Abstract
Northern regions have received considerable attention not only because the effects of climate change are amplified at high latitudes but also because this region holds vast amounts of carbon (C) stored in permafrost. These carbon stocks are vulnerable to warming temperatures and increased permafrost thaw and the breakdown and release of soil C in the form of carbon dioxide (CO2) and methane (CH4). The majority of research has focused on quantifying and upscaling the effects of thaw on CO2 and CH4 emissions from terrestrial systems. However, small ponds formed in permafrost wetlands following thawing have been recognized as hotspots for C emissions. Here, we examined the importance of small ponds for C fluxes in two permafrost wetland ecosystems in northern Sweden. Detailed flux estimates of thaw ponds during the growing season show that ponds emit, on average (±SD), 279 ± 415 and 7 ± 11 mmol C m−2 d−1 of CO2 and CH4, respectively. Importantly, addition of pond emissions to the total C budget of the wetland decreases the C sink by ~39%. Our results emphasize the need for integrated research linking C cycling on land and in water in order to make correct assessments of contemporary C balances.
Introduction
Climate warming accelerates permafrost thaw in northern regions, leading to the breakdown and release of soil carbon (C) in the form of carbon dioxide (CO2) and methane (CH4)1. Northern permafrost wetlands are of particular importance due to their vast C stocks2, which are vulnerable to changes in climate3. Increases in ambient air temperatures will result in permafrost degradation and the release of stored C as CO2 and CH4, thus contributing to a positive feedback on the climate1. Degradation of permafrost and the ground slumping that follows can often cause shifts in vegetation composition and expansion of wet areas4,5 that may lead to the formation and growth of thaw ponds6,7. Thaw lakes and ponds have been suggested to play a particularly significant role in the permafrost C feedback loop by acting as direct conduits for the release of CO2 and CH4 to the atmosphere1,8,9. However, few in-depth pond studies exist, especially so regarding their quantitative importance for the C exchange between land and atmosphere. Freshwater lakes are well known to be significant emitters of CO2 and CH4 on a landscape scale10–12, but previous studies do not include empirical measurements from small thaw ponds. Here, we examined the importance of small ponds for C fluxes in permafrost wetlands in northern Sweden.
On a few occasions when C exchange between water and air has been measured in thaw ponds, they are frequently found to be high emitters of CO2 and CH47,13–15. The importance of thaw ponds, however, is largely overlooked in large scale assessments of C cycling due to their small size and the difficulties in mapping them using satellite imagery. For example, most upscaling studies exclude ponds smaller than 0.001 km2 16. Integrated studies in which direct C fluxes from small ponds in permafrost regions are included into a catchment-scale C balance are thus still lacking. This study is the first to directly include C exchange from small thaw ponds in a wetland C exchange estimate.
In this study, we quantified atmospheric C exchange from 52 small thaw ponds (surface area 4–150 m2) in two permafrost wetlands to determine their role in the total (terrestrial + aquatic) net carbon balance (NCB). Of the 52 ponds, we carried out high-resolution chamber measurements of CO2 and CH4 fluxes over the full growing season of 15 ponds with different vegetation composition, shoreline erosion, and water sources (for map of sampling locations see Supplementary Figure S1). Furthermore, we measured dissolved CO2 and CH4 of the other 37 thaw ponds to assess large-scale spatial patterns. The study location in northern Sweden is a representative of regions with high permafrost vulnerability. The study region is characterized by discontinuous permafrost that has been experiencing substantial thawing during the last decades, mainly attributed to changes in air temperature and precipitation17 (Fig. 1). Detailed assessments of one of the wetlands have observed an increased wetness between 1970 and 200018. By using aerial color imagery from 2008, we found that this trend has persisted alongside increases in annual air temperature and active layer thickness (Fig. 1). This change emphasizes the need to better quantify the role of ponds in the landscape C exchange.
Figure 1.
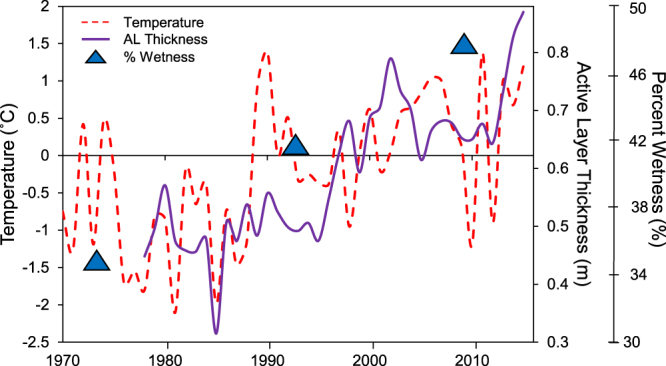
Decadal trends show increasing annual means for the Abisko area including annual air temperature (updated from Callaghan et al.31), autumnal active layer thickness (from 1978, updated from Akerman & Johansson17; bars represent standard deviations) and wetness data from Stordalen (based on three values from 1970, 1990 and 2008, updated from Malmer et al.18). Temperature data were collected at the Abisko Scientific Research Station, 10 km from Stordalen. Active layer thickness were measured in multiple mires in the Abisko area. The data on percent wetness from 2008 and ALT from 2007 is a contribution from this study.
Results and Discussion
The results show strong oversaturation and high exchange of CO2 and CH4 to the atmosphere from the thaw ponds. The concentrations (mean ± standard error) of CO2 (467 ± 21 μM, n = 52) and CH4 (24 ± 3 μM, n = 52) are approximately an order of magnitude higher than the mean values reported for thaw ponds in northern Canada4 (means = 34 and 2 μM for CO2 and CH4, respectively), but are in the same range as permafrost ponds in west-central Siberia19 (range = 70–770 and 0.6–48 μM for CO2 and CH4, respectively). The average CO2 exchange measured from the ponds in our study (279 ± 58 mmol C m−2 d−1) was greater than exchanges (max = 114 mmol C m−2 d−1) reported from thaw ponds in northern Canada7 and from lakes (average value 15 mmol C m−2 d−1) in the same catchment as in this study20. The mean diffusive CH4 exchange (7 ± 0.2 mmol C m−2 d−1) in the ponds was among the highest diffusive CH4 fluxes reported, but was the same magnitude as found in ponds in eastern Siberia (average = 4 mmol C m−2 d−1)14. Ebullition was highly variable between lakes and accounted for 71% the total CH4 flux when observed (17 ± 6 mmol C m−2 d−1).
Four pond types were identified during this study-including open water, moss-dominated, sedge-dominated, and lake-fed ponds (Supplementary Figure S2). Both CH4 and CO2 varied widely throughout the growing season and pond types showed different temporal variation. There were not significant differences in ebullition fluxes between pond types (Mann-Whitney U Statistical test, p-value = 0.44). Open-water systems had the highest mean diffusive emissions for both CO2 (610 ± 69 mmol C m−2 d−1) and CH4 (14 ± 2 mmol C m−2 d−1; Mann-Whitney U Statistical test, p-value < 0.001; Fig. 2b,d). These ponds had little to no vegetation at the surface or on the bottom and were surrounded by eroding banks, characteristics associated with high C exchange from ponds in northern Canada7. While this study was not designed to assess the importance of various drivers of C dynamics across ponds, the relatively high diffusive fluxes from open water ponds could be a result of high gas exchange velocities, relatively low photosynthetic CO2 fixation, and high terrestrial input of C and organic matter from adjacent thawing and eroding soils compared to vegetated ponds.
Figure 2.
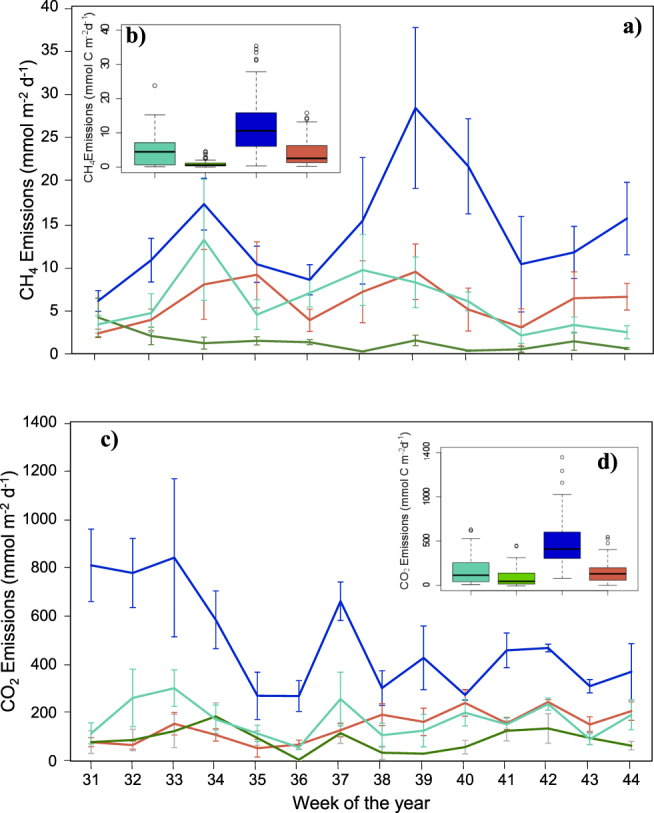
(a) Temporal, bi-weekly diffusive CH4 emissions for lake-fed (light green), moss-dominated (dark green), open-water (blue) and sedge-dominated (orange) ponds. (b) Mean CH4 emissions over the sampling period for each pond type. (c) Temporal, bi-weekly CO2 emissions. (d) Mean CO2 emissions over the sampling period for each pond type. Note the difference in scales. Error bars on bi-weekly plots represent the variation between mean fluxes from individual ponds within each pond type. Mean open-water pond emissions were the highest for both CO2 and CH4 compared to the rest of the pond types (Mann-Whitney U Statistical test, p-value < 0.001 for open-water and other pond type pairs).
A critical question is to what degree the emissions from thaw ponds are of quantitative importance compared to the overall C exchange from permafrost wetlands. One of the wetlands in this study (Stordalen) has been subject to extensive C exchange studies by chamber and eddy covariance techniques, enabling the first estimate of a complete NCB of a permafrost wetland and the specific importance of thaw ponds. Earlier studies in Stordalen found that the entire wetland is a large sink for CO2 and a source of CH421,22. However, these studies focused mainly on the terrestrial or vegetated (non-open water patches) wetland and did not measure ponds directly. To incorporate the C emissions from ponds in our study into the NCB for the entire wetland, we combined our data with the mean terrestrial C exchange over the green season from 2002 to 2007 from Bäckstrand et al.21, and the areal coverage of open water ponds (0.43 ha) and the vegetated wetland (16.5 ha) in 2008. In the study by Bäckstrand et al.22, based on automated chamber measurements, terrestrial wetland components were divided into dry palsa, sphagnum, and wet/minerotrophic eriophorum patches. While their C-budget for the wetland includes wet areas, it does not include diffusive emission estimates for ponds specifically. The addition of the pond C emissions from 2015 to the NCB increased the CH4 source and decreased the CO2 sink (Fig. 3). The overall NCB, including both CO2-C and CH4-C, decreased from −44 kg C d−1 to −27 kg C d−1. We do note that C fluxes are dependent on multiple physical parameters and these parameters are not exactly the same between 2000 and 2015.
Figure 3.
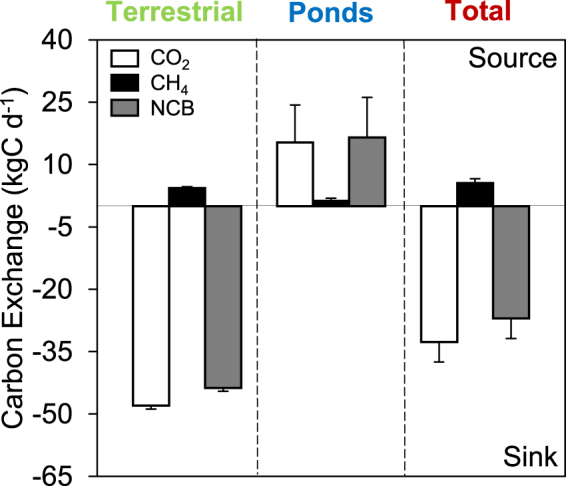
C exchange rate for CO2, CH4 (diffusion + ebullition), and CO2 + CH4 (NCB) for terrestrial wetland components, ponds, and the combination of terrestrial components and ponds over the growing season. The error bars for terrestrial components represent standard error in fluxes over a 7-year period17 (2002–2007), while the errors bars for ponds represent the uncertainty in areal coverage of ponds between 2000 and 2008.
Although the C emission from the thaw ponds are of quantitative importance, the exact contribution should be treated with caution given the uncertainty in area of ponds. We estimated a range in contribution (Fig. 3) of thaw ponds to the NCB by using the area of ponds in 2000 (~1%)18 and 2008 (~2.5%). It is highly likely that the ponds now occupy an even larger percentage of the wetland landscape due to further permafrost thaw and saturated hollow development23, however images from this study’s research season were not available. Assuming pond developing continuous to follow the trend observed between 2000 and 2008 (1.5% increase), total pond area in 2015 can be estimated to be around 4%. At 4% of the total land cover, C emissions from ponds would reduce the NCB from ~−27 kgC d−1 to only ~−3 kgC d−1. Additionally, available images used for upscaling in this study only identify open-water ponds and do not distinguish vegetated ponds from the surrounding terrestrial vegetation; therefore, we only use open-water emission means in the upscaling estimate. Not including vegetated ponds is most likely a conservative estimate of the NCB. To upscale C emissions from small ponds and improve estimates of larger scale NCB of high latitude peatland regions, there is a need for higher resolution areal data and improved techniques to estimate the areal C contribution from different ponds in the landscape.
Conclusion
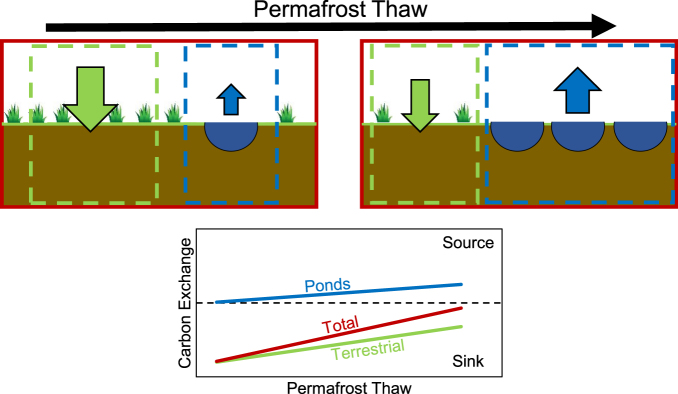
Our results suggest that ponds in northern permafrost wetlands are considerable sources of C to the atmosphere, significantly offsetting the net C uptake of wetlands, and thus reducing overall atmospheric C sink strength of the landscape. Thaw ponds cover a significant portion of land in the north6, and it is likely that those systems represent an important and sensitive component of the permafrost carbon feedback loop. The role of ponds as hotspots for C exchange following permafrost thaw and subsequent pond formation may be of increasing importance in permafrost regions in future decades following predicted permafrost thaw6. While projecting the trajectory of pond development and C exchange is highly uncertain, pond coverage may even decrease in some regions following thawing24,25, our results suggest that C release from thaw ponds following permafrost thaw may strongly contribute to a decrease in the overall C sink capacity of northern wetlands (Fig. 4). To reduce uncertainties, there is a need for more integrated research and further experiments or manipulations that focus on incorporated small ponds into regional NCB.
Figure 4.
A conceptual diagram outlining the possible impact of permafrost thaw on the overall C sink capacity of northern wetlands where thawing results in increased coverage by ponds. The diagram emphasizes the need to integrate studies of terrestrial (green) and pond (blue) ecosystems in order to understand and project the carbon exchange of whole wetlands (red).
Importantly, the type of pond and the vegetation that it is comprised of may be a driving factor. If ponds develop into sedge-dominated or deeper, open-water systems, more C may be released to the atmosphere. Vegetation shifts in this direction have already been observed during naturally induced permafrost thaw17,18 and experimentally due to increased snow cover26. However, if there is a trend towards moss-dominated ponds, atmospheric C emissions could be substantially less. Understanding and quantifying the C emissions from the different types of ponds is crucial to predicting and modeling future emissions from permafrost wetlands subject to thawing since the ponds are constantly changing and evolving. Our results emphasize the need for integrated research linking C cycling on land and in water to make correct assessments of contemporary and future C balances.
Methods
Site description
Sampling took place in two wetland complexes over the duration of the growing season (June-October) in 2015. The Stordalen (68 N, 19 E) and Storflaket (68 N, 19 E) wetlands are located in the same catchment, approximately 3 km apart, in a discontinuous permafrost zone east of Abisko, Sweden. Stordalen covers ~15 km2 and Storflaket ~3 km2. The wetland complexes contain raised patches of peat underlain by permafrost, which are surrounded by saturated hallows and ponds. The hollows are comprised of graminoids (Carex sp., Eriophorum sp.) and sphagnum sp., while the palsas are covered by woody shrubs (eg Betula nana), evergreen shrubs (eg, Andromeda polifolia, Empetrum hermaphroditum), moss (Sphagnum sp.) and lichen. The active layer thickness is approximately 0.5 m in the palsas and 1–3 meters in the hallows at both sites17. Yearly temperature and precipitation means for this region were recorded at the nearby Abisko Scientific Research Station (http://www.polar.se/abisko).
Ponds
In total, we sampled 52 ponds. Six ponds in Storflaket and nine ponds in Stordalen were sampled twice a week from the end of June until ice cover in early October. An additional 37 ponds were sampled in July. The ponds were chosen to cover a variety of morphologies, vegetation types, and degrees of erosion along the shoreline. Ponds were classified into moss dominated, sedge dominated and open water based on within-pond (1 × 1 meter grid) surface vegetation composition using a cluster analyses (R Company 3.2.1, pvclust Package). We also separated lake fed ponds that occasionally received external inputs of water from nearby lake.
Water sampling and analysis
Water temperature, dissolved oxygen, and barometric pressure were measured in situ four times a week using a YSI Professional Optical Dissolved Oxygen instrument (Yellow Springs Instruments, Ohio, USA), which was calibrated at the start of each week. Surface water samples were collected weekly in 0.5 L containers, pre-rinsed in the field with pond water. Samples were filtered the same day in the laboratory (Sarstedt Filtropur S 0.45 mm), acidified (DOC) thereafter refrigerated (DOC) until processing. Conductivity and pH were analyzed in the laboratory on the same day as sampling (MP220, Mettler Toledo International Inc.). Dissolved inorganic carbon (DIC) and dissolved CH4 samples were collected from the surface of the water twice a week using a five mL plastic syringe. Using a headspace method, 4 mL of water was injected into a pre-prepared, 22 mL vial (Wheaton) with 20 uL of 20% HCL27. Vials were stored in an upside down position at room temperature until analysis. Methane and DIC concentrations were determined using a gas chromatograph with a methanizer and flame-ionization detector (Clarus 500, Perkin Elmer Inc. U.S.). Internal standards were prepared with each batch of samples to correct for possible leakage of gas over time. Filtered DOC samples were run on a Shimadzu TOC-V CPH analyzer (Shimadzu Corporation, Japan).
Dissolved CO2 concentrations were measured twice a week using a Vaisala sensor attached to a handheld logger (Helsinki, Finland, GMM220 and GM70 series). Sensors were deployed at the water’s surface and were occasionally stirred until the concentration hit equilibrium (5–30 minutes). Concentrations were later back calibrated in the laboratory to correct for temperature and pressure settings28.
Carbon dioxide fluxes
Carbon dioxide fluxes were measured twice a week at seven of the fifteen ponds using a floating chamber (volume = 10.9 L). A plastic chamber was equipped with a CO2 sensor and attached by rope to a pole and handheld data logger (Vaisala Inc. Helsinki, Finland, GMM220 and GM70 series). The chamber was floated on the surface of the water between 9 am and 3 pm for 5–10 minutes at each pond while the concentration was recorded. Using the linear change in concentration and the dimensions of the chamber, the flux (F) was estimated using equation 1:
| 1 |
where Vch is the volume of the chamber (m3), A is the area of the chamber (m2), S is the linear change in concentration measured over the five-minute period (ppm/min), Kh is Henry’s law constant (dependent on temperature), P is the pressure (atm), and Q is a conversion factor to set the flux units to mmol Cm−2 d−1. To calculate the flux of CO2 from the ponds that were not directly measured with a chamber, we used the concentration of dissolved CO2 and a variation of Fick’s law (eq. 2) to determine the piston velocity coefficient (k):
| 2 |
which can be rearranged to solve for k as:
| 3 |
where F is the chamber flux (umol Cm−2 d−1), Cw is the concentration of dissolved gas at equilibrium with the headspace (umol Cm−3) and Ca is the concentration of CO2 in the atmosphere. All dissolved gas concentrations (CO2 and CH4) were calculated using Henry’s law:
| 4 |
where pGAS is the partial pressure of the gas in the headspace (ppm). The calculated k for both CO2 and CH4 values were normalized to 20 °C by calculating k600 values29.
Methane fluxes
A 24-hour static chamber (volume = 10.9 L) flux method was used to measure total CH4 emissions at each pond30. Flux measurements were taken on a biweekly schedule. To separate the contributions of ebullition events from the diffusive flux, we looked at the distribution of the experimentally derived k600 values from the CO2 chamber flux measurements. Measured CH4 fluxes with k600 values above the mean CO2 k600 value (0.79 m d−1) were not considered in the diffusive flux calculations. A total of 22% (61 of 280) measurements were determined to be effected by ebullition (based on k600 > 0.79 m d−1) and ebullition were estimated by subtracting the average diffusive flux from the total flux of ebullition-impacted chambers30. Given that ebullition is highly sporadic and spatially heterogeneous, we reported ebullition as a mean rate.
Statistics
Statistical analyses were done using the R statistics package (3.2.2, The R Company). Both CO2 and CH4 fluxes were normally distributed, however, the variances and sample sizes between pond types and monthly fluxes were not the same, therefore, we used the Kruskal-Wallis test for multiple group analyses and the Mann-Whitney U tests for follow-up pair-wise comparisons (p < 0.05). K600 values for CO2 and CH4 showed similar distributions.
GIS Analysis and Carbon Budget Estimate
Pond area within Stordalen was calculated using ArcGIS software (esri. ArcGIS Desktop. 10.1 Redlands, CA). We used a mosaic of real-color images (8 × 8 cm resolution) of Stordalen mire taken in August 2008. The mosaic was clipped to represent the same area as presented in Malmer et al.18 and as used in Bäckstrand et al.21. Then, digital classification of open water ponds was determined with the help of user-selected training locations. Once training sites were selected, we used supervised classification to identify all open water ponds in the mire. We then used the pixel count of all areas classified as a pond to determine the total pond area in the mire.
To incorporate C emissions from ponds into the terrestrial NCB for the wetland, we used pond flux measurements from Storflaket and Stordalen and terrestrial flux values reported in Bäckstrand et al.21. In this study, the wetland was split into dry palsa, sphagnum, and wet/minerotrophic eriophorum components. Fluxes were measured from these patch types over a period of 7 years. The average emission rates from each land-cover type and the average number of growing days over the 7-year period were used in our estimate of total C loss and uptake from the terrestrial components of the mire. Importantly, while Bäckstrand et al.’s C-budget for the wetland includes wet areas, it does not include fluxes from open patches of water. Here we included both diffusive and ebullition fluxes in the previous NBC estimate by multiplying the mean daily flux from our study for both CO2 and CH4 from open-water ponds with the total pond area of the wetland. Total CO2 and CH4 emissions over the growing season (averaged from the previous 7 year data set) from the ponds were then added to total C emission and uptake estimates for the terrestrial components of the mire. Only fluxes and areas of open water ponds were included in upscaling due to difficulties associated with separating moss and sedge dominated ponds from wet, minerotrophic patches in the aerial image.
Data availability
Data are available upon request.
Electronic supplementary material
Acknowledgements
Support for this work was funded by the Fulbright US Student Grant and the Climate Impacts Research Centre. We thank Kalle Heuchel, Albin Bjärhall, Keith Larson, and Anders Jonsson, for help with fieldwork and laboratory analyses. Thank you to the Abisko Scientific Research Station for supplying weather data and for use of the facilities.
Author Contributions
J.K. and M.K. designed the study; M.J. contributed data; M.K. performed fieldwork; E.L., M.K. did laboratory work; M.K., E.L., R.G., and J.K. analyzed and interpreted the data; and M.K., J.K., M.J., E.L., and R.G. contributed to the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27770-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schuur EAG, et al. Vulnerability of Permafrost Carbon to Climate Change: Implications for the Global Carbon Cycle. BioScience. 2008;58:701–714. doi: 10.1641/B580807. [DOI] [Google Scholar]
- 2.Turunen J, Tomppo E, Tolonen K, Reinikainen A. Estimating carbon accumulation rates of undrained mires in Finland – application to boreal and subarctic regions. The Holocene. 2002;12:69–80. doi: 10.1191/0959683602hl522rp. [DOI] [Google Scholar]
- 3.Fronzek S, Luoto M, Carter T. Potential effect of climate change on the distribution of palsa mires in subarctic Fennoscandia. Climate Research. 2006;32:1–12. doi: 10.3354/cr032001. [DOI] [Google Scholar]
- 4.McLaughlin J, Webster K. Effects of Climate Change on Peatlands in the Far North of Ontario, Canada: a Synthesis. Arctic, Antarctic, and Alpine Research. 2014;46:84–102. doi: 10.1657/1938-4246-46.1.84. [DOI] [Google Scholar]
- 5.Johansson C, Pohjola VA, Jonasson C, Callaghan TV. Multi-Decadal Changes in Snow Characteristics in Sub-Arctic Sweden. AMBIO. 2011;40:566–574. doi: 10.1007/s13280-011-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olefeldt D, et al. Circumpolar distribution and carbon storage of thermokarst landscapes. Nat Comms. 2016;7:13043. doi: 10.1038/ncomms13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurion I, et al. Variability in greenhouse gas emissions from permafrost thaw ponds. Limnol. Oceanogr., 55(1), 2010, 115–133. Limnol. Oceanogr. 2009;55:115–133. doi: 10.4319/lo.2010.55.1.0115. [DOI] [Google Scholar]
- 8.Walter, K. M., Chanton, J. P., Chapin, F. S. I., Schuur, E. A. G. & Zimov, S. A. Methane production and bubble emissions from arctic lakes: Isotopic implications for source pathways and ages. J. Geophys. Res. Biogeosci. 113 (2008).
- 9.Holgerson MA, Raymond PA. Drivers of carbon dioxide and methane supersaturation in small, temporary ponds. Biogeochemistry. 2015;124:1–14. doi: 10.1007/s10533-015-0099-y. [DOI] [Google Scholar]
- 10.Drake TW, Raymond PA, Spencer RGM. Terrestrial carbon inputs to inland waters: A current synthesis of estimates and uncertainty. Limnol. Oceanogr. 2017;19:395–11. [Google Scholar]
- 11.Cole JJ, et al. Plumbing the Global Carbon Cycle: Integrating Inland Waters into the Terrestrial Carbon Budget. Ecosystems. 2007;10:172–185. doi: 10.1007/s10021-006-9013-8. [DOI] [Google Scholar]
- 12.Kling GW, Kipphut GW, Miller MC. The flux of CO2 and CH4 from lakes and rivers in arctic Alaska. Hydrobiologia. 1992;240:23–36. doi: 10.1007/BF00013449. [DOI] [Google Scholar]
- 13.Wik M, Varner RK, Anthony KW, MacIntyre S, Bastviken D. Climate-sensitive northern lakes and ponds are critical components of methane release. Nature Geosci. 2016;9:99–106. doi: 10.1038/ngeo2578. [DOI] [Google Scholar]
- 14.Knoblauch C, Spott O, Evgrafova S, Kutzbach L, Pfeiffer E-M. Regulation of methane production, oxidation, and emission by vascular plants and bryophytes in ponds of the northeast Siberian polygonal tundra. J. Geophys. Res. 2016;120:2525–2541. doi: 10.1002/2015JG003053. [DOI] [Google Scholar]
- 15.Liebner S, et al. Methane oxidation associated with submerged brown mosses reduces methane emissions from Siberian polygonal tundra. Journal of Ecology. 2011;99:914–922. doi: 10.1111/j.1365-2745.2011.01823.x. [DOI] [Google Scholar]
- 16.Holgerson MA, Raymond PA. Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nature Geosci. 2016;9:222–226. doi: 10.1038/ngeo2654. [DOI] [Google Scholar]
- 17.Åkerman HJ, Johansson M. Thawing permafrost and thicker active layers in sub-arctic Sweden. Permafrost Periglac. Process. 2008;19:279–292. doi: 10.1002/ppp.626. [DOI] [Google Scholar]
- 18.Malmer., et al. Decadal vegetation changes in a northern peatland, greenhouse gas fluxes and net radiative forcing. Glob Change Biol. 2006;12:2352–2369. doi: 10.1111/j.1365-2486.2006.01267.x. [DOI] [Google Scholar]
- 19.Pokrovsky OS, Shirokova LS, Kirpotin SN, Kulizhsky SP, Vorobiev SN. Impact of western Siberia heat wave 2012 on greenhouse gases and trace metal concentration in thaw lakes of discontinuous permafrost zone. Biogeosciences. 2013;10:5349–5365. doi: 10.5194/bg-10-5349-2013. [DOI] [Google Scholar]
- 20.Lundin EJ, et al. Is the subarctic landscape still a carbon sink? Evidence from a detailed catchment balance. J. Geophys. Res. 2016;43:1988–1995. [Google Scholar]
- 21.Bäckstrand K, et al. Annual carbon gas budget for a subarctic peatland, Northern Sweden. Biogeosciences. 2010;7:95–108. doi: 10.5194/bg-7-95-2010. [DOI] [Google Scholar]
- 22.Christensen TR, et al. Monitoring the Multi-Year Carbon Balance of a Subarctic Palsa Mire with Micrometeorological Techniques. AMBIO. 2012;41:207–217. doi: 10.1007/s13280-012-0302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiegler, C. Surface energy exchange and land-atmosphere interactions of Arctic and subarctic tundra ecosystems under climate change. 1–56 (2016).
- 24.Smith LC, Sheng Y, MacDonald GM, Hinzman LD. Disappearing Arctic Lakes. Science. 2005;308:1429–1429. doi: 10.1126/science.1108142. [DOI] [PubMed] [Google Scholar]
- 25.Andresen CG, Lougheed VL. Disappearing Arctic tundra ponds: Fine-scale analysis of surface hydrology in drained thaw lake basins over a 65 year period (1948–2013) J. Geophys. Res. Biogeosci. 2015;120:466–479. doi: 10.1002/2014JG002778. [DOI] [Google Scholar]
- 26.Johansson M, et al. Rapid responses of permafrost and vegetation to experimentally increased snow cover in sub-arctic Sweden. Environ. Res. Lett. 2013;8:035025–11. doi: 10.1088/1748-9326/8/3/035025. [DOI] [Google Scholar]
- 27.Karlsson J, et al. Quantifying the relative importance of lake emissions in the carbon budget of a subarctic catchment. J. Geophys. Res. 2010;115:549–6. doi: 10.1029/2010JG001305. [DOI] [Google Scholar]
- 28.Johnson, M. S. et al. Direct and continuous measurement of dissolved carbon dioxide in freshwater aquatic systems-method and applications. Ecohydrol. n/a–n/a, 10.1002/eco.95 (2009).
- 29.Jahne B, Heinz G, Dietrich W. Measurement of the diffusion coefficients of sparingly soluble gases in water. J. Geophys. Res. 1987;92:10767–10776. doi: 10.1029/JC092iC10p10767. [DOI] [Google Scholar]
- 30.Bastviken, D., Cole, J., Pace, M. & Tranvik, L. Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Global Biogeochem. Cycles18, n/a–n/a (2004).
- 31.Callaghan, T. V. et al. A new climate era in the sub-Arctic: Accelerating climate changes and multiple impacts. Geophys. Res. Lett. 37, n/a–n/a (2010).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request.