As a group, mixed neuronal-glial tumors (MNGTs) exhibit genetic variability, including stable genomes, whole chromosome gains, BRAF-V600E, and FGFR1 mutations [8, 9, 11, 12]. While histologic criteria are described to distinguish MNGT types ganglioglioma (GG) and dysembryoplastic neuroepithelial tumor (DNT), non-specific features preclude confident classification in a high proportion of cases [2, 8, 10, 12]. Herein, we report the characterization of a novel FGFR2-INA fusion gene identified during clinical genomic profiling in two cases of MNGTs that could not be specifically classified as GG or DNT.
Clinical, imaging, histology, and fusion gene characteristics of each case are summarized in suppl. Table 1 (Online Resource 1). Both patients presented with seizures, cortical-based tumors, and one patient’s tumor was recurrent. By histology and immunohistochemistry, both cases consisted of oligodendrocyte-like cells and admixed neurons within microcytic spaces (Fig. 1a). GFAP-positive astrocytes, CD34 expression (MNGT-1), and calcification were observed. Both cases lacked pools of mucin, floating neurons, specific glioneuronal elements, eosinophilic granular bodies, and perivascular inflammation. Features were most similar to DNT; however, both lacked key criteria for this diagnosis.
Fig. 1.
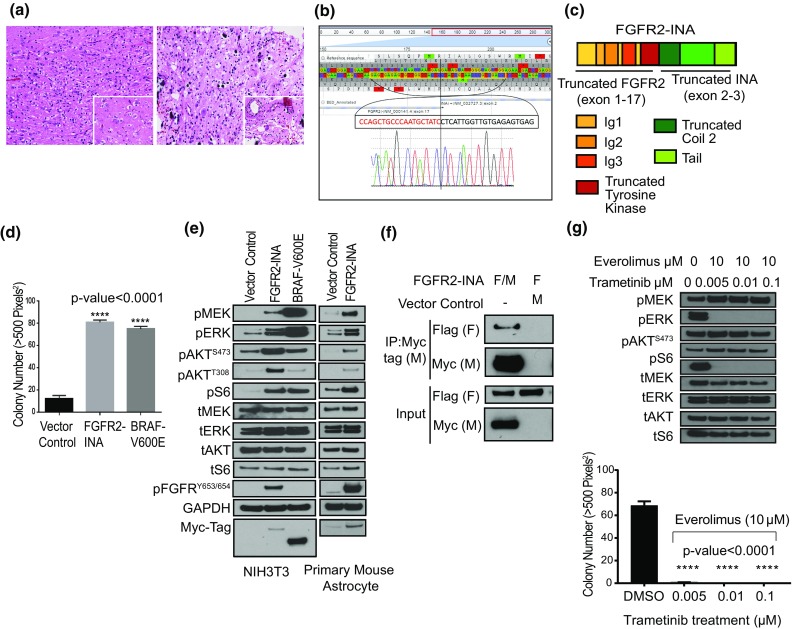
Histologic and sequencing characteristics of two MNGT harboring an FGFR2-INA fusion that activates the MAPK and PI3 K/mTOR pathways. a MNGT-1 (left) and MNGT-2 (right) contained small oligodendrocyte-like cells admixed with neurons surrounded by clear microcystic spaces (insets, 400X H&E), 200X H&E. b RNA-seq reads and confirmatory reverse complement Sanger sequencing of FGFR2-INA. c Structure of FGFR2-INA: FGFR2 exons 2–3 encode Ig-1, exons 4–5 encode Ig-2, exons 6–7 encode Ig-3 domains, and exons 9–17 encode a truncated tyrosine kinase domain (lacking three amino acids from FGFR2 exon-18). d Soft agar assay using NIH3T3 stably expressing FGFR2-INA, n = 10. Error bars represent SEM. e Western blot analysis of MAPK and PI3 K/mTOR pathway proteins in NIH3T3 and PMAs. ‘p’—phosphorylated; ‘t’—total protein. f Co-immunoprecipitation (Co-IP) assay with anti-Myc tag beads and co-transfecting HEK293 cells with Flag (F)- and Myc (M)-tagged FGFR2-INA, and F-FGFR2-INA with M- vector control. g Effect of combinatorial trametinib and everolimus treatment on FGFR2-INA-driven oncogenic signaling and growth in NIH3T3 cells
Targeted RNA-sequencing revealed a novel in-frame fusion between FGFR2 exon 17 and INA exon 2 (Fig. 1b) in both cases. Additional DNA sequence and copy number variants of clinical significance were also identified by targeted next-generation sequence panel [suppl. Tables 2, 3, 4 (Online Resource 1)] [7]. FGFR2, a receptor kinase, regulates several growth-related signaling pathways implicated in cancer progression, including RAS-RAF-MAPK and PI3K/AKT/mTOR [3]. INA encodes the alpha-internexin protein involved in cytoskeletal organization and neuronal morphogenesis [6]. The novel fusion retains the extracellular immunoglobin-like and tyrosine kinase domains of FGFR2, suggesting oncogenic activation of downstream signaling, and the truncated coil 2 and tail region of INA, suggesting dimerization (Fig. 1c).
We cloned FGFR2-INA and stably expressed it in NIH/3T3 and Tp53-null primary mouse astrocytes (PMAs) [1, 5] [suppl. Figure 1 (Online Resource 2)]. In soft agar proliferation assays, FGFR2-INA expressing NIH/3T3 showed a significant increase in colony count over control, similar to BRAF V600E (p < 0.0005) (Fig. 1d). Next, we assessed the signaling potential of FGFR2-INA. In serum starved conditions, we observed high-level activation of both the MAPK and PI3 K/mTOR pathways assessed via elevated levels of phosphorylated-ERK and -S6, respectively, compared to vector-controlled cells (Fig. 1e). Mechanistically, we found that FGFR2-INA homo-dimerizes in co-immunoprecipitation assays suggesting dimerization-induced activation of FGFR2-INA (Fig. 1f). Using combinatorial targeting of downstream MAPK and PI3K/mTOR pathways with trametinib and everolimus, respectively, we could suppress FGFR2-INA-driven oncogenic signaling and growth (Fig. 1f, suppl. Figure 2 (Online Resource 3)).
We identify and characterize a novel FGFR2-INA fusion associated with unclassified MNGT in two patients lacking other reported driver alterations (BRAF-V600E and FGFR1). Other FGFR2 fusions have been identified in epileptogenic tumors of the young with some overlapping histologic features to the current two cases [4]. It is possible that these tumors represent an emerging category of low-grade epileptogenic tumor. Our functional studies show that the FGFR2-INA fusion drives oncogenesis potentially via activation of the MAPK and PI3 K/mTOR pathways. Therefore, FGFR2-INA is the likely driver of tumorigenesis in at least a subset of MNGTs and is a potential target for small-molecule inhibitors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Children’s Brain Tumor Tissue Consortium (CBTTC).
References
- 1.Bandopadhayay P, Ramkissoon LA, et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet. 2016;48:273–282. doi: 10.1038/ng.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirose T, Scheithauer BW. Mixed dysembryoplastic neuroepithelial tumor and ganglioglioma. Acta Neuropathol. 1998;95:649–654. doi: 10.1007/s004010050852. [DOI] [PubMed] [Google Scholar]
- 3.Holbrook JD, Parker JS, et al. Deep sequencing of gastric carcinoma reveals somatic mutations relevant to personalized medicine. J Transl Med. 2011;9:119. doi: 10.1186/1479-5876-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huse JT, Snuderl M, et al. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): an epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol. 2017;133:417–429. doi: 10.1007/s00401-016-1639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain P, Fierst TM, et al. CRAF gene fusions in pediatric low-grade gliomas define a distinct drug response based on dimerization profiles. Oncogene. 2017;36:6348–6358. doi: 10.1038/onc.2017.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan MP, Chin SS, Fliegner KH, Liem RK. Alpha-internexin, a novel neuronal intermediate filament protein, precedes the low molecular weight neurofilament protein (NF-L) in the developing rat brain. J Neurosci. 1990;10:2735–2748. doi: 10.1523/JNEUROSCI.10-08-02735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li MM, Datto M, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the association for molecular pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis DN, Perry A, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 9.Prabowo AS, van Thuijl HF, et al. Landscape of chromosomal copy number aberrations in gangliogliomas and dysembryoplastic neuroepithelial tumours. Neuropathol Appl Neurobiol. 2015;41:743–755. doi: 10.1111/nan.12235. [DOI] [PubMed] [Google Scholar]
- 10.Prayson RA. Composite ganglioglioma and dysembryoplastic neuroepithelial tumor. Arch Pathol Lab Med. 1999;123:247–250. doi: 10.5858/1999-123-0247-CGADNT. [DOI] [PubMed] [Google Scholar]
- 11.Qaddoumi I, Orisme W, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016;131:833–845. doi: 10.1007/s00401-016-1539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone TJ, Keeley A, et al. Comprehensive molecular characterisation of epilepsy-associated glioneuronal tumours. Acta Neuropathol. 2018;135:115–129. doi: 10.1007/s00401-017-1773-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


