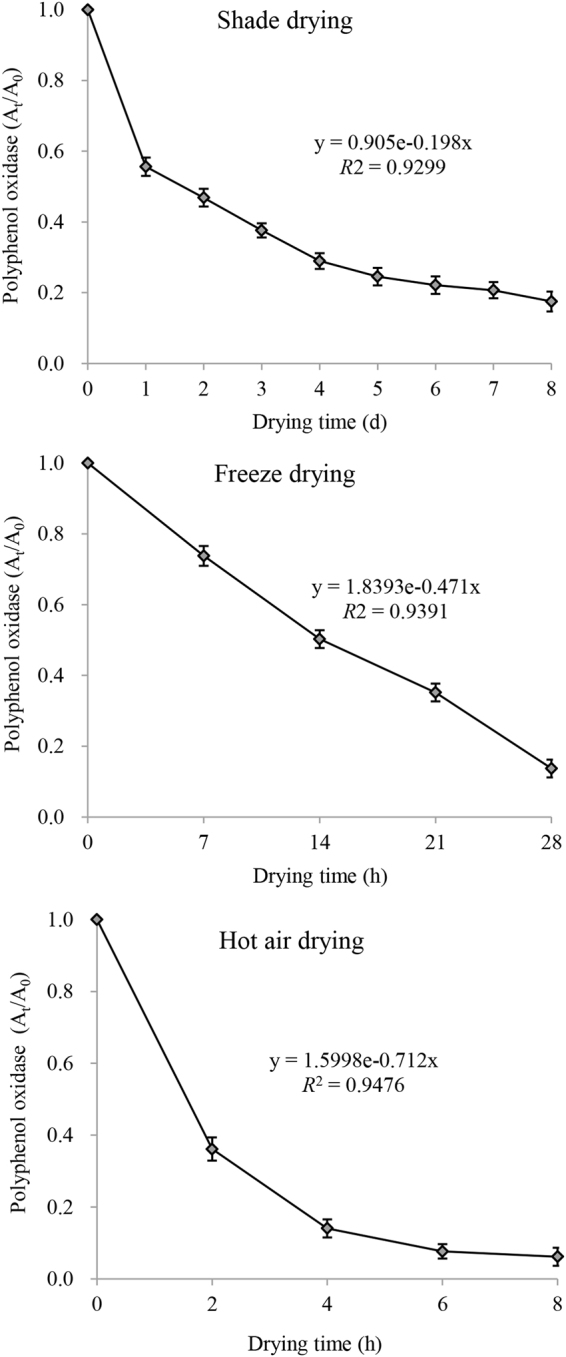
The kinetic parameters and residual activities of polyphenol oxidase in chicory leaves during different drying processes.
Phenolic compounds of chicory leaves dried by different methods
Different drying techniques had significantly effects on the total phenolics extraction yields of chicory leaves (P < 0.05). The total phenolics yields of leaves after drying with the different methods decreased in the following order: hot air-dried leaves (2.68 ± 0.09%) > freeze-dried leaves (2.34 ± 0.06%) > shade-dried leaves (1.35 ± 0.06%). Moreover, the influence of the drying method on the phenolic profile (chicoric acid, ferulic acid, chlorogenic acid, and caffeic acid) of chicory leaves was measured (Table 2). The content of chlorogenic acid in chicory leaves was highest among the tested phenolic compounds, followed by the contents of caffeic acid, ferulic acid and chicoric acid. The contents of chlorogenic acid and caffeic acid were significantly different among leaves dried by the three different methods (P < 0.05). The chlorogenic acid and caffeic acid contents in the leaves decreased in the following order: hot air-dried leaves > freeze-dried leaves > shade-dried leaves. The ferulic acid and chicoric acid contents in the leaves dried with hot air were also higher than those of leaves dried with the other two methods (P < 0.05). There were no significant differences in the contents of chicoric acid and ferulic acid between the shade-dried and freeze-dried leaves (P > 0.05). Hot air drying is recommended as a beneficial drying method for chicory leaves for preserving the phenolics constituents based on the current study.
Table 2.
Phenolic profile of chicory leaves after different drying methods (mg/g).
| Items | Shade drying | Freeze drying | Hot air drying |
|---|---|---|---|
| Chlorogenic acid | 1.756 ± 0.117c | 2.432 ± 0.186b | 4.558 ± 0.173a |
| Caffeic acid | 0.510 ± 0.009c | 0.580 ± 0.003b | 0.644 ± 0.032a |
| Chicoric acid | 0.033 ± 0.004b | 0.020 ± 0.005b | 0.137 ± 0.023a |
| Ferulic acid | 0.022 ± 0.001b | 0.036 ± 0.003b | 0.350 ± 0.043a |
Means in the same row (same phenolic compound) with different superscripts (a–c) differ significantly (P < 0.05). Data are the means ± SD (n = 3).
Hot air drying is one of the most commonly used dried methods in food industry and is inexpensive. Shade and freeze drying of chicory leaves caused losses of phenolics in this study. Losses of phenolics during drying can mainly be attributed to oxidative reactions. During shade drying and hot air drying, both non-enzymatic and enzymatic oxidative reactions are likely taking place in the plant material. However, hot air drying can inhibit enzymatic oxidative reactions to some extent due to the inactivation of enzymes at 60 °C (Fig. 5). Enzymatic oxidation by PPO and POD is more likely to occur during freeze drying due to the lower exposure to oxygen and to the damage to the cell structure caused by ice crystals formation12. Therefore, freeze drying caused a greater loss in the contents of total phenolics, chlorogenic acid, caffeic acid, ferulic acid and chicoric acid than hot air drying (Table 2). These differences can be explained by the formation of ice crystals within the tissue matrix during freeze drying, leading to cell rupture and exposure of the phenolic species to the aforementioned oxidative conditions15. Freeze drying of guava powders and pumpkin also led to the loss of greater contents of phenolic compounds than hot air drying12,14. However, in most cases, freeze dried products exhibit much higher contents of bioactive compounds than hot air dried products. Some studies have shown that the freeze drying of grape skin, tomatoes, ginger and citrus fruits leads to higher contents of phenolic compounds than hot air drying16–18. Therefore, whether hot air or freeze drying will be better for preserving the content of phenolic compounds in a given fruit or vegetable is difficult to predict based on data from other foods and needs to be supported by experimental data from the relevant plant.
Antioxidant activities of chicory leaves dried by different methods
The DPPH free radical is a kind of stable free radical. The ability to scavenge the DPPH radical is widely used to estimate the antioxidant activities of biological samples19. The antioxidant substances can reduce the DPPH radical to diphenyl-picrylhydrazine, which is yellow20. The antioxidant ability of a sample is highly correlated with the content of the yellow compound in the reaction system. The ABTS free radical is also stable, and the ABTS radical scavenging assay only needs a short reaction time (approximately 15 min), and this method has been widely used to assess the antioxidant capacities of various samples21. There is a positive correlation between the antioxidant capacity and the reducing power of a sample22. The free radical chain can be broken down by an antioxidant by providing a hydrogen atom or by the reaction with a peroxide precursor, which concomitantly inhibits the formation of peroxide23.
The DPPH radical scavenging activities, reducing powers, and ABTS radical scavenging activities of chicory leaves dehydrated by different methods are present in Table 3. Higher numerical values represent stronger antioxidant capacities. The leaves dried with hot air possessed stronger ferric reducing power and DPPH radical scavenging activity than leaves dehydrated by freeze drying or shade drying (P < 0.05). The ABTS radical scavenging activities of the hot air-dried and freeze-dried leaves were not significantly different (P > 0.05). The leaves dehydrated by shade drying exhibited lower antioxidant activities than the leaves dried by hot air or freeze drying (P < 0.05).
Table 3.
Antioxidant activities of chicory leaves after different drying methods (μmol Trolox equivalents/g).
| Items | Shade drying | Freeze drying | Hot air drying |
|---|---|---|---|
| DPPH radical scavenging activity | 165.0 ± 10.1c | 346.4 ± 12.5b | 389.7 ± 9.90a |
| ABTS radical scavenging activity | 139.2 ± 8.20b | 264.7 ± 14.3a | 280.3 ± 11.1a |
| Ferric reducing power | 232.2 ± 13.7c | 430.3 ± 13.6b | 458.2 ± 13.1a |
Means in the same row (same antioxidant assay) with different superscripts (a–c) differ significantly (P <0.05). Data are the means ± SD (n = 3).
The chicory leaves with higher total phenolics contents possessed greater antioxidant capacities. Correlation analysis indicated that the total phenolics contents of the leaves was positively correlated with their DPPH radical scavenging activity (r = 0.998, P < 0.05). These results showed that the preservation of phenolics was important for the retention of the antioxidant activity of chicory leaves.
In conclusion, severe enzymatic degradation can be catalyzed by PPO and POD in harvested fresh chicory leaves. For enzymatic inactivation, PPO is recommended as the reference enzyme for the heat treatments of chicory leaves because PPO has a higher activation energy than POD. Preliminary treatment with hot water for 3 min at 90 °C was beneficial for the retention of the phenolic compounds in fresh leaves. The phenolic profiles and antioxidant activities of chicory leaves were significantly influenced by the drying techniques. Hot air drying was better for the preservation of phenolic compounds in chicory leaves. The hot air-dried and freeze-dried leaves possessed good antioxidant activities. The leaves with higher phenolics contents had stronger antioxidant activities, which indicated that phenolics preservation is important to maintain the antioxidant activity of chicory leaves.
Materials and Methods
Chicory leaves and reagents
Fresh aboveground parts of chicory were harvested in Jilin Agricultural University (Jilin, China). The plants were in the bloom stage. Leaves were separated from the stems and cleaned with water without damaging the leaves. All the chemicals used in high-performance liquid chromatography (HPLC) were of HPLC-grade. DPPH, gallic acid, Folin-Ciocalteu reagent, ABTS, chicoric acid, ferulic acid, caffeic acid, and chlorogenic acid were bought from Sigma-Aldrich (St. Louis, USA). All other reagents were of analytical-grade and were obtained from local suppliers.
Thermal inactivation treatments
For the heat treatment of chicory leaves with hot water, 50 g of fresh leaves was treated at 75, 80, 85, 90 and 95 °C for 1 to 6 min. After treatment in a hot water bath, the leaves were quickly cooled with ice water to terminate the thermal inactivation. A twenty-gram sample of fresh or blanched leaves was homogenized with a lab blender (JJ-2, Changzhou Lang Yue Instrument Manufacturing Co., Ltd., Changzhou, China) with 200 mL of 95% (v/v) ethanol. The homogenate was subjected to ultrasonic treatment using an ultrasonic processer (KQ-100KDE, Kunshan ultrasonic instruments Co., Ltd., Kunshan, China) at room temperature for 60 min to extract the total phenolics. Next, centrifugation (3K30, Sigma, Germany) at 4 °C and 3000 g for 10 min was used to separate the supernatant from the extract homogenate. The supernatant was adjusted to a volume of 200 mL by 95% (v/v) ethanol and prepared for the determination of total phenolics content and DPPH radical scavenging activity following thermal inactivation treatment. The DPPH radical scavenging activity is reported in micromole Trolox per gram of chicory leaves (dry basis). The total phenolics extraction yield was calculated by using the following equation (1):
| 1 |
where C is the total phenolics concentration by mass in the chicory leaf extract, V is the volume of chicory leaf extract, and W is the weight of chicory leaves (dry basis) used to prepare the chicory leaf extract.
Measurement of PPO and POD activities
The enzymes in the blanched chicory leaves were extracted and purified based on the method reported by Ünal and Sener24. The extraction and purification processes were carried out at 4 °C. The crude extract was purified by DEAE-Toyopearl 650 M and Sephadex G-100 gel column chromatography. The PPO and POD activities and protein content were monitored in the collected fractions (3 mL). The fractions showing maximum PPO activity and POD activity were separately pooled and used to analyze the enzyme inactivation kinetics of PPO and POD.
The method used for measuring the PPO activity was adapted from Augusto et al.25 using pyrogallol as the substrate25. Pyrogallol (40 mM) was dissolved in phosphate buffer (0.2 M, pH 7). The reaction system was composed of enzyme extract (0.25 mL) and pyrogallol solution (2.75 mL). The blank was the same mixture except the enzyme extract was replaced with the same volume of phosphate buffer (0.2 M, pH 7). The absorbance was recorded at a wavelength of 420 nm.
The method used for measuring the POD activity was adapted from Tan et al.26. Guaiacol was used as the substrate. The reaction system was composed of phosphate buffer (0.2 M, 1.7 mL, pH 6), guaiacol (40 mM, 1 mL), hydrogen peroxide (40 mM, 1 mL), and the enzyme extract (0.3 mL). The blank was composed of the same reagents except additional phosphate buffer (0.2 M, 0.3 mL, pH 6) was used instead of the enzyme extract. The absorbance was measured at 470 nm.
To determine the enzymes activities of PPO and POD, absorbance values were read every 20 seconds for 30 min. A curve was established using time as the abscissa and absorbance as the ordinate. The rate of the enzymatic reaction was calculated from the linear portion of the curve. The amount of enzyme that induced a 0.01 increase in the absorbance every minute was defined as one unit of PPO or POD activity27.
Enzyme inactivation kinetic models
It was reported that a first-order inactivation kinetic model was suitable for describing the enzyme inactivation of PPO and POD during hot water treatment of the plant material28. The kinetic parameters of PPO and POD inactivation were calculated according to the following equation (2):
| 2 |
where At is the enzyme activity at time t; A0 is the enzyme activity at time = 0; k is the first-order rate constant; and t is the hot water treatment time.
In addition, the thermal treatment temperature and k are related by the Arrhenius equation as follows (3):
| 3 |
where kref is the rate constant of a reference temperature (Tref); Ea is the activation energy of the enzymatic reaction; R is the gas constant; and T is the absolute temperature.
Drying methods of fresh leaves
Three dehydration methods (shade drying, hot air drying, and freeze drying) were used to dry the chicory leaves. Shade drying was done in a shady and drafty room (20 °C, relative humidity of 45%) for 8 days. Hot air drying was done in a drying oven at 60 °C for 8 h, and the air flow rate was 2.0 m·s−1. Freeze drying was done in a vacuum freeze dryer (SCIENTZ-12N, Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) at −70 °C for 28 h. The PPO activity of the chicory leaves was measured as described in the ‘Measurement of PPO and POD activities’ section above following the thermal inactivation treatments. The moisture contents of the chicory leaf samples were measured by oven drying at 105 °C.
Extraction of phenolic compounds from chicory leaves after drying
The dried chicory leaves were ground into powders and passed through a 1 mm sieve. One gram of the dried chicory leaf powder was mixed with 95% aqueous ethanol (25 mL), and the mixture was subjected to ultrasonic treatment in an ultrasonic bath (KQ-100KDE, Kunshan ultrasonic instruments Co., Ltd., Kunshan, China) at room temperature for 60 min. After extraction, the samples were centrifuged for 10 min (4 °C, 3000 g). The supernatant was adjusted to 25 mL by aqueous ethanol (95%) and was prepared for the determination of the total phenolics content and antioxidant activities and HPLC analysis of phenolic compounds.
Measurement of the total phenolics content
The Folin–Ciocalteu method was used to assay the total phenolics contents of the chicory leaf extracts29,30. Distilled water (6 mL) and Folin–Ciocalteu reagent (0.5 mL) were mixed with chicory leaf extract (0.1 mL). The mixture was kept at room temperature for 3 min. Aqueous sodium carbonate solution (20%, 1.5 mL) and distilled water (1.9 mL) were added into the mixture. The mixture was incubated at room temperature for 60 min in a dark place. The absorption was assayed at 760 nm against a blank. The blank consisted of the same mixture except the chicory leaf extract was replaced with 0.1 mL of aqueous ethanol (95%). The standard curve used for the calculation of the total phenolics content was calibrated with gallic acid.
Analysis of the phenolic compounds
The method used to determine the phenolic compounds was adapted from Kaewnarin et al.31 and used an LC-2010ATH HPLC instrument (Shimadzu, Japan) coupled to a UV detector31. The chicory leaf extract was separated on an Amethyst C18-H column (250 × 4.6 mm, 5.0 μm). The mobile phase was composed of 0.1% aqueous phosphoric acid and 100% acetonitrile (20:80, v:v). The column oven temperature, detection wavelength, injection volume, and flow rate were 25 °C, 327 nm, 20 μL, and 1 mL/min, respectively. All the phenolic acids, including chicoric acid, caffeic acid, chlorogenic acid, and ferulic acid, were quantified using an external standard (Fig. S1).
Antioxidant activities
The antioxidant activities of the chicory leaf extracts were evaluated using the DPPH radical scavenging activity32, ferric reducing power33, and ABTS radical scavenging activity assays34. The equivalent antioxidant activities were determined relative to a standard curve of Trolox, and the values are reported in micromole Trolox per gram of the chicory leaf sample (dry basis).
Statistical analysis
All treatments were carried out in triplicate, and all sample analyses were performed three times and averaged. The results are reported as the mean ± standard deviation (SD). One-way ANOVA in SPSS 17.0 for Windows (SPSS Inc., Chicago, IL) was performed for statistical analysis. Differences at P < 0.05 were considered to be statistically significant by Duncan’s multiple-range test. Linear regressions were used to estimate the linear correlation between two sets of parameters. Regression parameters at 0.05 probability were regarded as a significant correlation.
Data Availability
All data generated or analyzed during this study are included in this published article.
Electronic supplementary material
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 31601972), and the Project Funded by China Postdoctoral Science Foundation (No. 2017M621224).
Author Contributions
All authors had reviewed the manuscript. H.M.S. and R.L. designed the research. R.L., M.H.W., J.Y.Y. and M.Y.D. performed the experiments. H.M.S., R.L. and H.X.W. drafted the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27874-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nishimura M, et al. Effects of the extract from roasted chicory (Cichorium intybus L.) root containing inulin-type fructans on blood glucose, lipid metabolism, and fecal properties. Journal of Traditional and Complementary Medicine. 2015;5:161–167. doi: 10.1016/j.jtcme.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saggu S, et al. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food and Chemical Toxicology. 2014;72:138–146. doi: 10.1016/j.fct.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Fan H, et al. Isolation and identification of terpenoids from chicory roots and their inhibitory activities against yeast α-glucosidase. European Food Research and Technology. 2017;246:1009–1017. doi: 10.1007/s00217-016-2810-1. [DOI] [Google Scholar]
- 4.Mulabagal V, Wang HB, Ngouajio M, Nair MG. Characterization and quantification of health beneficial anthocyanins in leaf chicory (Cichorium intybus) varieties. European Food Research and Technology. 2009;230:47–53. doi: 10.1007/s00217-009-1144-7. [DOI] [Google Scholar]
- 5.Conforti F, et al. The protective ability of Mediterranean dietary plants against the oxidative damage: The role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chemistry. 2009;112:587–594. doi: 10.1016/j.foodchem.2008.06.013. [DOI] [Google Scholar]
- 6.Cai YZ, Luo Q, Sun M, Corke H. Antioxidant capacity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sciences. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin LZ, et al. Thermal inactivation kinetics of Rabdosia serra (Maxim.) Hara leaf peroxidase and polyphenol oxidase and comparative evaluation of drying methods on leaf phenolic profile and bioactivities. Food Chemistry. 2012;134:2021–2029. doi: 10.1016/j.foodchem.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Duangmal K, Apenten RKO. A comparative study of polyphenoloxidases from taro (Colocasia esculenta) and potato (Solanum tuberosum var. Romano) Food Chemistry. 1999;64:351–359. doi: 10.1016/S0308-8146(98)00127-7. [DOI] [Google Scholar]
- 9.Rudra SG, Shivhare US, Basu S, Sarkar BC. Thermal inactivation kinetics of peroxidase in coriander leaves. Food and Bioprocess Technology. 2008;1:187–195. doi: 10.1007/s11947-007-0013-2. [DOI] [Google Scholar]
- 10.Zhu Y, Pan ZL, McHugh TH, Barrett DM. Processing and quality characteristics of apple slices processed under simultaneous infrared dry-blanching and dehydration with intermittent heating. Journal of Food Engineering. 2010;97:8–16. doi: 10.1016/j.jfoodeng.2009.07.021. [DOI] [Google Scholar]
- 11.Periche A, Castelló ML, Heredia A, Escriche I. Effect of different drying methods on the phenolic, flavonoid and volatile compounds of Stevia rebaudiana leaves. Flavour and Fragrance Journal. 2016;31:173–177. doi: 10.1002/ffj.3298. [DOI] [Google Scholar]
- 12.Nunes JC, et al. Effect of drying method on volatile compounds, phenolic profile and antioxidant capacity of guava powders. Food Chemistry. 2016;197:881–890. doi: 10.1016/j.foodchem.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 13.Borchani C, et al. Effect of drying methods on physico-chemical and antioxidant properties of date fibre concentrates. Food Chemistry. 2011;125:1194–1201. doi: 10.1016/j.foodchem.2010.10.030. [DOI] [Google Scholar]
- 14.Aydin E, Gocmen D. The influences of drying method and metabisulfite pre-treatment on the color, functional properties and phenolic acids contents and bioaccessibility of pumpkin flour. LWT – Food Science and Technology. 2015;60:385–392. doi: 10.1016/j.lwt.2014.08.025. [DOI] [Google Scholar]
- 15.Sogi DS, Siddiq M, Dolan KD. Total phenolics, carotenoids and antioxidant properties of Tommy Atkin mango cubes as affected by drying techniques. LWT-Food Science and Technology. 2015;62:564–568. doi: 10.1016/j.lwt.2014.04.015. [DOI] [Google Scholar]
- 16.de Torres C, Díaz-Maroto MC, Hermosín-Gutiérrez I, Pérez-Coello MS. Effect of freeze-drying and oven-drying on volatiles and phenolics composition of grape skin. Analytica Chimica Acta. 2010;660:177–182. doi: 10.1016/j.aca.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Gümüşay ÖA, Borazan AA, Ercal N, Demirkol O. Drying effects on the antioxidant properties of tomatoes and ginger. Food Chemistry. 2015;173:156–162. doi: 10.1016/j.foodchem.2014.09.162. [DOI] [PubMed] [Google Scholar]
- 18.Sun YJ, Shen Y, Liu DH, Ye XQ. Effects of drying methods on phytochemical compounds and antioxidant activity of physiologically dropped un-matured citrus fruits. LWT- Food Science and Technology. 2015;60:1269–1275. doi: 10.1016/j.lwt.2014.09.001. [DOI] [Google Scholar]
- 19.Hu FL, Lu RL, Huang B, Ming L. Free radical scavenging activity of extracts prepared from fresh leaves of selected Chinese medicinal plants. Fitoterapia. 2004;75:14–23. doi: 10.1016/j.fitote.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Luo AX, et al. In Vitro and In vivo antioxidant activity of a water-soluble polysaccharide from Dendrobium enneanum. Molecules. 2011;16:1579–1592. doi: 10.3390/molecules16021579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu LC, et al. Antioxidant and antiproliferative activities of red pitaya. Food Chemistry. 2006;95:319–327. doi: 10.1016/j.foodchem.2005.01.002. [DOI] [Google Scholar]
- 22.Duh PD, Yen GC. Antioxidant activity of three herbal water extracts. Food Chemistry. 1997;60:639–645. doi: 10.1016/S0308-8146(97)00049-6. [DOI] [Google Scholar]
- 23.Xing RE, et al. Antioxidant activity of differently regioselective chitosan sulfates in vitro. Bioorganic and Medical. Chemistry. 2005;13:1387–1392. doi: 10.1016/j.bmc.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Ünal MÜ, Sener A. Two-year comparison of the biochemical properties of polyphenol oxidase from Turkish Alyanak apricot (Prunus armenica L.) Food Chemistry. 2016;190:741–747. doi: 10.1016/j.foodchem.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Augusto PED, Ibarz R, Garvín A, Ibarz A. Peroxidase (POD) and polyphenol oxidase (PPO) photo-inactivation in a coconut water model solution using ultraviolet (UV) Food Research International. 2015;74:151–159. doi: 10.1016/j.foodres.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 26.Tan TC, et al. Composition, physicochemical properties and thermal inactivation kinetics of polyphenol oxidase and peroxidase from coconut (Cocos nucifera) water obtained from immature, mature and overly-mature coconut. Food Chemistry. 2014;142:121–128. doi: 10.1016/j.foodchem.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 27.Zhang YL, et al. Influence of several postharvest processing methods on polyphenol oxidase activity and cichoric acid content of Echinacea purpurea roots. Industrial Crops and Products. 2011;34:873–881. doi: 10.1016/j.indcrop.2011.02.010. [DOI] [Google Scholar]
- 28.Zheng H, Lu HF. Effect of microwave pretreatment on the kinetics of ascorbic acid degradation and peroxidase inactivation in different parts of green asparagus (Asparagus officinalis L.) during water blanching. Food Chemistry. 2011;128:1087–1093. doi: 10.1016/j.foodchem.2011.03.130. [DOI] [Google Scholar]
- 29.Heimler D, Isolani L, Vignolini P, Romani A. Polyphenol content and antiradical activity of Cichorium intybus L. from biodynamic and conventional farming. Food Chemistry. 2009;114:765–770. doi: 10.1016/j.foodchem.2008.10.010. [DOI] [Google Scholar]
- 30.Değirmencioğlu N, Gürbüz O, Herken EN, Yıldız AY. The impact of drying techniques on phenolic compound, total phenolic content and antioxidant capacity of oat flour tarhana. Food Chemistry. 2016;194:587–594. doi: 10.1016/j.foodchem.2015.08.065. [DOI] [PubMed] [Google Scholar]
- 31.Kaewnarin K, Suwannarach N, Kumla J, Lumyong S. Phenolic profile of various wild edible mushroom extracts from Thailand and their antioxidant properties, anti-tyrosinase and hyperglycaemic inhibitory activities. Journal of Functional Foods. 2016;27:352–364. doi: 10.1016/j.jff.2016.09.008. [DOI] [Google Scholar]
- 32.Bachiega P, et al. Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chemistry. 2016;190:771–776. doi: 10.1016/j.foodchem.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Ma LS, Chen HX, Zhu WC, Wang ZH. Effect of different drying methods on physicochemical properties and antioxidant activities of polysaccharides extracted from mushroom Inonotus obliquus. Food Research International. 2013;50:633–640. doi: 10.1016/j.foodres.2011.05.005. [DOI] [Google Scholar]
- 34.Liang NJ, Xue W, Kennepohl P, Kitts DD. Interactions between major chlorogenic acid isomers and chemical changes in coffee brew that affect antioxidant activities. Food Chemistry. 2016;213:251–259. doi: 10.1016/j.foodchem.2016.06.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


