Abstract
To identify the incidence, risk factors and impact on long-term survival of invasive pulmonary aspergillosis (IPA) and Aspergillus colonisation in patients receiving vv-extracorporeal membrane oxygenation (ECMO). A retrospective evaluation was performed of patients receiving vv-ECMO at a tertiary hospital in Manchester (UK) between January 2012 and December 2016. Data collected included epidemiological data, microbiological cultures, radiographic findings and outcomes. Cases were classified as proven IPA, putative IPA or Aspergillus colonisation according to a validated clinical algorithm. One hundred thirty-four patients were supported with vv-ECMO, median age of 45.5 years (range 16.4–73.4). Ten (7%) patients had putative IPA and nine (7%) had Aspergillus colonisation. Half of the patients with putative IPA lacked classical host risk factors for IPA. The median number of days on ECMO prior to Aspergillus isolation was 5 days. Immunosuppression and influenza A infection were significantly associated with developing IPA in a logistic regression model. Cox regression model demonstrates a three times greater hazard of death associated with IPA. Overall 6-month mortality rate was 38%. Patients with putative IPA and colonised patients had a 6-month mortality rate of 80 and 11%, respectively. Immunosuppression and influenza A infection are independent risk factors for IPA. IPA, but not Aspergillus colonisation, is associated with high long-term mortality in patients supported with vv-ECMO.
Keywords: Aspergillus fumigatus, ECMO, Galactomannan, Voriconazole, Outcome
Introduction
Veno-venous extracorporeal membrane oxygenation (vv-ECMO) is an effective treatment to support patients with severe respiratory failure unresponsive to conventional therapies [1]. Its use is increasing but survival to hospital discharge remains between 50 and 60% [2]. Older age, being immunocompromised, longer duration of mechanical ventilation before vv-ECMO, neuromuscular blockade agents, nitric oxide use and increased extra-pulmonary organ failure are associated with worse outcomes [3–5].
Healthcare-associated infections occur in 26–45% of patients on vv-ECMO and are associated with high mortality and increased hospital stay [6–8]. Fungal pathogens are commonly isolated from adults supported with vv-ECMO [9, 10]. Aspergillus spp. are identified more frequently in this group compared with those in other groups of critically ill patients [7]. Aspergillus in the airways of patients supported with ECMO may be associated with poorer outcomes [10–12]. Several major risk factors have been identified for developing invasive pulmonary aspergillosis (IPA) in critically ill patients not receiving vv-ECMO, such as influenza A infection, higher SOFA score and previous broad-spectrum antibiotic therapy [13–15].
The diagnosis of IPA in critically ill patients is challenging. Severe respiratory failure and coagulopathy make invasive sampling techniques such as lung biopsy difficult. Non-invasive diagnostic tests have not been well validated in immunocompetent patients [16]. A simple, clinical diagnostic algorithm for the diagnosis of IPA in critically ill patients has been externally validated in a large, multi-centre cohort and may have utility in patients supported with vv-ECMO [17, 18]. The algorithm incorporates mycological culture and microscopy of broncho-alveolar lavage (BAL) samples from critically ill patients as a tool to discriminate Aspergillus colonisation from IPA.
Here, we describe the incidence of Aspergillus infection and colonisation in patients supported with vv-ECMO and identify potential risk factors and the effect of Aspergillus infection on long-term survival.
Materials and methods
Study design and setting
A retrospective, observational study of all critically ill patients who received vv-ECMO support between January 2012 and December 2016 was performed. As the study was a retrospective service evaluation, ethical approval was not required.
Study definitions
Patients, aged 16 years or older and with potentially reversible severe acute respiratory failure despite optimisation of conservative therapy, were considered for support with vv-ECMO. Patients with positive Aspergillus spp. culture from BAL were classified as proven IPA, putative IPA or Aspergillus colonisation according to a validated clinical algorithm [17, 18]. A diagnosis of proven IPA requires histopathological evidence of fungal invasion. For a diagnosis of putative IPA, a patient had a positive culture for Aspergillus spp. from any lower respiratory tract (LRT) sample, together with meeting three criteria: (1) compatible signs and symptoms, (2) any abnormality on medical imaging and (3) either a positive semi-quantitative Aspergillus culture from BAL without bacterial growth and a positive cytological smear showing branching hyphae or any of the host risk factor for IPA according to EORTC/MSG criteria [19]. Aspergillus colonisation was considered when ≥ 1 criteria necessary for a diagnosis of putative IPA were not met. Patients without Aspergillus spp. in the airways were included in the non-Aspergillus group.
Data collection
Clinical data
Clinical data were collected from electronic medical records including demographics (age, sex), underlying medical conditions, indication for vv-ECMO, duration of ventilation prior to vv-ECMO and duration of vv-ECMO support, Aspergillus diagnosis, antifungal therapy and clinical outcome.
Microbiological and radiological data
Bacterial and fungal culture findings were recorded. Galactomannan (GM) was measured in BAL [20]. All chest computed tomography (CT) was reported by specialised chest radiologists. Chest CT scans were considered to be “suggestive” of IPA if any of the following were seen: (1) lung cavitation, (2) air-crescent sign or (3) dense, well-circumscribed lesion(s) with or without a halo sign.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 7.0 and IBM SPSS Statistics version 22. The Mann-Whitney test was used to compare GM indexes in patients with IPA with those without. A logistic regression model was used to identify risk factors (bacterial pneumonia, influenza A infection, chronic obstructive pulmonary disease and immunosuppression) for acquiring IPA. A multivariate Cox proportional hazards regression model was used to establish factors affecting outcome in patients receiving vv-ECMO (age, days of mechanical ventilation prior to receiving vv-ECMO support, bacterial or viral pneumonia and Aspergillus colonisation or IPA). These factors were included as they have previously been shown to affect outcome in patients supported with vv-ECMO [4]. Kaplan-Meier curves were used to compare survival of patients with Aspergillus infection, colonised patients and patients without any evidence of Aspergillus over time. Statistical significance was defined as p < 0.05.
Environmental screening
During the study period, environmental air sampling was performed periodically according to local policy and published protocols [21]. Samples were collected from multiple locations within the Cardiothoracic Critical Care Unit. The agar plates were incubated at 30 °C for 5 days.
Results
Patients’ demographics and clinical characteristics
A total of 134 patients, 73 (54%) male, were included. The mean age was 44.3 years (range 16.4–73.4) (Table 1). Respiratory infection was the most common indication for receiving vv-ECMO support with 74/134 (55%) patients with bacterial pneumonia, 23/74 (31%) of whom had Streptococcus pneumoniae isolated from BAL. Forty (30%) patients were admitted with viral pneumonia of whom 24/40 (60%) had influenza A infection. One patient with Streptococcus pneumoniae pneumonia developed putative IPA. The most common underlying respiratory diagnosis was asthma, 18/134 (13%) of patients. No patients had a diagnosis of allergic bronchopulmonary aspergillosis. The median length of stay on mechanical ventilation prior to vv-ECMO was 2 days (range 1–22) and the median duration on vv-ECMO support was 13 days (range 1–57).
Table 1.
Patient demographics and clinical, microbiological and radiological findings
| Non-Aspergillus group (n = 115) | Putative IPA (n = 10) | Aspergillus colonisation (n = 9) | |
|---|---|---|---|
| Age, years [median, (range)] | 45.4 (16.4–73.4) | 51.2 (23.9–64.6) | 35.3 (17.8–46.8) |
| Male [n (%)] | 60 (52) | 8 (80) | 5 (63) |
| Time ventilated before vv-ECMO, days [median, (range)] | 3 (1–22) | 1 (1–7) | 1 (1–6) |
| Duration of vv-ECMO support, days [median, (range)] | 13 (1–57) | 26 (7–45) | 10 (4–29) |
| Underlying conditions [n (%)] | |||
| COPD | 7 (6) | 1 (10) | 1 (13) |
| Asthma | 13 (11) | 1 (10) | 4 (50) |
| Diabetes | 7 (6) | 1 (10) | 0 |
| Solid tumour | 2 (2) | 1 (10) | 0 |
| HIV | 0 | 1 (10) | 0 |
| Active TB | 0 | 1 (10) | 0 |
| Autoimmune disease | 3 (3) | 1 (10) | 0 |
| Solid organ transplant | 1 (< 1) | 1 (10) | 0 |
| Current pregnancy | 1 (< 1) | 0 | 0 |
| Smoking | 15 (13) | 0 | 2 (25) |
| Heavy alcohol consumption | 10 (9) | 0 | 1 (13) |
| IVDU | 3 (3) | 0 | 0 |
| Immunosuppressiona | 15 (13) | 5 (50) | 1 (11) |
| Others | 37 (32) | 2 (20) | 1 (11) |
| EORTC host factors [n (%)] | 4 (40) | 0 | |
| Prolonged steroid therapy | 3 (30) | 0 | |
| Chemotherapy | 1 (10) | 0 | |
| Indication for ECMO [n (%)] | |||
| Bacterial pneumonia | 68 (59) | 5 (50) | 1 (11) |
| Streptococcus pneumoniae | 22 (19) | 1 (10) | 0 |
| Viral pneumonia | 31 (27) | 5 (50) | 4 (44%) |
| Influenza A | 18 (16) | 5 (50) | 1 (13) |
| Asthma | 7 (6) | 1 (10) | 2 (22) |
| Burns | 5 (4) | 0 | 0 |
| Eosinophilic pneumonia | 4 (4) | 0 | 0 |
| Other | 0 | 0 | 2 (22) |
| Isolated species [n (%)] | |||
| Aspergillus fumigatus | 0 | 10 (100) | 9 (100) |
| Laboratory findings | |||
| BAL GM, OD index > 0.5 [n (%)] | 21/53 (39) | 6/8 (75) | 4/8 (50) |
| BAL GM, OD index [median, (range)] | 0.34 (0.03–9.30) | 7.4 (0.53–10.08) | 0.54 (0.04–9.44) |
| Abnormal radiological findings | |||
| Non-specific chest CT scan findings [n (%)] | 106 (92) | 6 (60) | 8 (100) |
| “Suggestive” chest CT scan findings of IPA [n (%)] | 9 (8) | 4 (40) | 0 |
Data are numbers or proportion (%)
IPA invasive pulmonary aspergillosis, ECMO extracorporeal membrane oxygenation, COPD chronic obstructive pulmonary disease, HIV human immunodeficiency virus, TB tuberculosis, IVDU intravenous drug user, EORTC European Organisation for Research and Treatment of Cancer, BAL broncho-alveolar lavage, GM galactomannan, OD optical density, CT computed tomography
aIncludes patients who are neutropenic or receiving corticosteroids or chemotherapy
Ten (7%) patients had putative IPA and nine (7%) patients had Aspergillus colonisation. Six of ten (60%) patients with putative IPA fulfilled the diagnostic criteria within the first 2 days of receiving vv-ECMO support whilst the median number of days supported with vv-ECMO prior to Aspergillus isolation was 5 days (range 0–27). One hundred fifteen (86%) patients never had a positive sputum or BAL culture for Aspergillus spp. Four of ten (40%) patients with putative IPA had “classical” diagnostic host criteria for IPA according to EORTC criteria [19].
Risk factors for acquiring IPA and patient outcomes
Influenza A infection (HR 11.4, 95% CI 1.97–65.86; p = 0.007) and immunosuppression (HR 10.75, 95% CI 2.23–51.72; p = 0.003) were associated with an increased risk of patients developing IPA (Table 2). The overall 6-month all-cause mortality of all patients who were supported with vv-ECMO during the study period was 38% (51/134). Eight of ten (80%) patients with putative IPA and 1/9 (11%) with Aspergillus colonisation had died at 6 months (Fig. 1). Cox regression analysis found mortality was over three times higher in patients with putative IPA compared with that of patients with either colonisation or patients with no evidence of Aspergillus spp. (p = 0.036) (Fig. 2, Table 3). Additionally, both age and length of mechanical ventilation prior to vv-ECMO were also found to be associated with an increased risk of death. Aspergillus colonisation during vv-ECMO, bacterial pneumonia or viral pneumonia was not associated with an increased hazard ratio in the Cox regression model.
Table 2.
Risk factors for acquiring IPA in critically ill patients receiving vv-ECMO
| Risk factor | Hazard ratio (95% CI) | p value |
|---|---|---|
| Bacterial pneumonia | 1.51 (0.30–7.60) | 0.615 |
| Influenza A | 11.40 (1.97–65.86) | 0.007 |
| Chronic obstructive pulmonary diseases | 4.83 (0.351–66.49) | 0.239 |
| Immunosuppressiona | 10.75 (2.23–51.72) | 0.003 |
aImmunosuppression includes patients who are neutropenic or receiving corticosteroids or chemotherapy
Fig. 1.
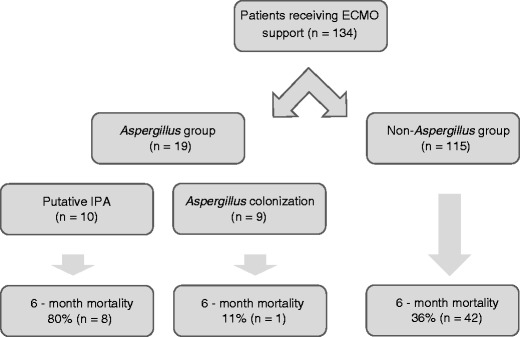
Flowchart and mortality in all groups of patients included in the study
Fig. 2.
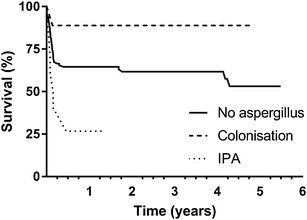
Kaplan-Meier plots showing long-term outcome of patients who received vv-ECMO with putative IPA and Aspergillus colonisation and patients with no evidence of Aspergillus infection during their admission
Table 3.
Effect of risk factors on survival of critically ill patients receiving vv-ECMO
| Risk factor | Hazard ratio (95% CI) | p value |
|---|---|---|
| Viral pneumonia | 0.49 (0.17–1.42) | 0.188 |
| Bacterial pneumonia | 0.63 (0.317–1.27) | 0.199 |
| Age | 0.003 | |
| 18–49 years | 1 (reference category) | |
| 50–59 years | 3.44 (1.42–8.33) | 0.006 |
| ≥ 60 years | 3.85 (1.55–9.56) | 0.004 |
| Aspergillus diagnosis | ||
| Non-Aspergillus infection | 1 (reference category) | 0.067 |
| Aspergillus colonisation | 0.43 (0.06–3.32) | 0.421 |
| Putative IPA | 3.31 (1.08–10.14) | 0.036 |
| Time on mechanical ventilation prior to vv-ECMO | ||
| < 48 h | 1 (reference category) | 0.028 |
| 48 h to 7 days | 2.02 (0.91–4.54) | 0.085 |
| > 7 days | 3.70 (1.37–9.98) | 0.010 |
Laboratory and radiological findings
From those patients who had a positive BAL Aspergillus culture, all had Aspergillus fumigatus identified. GM was measured in the BAL fluid from 53/115 (46%) patients in the non-Aspergillus group, 8/10 (80%) patient with putative IPA and 8/9 (88%) patients with Aspergillus colonisation (Table 1). Patients with putative IPA had significantly higher BAL GM OD indexes compared to patients without putative IPA
(Mann-Whitney test p = 0.029) (Fig. 3). Using a GM index threshold of ≥ 0.5 for a diagnosis of putative IPA, the sensitivity, specificity and positive and negative predictive values of the BAL GM assay were 75, 59, 19 and 95% respectively.
Fig. 3.
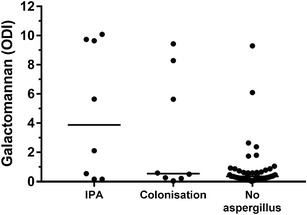
BAL GM mean optical indexes (ODI) measured in patients with putative IPA and Aspergillus colonisation and patients with no evidence of Aspergillus in their airways
All patients with Aspergillus in BAL had a chest CT scan performed. Four out of ten (40%) patients with putative IPA had typical radiology changes suggestive of IPA; 1/4 (25%) had a classical host risk factor.
Antifungal therapy
Seven of ten (70%) patients with putative IPA were treated with a combination of micafungin and voriconazole, 2/10 (20%) patients received voriconazole and 1/10 (10%) received liposomal amphotericin B. Seven of the nine (77%) patients colonised with Aspergillus and 51 of 115 (44%) patients from the non-Aspergillus group received antifungal therapy either empirically or to treat candidaemia.
Environmental screening
A total of 46 air samples were collected over eight separate occasions. A. fumigatus was isolated on three occasions (range 1–3 colonies forming units (CFU) per cubic metre of air) whilst A. niger was isolated on one occasion. Throughout the study period, the level of A. fumigatus in the adjacent outside air was in the range of 2 to 23 CFU per cubic metre of air.
Discussion
During a 5-year period, we found an incidence of putative IPA and Aspergillus colonisation in critically ill patients receiving vv-ECMO support of 7% each. The combined incidence (14%) of Aspergillus disease in patients receiving vv-ECMO support is higher than that reported in critically ill adults, which is typically between 0.05 and 7.5% [7, 12, 22, 23]. Many of these trials did not differentiate between Aspergillus infection and colonisation and include patients receiving both vv-ECMO and veno-arterial ECMO as a combined category [7, 24], making true comparison difficult. Aubron et al. [11] described 11 patients from 151 receiving ECMO support with Aspergillus disease but just 2 (18%) patients were supported with vv-ECMO. Hospital-acquired Aspergillus infection is very unlikely as environmental screening demonstrated an acceptable number of environmental Aspergillus conidia with no evidence of excessive fungal contamination. Our study has identified immunosuppression and influenza A infection as independent risk factors for IPA. Thus, the high incidence of Aspergillus infection in our patients may be related to the high rate of patients with influenza A.
IPA, but not Aspergillus colonisation, was associated with a significantly higher mortality at 6 months in patients receiving vv-ECMO (80 vs 11%). This difference in outcomes between infection and colonisation was also previously described by Contou et al. in critically ill patients with acute respiratory distress syndrome (ARDS) [25]. The multivariate Cox regression model, which incorporated risk factors known to be associated with excess mortality in patients receiving vv-ECMO, demonstrated a three times greater hazard of death in patients with putative IPA compared to non-Aspergillus group. Previous studies on critically ill patients, which include immunocompromised patients, have shown mortality rates due to IPA of over 75% [11, 14, 22]. In comparison to previous studies, which only focused on hospital mortality, we have provided long-term follow-up data which shows that patients die early in their critical illness from IPA.
Diagnosing Aspergillus infection in critically ill patients remains challenging. We found that 60% of patients with putative IPA had non-specific changes of IPA on the CT scan. Higher levels of GM in BAL were observed in patients with putative IPA than in colonised patients or patients with no evidence of Aspergillus in their airways. A GM in BAL with an OD index ≥ 0.5 showed a negative predictive value of 95% suggesting that this test may be useful as a rule-out test for IPA in this patient population.
The most appropriate antifungal therapy for IPA on vv-ECMO is still unclear. In the present study, the number of patients with Aspergillus infection and colonisation was too small to find significant differences with different treatment regimes. The role of antifungal prophylaxis is still unclear but it may be beneficial in high-risk patients supported on vv-ECMO (e.g. influenza infection, age over 60 years and prolonged previous mechanical ventilation). This strategy has been previously used in high-risk immunocompromised patients [26] but further studies are needed to specify its role in patients with vv-ECMO support.
This study has some limitations mostly related with the retrospective analysis of the data at a single centre. The relatively small number of putative IPA and colonised patients indicates that these results of both regression models should be viewed as exploratory rather than confirmatory. Severity of illness score was not recorded on the medical records of patients who received vv-ECMO support. GM was collected in only 69/134 (51%) of patients, so care is required interpreting sensitivity and specificity analysis. Larger prospective studies are required to identify clinically important biomarkers of IPA and optimal antifungal regimens.
In summary, Aspergillus infection in patients undergoing vv-ECMO support is associated with poor long-term outcome. Prolonged mechanical ventilation and older age were associated with worse outcome. Previous influenza infection was identified as a risk factor for developing IPA. GM in BAL may have a role as a rule-out test for IPA in this population. Optimal antifungal therapy remains unclear and further studies are required to optimise diagnosis and management in patients receiving vv-ECMO.
Acknowledgements
This project was supported by the National Institute of Health Research (NIHR) Manchester Biochemical Research Centre, Manchester University NHS Foundation Trust.
Author contributions
IRG participated in the study concept, data collection and manuscript writing, drafting and reviewing. ST, MDR, AA and JB participated in manuscript editing and reviewing. PF participated in data analysis, interpretation and manuscript reviewing. CG participated in data collection and manuscript reviewing. EM participated in the study concept, manuscript design, data interpretation, editing and reviewing. TF participated in the manuscript design, writing, editing and reviewing as well as data analysis and interpretation. All authors read and approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study, formal consent is not required.
Informed consent
Informed consent was not required.
Footnotes
E. G. Muldoon and T. W. Felton are joint last authors.
A part of this study was presented as an oral presentation at the 27th European Congress of Clinical Microbiology and Infectious Diseases (Vienna, Austria, 2017).
Contributor Information
E. G. Muldoon, Email: EavanMuldoon@mater.ie
T. W. Felton, Email: timothy.felton@manchester.ac.uk
References
- 1.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM et al (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet [Internet] Elsevier Ltd; 374(9698):1351–1363. Available from: 10.1016/S0140-6736(09)61069-2 [DOI] [PubMed]
- 2.Smith M, Vukomanovic A, Brodie D, Thiagarajan R, Rycus P, Buscher H. Duration of veno-arterial extracorporeal life support (VA ECMO) and outcome: an analysis of the Extracorporeal Life Support Organization (ELSO) registry. Crit Care [Internet] 2017;21(1):45. doi: 10.1186/s13054-017-1633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francischetti IMB, Szymanski J, Rodriguez D, Heo M, Wolgast LR (2017) Laboratory and clinical predictors of 30-day survival for patients on extracorporeal membrane oxygenation (ECMO): 8-year experience at Albert Einstein College of Medicine, Montefiore Medical Center. J Crit Care [Internet] Elsevier Inc. 40:136–144. Available from: 10.1016/j.jcrc.2017.03.027 [DOI] [PubMed]
- 4.Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure: the respiratory extracorporeal membrane oxygenation survival prediction (RESP) score. Am J Respir Crit Care Med. 2014;189(11):1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 5.Hirshberg E, Miller 3rd RR, Morris AH (2013) Extracorporeal membrane oxygenation in adults with acute respiratory distress syndrome. Curr Opin Crit Care [Internet] Elsevier Inc; 19(1):38–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23222676 [DOI] [PubMed]
- 6.Haneke F, Schildhauer TA, Schlebes AD, Strauch J, Swol J (2015) Infections and extracorporeal membrane oxygenation: incidence, therapy and outcome. ASAIO J [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26579980 [DOI] [PubMed]
- 7.Aubron C, Cheng AC, Pilcher D, Leong T, Magrin G, Cooper DJ, et al. Infections acquired by adults who receive extracorporeal membrane oxygenation risk factors and outcome. Infect Control Hosp Epidemiol [Internet] 2013;34(1):24–30. doi: 10.1086/668439. [DOI] [PubMed] [Google Scholar]
- 8.Sun G, Li B, Lan H, Wang J, Lu L, Feng X et al (2017) Factores de riesgo de las infecciones nosocomiales en pacientes que reciben oxigenación por membrana extracorpórea. Med Clin (Barc) [Internet] SEGO; (xx). Available from: http://linkinghub.elsevier.com/retrieve/pii/S0025775317302841 [DOI] [PubMed]
- 9.Pieri M, Agracheva N, Fumagalli L, Greco T, De BM, Calabrese MC. Infections occurring in adult patients receiving mechanical circulatory support: the two-year experience of an Italian National Referral Tertiary Care Center |. Med Int. 2013;37(7):468–475. doi: 10.1016/j.medin.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011;12(3):277–281. doi: 10.1097/PCC.0b013e3181e28894. [DOI] [PubMed] [Google Scholar]
- 11.Aubron C, Pilcher D, Leong T, Cooper DJ, Scheinkestel C, Pellegrino V, et al. Aspergillus sp. isolated in critically ill patients with extracorporeal membrane oxygenation support. Scand J Infect Dis[Internet] 2013;45(9):715–721. doi: 10.3109/00365548.2013.797598. [DOI] [PubMed] [Google Scholar]
- 12.Thomas P, et al. The morbidity and mortality of patients with fungal infections before and during extracorporeal membrane oxygenation support. Pediatr Crit Care. 2013;13(5):82–89. doi: 10.1097/PCC.0b013e31824fbaf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crum-Cianflone NF. Invasive aspergillosis associated with severe influenza infections. Open Forum Infect Dis. 2016;3(3):1–8. doi: 10.1093/ofid/ofw171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wauters J, Baar I, Meersseman P, Meersseman W, Dams K, De Paep R, et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med. 2012;38(11):1761–1768. doi: 10.1007/s00134-012-2673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otterspoor LC, Smit FH, van Laar TJ, Kesecioglu J, van Dijk D. Prolonged use of extracorporeal membrane oxygenation combined with prone positioning in patients with acute respiratory distress syndrome and invasive aspergillosis. Perfusion [Internet] 2012;27(4):335–337. doi: 10.1177/0267659112442098. [DOI] [PubMed] [Google Scholar]
- 16.Meersseman W, Lagrou K, Maertens J, Wilmer A, Hermans G, Vanderschueren S, et al. Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patients. Am J Respir Crit Care Med. 2008;177(1):27–34. doi: 10.1164/rccm.200704-606OC. [DOI] [PubMed] [Google Scholar]
- 17.Vandewoude K, Blot S, Benoit D, Depuydt P, Vogelaers D, Colardyn F. Invasive aspergillosis in critically ill patients: analysis of risk factors for acquisition and mortality. Acta Clin Belg. 2004;59(5):251–257. doi: 10.1179/acb.2004.037. [DOI] [PubMed] [Google Scholar]
- 18.Blot SI, Taccone FS, Van Den Abeele AM, Bulpa P, Meersseman W, Brusselaers N, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186(1):56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 19.De Pauw B, Walsha TJ, Donnellya JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive. Clin Infect Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amur S (2015) Biomarker qualification. Present FDA Public Work. 1–28
- 21.Morris G, Kokki MH, Anderson K, Richardson MD. Sampling of Aspergillus spores in air. J Hosp Infect. 2000;44(2):81–92. doi: 10.1053/jhin.1999.0688. [DOI] [PubMed] [Google Scholar]
- 22.Taccone FS, Van den Abeele A-M, Bulpa P, Misset B, Meersseman W, Cardoso T et al (2015) Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care [Internet] 19:7 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4344741/ [DOI] [PMC free article] [PubMed]
- 23.Mosca MS, Narotsky DL, Liao M, Mochari-Greenberger H, Beck J, Mongero L, et al. Survival following veno-venous extracorporeal membrane oxygenation and mortality in a diverse patient population. J Extra Corpor Technol [Internet] 2015;47(4):217–222. [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia X, Mian a, Mendiratta P, Gupta P, Rycus P, Prodhan P. Aspergillus infection and extracorporeal membrane oxygenation support. J Intensive Care Med. 2012;28(3):178–184. doi: 10.1177/0885066611432542. [DOI] [PubMed] [Google Scholar]
- 25.Contou D, Dorison M, Rosman J, Schlemmer F, Gibelin A, Foulet F, et al. Aspergillus-positive lower respiratory tract samples in patients with the acute respiratory distress syndrome: a 10-year retrospective study. Ann Intensive Care Springer Paris. 2016;6(1):1–11. doi: 10.1186/s13613-015-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner AH, Prodhan P, Stovall SH, Gossett JM, Stern JE, Wilson CD et al (2012) Fungal infections and antifungal prophylaxis in pediatric cardiac extracorporeal life support. J Thorac Cardiovasc Surg [Internet]. The American Association for Thoracic Surgery; 143(3):689–695. Available from: 10.1016/j.jtcvs.2011.12.001 [DOI] [PubMed]


