Abstract
Ovarian cancer is the second most common gynaecologic malignancy and the main cause of death from gynaecologic cancer, due to late diagnosis and chemoresistance. Studies have reported the role of cysteine in cancer, by contributing for hydrogen sulphide (H2S) generation and as a precursor of glutathione (GSH). However, the role of cysteine in the adaptation to hypoxia and therapy response remains unclear. We used several ovarian cancer cell lines, ES2, OVCAR3, OVCAR8, A2780 and A2780cisR, to clarify cysteine relevance in ovarian cancer cells survival upon hypoxia and carboplatin. Results show that ES2 and OVCAR8 cells presented a stronger dependence on cysteine availability upon hypoxia and carboplatin exposure than OVCAR3 cells. Interestingly, the A2780 cisR, but not A2780 parental cells, benefits from cysteine upon carboplatin exposure, showing that cysteine is crucial for chemoresistance. Moreover, GSH degradation and subsequent cysteine recycling pathway is associated with ovarian cancer as seen in peripheral blood serum from patients. Higher levels of total free cysteine (Cys) and homocysteine (HCys) were found in ovarian cancer patients in comparison with benign tumours and lower levels of GSH were found in ovarian neoplasms patients in comparison with healthy individuals. Importantly, the total and S-Homocysteinylated levels distinguished blood donors from patients with neoplasms as well as patients with benign from patients with malignant tumours. The levels of S-cysteinylated proteins distinguish blood donors from patients with neoplasms and the free levels of Cys in serum distinguish blood from patients with benign tumours from patients with malignant tumours. Herein we disclosed that cysteine contributes for a worse disease prognosis, allowing faster adaptation to hypoxia and protecting cells from carboplatin. The measurement of serum cysteine levels can be an effective tool for early diagnosis, for outcome prediction and follow up of disease progression.
Introduction
Ovarian cancer is a group of distinct diseases that have a common anatomical location1 and it is the major cause of death from gynaecologic cancer and the second most common gynaecologic malignancy worldwide2,3. The diagnosis at an advanced stage, when a cure is rare, together with resistance to conventional therapy, have a dramatic impact in patient survival4. Epithelial ovarian cancer (EOC) includes the majority of malignant ovarian neoplasms5, and the carcinoma histotypes are serous (OSC), endometrioid, clear cell (OCCC) and mucinous. The high-grade OSC is the prevalent histotype4 with diagnosis at an advanced stage in approximately 70% of patients1. The OCCC is a rather uncommon histotype that is frequently diagnosed at an initial stage but highly chemoresistant6. The standard care for ovarian cancer is a combination of surgery and paclitaxel-carboplatin combined chemotherapy7. However, despite an initial response, the disease recurs in over 85% of cases with advanced ovarian cancer8. The development of ascites is a common characteristic of ovarian cancer9. The ascitic fluid contains growth factors secreted by both cancer and stromal cells9 and these factors are mitogenic to cancer cells, contributing for an ideal microenvironment for tumour growth10,11.
Metabolism reprogramming is a common feature of cancer cells, providing enough sources of energy and biomass to support cancer cell survival and proliferation12. Serpa and Dias proposed a model in which cells not adapted to microenvironment would undergo cell death whereas cells metabolically fitted would be positively selected and carry on cancer progression and metastasis13. Soon after this report, Hanahan and Weinberg also included reprogramming of energy metabolism as an emerging hallmark of cancer14.
Expanding evidence exists on the dependence of these processes on cysteine and its metabolism, as cysteine contributes to the generation of hydrogen sulphide (H2S)15–20 and glutathione (GSH)21–23. It is known that tumours are subjected to intermittent hypoxia24,25 and that hypoxia-inducible factors (HIFs) mediate adaptive pathophysiological responses underlying resistance to radiation therapy and chemotherapy26. In the context of ovarian cancer, Cutter et al.27, have recently reported that cancer cell lines subject to hypoxia are more invasive, have increased migratory ability and an epithelial-mesenchymal transition (EMT) phenotype27. Additionally, higher levels of cytoplasmic thiol-containing species, including GSH or metallothioneins have been associated with resistance to platinum-based chemotherapy23,28. These species are rich in cysteine and result in decreased drug availability and increased intracellular drug detoxification/inactivation, since platinum binds readily to sulfhydryl groups29. Lopes-Coelho et al.23 have shown that different ovarian cancer histological types present different metabolic outcomes concerning thiols and chemoresistance. Under normoxic conditions the OCCC cells were more resistant to carboplatin than OSC cells. Moreover, the inhibition of GSH synthesis by buthionine sulphoximine (BSO) sensitized OCCC cells to carboplatin23. Therefore, chemoresistance in those cells might be associated with thiols dynamics. However, the role of cysteine in hypoxic ovarian cancer cells survival and its influence on cells’ response to chemotherapy remains unknown. As hypoxia can be a mean of selecting more aggressive resistant cancer cells, we hypothesised that cysteine favours the adaptation to hypoxic conditions and chemotherapeutic agents, having a pivotal role in tumour progression, recurrence and chemoresistance. To test this hypothesis, we used several ovarian cancer cell lines, including serous carcinoma (OSC - OVCAR3 and OVCAR8), sensitive and resistant to Cisplatin A2780 (A2780 parental and A2780 cisR) unspecified histotype ovarian carcinoma cells and clear cell carcinoma (OCCC-ES2) cells. To better clarify the relevance of cysteine and other thiols in ovarian cancer, we analysed their levels in peripheral blood serum from patients with benign and malignant tumours, from healthy individuals and in ascitic fluid from ovarian cancer patients.
Results
Cysteine has a widespread protective effect under hypoxia in ovarian cancer cells
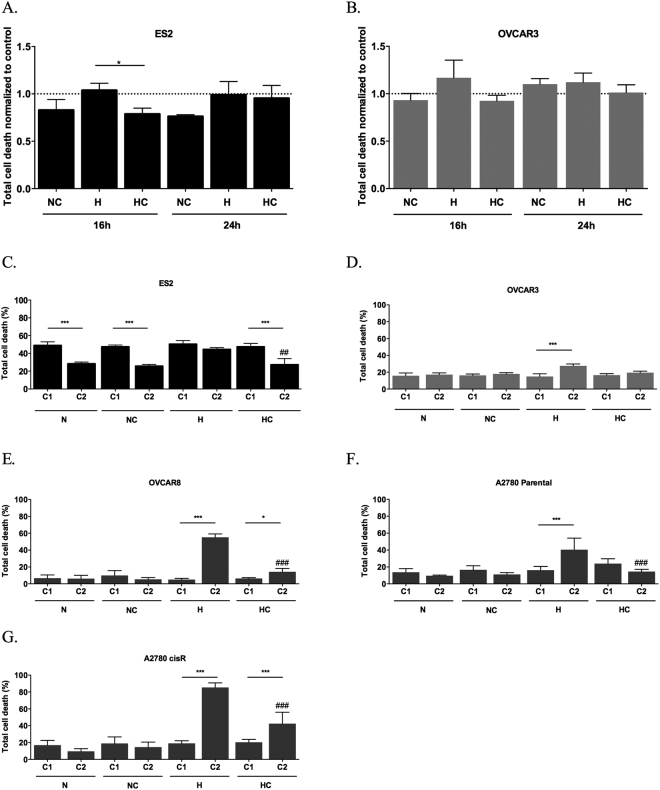
Cysteine protected ES2 cells from death under hypoxia (H) after 16 h (p = 0.026) (Fig. 1A), but the effect was not maintained after 24 h (Fig. 1A). Regarding OVCAR3 cells, no differences were found in total cell death levels in both time-points (Fig. 1B). However, in early apoptosis (p = 0.01) after 24 h of assay cysteine was advantageous for cells under H (Suppl. Fig. 1).
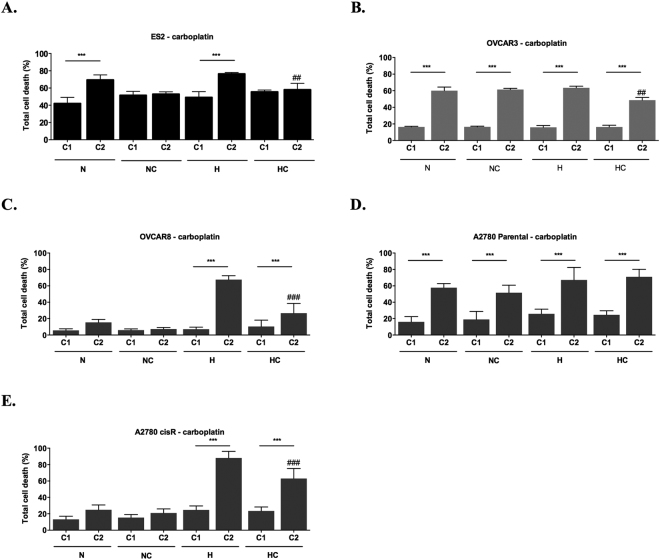
Figure 1.
Cysteine has a widespread protective effect under hypoxia in ovarian cancer cells. Total cell death normalized by control for 16 and 24 h of assay for (A) ES2 cells and (B) OVCAR3 cells and total cell death in the first cycle (C1) and in the second cycle (C2) of treatments in (C) ES2, (D) OVCAR3, (E) OVCAR8, (F) A2780 parental cells and (G) A2780 cisR cells. N - Normoxia; NC - Normoxia supplemented with cysteine; H - Hypoxia; HC - Hypoxia supplemented with cysteine. Results are shown as mean ± SD. Cardinals represent statistical significance among hypoxia with and without cysteine supplementation within the same cycle of treatments. *p < 0.05, **p < 0.01, ***p < 0.001 (One-way ANOVA with post hoc Tukey tests).
The effect of normoxia (N) with cysteine and H without cysteine in total cell death was similar between cell lines. However, there was no similarity for the effect of H plus cysteine on cell death in ES2 and OVCAR3 cells (p = 0.049). As observed, ES2 cells presented lower death levels compared to its control than OVCAR3 cells (Fig. 1A and B). With 24 h of incubation under N, cysteine induced higher levels of cell death in OVCAR3 cells compared to ES2 cells (p = 0.001). However under H, there were no significant differences among cell lines.
Next, we addressed the long-term effect of cysteine in ovarian cancer cells, also clarifying if the absence of protection by cysteine under H with 24 h of assay in ES2 was due to cysteine consumption or CoCl2 exhaustion. The exposure of ES2 cells to two experimental cycles revealed a significant decrease in total cell death levels in all conditions from the first to the second cycle (p = 0.000 for N, NC and HC), with the exception of H without cysteine (Fig. 1C).
No major alterations were registered from the first to the second cycle in total cell death for OVCAR3 cell line with the exception of total cell death under H without cysteine (p < 0.001; Fig. 1D).
We also investigated the effect of cysteine in the adaptation to hypoxia in other ovarian cancer cell lines. In OVCAR8 cell line, total cell death levels increased from the first to the second cycle under hypoxia, especially without cysteine, with no differences in the normoxia conditions (under H p < 0.001, under HC p = 0.020) (Fig. 1E). Nonetheless, in the second, cysteine revealed to be also advantageous for this cell line (H vs HC p < 0.001).
We also addressed if cysteine impacts differently the adaptive potential to hypoxia in sensitive and cisplatin resistant A2780 cells. In hypoxia condition, both A2780 and A2780 cisR cell lines died more at the second cycle of culture without cysteine and A2780 cisR with cysteine (A2780 cells p < 0.001; A2780 cisR cells p < 0.001 under H and HC). Nevertheless, cysteine revealed to be advantageous under hypoxia in the second cycle for both cell lines (H vs HC for A2780 parental cells p < 0.001, for A2780 cisR cells p < 0.001) (Fig. 1F and G).
So different ovarian cancer cells present different abilities to deal with metabolize cysteine Moreover, results have shown a widespread protective effect of cysteine upon hypoxic stress in ovarian cancer cells from different origins.
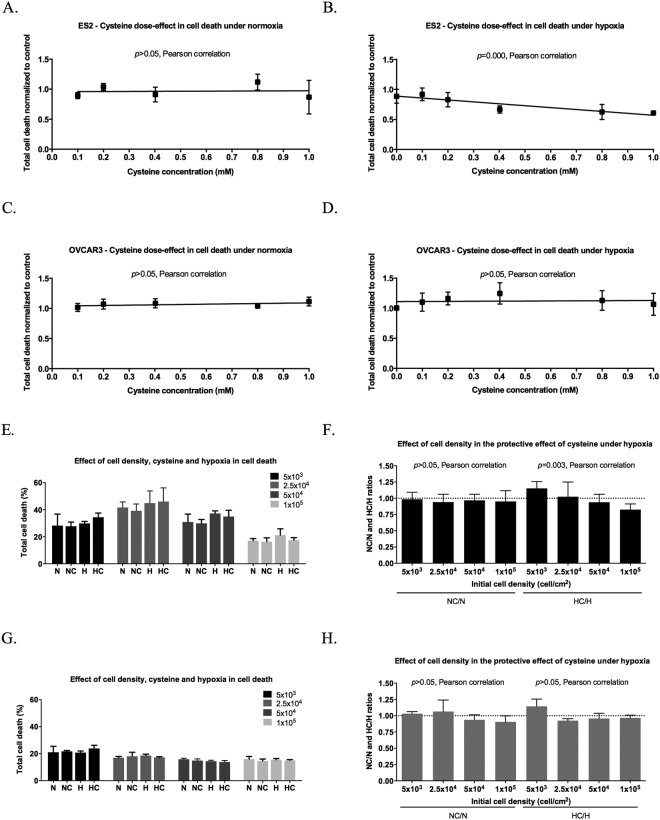
The protective effect of cysteine under hypoxia (H) in ES2 (OCCC) is dose dependent
To test whether the protective effect of cysteine in ES2 cells in H was dose-dependent, we performed an assay under N and H without cysteine and with increasing cysteine concentrations. While there were no dose-dependence effects in ES2 in N (Fig. 2A), cell death decreased in H with increasing cysteine concentrations (Pearson r = −0.777, p < 0.001) (Fig. 2B).
Figure 2.
The dose dependent and cell density dependent effects of cysteine in hypoxia adaptation in ES2 (OCCC) and OVCAR3 (OSC) cells. (A) Pearson correlation under normoxia for ES2 cell line. (B) Pearson correlation under hypoxia for ES2 cell line. (C) Pearson correlation under normoxia for OVCAR3 cell line and (D) Pearson correlation under hypoxia for OVCAR3 cell line for 16 h of assay. Treatments were normalized by the control values (normoxia without cysteine supplementation). (E) Percentage of total cell death for ES2 cells for 16 h of assay for different initial cell densities. (F) Pearson correlation for NC/N and HC/H ratios for ES2 cells. (G) Percentage of total cell death for OVCAR3 cells for 16 h of assay for different initial cell densities and (H) Pearson correlation for NC/N and HC/H ratios for OVCAR3 cells. N – Normoxia; NC – Normoxia supplemented with cysteine; H – Hypoxia; HC – Hypoxia supplemented with cysteine. 5 × 103, 2.5 × 104, 5 × 104 and 1 × 105 refers to initial cell densities expressed as cells/cm2. Results are shown as mean ± SD.
Regarding OVCAR3 cells, there was no correlation between cell death and cysteine concentration neither under N nor under H (Fig. 2C and D).
The protective effect of cysteine under hypoxia (H) in ES2 (OCCC) is cell density-dependent
We also asked if this protective effect of cysteine under H in ES2 cells was dependent on cell density. In a general way, results showed a decreased cell death trend with increased cell density (p < 0.001 for all conditions) (Fig. 2E). Moreover, results suggest that the protective effect of cysteine under H is dependent on cell density, as demonstrated by the decreased cell death ratio HC/H with the increase of cell density (Pearson r = −0.628, p = 0.003) (Fig. 2F) whereas cell density did not impact on the cell death ratio NC/N (Fig. 2F).
Regarding OVCAR3 cells, the results also showed lower levels of cell death with higher cell densities in all conditions (p = 0.004), with the exception of the control (Fig. 2G). However, there was no impact of cell density neither on the NC/N nor on the HC/H ratios (Fig. 2H).
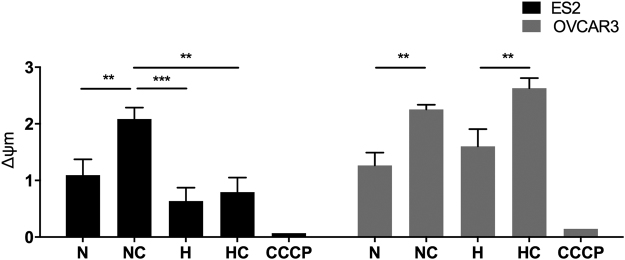
Cysteine increases mitochondrial membrane potential in ES2 (OCCC) under normoxia and in OVCAR3 (OSC) under normoxia and hypoxia
Mitochondrial membrane potential (Δψm) is associated with ATP production by oxidative phosphorylation, being a good indicator of cells health and functional status30. Δψm changes can be measured by red/green fluorescence intensity ratio using JC-1 probe, in which Δψm loss can be detected by a decrease in this ratio.
For ES2 cells, the Δψm loss was lower under N plus cysteine compared to the other conditions (p = 0.005 compared to N, p < 0.001 compared to H and p = 0.001 compared to HC) (Fig. 3). However, there were no differences among H with and without cysteine.
Figure 3.
Mitochondrial membrane potential under normoxia and hypoxia with and without cysteine, in ES2 (OCCC) and OVCAR3 (OSC) cells. Quantification of Δψm expressed as a ratio of red/green fluorescence intensity ratio in the different treatments (ratio of geometrical means) for ES2 and OVCAR3 cell lines for 16 h of assay. CCCP is a positive control for depolarization. N – Normoxia; NC – Normoxia supplemented with cysteine; H – Hypoxia; HC – Hypoxia supplemented with cysteine. Results are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 (One-way ANOVA with post hoc Tukey tests).
Regarding OVCAR3 cells, the Δψm loss was always lower in the presence of cysteine (NC vs N p = 0.002, NC vs H p = 0.024, HC vs N p < 0.001 and HC vs H p = 0.002) (Fig. 3).
Together, results suggest that cysteine allows mitochondrial function and cell health, especially under normoxia for ES2 cells and both under normoxia and hypoxia for OVCAR3 cells.
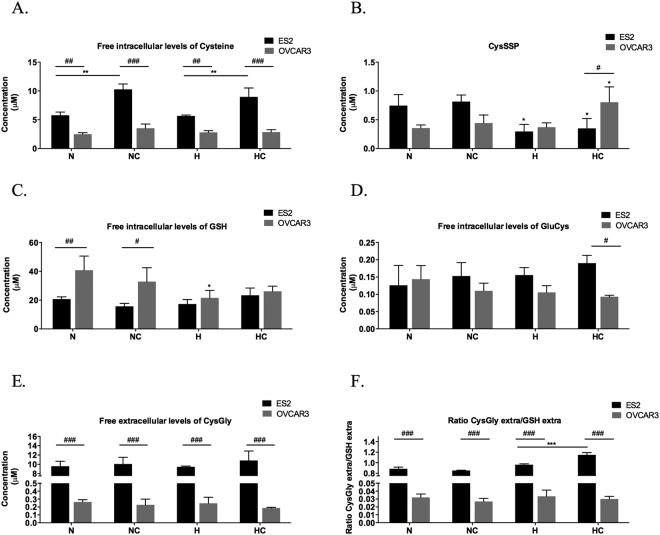
ES2 (OCCC) adaptation to hypoxia relies on free intracellular cysteine availability
Cysteine intracellular pool has the contribution of the free cysteine fraction and the protein-bound fraction (S-cysteinylated proteins-CysSSP).
ES2 (OCCC) cells presented higher free intracellular levels of cysteine in all treatments than OVCAR3 (OSC) cells (p = 0.001 in N, p < 0.001 in NC, p = 0.003 in H, p < 0.001 in HC) (Fig. 4A). Also, cysteine supplementation induced higher free intracellular levels of cysteine both in N (p = 0.001) and in H (p = 0.008). This result might indicate that H did not impair ES2 cells capacity to uptake this amino acid or that in this cells cysteine is not channelled for protein-S-cysteinylation pool or to de novo GSH synthesis.
Figure 4.
ES2 (OCCC) adaptation to hypoxia relies on free intracellular cysteine availability. Free intracellular levels of (A) Cys– cysteine, (B) S-cysteinylated proteins – CysSSP, (C) GSH – Glutathione, (D) GluCys – Glutamylcystein, (E) CysGly – Cysteinylglycine and (F) free extracellular CysGly/free extracellular GSH ratio in ES2 (black bars) and OVCAR3 (grey bars) cells. N – Normoxia; NC – Normoxia supplemented with cysteine; H – Hypoxia; HC – Hypoxia supplemented with cysteine. Results are shown as mean ± SD. Cardinals represent statistical significance between cell lines. Asteriscs represent statistical significance among treatments within the same cell line or in comparison with the control (normoxia without cysteine supplementation). *p < 0.05, **p < 0.01, ***p < 0.001 (One-way ANOVA with post hoc Tukey tests).
Thus, we asked how CysSSP was comparable between the two cell lines. We observed that hypoxia reduces CysSSP in ES2 cells (p = 0.02, for N vs H, p = 0.037 for N vs HC, p = 0.009 for NC vs H and p = 0.016 for NC vs HC) (Fig. 4B). This might suggest that this intracellular pool of cysteine is reduced to supply free cysteine for cells adaptation to hypoxia. In fact, cysteine in these cells does not increase CysSSP indicating the need of free cysteine availability and that the supplemented cysteine is not targeting proteins by S-cysteinylation.
To clarify if this will be important for maintaining GSH pool, we next evaluated the effect of tested conditions in GSH availability. No changes were observed for GSH intracellular levels in conditions tested (Fig. 4C). Results suggest that ES2 maintain high levels of available cysteine that are not being canalized to GSH synthesis as no changes were observed for GluCys, the first product of GSH synthesis, even with supplementation of cysteine (Fig. 4D). Moreover, in hypoxia conditions, the supplementation of cysteine increased GSH catabolism and cysteine recycling, as seen by the increased ratio CysGly/GSH, being CysGly the first product of GSH degradation. Thus, hypoxia decreases CysSSP supplying free Cys (Fig. 4B) and cysteine supplementation in hypoxia increases intracellular pool of free Cys (Fig. 4A) and the recycling of cysteine through GSH catabolism (Fig. 4F). In accordance, the protective effect of cysteine under hypoxia was dependent on its concentration and cell metabolic profile, indicating that intracellular pool of free cysteine in ES2 plays an important role in adaptation to hypoxia.
A different scenario was observed in OVCAR3. This cell line presented lower intracellular levels of cysteine than ES2 and the supplementation of cysteine did not have any effect on its free intracellular levels (Fig. 4A). This might suggest alteration in cysteine import or that imported cysteine is being channelled for CySSP pool or GSH synthesis. In addition, in this cell line H did not changed CysSSP and cells exposed to cysteine supplementation and H experienced an increase in CysSSP (p = 0.032 compared to N and p = 0.038 compared to H) (Fig. 4B). Also, under H with cysteine, ES2 cells presented lower levels of S-cysteinylated proteins (CysSSP) than OVCAR3 cells (p = 0.030) (Fig. 4B). Moreover, the recycling of cysteine through GSH catabolism is much lower than in ES2 as evaluated by the ratio CysGly/GSH (p < 0.001) (Fig. 4F). Together, results suggest that OVCAR3 are not so dependent on free cysteine as ES2.
Cysteine and hypoxia (H) have a role on the response to carboplatin by ovarian cancer cells
Usually, ovarian cancer therapy protocols include platinum salts and taxanes7. However, we tested paclitaxel with and without carboplatin and no relevant alterations were found related to H (Suppl. Fig. 2). In N it was only observed an increase in cell death due to paclitaxel in the presence of cysteine in the first cycle of ES2 cells exposure to drugs (Suppl. Fig. 2). So, the experimental course was developed using carboplatin, as chemically thiols react directly with platinum salts (oxidative compounds) and under H a protective effect was noticed in OCCC cells which are intrinsically resistant to carboplatin23.
Herein, we addressed the role of H and cysteine in ovarian cancer cells response and adaptation to carboplatin, by exposing cells to two cycles of carboplatin and assessing the effects on cell death from the first to the second cycle of drug exposure.
With one cycle of carboplatin, no differences were found among treatments in ES2 cells (Fig. 5A). Regarding the effect of two cycles of carboplatin, it was possible to observe differences among treatments (p = 0.002). Results showed that cysteine protects cells from carboplatin-induced death both in H and N (p = 0.008 in H and p = 0.015 in N) (Fig. 5A). When analysing the alterations in ES2 total cell death in consecutive cycles, results showed that carboplatin induced higher cell death at the second cycle only in the absence of cysteine treatment (p < 0.001 for N and H) (Fig. 5A). This result suggests that for ES2 cells, cysteine is not only a protective factor from death under H but it also allows faster adaptation to carboplatin both in N and H.
Figure 5.
Cysteine and hypoxia (H) have a role on the response to carboplatin by ovarian cancer cells. Total cell death in the first (C1) and in the second (C2) cycles of treatments in the presence of Carboplatin for (A) ES2, (B) OVCAR3, (C) OVCAR8, (D) A2780 parental cells and (E) A2780 cisR cells. N – Normoxia; NC – Normoxia supplemented with cysteine; H – Hypoxia; HC – Hypoxia supplemented with cysteine. Results are shown as mean ± SD. Cardinals represent statistical significance among hypoxia with and without cysteine supplementation within the same cycle of treatments. *p < 0.05, **p < 0.01, ***p < 0.001 (One-way ANOVA with post hoc Tukey tests).
In OVCAR3 cells (Fig. 5B), with one cycle of carboplatin there were no differences among treatments in cell death levels. However, with two cycles, there were differences among conditions (p = 0.001), in which cell death levels were lower under H supplemented with cysteine compared to the other treatments (p = 0.007 compared to N; p = 0.003 compared to NC and p = 0.001 compared to H). Regarding changes from the first to the second cycle of carboplatin in OVCAR3 cells, there was an increase in total cell death in all treatments in the second cycle (p < 0.001) (Fig. 5B). In this cell line, under hypoxia, cysteine protected cells from death in the second cycle of carboplatin (p = 0.001). Nevertheless, total cell death was higher in this cycle compared to the first one (p < 0.001). Under N, cysteine did not confer any advantage for cells in the presence of carboplatin in the second cycle.
We also asked the effect of hypoxia and cysteine in other ovarian cancer cell lines response to carboplatin. For OVCAR8 cells, no differences were observed with one cycle of carboplatin exposure among experimental conditions (Fig. 5C). However, with two cycles, hypoxia was especially disadvantageous without cysteine for OVCAR8 cells (N vs H p < 0.001, NC vs H p < 0.001, N vs HC p = 0.041, H vs HC p < 0.001). When analysing the alterations in total cell death levels from the first to the second cycle in OVCAR8 cells, increased levels were observed under hypoxia with and without cysteine (under H and HC p < 0.001), without significant differences under normoxia (Fig. 5C). Nevertheless, cysteine was advantageous under hypoxia in the second cycle (H vs HC p < 0.001).
For A2780 parental cells, in the first cycle, no differences were observed among treatments in the presence of carboplatin. In the second cycle of experimental conditions, increased cell death levels were observed under hypoxia with cysteine (p = 0.02) compared to normoxia with cysteine. When analysing the alterations in total cell death levels from the first to the second cycle, results showed an increase in cell death between cycles in all the treatments (Fig. 5D).
Regarding A2780 cisR cells, in the first cycle it was observed an increase in cell death levels under hypoxia with and without cysteine supplementation compared to the control and to normoxia with cysteine (N vs H p = 0.001, N vs HC p = 0.005, NC vs H p = 0.007 and NC vs HC p = 0.026), with no differences in the other conditions (Fig. 5E). In the second cycle, hypoxia was disadvantageous for these cells, especially without cysteine (N/NC vs H/HC, p < 0.001). When analysing the alterations in total cell death levels from the first to the second cycle, results showed increased cell death levels only under hypoxia, both with (p < 0.001) and without (p < 0.001) cysteine (Fig. 5F). Nevertheless, cysteine was advantageous in the second cycle of experimental conditions for A2780 cisR under hypoxia also in the presence of carboplatin (H vs HC, p < 0.001), in contrast to A2780, the parental cell line.
Altogether, results revealed a positive effect of cysteine under hypoxia and in the presence of carboplatin in ovarian cancer cells from different origins, thus suggesting a widespread role for cysteine in the selection of more aggressive phenotypes in ovarian cancer cells.
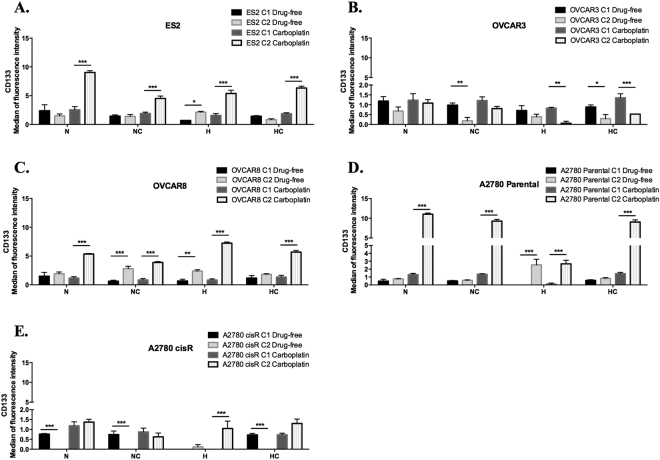
In ES2, OVCAR8 and A2780 cancer stem CD133+ cells command resistance to hypoxia and carboplatin
We next asked if the cancer stem CD133+ cells could be responsible for cell resistance to carboplatin.
For that, we compared the changes from the first cycle to the second cycle of treatments in the levels of CD133+ cells, based on the intensity of fluorescence.
In ES2 in a drug-free environment, results showed that hypoxia induced CD133 expression (Fig. 6) (p = 0.013). Upon carboplatin exposure, from the first cycle to the second cycle, there was an increase in CD133 expression in all treatments (under N, NC, H and HC p < 0.001) (Fig. 6A).
Figure 6.
In ES2, OVCAR8 and A2780 cancer stem CD133+ cells command resistance to hypoxia and carboplatin. CD133 expression levels in a drug-free environment and upon carboplatin exposure for (A) ES2 cells, (B) OVCAR3 cells, (C) OVCAR8 cells, (D) A2780 parental cells and (E) A2780 cisR cells in the first and in the second cycles of treatments. N – Normoxia; NC – Normoxia supplemented with cysteine; H – Hypoxia; HC – Hypoxia supplemented with cysteine. Results are shown as mean ± SD. Asterisks represent statistical significance among the first and the second cycle within the same treatment. *p < 0.05, **p < 0.01, ***p < 0.001 (One-way ANOVA with post hoc Tukey tests).
Interestingly, for OVCAR3 cells and in a drug-free environment, CD133 levels decreased in treatments with cysteine (under NC p = 0.001; under HC p = 0.02). Upon carboplatin exposure, there was a decrease in CD133 expression levels in treatments under hypoxia (under H p = 0.001; under HC p < 0.001), with no differences among cycles in treatments under normoxic conditions (Fig. 6B).
Regarding OVCAR8 cells, in a drug-free environment, results showed that hypoxia without cysteine induced CD133 expression (p = 0.001). Moreover, this effect was also observed under normoxia with cysteine supplementation (p < 0.001) (Fig. 6C). Upon carboplatin exposure, from the first cycle to the second cycle, there was an increase in CD133 expression in all treatments (under N, NC, H and HC p < 0.001) (Fig. 6C).
A2780 parental cells, in a drug-free environment, express more CD133 in H without cysteine (p < 0.000), with no significant differences among cycles in the other conditions (Fig. 6D). Upon carboplatin exposure, from the first cycle to the second cycle, an increase in CD133 expression was observed in all conditions (under N, NC, H and HC p < 0.001) (Fig. 6D). A different scenario was observed for A2780 cisR cells. In a drug-free environment, there was a decrease of CD133 expression from the first to the second cycle in all conditions (under N, NC and HC p < 0.001) (Fig. 6E) with the exception of hypoxia, in which there were no significant differences among cycles (Fig. 6E). Upon carboplatin exposure, hypoxia without cysteine induced CD133 levels (p < 0.001) from the first to the second cycle. Under hypoxia with cysteine, there was a trend to increase CD133 expression among cycles (p = 0.053) (Fig. 6E).
Together, results show that the levels of CD133 do not influence OVCAR3 cells resistance to neither hypoxia nor carboplatin, contrarily to what was observed in ES2, OVCAR8 and A2780 parental cells. Regarding the already resistant A2780 cisR cells, results point out that overall carboplatin resistance is not related with CD133 levels, only when combined with hypoxic stress.
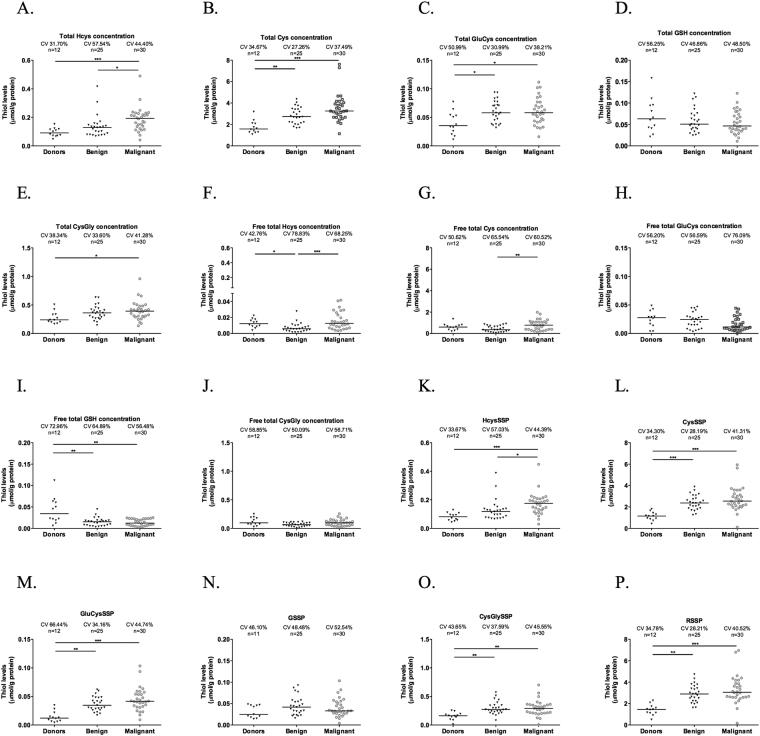
All thiols but GSH are increased in patients with ovarian tumours- high GSH turnover
Given the role of cysteine in the adaptation of ovarian cancer cell lines to hypoxic conditions and carboplatin exposure, we next assessed if higher serum levels of thiols were associated with ovarian tumours malignancy. Strikingly, total HCys levels were effective in distinguishing patients with malignant tumours from benign tumours and both of them from healthy individuals (Fig. 7A). Results showed that increased total levels of Cys and GluCys (GSH precursor) were associated with ovarian tumours regardless malignancy (Fig. 7B and C). Total GSH levels did not differ among groups (p > 0.05) and CysGly (GSH degradation product) was increased in patients with malignant tumours compared only to healthy individuals (p = 0.028) (Fig. 7D and E). Regarding free thiol content in plasma, Cys levels were increased in patients with malignant tumours compared to patients with benign tumours (p = 0.005) but without significant differences compared to healthy individuals (Fig. 7G).
Figure 7.
All thiols but GSH are increased in patients with ovarian tumours- high GSH turnover. Total thiols quantification in serum from healthy individuals (donors) and in serum from patients with benign and malignant ovarian tumours for (A) HCys- Homocysteine, (B) Cys – cysteine, (C) GluCys – Glutamylcysteine, (D) GSH – Glutathione, (E) CysGly – Cysteinylglycine. Thiol concentration was normalized by protein concentration. Free total thiols quantification in serum from healthy individuals (donors) and in serum from patients with benign and malignant ovarian tumours for (F) HCys- Homocysteine, (G) Cys – cysteine, (H) GluCys – Glutamylcysteine, (I) GSH – Glutathione, (J) CysGly – Cysteinylglycine. Thiol concentration was normalized by protein concentration. Thiols concentration bound to proteins quantification in serum from healthy individuals (donors) and in serum from patients with benign and malignant ovarian tumours for (K) HcySSP – S-homocysteinylated proteins, (L) CysSSP – S-cysteinylated proteins, (M) GluCysSSPS – S-Glutamylcysteinylated proteins, (N) GSSP – S-glutathionylated proteins, (O) CysGlySSP – S-cysteinylglycinylated proteins. (P) RSSP – total S-thiolated proteins. Thiol concentration was normalized by protein concentration. Results are shown as median. The *represent the statistical significance among groups. *p < 0.05, **p < 0.01, ***p < 0.001 (independent samples Kruskal Wallis One-way ANOVA with multiple comparisons).
Free HCys levels were able to distinguish patients with malignant tumours from benign tumours (p < 0.001) and patients with benign tumours from healthy individuals (p = 0.02) without significant differences between malignant tumours and healthy individuals (Fig. 7F). Lower free GSH levels were associated with ovarian neoplasms regardless malignancy (p = 0.006 donors vs benign tumours and p = 0.005 donors vs malignant tumours) (Fig. 7I). Free GluCys and free CysGly levels did not differ among groups (p > 0.05) (Fig. 7H and J).
Higher levels of S-cysteinylated, S-glutamylcysteinylated, and S-cysteinylglycinylated proteins (CysSSP, GluCysSSP and CysGlySSP) were associated with ovarian neoplasms regardless malignancy (CysSSP: donors vs benign p < 0.001, donors vs malignant p < 0.001; GluCysSSP: donors vs benign p = 0.001, donors vs malignant p < 0.001; CysGlySSP: donors vs benign p = 0.001, donors vs malignant p = 0.001) (Fig. 7L,M and O). Higher levels of S-Homocysteinylated proteins (HCysSSP) were associated only with malignant ovarian tumours (p = 0.019 benign vs malignant, p < 0.001 donors vs malignant) (Fig. 7K). There were no differences in levels of S-glutathionylated proteins (GSSP) among groups (Fig. 7N).
With the exception of GSH total levels, in which there was a trend to lower levels in serum from patients with ovarian neoplasms compared to healthy individuals, other thiols were mainly in the protein bound form and not free in plasma. Together, results suggested that higher levels of thiols are associated with ovarian neoplasms, regardless malignancy, showing that patients with ovarian neoplasms present different thiol dynamics.
Noticeably, total CysSSP was capable of distinguishing serum of healthy donors from patients with ovarian tumours (benign and malignant) and total free Cys levels distinguished serum of patients with malignant tumours from patients with benign tumours. Cysteine, free and protein- bound, are for sure a suitable marker for ovarian cancer.
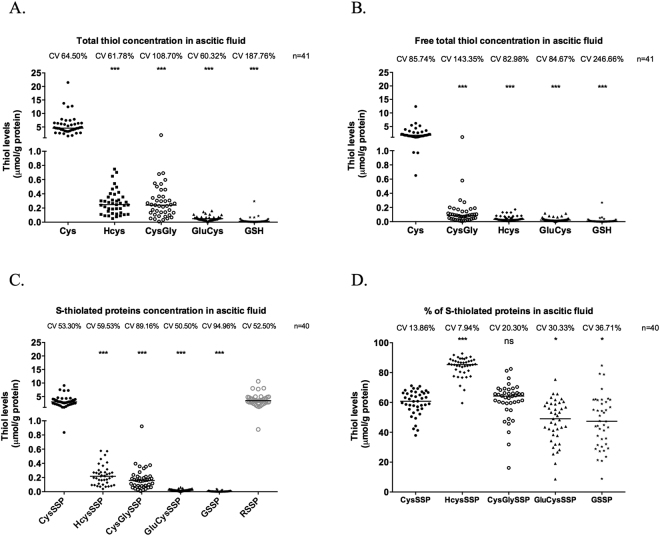
Cysteine is the prevalent thiol in ascitic fluid from patients with advanced ovarian cancer
As the ascitic fluid is an important component of ovarian tumour cells microenvironment, we disclosed if cysteine and other thiols were present in this biofluid derived from patients with ovarian cancer. Cysteine was the most prevalent thiol in the three pools, total (Fig. 8A), free (Fig. 8B) and S-cysteinylated proteins (Fig. 8C). HCys was mainly present bound to proteins, sequentially followed by Cys and CysGly, and then GluCys and GSH (Fig. 8D).
Figure 8.
Cysteine is the prevalent thiol in ascitic fluid from patients with ovarian cancer. Thiols quantification in ascitic fluid from patients with ovarian cancer. (A) Total thiols concentration. (B) Free total thiols concentration. (C) Thiols concentration bound to proteins. (D) Percentage of thiols concentration bound to proteins. Cys – cysteine, HCys- Homocysteine, CysGly – Cysteinylglycine, GluCys – Glutamylcystein, GSH – Glutathione, CysSSP – S-cysteinylated proteins, HcySSP – S-homocysteinylated proteins, CysGlySSP – S-cysteinylglycinylated proteins, GluCysSSPS – Glutamylcysteinylated proteins, GSSP – S-glutathionylated proteins, RSSP – total S-thiolated proteins. Thiol concentration was normalized by protein concentration. Results are shown as median. The *represent the statistical significance in relation to Cysteine concentration. *p < 0.05, **p < 0.01, ***p < 0.001 (independent samples Kruskal Wallis One-way ANOVA with multiple comparisons).
As the prevalent thiol in the ascitic fluid, cysteine might promote an ideal tumour microenvironment for cancer cells, possibly acting both as a redox buffer and as a modifier of proteins, mediating the adaptation or abrogating the effect of platinum salts, such as carboplatin.
Discussion
Although the outcome prognosis of different histological types of ovarian cancer had been a matter of debate, it was shown that patients with OCCC had a significantly worse prognosis than patients with OSC when matched for age, stage, and level of primary cytoreduction31. In 2011, Lee et al. have shown that OCCC patients had a poorer prognosis than those with other histological subtypes of carcinoma, especially in advanced stages32. Moreover, while OCCC show intrinsic resistance to conventional platinum-based chemotherapy, OSC exhibit at first sensitiveness to platinum-based chemotherapy, though the majority of cases develop chemoresistance during the course of treatments33,34. In the present work, several ovarian cancer cell lines were used and major differences were found on cysteine dependence on copping with hypoxia and carboplatin. Importantly, ES2 cells and OVCAR8 presented a stronger dependence on cysteine metabolism in handling with hypoxic stress and carboplatin than OVCAR3. Considering A2780 and A2780 cisR cells under hypoxia upon carboplatin exposure, cysteine was advantageous for A2780 cisR but not for A2780 parental cells, reinforcing that cysteine accounts for the selective process of platinum resistant ovarian cancer cells. We must underline that OVCAR3 cells did not die more under hypoxia than ES2 cells. Instead, our results show that these cells manage hypoxic stress by different mechanisms. In fact, we observed that ES2 cells express higher levels of hypoxia-inducible factor 1-α (HIF-1α than OVCAR3 cells under both normoxia and hypoxia (Suppl. Fig. 3). Nevertheless, the ability to use cysteine proves to be advantageous under carboplatin exposure and allows a faster adaptive response to hypoxia, indicating that cysteine has a role in ovarian cancer cells survival in adverse environments. This evidence, on the other hand, points that cysteine metabolism is strongly related to a worse prognosis of ovarian cancer. It is plausible that the worse prognosis associated to the OCCC histological type is related to cysteine metabolism and or cysteine-dependent redox signalling process. However, we must test other OCCC cell lines in order to clarify the hypothesis that OCCC and OSC tumours are a priori metabolically different.
Moreover, we observed that the expression of CD133, a cancer stem cell marker35, was also increased in ES2, OVCAR8 and A2780 parental cells but not in OVCAR3 cells, upon cyclic exposure to hypoxia. Moreover, in these cells, carboplatin induced CD133 expression. This fact indicates that the activation of a pool of cancer stem cells underlies the adaptive capacity of these cells to hypoxia and drug resistance, where cysteine acts as a facilitator. Interestingly, upon carboplatin exposure, the already resistant A2780 cisR cells did not induce CD133 expression in the majority of treatments, whereas A2780 parental cells did. Those results support that CD133 expression is involved in the process of adaptation to carboplatin but it is lost when cells become chemoresistant, thus suggesting a role for CD133 in the adaptation to adverse environments.
Lopes-Coelho et al. have shown that, under normoxia, ES2 cells are more resistant to carboplatin than OVCAR3 and that the inhibition of GSH production by buthionine sulphoximine (BSO), sensitizes ES2 cells to carboplatin23. Our results suggest that the protective effect against carboplatin under hypoxia is mediated by cysteine. However, it is still unclear if GSH is involved in this protection under hypoxia. Moreover, our team has described recently that in normoxia and in glucose free media ES2 has higher levels of thiols than OVCAR323. In the present study we cannot see that difference since we used different culture conditions, namely high glucose culture media. It is known that glucose can form adducts with glutathione, which can in part explain the observed differences36. Despite we have detected lower or equal levels of GSH in ES2 than in OVCAR3 cells, we also detected higher degradation of GSH in ES2, pointing a faster thiols’ turnover in this cell line indicated by the levels of cysteinylglycine (Cys-Gly)37.
In a general way, our results pointed to a tendency to decreased cell death levels with increased cell density. Recently, it was shown that cytosolic lipid droplets, which are known to protect cancer cells from starvation-induced death, increased in content with increased cell density in HeLa cells38. This observation could, in part, explain the protective effect of high initial cell densities observed in our cell models. However, in the initial cell density 5 × 103 cells, we observed lower cell death levels than 2.5 × 104. This could be due to cells dormancy that could ensure cells survival in a quiescent metabolic state, due to stressful conditions39 that could be imposed by such a decreased cell density. Moreover, the effect of cell density in the response to carboplatin is an issue that must be addressed in future studies using 3D models. One may expect that increased cell density will have an impact on cells capacity to adapt to carboplatin, since a larger cell population could present more heterogeneity, thus more adaptive potential. In fact, our preliminary results of spheroids models point out a protective effect of cysteine in ES2 but not in OVCAR3 upon carboplatin exposure (Suppl. Fig. 4). Interestingly, Lavi and colleagues40, through a mathematical model, showed how cancer cell density and cells alteration rate impact the heterogeneity over time, and its consequences on multidrug resistance. Moreover, in the context of ovarian cancer, Greene et al.41 have shown that local cell density and initial global cell density, are able to lead to significant differences in spatial growth, proliferation, and paclitaxel-induced apoptosis rates in OVCAR8 cells, thus having a role not only in cancer cells growth but also in drug response.
In the present work, a lower loss of Δψm under hypoxia plus cysteine compared to cysteine absence was expected. Although, it was not observed. Mitochondrial membrane potential (Δψm) has been considered a good indicator of cells health30, as it is critical for maintaining the physiological function of the respiratory chain to generate ATP in mitochondria. A significant loss of ΔΨm will deplete cells of energy with subsequent death. The obtained results are apparently contradictory to the data obtained with Annexin V/PI staining for cell death, in which, under hypoxia, cysteine was able to protect cells from death. However, annexin V labels apoptotic cells independently of the apoptotic pathways they underwent and as extrinsic apoptosis does not involve mitochondrial disruption it can be a plausible explanation for these results. On the other hand, to assess Δψm only adherent cells were used. Hence, cells that were dying faster under hypoxia without cysteine were not used to assess Δψm. Additionally, Tang et al. found that cancer cells were able to survive even after the cells had undergone critical apoptotic events such as mitochondrial fragmentation and dysfunction, nuclear condensation, cell shrinkage and activation of caspases42. They observed this apoptosis reversibility in various cancer cell lines and after different apoptotic stimuli42. Therefore, another interesting possibility is that, under hypoxia, cysteine is able to reverse apoptosis in ES2 cells.
With the exception of GSH, our results shown an overall increase of thiols concentration in serum from patients with ovarian neoplasms, regardless malignancy, compared to healthy donors. Strikingly, total and free levels of HCys distinguished all the three groups of individuals and free levels of Cys showed to be effective in distinguishing malignant from benign neoplasms. In disease, the literature has already shown that increased levels of HCys, Cys and CysGly in peripheral blood serum were already associated with ischemic heart disease43 and increased levels of CysGly44 and Cys45 were associated with increased risk of breast cancer44. Moreover, higher HCys levels were associated with greater risk of breast cancer only when combined with higher levels of Cys and with low levels of folate45. Differently, high plasma HCys levels were also associated with increased risk of colorectal cancer, whereas high Cys levels were associated with decreased risk for this disease46. Chiang and colleagues have confirmed the association between increased HCys levels and the risk of colorectal cancer adding that this increased risk was independent of oxidative stress indicators and antioxidant capacities47. In contrast, higher serum concentrations of Cys were associated with a significantly reduced risk of oesophageal squamous cell carcinomas and gastric cardia adenocarcinomas48. The assessment of different thiol pools total, free and protein linked confers an innovative approach in our work. We observed that the contribution of protein-S-thiolation was augmented in patients with ovarian neoplasms compared to healthy individuals. The reversible thiolation of proteins was already associated with the regulation of several metabolic processes such as enzyme activity, transport activity, signal transduction and gene expression49. Several proteins - such as Ras, Jun N-terminal kinase (JNK)- 2, AP-1, NF-kappaB, PKC, caspase, thioredoxin and p5349,50 - which are known to have important roles in cancer, are regulated by thiol oxidation50. Visscher et al. reported that many oncogenic mutations consist in an insertion of a novel cysteine in the protein sequence, as it happens in 12% of KRAS and 88% of FGFR mutations51. These facts suggest that newly introduced cysteines should play a role in tumourigenesis by contributing for tumour suppressor genes inactivation and oncogenes activation. Moreover, thiols can also interfere with epigenetic modulation of genes expression, which is pivotal in carcinogenesis52.
Importantly, our results have shown that cysteine levels are putative biomarkers for ovarian cancer early diagnosis. As seen, the total levels in peripheral blood of protein-S-cysteinylation distinguished healthy donors from patients with ovarian neoplasms (benign and malignant) and free cysteine distinguishes patients with ovarian benign tumours from patients with malignant tumours. This is undoubtedly a step forward in ovarian cancer research, as the late diagnosis is one of the most important barriers accounting for the poor outcome and high mortality.
We also observed that cysteine was the prevalent thiol in ascitic fluid from patients with ovarian cancer and that S- cysteinylation was the most abundant form of S-thiolated proteins showing, again, that cysteine is a relevant organic compound in ovarian cancer cells microenvironment.
We must emphasize that most samples analysed from patients with ovarian cancer were from OSC histotype. It would be interesting to assess thiols levels also in patients with OCCC. Albeit the limitation of the in vitro studies with a single OCCC cell line, we believe that the levels of cysteine and other thiols would be even higher in patients with this histological type compared to OSC, allowing faster disease progression and recurrence. Despite OCCC is frequently diagnosed at an initial stage6, in an extraovarian advanced stage the disease has poor prognosis given intrinsic chemoresistance to conventional platinum drugs6. However, what we undoubtably observe is that ovarian cancer cells that are better adapted to cysteine are more resistant to carboplatin effects mainly in hypoxia. The ability to take advantage of cysteine accounting for chemoresistance can be intrinsic, as OCCC, or acquired upon cyclic exposure to chemotherapy as happens with recurrent OSC.
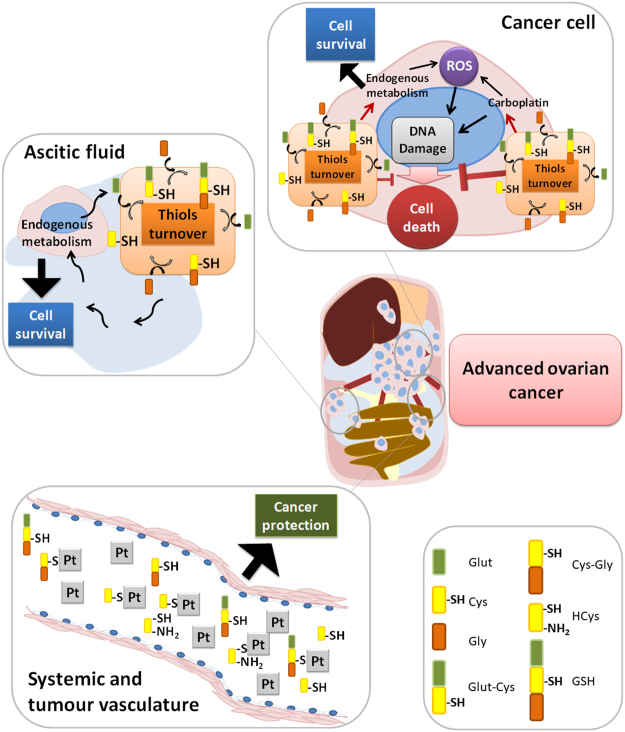
Taken together, results suggest that cysteine protects cells from the adverse hypoxic microenvironment and from platinum-based chemotherapy, thus having a role in cancer progression. The high cysteine concentrations in ascitic fluid and in serum from patients with ovarian malignant tumours leads us to propose that cysteine acts as a first protection barrier for neoplastic cells against hypoxia, having a role as a redox buffer, and against chemotherapy, as sulphur rapidly binds to platinum-based cancer chemotherapy, thus preventing drug efficacy (Fig. 9). In metastatic peritoneal disease, cysteine rich ascites contributes for the perpetual maintenance of resistance to therapy, as cancer cells proliferate directly embedded in ascitic fluid (Fig. 9).
Figure 9.
Role of cysteine in cells response to carboplatin and hypoxia adaptation. In cancer cells, cysteine allows adaptation to an adverse hypoxic microenvironment acting as a redox buffer, and to platinum-based chemotherapy as sulphur rapidly binds to carboplatin, avoiding DNA damage and evading apoptosis. Because of the presence of high cysteine concentrations in ascitic fluid from patients with ovarian cancer and also in serum from patients with ovarian malignant neoplasms, we propose that cysteine acts as a first protection barrier for malignant cells against hypoxia, having a role as a redox buffer. Results suggest a role of cysteine in ovarian malignant tumours and in disease progression, as cysteine is the prevalent thiol in ascitic fluid, an important compartment of tumour cells microenvironment.
This paper highlights the role of cysteine underlying the resistance of ovarian cancer cells to hypoxia and carboplatin, which hinders cancer fighting. We are currently developing more studies to disclose if cysteine metabolic pathways can be elected as therapeutic targets to reverse resistance to carboplatin. Nevertheless, the best achievement of our study was the assessment of total free-cysteine and total protein-S-cysteinylation levels as promising strategy for ovarian cancer screening and early diagnosis. Moreover, cysteine levels in peripheral blood serum from ovarian cancer patients can be used to stratify patients according to risk therapy resistance allowing a more accurate prediction of disease outcome.
Material and Methods
Cell culture
Cell lines ES2 (CRL-1978), OVCAR-3 (HTB-161) and OVCAR-8 (CVCL-1629) were obtained from American Type Culture Collection (ATCC). Cell line A2780 sensitive (93112519) and A2780 cisplatin resistant (93112517) were obtained from Sigma Aldrich. Cells were maintained at 37 °C in a humidified 5% CO2 atmosphere. ES2, OVCAR3 and OVCAR8 cell lines were cultured in DMEM (41965–039, Gibco, Life Technologies) supplemented with 1% FBS (S 0615, Merck), 1% antibiotic-antimycotic (AA) (P06–07300, PAN Biotech). A2780 parental and A2780 cisR cells were cultured in RPMI 1640 (BE12-167F, Lonza) supplemented with 0.58 g/L of L-glutamine, 1% FBS (S 0615, Merck) and 1% antibiotic-antimycotic (AA) (P06-07300, PAN Biotech).
Cells were exposed to 0.4 mM L-Cysteine (102839, Merck) and/or exposed to hypoxia-induced conditions with 0.1 mM cobalt chloride (C8661, Sigma-Aldrich). Cobalt is a hypoxia mimicking agent used in in vivo53 and in vitro54–56 studies. Chemically, CoCl2 reacts with oxygen impairing its dissolution and oxygenation of aqueous solutions57 and is a way of impairing the availability of oxygen in culture media.
Prior to any experiment, cells were synchronized under starvation (culture medium without FBS) for 8 h at 37 °C and 5% CO2.
Peripheral blood serum and ascitic fluid
After collection, the blood was allowed to clot at room temperature, during 15–30 min. The clot was removed by centrifuging at 3000 rpm for 5 min at 4 °C. Serum is the resulting supernatant, which were preserved at −80 °C.
After collection of the ascitic fluid, it was centrifuged at 1200 rpm for 2 min at room temperature, to remove the cells avoiding cell lysis. The supernatant was preserved at −80 °C.
The serum and ascitic fluid samples were collected from different individuals, with the exception of four serum and ascitic fluid samples, in which the patients were the same. The levels of thiols were determined in these biological samples, as described below.
Cell death analysis
Cells (5 × 105 cells/well) were seeded in 6-well plates and cultured in control condition and exposed to 0.4 mM L-cysteine and/or hypoxia induced with 0.1 mM cobalt chloride. Cells were collected at 16 h and 24 h after stimulation.
To test whether the protective effect of cysteine in ES2 and OVCAR3 cells is concentration-dependent, cells (5 × 105 cells/well) were seeded in 6-well plates and cultured in control condition and exposed to 0.1 mM, 0.2 mM, 0.4 mM, 0.8 mM and 1.0 mM L-cysteine and/or hypoxia induced with 0.1 mM cobalt chloride. Cells were collected at 16 h after stimulation.
To test whether the protective effect of cysteine under hypoxia was dependent on initial cell density, 5 × 103, 2.5 × 104, 5 × 104 and 1 × 105 cells/cm2 were seeded in 24-well plates and cultured in control condition and exposed to 0.4 mM L-cysteine and/or hypoxia induced with 0.1 mM cobalt chloride. Cells were collected at 16 h after stimulation.
Cells were harvested and centrifuged at 1200 rpm for 3 min, followed by incubation with 1 μL annexin V-Alexa Fluor 488 (640906, BioLegend) in 100 μL annexin V binding buffer 1× (10 mM HEPES (pH 7.4), 0.14 M sodium chloride (NaCl), 2.5 mM calcium chloride (CaCl2) and incubated at room temperature in the dark for 15 min. After incubation, samples were rinsed with 0.1% (w/v) BSA (A9647, Sigma) in PBS 1× and centrifuged at 1200 rpm for 3 min. Cells were suspended in 200 μL of annexin V binding buffer 1× and 5 μL propidium iodide (50 μg/mL). Acquisition was performed with a FACScalibur (Becton Dickinson). Data were analysed with FlowJo software.
Mitochondrial membrane potential (MMP)
The MMP was measured in ES2 and OVCAR3 cell lines using JC-1 (M34152, Molecular Probes, Life Technologies)58, a specific mitochondrial probe, which becomes red when it enters polarized mitochondria and it is green in cytoplasm when mitochondrial potential is lost avoiding its entrance.
Cells (5 × 105 cells/well) were seeded in 6-well plates and cultured in control condition and exposed to 0.4 mM L-cysteine and/or 0.1 mM cobalt chloride and were collected at 16 h after stimulation. Live cells were then washed with phosphate-buffered saline 1×, incubated for 30 min with 5 μM JC-1 (37 °C, 5% CO2), washed with PBS 1×, trypsinized, and recovered in a final volume of 300 μL of PBS. A positive depolarized control was used, in which cells were previously incubated with 50 μM CCCP for 5 min (37 °C, 5% CO2) and then incubated with JC-1. Acquisition was performed in a FACScalibur (Becton Dickinson). Data were analysed with FlowJo software.
Cells response to carboplatin
Cells (1 × 105 cells/well) were seeded in 24-well plates and cultured in control condition and exposed to 0.4 mM L-cysteine and/or 0.1 mM cobalt chloride. Cells were also exposed to the previous conditions combined with carboplatin 25 μg/mL. Cells were collected after one cycle of stimulation (16 h per cycle) and two cycles of stimulation (which was preceded by a period without drugs of proximately 32 h between cycles). Cell death analysis was performed.
CD133 quantification
Cells (2 × 105 cells/well) were seeded in 12-well plates (ES2, OVCAR3 and OVCAR8) or 24-well plates (1 × 105 cells/well) (A2780 and A2780 cisR) and cultured in control condition and exposed to 0.4 mM L-cysteine and/or 0.1 mM cobalt chloride. Cells were also exposed to the previous conditions combined with carboplatin 25 μg/mL. Cells were collected after one cycle of stimulation (16 h per cycle) and two cycles of stimulation (which was preceded by a period without drugs of proximately 32 h between cycles). Cells were collected with PBS 1x -EDTA 2 mM, centrifuged at 1200 rpm for 3 min, re-suspended and incubated with 1 μL of CD133 (AC133-PE human, Miltenyi Biotec) and 99 μL PBS-BSA 0.1% for 20 min in the dark 4 °C. Acquisition was performed in a FACScalibur (Becton Dickinson). Data were analysed with FlowJo software.
Thiols quantification
Because thiols can impact protein function by the formation of intra- and inter-molecular disulfide bond(s) with proteins59, or may exist as free thiols, we measured both total and free thiol levels.
Thiols quantification was performed by high-performance liquid chromatography (HPLC). Cells (4 × 106) were cultured in 75 cm2 tissue culture flasks in control conditions and exposed either to 0.4 mM L-cysteine and/or 0.1 mM cobalt chloride for 16 h. The supernatants and the lysates were stored at −80 °C. The assessment of the levels of cysteine (Cys), homocysteine (HCys), glutamylcysteine (GluCys), glutathione (GSH) and cysteinylglycine (CysGly) was performed according to Grilo and co-authors60 and adapted to cell culture. The detector was set at excitation and emission wavelengths of 385 and 515 nm, respectively. The mobile phase consisted of 100 mM acetate buffer (pH 4.5) and methanol [98:2 (v/v)]. The analytes were separated in an isocratic elution mode for 20 min, at a flow rate of 0.6 mL/min. The thiolomic profile was also quantified in peripheral blood serum from healthy individuals and patients with malignant and benign ovarian tumours and in ascitic fluid from patients with ovarian cancer, according to Grilo et al.60.
Regarding serum samples, 12 samples of healthy donors, 25 samples of patients with benign ovarian tumours and 30 samples of patients with malignant ovarian tumours were analysed.
Regarding ascitic fluid samples, 41 samples were analysed, with patients diagnosed with the following ovarian cancer histotypes: serous, (n = 32), mucinous (n = 3), endometriod (n = 1,) and without histotype information (n = 5).
Statistical analysis
Data are presented as the mean ± SD and all the graphics were done using the PRISM software package (PRISM 6.0 for Mac OS X; GraphPad software, USA, 2013). Assays were performed with, at least, 3 replicates per experimental condition. For comparisons of two groups, two-tailed independent-samples T-test was used. For comparison of more than two groups, One-way analysis of variance (ANOVA) with Tukey’s multiple-comparisons post hoc test was used. To assess the existence of a linear relationship between two variables, two-tailed Pearson correlation was used. To assess differences in cysteine and GSH levels in peripheral blood and in ascitic fluid, 2-sided independent samples Kruskal-Wallis 1-way ANOVA with pairwise comparisons was performed, where the adjusted significance was considered. Statistical significance was established as p < 0.05. All statistical analyses were performed using the IBM Corp. Released 2013. IBM SPSS Statistics for Macintosh, Version 22.0. Armonk, NY: IBM Corp. software.
Study approval
All the experiments were developed according to relevant guidelines and regulations.
The human samples were collected under informed consent of patients and blood donors from Instituto Português de Oncologia de Lisboa, Francisco Gentil, EPE. The study protocol was approved by the Ethical Committee IPOLFG (references: UIC/1080 and UIC/1082).
Data availability
All data generated and analysed during this study are included in the article and in supplementary information. Cell lines and reagents are commercially available.
Electronic supplementary material
Acknowledgements
The authors would like to acknowledge the Instituto Português de Oncologia de Lisboa Francisco Gentil (IPOLFG) for partially funding the project. We would also like to acknowledge Dr Dialina Brilhante and Dr. Teresa Guerreiro (Serviço de Imuno-hemoterapia, IPOLFG) for providing blood donors samples; to Dr Humberto Gonçalves (Pharmacy, IPOLFG) for paclitaxel and carboplatin preparation, and Marta Teixeira (IBET) for the technical support in 3D models. The study was also funded by Projecto TVI. This research was supported by Fundação para a Ciência e Tecnologia (FCT) (PhD ProRegeM program, PD/BD/105893/2014, FCT fellowship, PD/BD/105768/2014). iNOVA4Health - UID/Multi/04462/2013, a program financially supported by Fundação para a Ciência e Tecnologia/Ministério da Educação e Ciência, through national funds and co-funded by FEDER under the PT2020 Partnership Agreement is acknowledged.
Author Contributions
Sofia C. Nunes- Planning and execution of the experimental study, responsible for more than 90% of results. Writing of the manuscript. Cristiano Ramos, Filipa Lopes-Coelho, Catarina Sequeira, Fernanda Silva, Sofia Gouveia-Fernandes and Armanda Rodrigues- Execution of some experimental work and revision of the manuscript. António Guimarães and Margarida Silveira- Selection of patients, collection of samples and revision of the manuscript. Sofia Abreu, Vitor E Santo and Catarina Brito- Preliminary studies in 3D models and revision of the manuscript. Ana Félix- Discussion of the results and revision of the manuscript. Sofia A. Pereira- Project planning and discussion of the results and revision of the manuscript. Jacinta Serpa- Responsible for the whole project. Project planning and discussion of the results and revision of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27753-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vaughan S, et al. Rethinking ovarian cancer: recommendations for improving outcomes Sebastian. Nat. Rev. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Miller K, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, et al. Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10:803–808. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 5.Chan JK, et al. Patterns and progress in ovarian cancer over 14 years. Obstet. Gynecol. 2006;108:521–528. doi: 10.1097/01.AOG.0000231680.58221.a7. [DOI] [PubMed] [Google Scholar]
- 6.Itamochi, H., Kigawa, J. & Terakawa, N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. 99, 653–658 (2008). [DOI] [PMC free article] [PubMed]
- 7.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 8.Ip CKM, Li S, Tang MH, Sy SKH, Ren Y. Stemness and chemoresistance in epithelial ovarian carcinoma cells under shear stress. Sci. Rep. 2016;6:1. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moradi MM, et al. Serum and Ascitic Fluid Levels of Necrosis Factor-Alpha in Patients with Ovarian Epithelial Cancer. Cancer. 1993;15:2433–2440. doi: 10.1002/1097-0142(19931015)72:8<2433::AID-CNCR2820720822>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Mills B, Roifman M, Mellors A. A Putative New Growth Factor in Ascitic Fluid from Ovarian Cancer Patients: Identification, Characterization, and Mechanism of Action’. Cancer Res. 1988;48:1066–1071. [PubMed] [Google Scholar]
- 11.Mills GB, et al. Ascitic Fluid from Human Ovarian Cancer Patients Contains Growth Factors Necessary for Intraperitoneal Growth of Human Ovarian Adenocarcinoma Cells. J.Clin. Invest. 1990;86:851–855. doi: 10.1172/JCI114784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serpa J, Dias S. Metabolic cues from the microenvironment act as a major selective factor for cancer progression and metastases formation. Cell Cycle. 2011;10:180–181. doi: 10.4161/cc.10.2.14476. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharyya, S. et al. Cystathionine Beta-Synthase (CBS) Contributes to Advanced Ovarian Cancer Progression and Drug Resistance. Plos One8 (2013). [DOI] [PMC free article] [PubMed]
- 16.Szabo C, et al. Tumor-derived hydrogen sulfide, produced by cystathionine- β -synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. PNAS Pharmacol. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sen S, et al. Role of cystathionine β -synthase in human breast Cancer. Free Radic. Biol. Med. 2015;86:228–238. doi: 10.1016/j.freeradbiomed.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Panza E, et al. Role of the cystathionine γ lyase/hydrogen sulfide pathway in human melanoma progression. Pigment Cell Melanoma Res. 2015;28:61–72. doi: 10.1111/pcmr.12312. [DOI] [PubMed] [Google Scholar]
- 19.Gai JW, et al. Expression profile of hydrogen sulfide and its synthases correlates with tumor stage and grade in urothelial cell carcinoma of bladder. Urol. Oncol. Semin. Orig. Investig. 2016;34:166.e15–166.e20. doi: 10.1016/j.urolonc.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Pan Y, Zhou C, Yuan D, Zhang J, Shao C. Radiation Exposure Promotes Hepatocarcinoma Cell Invasion through Epithelial Mesenchymal Transition Mediated by H2S/CSE Pathway. Radiat. Res. 2015;185:96–105. doi: 10.1667/RR14177.1. [DOI] [PubMed] [Google Scholar]
- 21.Schnelldorfer T, et al. Glutathione depletion causes cell growth inhibition and enhanced apoptosis in pancreatic cancer cells. Cancer. 2000;89:1440–7. doi: 10.1002/1097-0142(20001001)89:7<1440::AID-CNCR5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Balendiran GK, Dabur R, Fraser D. The role of glutathione in cancer. cell Biochem. Funct. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- 23.Lopes-Coelho F, et al. HNF1B drives glutathione (GSH) synthesis underlying intrinsic carboplatin resistance of ovarian clear cell carcinoma (OCCC) Tumor Biol. 2016;37:4813–4829. doi: 10.1007/s13277-015-4290-5. [DOI] [PubMed] [Google Scholar]
- 24.Mikalsen SG, Jeppesen Edin N, Sandvik JA, Pettersen EO. Separation of two sub-groups with different DNA content after treatment of T-47D breast cancer cells with low dose-rate irradiation and intermittent hypoxia. Acta radiol. 2017;0:28418511769999. doi: 10.1177/0284185117699999. [DOI] [PubMed] [Google Scholar]
- 25.Gutsche K, et al. Intermittent hypoxia confers pro-metastatic gene expression selectively through NF-κB in inflammatory breast cancer cells. Free Radic. Biol. Med. 2016;101:129–142. doi: 10.1016/j.freeradbiomed.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Semenza GL. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutter, N. L., Walther, T., Gallagher, L., Lucito, R. & Wrzeszczynski, K. Hypoxia signaling pathway plays a role in ovarian cancer chemoresistance. In Advances in Ovarian Cancer Research: Exploiting Vulnerabilities, 10.1158/1557-3265 (2016).
- 28.Kigawa J, Minagawa Y, Cheng X, Terakawa N. y-Glutamyl cysteine synthetase up-regulates Multidrug resistance-associated protein in Patients with chemorresistant epithelial ovarian cancer. Clin. Cancer Res. 1998;4:1737–1741. [PubMed] [Google Scholar]
- 29.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 30.Perry SW, Norman JP, Barbieri J, Brown EB, Harris A. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tammela J, Geisler J, Eskew PJ, Geisler H. Clear cell carcinoma of the ovary: poor prognosis compared to serous carcinoma. Eur. J. Gynaecol. Oncol. 1998;19:438–440. [PubMed] [Google Scholar]
- 32.Lee Y, et al. Gynecologic Oncology Prognosis of ovarian clear cell carcinoma compared to other histological subtypes: A meta-analysis. Gynecol. Oncol. 2011;122:541–547. doi: 10.1016/j.ygyno.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Goff BA, et al. Clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy in stage III disease. Gynecol. Oncol. 1996;417:412–417. doi: 10.1006/gyno.1996.0065. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama, T. et al. Clinical characteristics of clear cell carcinoma of the ovary. Cancer88 (2000).
- 35.Burgos-ojeda, D., Rueda, B. R. & Buckanovich, R. J. Ovarian cancer stem cell markers: Prognostic and therapeutic implications. 322, 1–7 (2012). [DOI] [PMC free article] [PubMed]
- 36.Szwergold BS, Howell SK, Beisswenger PJ. Transglycation—A Potential New Mechanism for Deglycation of Schiff’s Bases. Ann. N. Y. Acad. Sci. 2006;1043:845–864. doi: 10.1196/annals.1333.097. [DOI] [PubMed] [Google Scholar]
- 37.Miranti EH, et al. Prospective study of serum cysteine and cysteinylglycine and cancer of the head and neck, esophagus, and stomach in a cohort of male smokers. Am. J. Clin. Nutr. 2016;104:686–693. doi: 10.3945/ajcn.115.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guštin, E., Jarc, E., Kump, A. & Petan, T. Lipid Droplet Formation in HeLa Cervical Cancer Cells Depends on Cell Density and the Concentration of Exogenous Unsaturated Fatty Acids. Acta Chim. Slov. 549–554, 10.17344/acsi.2016.2908 (2017). [DOI] [PubMed]
- 39.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavi O, Greene JM, Levy D, Gottesman MM. The role of cell density and intratumoral heterogeneity in multidrug resistance. Cancer Res. 2013;73:7168–7175. doi: 10.1158/0008-5472.CAN-13-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greene JM, Levy D, Herrada SP, Gottesman MM, Lavi O. Mathematical modeling reveals that changes to local cell density dynamically modulate baseline variations in cell growth and drug response. Cancer Res. 2016;76:2882–2890. doi: 10.1158/0008-5472.CAN-15-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang HL, Yuen KL, Tang HM, Fung MC. Reversibility of apoptosis in cancer cells. Br. J. Cancer. 2009;100:118–122. doi: 10.1038/sj.bjc.6604802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendis, S., Athauda, S. B. P. & Kenji, T. Association between hyperhomocysteinaemia and ischaemic heart disease in Sri Lankans. Int. J. Cardiol., 97–103 (1997). [DOI] [PubMed]
- 44.Lin J, Manson JE, Selhub J, Buring JE, Zhang SM. Plasma cysteinylglycine levels and breast cancer risk in women. Cancer Res. 2007;67:11123–11127. doi: 10.1158/0008-5472.CAN-07-3061. [DOI] [PubMed] [Google Scholar]
- 45.Lin J, et al. Plasma homocysteine and cysteine and risk of breast cancer in women. Cancer Res. 2010;70:2397–405. doi: 10.1158/0008-5472.CAN-09-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller JW, et al. Homocysteine, cysteine, and risk of incident colorectal cancer in the Women’ s Health Initiative observational cohort 1–3. Am J Clin Nutr. 2013;97:827–34. doi: 10.3945/ajcn.112.049932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiang FF, et al. High homocysteine is associated with increased risk of colorectal cancer independently of oxidative stress and antioxidant capacities. Clin. Nutr. 2014;33:1054–1060. doi: 10.1016/j.clnu.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Murphy G, et al. Prospective study of serum cysteine levels and oesophageal and gastric cancers in China. Gut. 2011;60:1–6. doi: 10.1136/gut.2010.225854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Traverso, N. et al. Role of Glutathione in Cancer Progression and Chemoresistance. Oxid. Med. Cell. Longev. 2013 (2013). [DOI] [PMC free article] [PubMed]
- 50.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular Mechanisms and Clinical Implications of Reversible Protein S -Glutathionylation. Antioxid. Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Visscher M, Arkin MR, Dansen TB. Covalent targeting of acquired cysteines in cancer. Curr. Opin. Chem. Biol. 2016;30:61–67. doi: 10.1016/j.cbpa.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.García-Giménez JL, Romá-Mateo C, Pérez-Machado G, Peiró-Chova L, Pallardó FV. Role of glutathione in the regulation of epigenetic mechanisms in disease. Free Radic. Biol. Med. 2017;112:36–48. doi: 10.1016/j.freeradbiomed.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Epstein ACR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/S0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 54.Wu, D. & Yotnda, P. Induction and Testing of Hypoxia in Cell Culture. J. Vis. Exp., 4–7, 10.3791/2899 (2011). [DOI] [PMC free article] [PubMed]
- 55.Goldberg MA, Dunning SP, Bunn HF. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science (80-.) 1988;242:1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- 56.Al Okail MS. Cobalt chloride, a chemical inducer of hypoxia-inducible factor-1α in U251 human glioblastoma cell line. J. Saudi Chem. Soc. 2010;14:197–201. doi: 10.1016/j.jscs.2010.02.005. [DOI] [Google Scholar]
- 57.Ghaly, A. E. & Kok, R. The effect of sodium sulfite and cobalt chloride on the oxygen transfer coefficient (1988).
- 58.Reers M, et al. Mitochondrial Membrane potential monitored by JC-1 Dye. Methods Enzymol. 1995;260:406–417. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- 59.Travedi MV, Laurence JS, Siahann TJ. The role of thiols and disulfides in protein chemical and physical stability. Curr Protein Pept Sci. 2009;10:614–625. doi: 10.2174/138920309789630534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grilo NM, et al. Unmasking efavirenz neurotoxicity: Time matters to the underlying mechanisms. Eur. J. Pharm. Sci. 2017;105:47–54. doi: 10.1016/j.ejps.2017.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analysed during this study are included in the article and in supplementary information. Cell lines and reagents are commercially available.