Summary
Background
Very late (aged ≥60 years) onset schizophrenia-like psychosis occurs frequently but no placebo-controlled, randomised trials have assessed the efficacy and risks of antipsychotic treatment. We investigated whether low-dose amisulpride (100 mg daily) is superior to placebo in reducing psychosis symptoms over 12 weeks and whether any benefit is maintained by continuing treatment after 12 weeks.
Methods
The ATLAS double-blind controlled trial enrolled participants from 25 old age psychiatry services in the UK. Eligible participants (ie, those with a diagnosis of very late-onset schizophrenia-like psychosis and a Brief Psychiatric Rating Scale [BPRS] score of ≥30, without cognitive impairment) were randomly assigned (1:1:1) to one of three groups in a two-stage trial: amisulpride in stage 1 and 2 (group A), amisulpride then placebo (group B), or placebo then amisulpride (group C). Treatment (100 mg oral amisulpride daily vs placebo) was given for 12 weeks in stage 1 and, initially, 24 weeks then reduced to 12 weeks in stage 2. Participants, investigators, and outcome assessors were masked to treatment allocation. Primary outcomes were psychosis symptoms assessed by the BPRS at 4, 12, and 24, or 36 weeks, and trial treatment discontinuation for non-efficacy. The primary, secondary, and safety endpoints were all analysed in participants given at least one dose of study treatment in modified intention-to-treat analyses. This study is registered with EudraCT, number 2010-022184-35, and ISRCTN, number ISRCTN45593573.
Findings
Between Sept 27, 2012, and June 28, 2016, we recruited 101 participants. 92 (91%) of 101 participants took trial medication, of whom 59 (64%) completed stage 1 and 34 (58%) of these 59 participants completed stage 2 treatment. Despite suboptimal compliance, improvements in BPRS scores at 12 weeks were 7·7 points (95% CI 3·8–11·5, p=0·0002) greater with amisulpride (mean 11·9 points [SE 1·3]) than with placebo (4·2 points [1·0]). In stage 2, BPRS scores improved by a mean of 1·1 points (1·6) from 12 weeks to the final assessment in those who continued amisulpride but deteriorated by 5·2 points (2·0) in those who switched from amisulpride to placebo (difference 6·3 points [95% CI 0·9–11·7], p=0·024). Fewer participants who were allocated amisulpride than placebo stopped treatment because of non-efficacy in stage 1 (p=0·010) and stage 2 (p=0·031). Serious adverse events were reported more frequently in the amisulpride group than in the placebo group in stage 1 (p=0·057) and stage 2 (p=0·19). The most common serious adverse events were infection (five patients in the amisulpride group, three in the placebo group) and extrapyramidal side-effects (three patients in the amisulpride group, none in the placebo group). Five patients died during the study, one from a gastric ulcer bleed before treatment started (group B), two while taking stage 2 treatment (one in group A and one in group C), and two who stopped trial treatment in stage 1 and died many weeks later (one in group B and one in group C). No deaths were related to treatment.
Interpretation
Low-dose amisulpride is effective and well tolerated as a treatment for very late-onset schizophrenia-like psychosis, with benefits maintained by prolonging treatment.
Funding
UK National Institute for Health Research.
Introduction
Onset of schizophrenia spectrum psychosis most commonly occurs in late adolescence or early adult life, although a late-onset (aged ≥60 years) variant of non-affective functional psychosis without dementia has long been recognised,1 and is classified as very late-onset schizophrenia-like psychosis.2 After dementia and depression, very late-onset schizophrenia-like psychosis is the largest diagnostic group seen by older people's mental health services, with a reported annual incidence of 27 per 100 000 men and 48 per 100 000 women.3 Female sex, increasing age, sensory impairment, and migrant status are risk factors but, in contrast to schizophrenia, genetic factors have not been implicated in the aetiology of very late-onset schizophrenia-like psychosis.2, 3, 4, 5 Older people with very late-onset schizophrenia-like psychosis present with positive psychosis symptoms, typically persecutory delusions with or without multimodal hallucinations, which are distressing, persist for many years, and are associated with increased risks of social dysfunction, institutionalisation, and death.6, 7
Research in context.
Evidence before this study
We searched PubMed and the Cochrane database on Jan 13, 2017, for relevant articles, using the search terms “late-onset schizophrenia”, “very late-onset schizophrenia-like”, “clinical trial”, and “treatment”, with no date or language restrictions. We did not find any randomised, controlled, double-blind trials in this patient group with onset of a schizophrenia-like illness at age 60 years or older. Available evidence for the use of antipsychotics in very late-onset schizophrenia-like psychosis comes from one single-arm trial of 5 weeks of treatment with amisulpride that reported improvements in psychosis symptoms without significant extrapyramidal side-effects and from retrospective case-note reviews and published expert opinion. Because of the absence of reliable evidence on the benefits of antipsychotic treatment and high-profile reports of questionable efficacy and adverse safety of such treatment in patients with Alzheimer's disease and psychosis, there are concerns that the risks of antipsychotics in an older patient group, who are potentially vulnerable to such side-effects, might outweigh any benefits. Furthermore, patients with very late-onset schizophrenia-like psychosis usually have scarce insight into the potential value of taking antipsychotic treatment, hence most patients with such psychosis have not engaged with antipsychotic treatment.
Added value of this study
This randomised, placebo-controlled, double-blind trial provides the first reliable evidence that an antipsychotic drug (amisulpride 100 mg daily) is superior to placebo in reducing psychosis symptoms over 12 weeks and 24 weeks in patients with very late-onset schizophrenia-like psychosis. The treatment effect size from 24 weeks of amisulpride was large despite suboptimal compliance. Treatment was well tolerated with similar discontinuation rates for side-effects with amisulpride and placebo, despite a modest increase in extrapyramidal symptoms in patients given amisulpride.
Implications of all the available evidence
Psychosis symptoms in patients with very late-onset schizophrenia-like psychosis show a good and sustained response to amisulpride (100 mg/day) and this treatment is well tolerated. This randomised controlled trial is the first to show evidence of clear benefits from antipsychotic treatment in a situation where physicians might previously have shared the reluctance or ambivalence of their patients about such treatment.
Although atypical antipsychotics are used to treat very late-onset schizophrenia-like psychosis, their benefits and risks have not been properly evaluated. To our knowledge, the only evidence for efficacy is from a retrospective review of case notes8 and one single-arm, 5-week trial of amisulpride that reported improvements in psychosis without worsening of extrapyramidal symptoms.9 No randomised placebo-controlled trial data are available to guide antipsychotic treatment in very late-onset schizophrenia-like psychosis.10 Clinicians' concerns about the poor efficacy and substantial risks associated with the prescribing of antipsychotic drugs for older people with Alzheimer's disease,11, 12 compounded by the characteristic impairment of insight into the potential value of treatment among patients with very late-onset schizophrenia-like psychosis, have resulted in less than half of eligible patients currently receiving treatment.13
The primary aims of the antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS) trial were to determine whether 12 weeks of treatment for very late-onset schizophrenia-like psychosis with low-dose amisulpride (100 mg daily) improves psychiatric symptom scores compared with placebo, and whether prolonged treatment with 100 mg amisulpride beyond 12 weeks confers additional benefit. We also aimed to assess side-effects and serious adverse events associated with amisulpride treatment, compliance with allocated treatment in this difficult-to-treat population, and the effects of treatment on quality of life.
Methods
Study design
ATLAS was a pragmatic, randomised, three-arm, double-blind, placebo-controlled trial with two stages. In stage 1, we investigated the efficacy and tolerability of oral amisulpride over 12 weeks compared with placebo. In stage 2, we investigated the effects of continuing amisulpride versus withdrawal to placebo. Stage 2 treatment was initially planned to last 24 weeks, but the protocol was amended to reduce the duration to 12 weeks to increase the number of patients still being treated at the final assessment.
People with very late-onset schizophrenia-like psychosis often have little insight into their illness or the possibility that antipsychotic treatment could improve their symptoms; therefore we did not consider it practicable to involve patients directly in the design or management of the trial. Instead, the Service User Research Enterprise at the Institute of Psychiatry, Psychology and Neuroscience, King's College London (London, UK) advised on the choice of outcome measures, trial procedures, and on the use of language in trial patient information and consenting materials.
The study was done in compliance with the principles of the Declaration of Helsinki (1996) and the principles of Good Clinical Practice, and was reviewed and approved by The London and Surrey Borders Multicentre Research Ethics Committee (reference 11/LO/1267) and the Medicines and Healthcare products Regulatory Agency. The protocol is given in the appendix.
Participants
We recruited patients with very late-onset schizophrenia-like psychosis from community and inpatient specialist old age psychiatry services within the UK National Health Service. Eligibility criteria were diagnosis of very late-onset schizophrenia-like psychosis according to International Consensus Group Criteria,2 including onset of delusions or hallucinations, or both, at age 60 years or older, BPRS score14 of 30 or greater, and capacity to give informed consent to participate in the trial. Exclusion criteria were cognitive impairment or a standardised Mini Mental State Examination15 score of less than 25, diagnosis of affective disorder, uncontrolled serious physical illness, prescribed amisulpride in the previous 28 days, contraindications to amisulpride, and participation in another clinical trial of an investigational medicinal product in the previous 28 days. Participants with conditions that would prevent them from having a blood test were excluded from the optional blood tests, but not the full trial. People treated with antipsychotics other than amisulpride in the 28 days before enrolment but who still satisfied eligibility criteria, and for whom stopping their current antipsychotic was considered appropriate, could participate. These participants stopped antipsychotic treatment the day before starting trial medication. Participants were given an information sheet about the study and the trial procedures were explained to them, after which participants gave written informed consent.
Randomisation and masking
Once informed consent was given and baseline assessments were done, we randomly allocated participants in a 1:1:1 ratio, using a centralised randomisation service, to one of three groups: amisulpride (group A); amisulpride followed by placebo (group B); or placebo followed by amisulpride (group C). Treatment packs were allocated centrally by the ATLAS study office. The minimised randomisation procedure aimed to balance treatment allocation (as much as possible given available packs at each centre) overall, and by six stratification variables: age (60–69, 70–79, ≥80 years), sex, living circumstances (living with spouse or partner, living alone, other), time since onset of symptoms (<6 months, ≥6 months), previous antipsychotic treatment (no, yes >1 month previously, yes ≤28 days ago), and BPRS score (30–39, 40–49, ≥50). Participants were enrolled by their clinicians, clinical study officers, or ATLAS trial research nurses. These staff did outcome assessments. Amisulpride and placebo were provided in identical overencapsulated treatment cartons to maintain blinding. Participants, prescribing clinicians, outcome assessors, and all ATLAS trial staff (except statisticians) were masked to group assignment.
Procedures
Participants were given overencapsulated 100 mg amisulpride or placebo in 12-week treatment cartons, each containing three 28-day blister packs. Group A took one capsule containing 100 mg amisulpride daily for 36 weeks (24 weeks for patients enrolled after the protocol amendment). Group B took one capsule containing 100 mg amisulpride daily for 12 weeks followed by one matching placebo capsule daily for a further 24 weeks (12 weeks for patients enrolled after the protocol amendment). Group C took one placebo capsule daily for 12 weeks followed by one 100 mg amisulpride capsule daily for a further 24 weeks (12 weeks for patients enrolled after the protocol amendment). Data from participants in group C were not used for trial analyses in stage 2 because there was not an appropriate comparator group. Information on compliance and the number of tablets taken was collected at each follow-up assessment and pills were counted to confirm reported compliance. Adverse events were recorded at each visit from a checklist of anticipated side-effects of amisulpride and free text recording of adverse events.
To determine whether there was an association between compliance and baseline characteristics, we compared the baseline characteristics of completers and dropouts using time to dropout.
Outcomes
Outcomes were assessed at 4 and 12 weeks and at 24 or 36 weeks for all participants regardless of whether or not they continued to take trial medication. The first primary outcome was change in psychosis symptoms, assessed by the BPRS score, between baseline and week 12 and between week 12 and the final assessment (week 24 or 36). The BPRS is a widely used clinician-rated instrument for the assessment of positive, negative, and affective symptoms in patients with psychotic disorders and was chosen because it covers the important symptoms elicited in patients with very late-onset schizophrenia-like psychosis.16 It has been shown to be sensitive to improvements in symptoms associated with antipsychotic treatment in patients with very late-onset schizophrenia-like psychosis,9 and even clinically inexperienced raters can show test-retest reliability of over 90% if provided with guidance for the structured interview.17 Each of the 18 BPRS symptom constructs is assessed by the rater on a seven-point scale ranging from 1 (absent) to 7 (extremely severe). The total score (18–126) is calculated by summing the scores from the 18 items, with higher scores indicating more severe psychopathology. The BPRS was developed as a scale to measure general psychopathology in a wide range of psychiatric disorders and our prior hypothesis was that benefits of amisulpride would be most apparent on the six items (hostility, suspiciousness, hallucinations, unusual thought content, tension, and uncooperativeness) that most closely reflect the symptomatology of very late-onset schizophrenia-like psychosis; therefore we did a prespecified analysis of the change in the combined scores from these six BPRS domains.
The second primary outcome was the proportion of patients who withdrew from trial treatment because of a perceived absence of efficacy between baseline and week 12 and between week 13 and the final assessment.
Secondary outcomes were extrapyramidal side-effects, measured with the Simpson Angus Scale, which assesses nine extrapyramidal signs (gait, arm dropping, shoulder shaking, elbow rigidity, wrist rigidity, leg pendulousness, glabellar tap, tremor, and salivation) by direct examination;18 compliance expressed as proportion of participants who discontinued treatment and as percentage of prescribed medication taken between weeks 1 and 12 and between weeks 13 and 24; quality of life measured with the WHO Quality of Life Scale19 and the EuroQol-5D;20 and resource usage measured with the Client Service Receipt Inventory.21 A full economic analysis will be reported separately. Pharmacokinetics (ie, blood concentrations of amisulpride and the hormone prolactin) were analysed in patients who consented to an optional blood test. These data will be published separately.
Statistical analysis
Since patients with very late-onset schizophrenia-like psychosis have rarely been recruited to randomised controlled trials, we used an initial feasibility phase to assess recruitment and retention, after which the initial target sample size of 300 randomised participants was pragmatically reduced to 100. This reduction was made because of the practical difficulties involved in the recruitment of people with this diagnosis, who are largely without insight into the presence of illness or the possible benefits of treatment. On the basis of a 5-point improvement on the BPRS being a minimal clinically important difference and the assumption that the SD of BPRS measures would be 9 points, we powered the trial to detect a moderate treatment effect of 0·56 SDs.22 If 100 patients were randomised and 90% of participants completed outcome assessments at 12 weeks, this design would provide 70% power at p<0·05 (two-sided) to detect the 5-point minimal clinically important difference in BPRS, and over 80% power to detect a 6-point difference in BPRS in stage 1.
Participants who received at least one dose of study treatment were included in the modified intention to treat analyses of all primary and secondary outcomes using standard Student's t test and repeated measures regression methods.23 For patients who were assessed at 4 weeks but not at 12 weeks, the 4-week assessment was carried forward for the 12-week comparisons of change from baseline. For sensitivity analyses, we analysed the data with and without using last observation carried forward to check for any differences. We used SAS software (version 9.3) for all statistical analyses. An independent data monitoring and ethics committee reviewed and monitored the unblinded accumulating data and the safety of patients in the study. This study is registered with EudraCT, number 2010-022184-35, and ISRCTN, number ISRCTN45593573.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
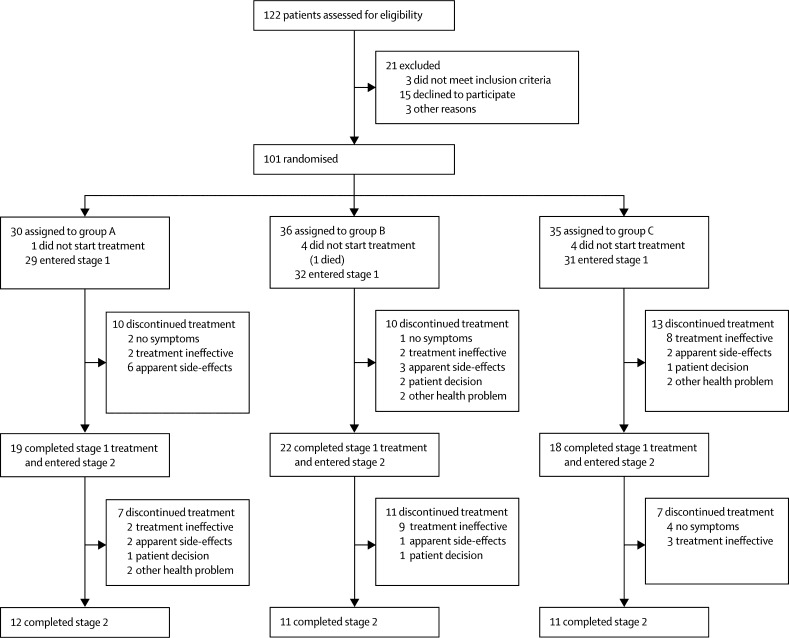
Between Sept 27, 2012, and June 28, 2016, we recruited 101 participants onto the ATLAS trial from 25 National Health Service mental health trusts in England and Scotland. Nine randomised patients did not start trial medication and, as prespecified in the protocol, were excluded from all analyses; one participant had been allocated to group A, four to group B, and four to group C (figure 1). Baseline characteristics were well balanced across all treatment groups at stage 1 baseline. Mean age was 80·2 years (SD 6·9), and most participants were female, lived alone, and had symptoms for longer than 6 months; about half of patients had taken antipsychotic treatment previously and BPRS scores averaged 41·3 (7·9; table 1) at stage 1 baseline. BPRS assessments were done for 92 (100%) of 92 participants at baseline, 87 (95%) of 92 at 4 weeks, 83 (90%) of 92 at 12 weeks, and 54 (92%) of 59 at final assessment.
Figure 1.
Trial profile
Group A=amisulpride 100 mg daily for 36 weeks (24 weeks after protocol amendment). Group B=amisulpride 100 mg daily for 12 weeks followed by placebo daily for 24 weeks (12 weeks after protocol amendment). Group C=placebo daily for 12 weeks followed by amisulpride 100 mg daily for 24 weeks (12 weeks after protocol amendment).
Table 1.
Baseline characteristics
|
Stage 1 (n=92) |
Stage 2 (n=59) |
|||||
|---|---|---|---|---|---|---|
| Group A (n=29) | Group B (n=32) | Group C (n=31) | Group A (n=19) | Group B (n=22) | Group C (n=18) | |
| Age, years | ||||||
| 60–69 | 0 | 5 (16%) | 0 | 0 | 4 (18%) | 0 |
| 70–79 | 13 (45%) | 14 (44%) | 12 (39%) | 10 (53%) | 10 (45%) | 5 (28%) |
| ≥80 | 16 (55%) | 13 (41%) | 19 (61%) | 9 (47%) | 8 (36%) | 13 (72%) |
| Mean age (SD) | 81·2 (6·8) | 78·8 (8·3) | 80·6 (5·4) | 80·6 (7·4) | 77·6 (7·7) | 80·9 (5·3) |
| Sex | ||||||
| Male | 8 (28%) | 7 (22%) | 6 (19%) | 5 (26%) | 6 (27%) | 4 (22%) |
| Female | 21 (72%) | 25 (78%) | 25 (81%) | 14 (74%) | 16 (73%) | 14 (78%) |
| Ethnicity | ||||||
| White | 22 (76%) | 22 (71%) | 22 (73%) | 16 (84%) | 15 (71%) | 11 (65%) |
| Black | 7 (24%) | 7 (23%) | 6 (20%) | 3 (16%) | 5 (24%) | 4 (24%) |
| Mixed | 0 | 1 (3%) | 0 | 0 | 0 | 0 |
| Other | 0 | 1 (3%) | 2 (7%) | 0 | 1 (5%) | 2 (12%) |
| Living circumstances | ||||||
| Alone | 23 (79%) | 20 (63%) | 20 (65%) | 14 (74%) | 12 (55%) | 12 (67%) |
| With spouse or partner | 4 (14%) | 6 (19%) | 6 (19%) | 4 (21%) | 5 (23%) | 2 (11%) |
| Other | 2 (7%) | 6 (19%) | 5 (16%) | 1 (5%) | 5 (23%) | 4 (22%) |
| BPRS score | ||||||
| 30–39 | 11 (38%) | 13 (41%) | 18 (58%) | 6 (32%) | 8 (36%) | 12 (67%) |
| 40–49 | 15 (52%) | 12 (38%) | 11 (35%) | 12 (63%) | 9 (41%) | 6 (33%) |
| ≥50 | 3 (10%) | 7 (22%) | 2 (6%) | 1 (5%) | 5 (23%) | .. |
| Mean BPRS score (SD) | 41·4 (7·2) | 43·5 (9·4) | 38·9 (6·2) | 41·8 (7·5) | 44·1 (9·4) | 37·7 (4·6) |
| Time with symptoms* | ||||||
| <6 months | 11/28 (39%) | 4 (12%) | 8 (26%) | 8/18 (44%) | 2 (9%) | 3 (17%) |
| ≥6 months | 17/28 (61%) | 28 (88%) | 23 (74%) | 10/18 (56%) | 20 (91%) | 15 (83%) |
| Use of antipsychotics | ||||||
| None previously | 13 (45%) | 17 (53%) | 15 (48%) | 7 (37%) | 12 (55%) | 9 (50%) |
| Yes, >1 month previously | 2 (7%) | 1 (3%) | 6 (19%) | 2 (11%) | 1 (5%) | 3 (17%) |
| Yes, in last month | 14 (48%) | 14 (44%) | 10 (32%) | 10 (53%) | 9 (41%) | 6 (33%) |
| MMSE score | ||||||
| 25–27 | 15 (52%) | 15 (47%) | 15 (48%) | 9 (47%) | 11 (50%) | 11 (61%) |
| 28–30 | 14 (48%) | 17 (53%) | 16 (52%) | 10 (53%) | 11 (50%) | 7 (39%) |
| Mean MMSE score (SD) | 27·2 (1·5) | 27·6 (1·6) | 27·8 (1·7) | 27·4 (1·4) | 27·5 (1·7) | 27·4 (1·9) |
Data are n (%) unless otherwise stated. BPRS=Brief Psychiatric Rating Scale. MMSE=Mini Mental State Examination.
Data were missing for one patient in group A.
14 (15%) of 92 participants had stopped taking trial medication by 4 weeks and a further 19 (21%) of 92 participants stopped between weeks 4 and 12 of stage 1 treatment. Of the remaining 59 participants who entered stage 2, 34 (58%) completed the 24 weeks (or later, 12 weeks) of stage 2 treatment (figure 1).
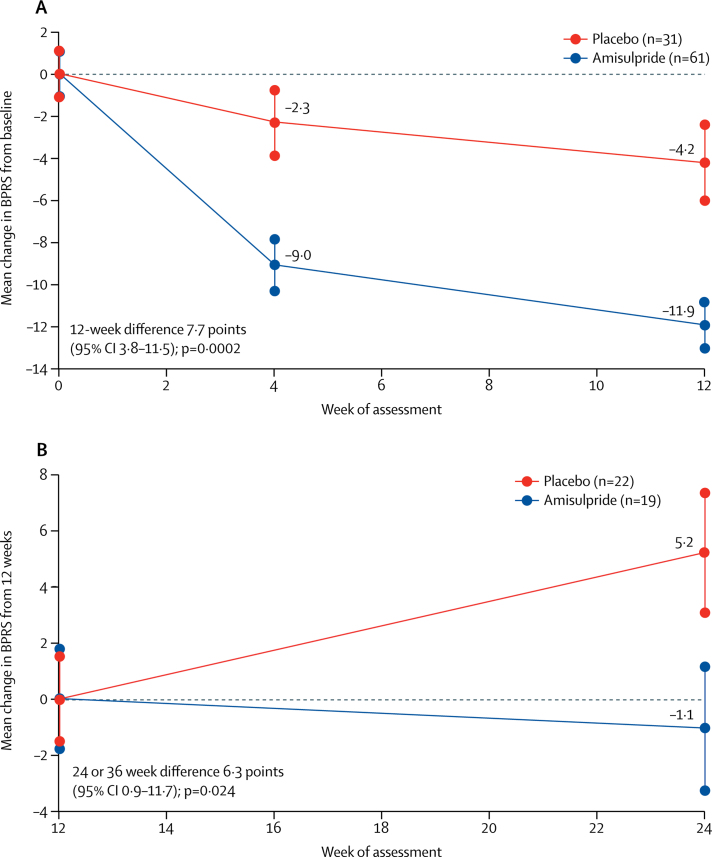
BPRS scores were significantly lower at weeks 4 and 12 than at baseline in both the amisulpride and placebo groups (figure 2; appendix) Improvements in BPRS scores over the 12 weeks of stage 1 treatment were significantly greater with amisulpride (groups A and B) than with placebo (group C). The difference in change in BPRS scores in favour of amisulpride versus placebo was clear at 4 weeks (6·7 point difference, 95% CI 3·2–10·3; p=0·0003) and increased to 7·7 points (3·8–11·5) at 12 weeks, which is a mean improvement of 11·9 points (SE 1·3) with amisulpride versus 4·2 points (1·0) with placebo between baseline and week 12 (p=0·0002). The average difference over the first 12 weeks was similar in a repeated measures multilevel model (6·1 points, 95% CI 2·4–9·8; p=0·001), and in sensitivity analyses that did not include the observations at week 4 that were carried forward for six patients without assessments at week 12 (data not shown).
Figure 2.
Change in BPRS scores from baseline* to 12 weeks in stage 1 (A) and from 12 weeks to final assessment in stage 2 (B)
Data are mean BPRS scores and SE, with baseline scores set to zero. BPRS=Brief Psychiatric Rating Scale. *Baseline scores in stage 1 were 42·5 (SE 1·1) for amisulpride and 38·9 (1·1) for placebo. Baseline scores in stage 2 were 28·5 (1·8) for amisulpride and 29·7 (1·5) for placebo.
Of the 41 patients in the stage 2 comparison of continuing versus stopping amisulpride, 30 were allocated to the 36-week schedule (15 continued amisulpride, 15 stopped amisulpride) and 11 were allocated to the 24-week schedule (four continued amisulpride, seven stopped amisulpride). BPRS scores improved by a mean of 1·1 points (SE 1·6) from 12 weeks to the final assessment in patients continuing amisulpride (group A), but deteriorated by 5·2 points (2·0) in those switching from amisulpride to placebo (group B; difference 6·3 points [95% CI 0·9–11·7]; p=0·024; figure 2). An analysis of outcomes in stage 2 stratified by treatment period (ie, 24 weeks or 36 weeks) produced similar findings to those of the unstratified analysis.
Examination of change in the combined scores from the six BPRS domains that were hypothesised to be most likely to show change in response to effective treatment showed that most of the benefit from amisulpride was seen in these domains. The difference in the 6-item score between amisulpride and placebo was 4·1 points (95% CI 1·9–6·2, p=0·0003) at 4 weeks, 5·3 points (95% CI 2·9–8·7; p<0·0001) at 12 weeks, and 4·6 points (95% CI 1·3–8·0; p=0·008) at the final assessment (appendix). The effect of amisulpride on the remaining 12 symptoms on the BPRS at 12 weeks was small, with a reduction of 2·4 points (95% CI 0·0–4·8; p=0·053).
41 (67%) of 61 participants allocated to amisulpride and 18 (58%) of 31 allocated to placebo completed stage 1 treatment (p=0·39, table 2). When reasons for stopping treatment were compared, fewer participants allocated to amisulpride than to placebo stopped because of non-efficacy (p=0·010). Similarly, fewer participants allocated to continue amisulpride in stage 2 stopped because of perceived non-efficacy than those who switched to placebo (p=0·031). No association was found between compliance and baseline characteristics (data not shown).
Table 2.
Reasons for stopping treatment
| Amisulpride | Placebo | p value | ||
|---|---|---|---|---|
| Stage 1 | Groups A and B (n=61) | Group C (n=31) | .. | |
| No symptoms | 3 (5%) | 0 | 0·21 | |
| Treatment ineffective | 4 (7%) | 8 (26%) | 0·010 | |
| Apparent side-effects | 9 (15%) | 2 (6%) | 0·25 | |
| Patient decision | 2 (3%) | 1 (3%) | 0·99 | |
| Other health problem | 2 (3%) | 2 (6%) | 0·48 | |
| Total | 20 (33%) | 13 (42%) | 0·39 | |
| Stage 2 | Group A (n=19) | Group B (n=22) | .. | |
| No symptoms | 0 | 0 | ||
| Treatment ineffective | 2 (11%) | 9 (4%) | 0·031 | |
| Apparent side-effects | 2 (11%) | 1 (5%) | 0·47 | |
| Patient decision | 1 (5%) | 1 (5%) | 0·92 | |
| Other health problem | 2 (11%) | 0 | 0·12 | |
| Total | 7 (37%) | 11 (50%) | 0·40 | |
Differences were assessed by χ2 test with associated p values (two-sided).
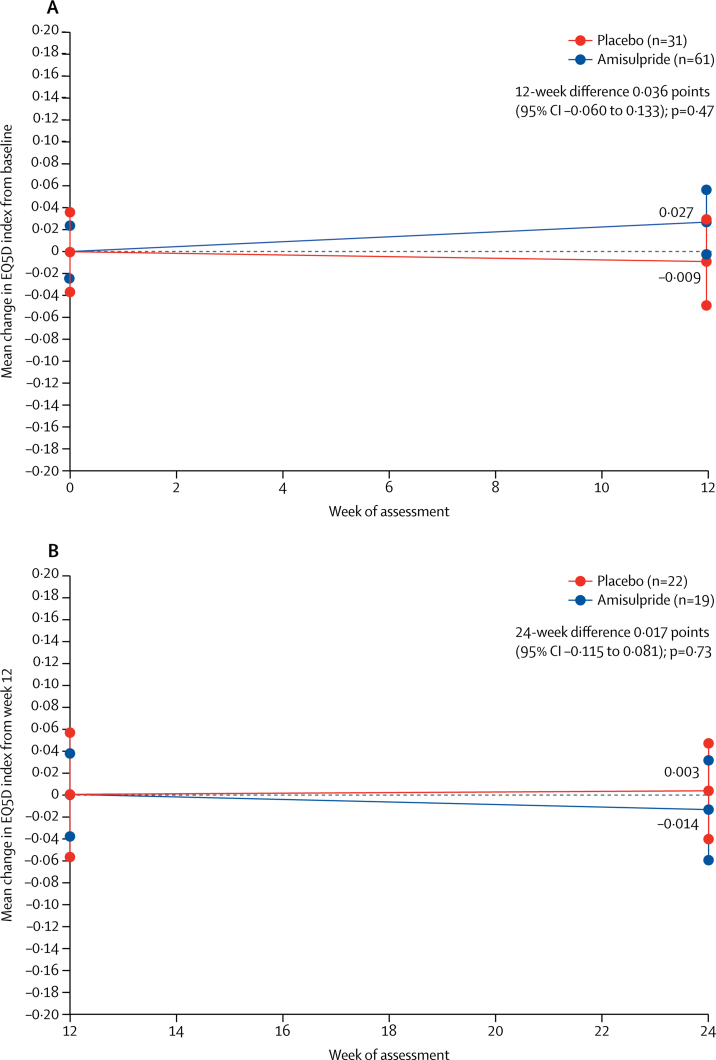
There were no significant differences between amisulpride and placebo in any of the secondary outcome measures in stage 1 or stage 2. Neither group had an improvement from baseline on either the EuroQol-5D utility score (figure 3) or any of the four WHO Quality of Life Scale domains (appendix), nor were differences in change from baseline between the amisulpride and placebo groups significant. For example, at week 12 in stage 1, EuroQol-5D utility scores improved by a mean of 0·027 (SE 0·029) with amisulpride and deteriorated by 0·009 (0·038) with placebo, but this difference was not significant (0·036 [95% CI −0·060 to 0·133], p=0·47; figure 3). Similarly, no significant differences between the two groups were seen for any of the subscales of the WHO Quality of Life Scale (appendix).
Figure 3.
Change in EuroQol-5D utility score from baseline* to 12 weeks in stage 1 (A) and from 12 weeks to final assessment in stage 2 (B)
Data are change in mean EuroQol-5D utility scores and SE, with baseline scores set to zero. *Baseline scores in stage 1 were 0·711 (SE 0·02) for amisulpride and 0·755 (0·04) for placebo. Baseline scores in stage 2 were 0·788 (0·04) for amisulpride and 0·743 (0·06) for placebo.
No significant differences in change in Simpson Angus Scale scores were seen between participants given amisulpride versus placebo (appendix). However, seven (11%) of 61 patients allocated to amisulpride developed clinically significant (ie, Simpson Angus Scale scores ≥6)18 extrapyramidal side-effects in stage 1, compared with none of the 31 patients given placebo (p=0·051). Serious adverse events were reported more frequently in the amisulpride group than in the placebo group in both stages (table 3). Three participants were admitted to hospital because of extrapyramidal symptoms that were considered to be related to treatment; all of these patients received amisulpride. More patients who received amisulpride reported side-effects deemed to be treatment-related by the study investigators than did those given placebo, with most of the excess due to potentially extrapyramidal symptoms (appendix). Falls were also more common in the amisulpride group than in the placebo group, but this difference was not significant (table 3). Five patients died during the study, one from a gastric ulcer bleed before treatment started, two while taking stage 2 treatment (chest infection in group A and myocardial infarction in group C), and two who stopped trial treatment in stage 1 and died many weeks later (hypertensive disease in group B and septicaemia in group C). No deaths were related to treatment.
Table 3.
Serious adverse events
| Amisulpride | Placebo | p value | ||
|---|---|---|---|---|
| Stage 1 | Groups A and B (n=61) | Group C (n=31) | .. | |
| Worsening EPSE | 2 (3%) | 0 | 0·31 | |
| Gastrointestinal | 1 (2%) | 0 | 0·47 | |
| Infection | 4 (7%) | 1 (3%) | 0·48 | |
| Cardiovascular | 1 (2%) | 0 | 0·47 | |
| Falls | 4 (7%) | 0 | 0·14 | |
| Genitourinary | 1 (2%) | 0 | 0·47 | |
| Psychiatric symptoms | 2 (3%) | 0 | 0·31 | |
| Total | 10 (16%) | 1 (3%) | 0·057 | |
| Stage 2 | Group A (n=19) | Group B (n=22) | .. | |
| Worsening EPSE | 1 (5%) | 0 | 0·28 | |
| Infection | 1 (5%) | 2 (9%) | 0·64 | |
| Cardiovascular | 0 | 1 (5%) | 0·35 | |
| Falls | 2 (11%) | 1 (5%) | 0·47 | |
| Genitourinary | 1 (5%) | 0 | 0·28 | |
| Psychiatric symptoms | 1 (5%) | 1 (5%) | 0·92 | |
| Other | 4 (21%) | 1 (5%) | 0·11 | |
| Total | 9 (47%) | 6 (27%) | 0·19 | |
Data are number of participants who experienced a serious adverse event. Differences were assessed by χ2 test with associated p values (two-sided). EPSE=extrapyramidal side-effects.
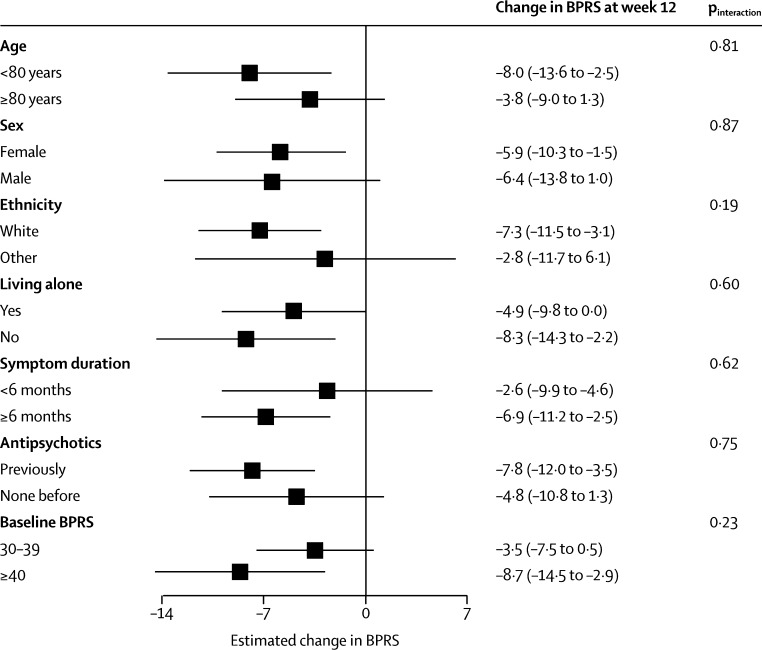
Treatment efficacy in the amisulpride group, as measured by changes in BPRS over the first 12 weeks, did not differ according to baseline characteristics (figure 4). Significant benefits were seen in patients who had taken antipsychotics previously, those with a longer duration of symptoms, and those with more severe symptoms.
Figure 4.
Change in BPRS from baseline to week 12
Datapoints show estimated change in BPRS from baseline to week 12; error bars show 95% CI. Subgroup analyses were generated from repeated measures model. p values were derived from the test for differing treatment efficacy between subgroups in the repeated measures model analyses. BPRS=Brief Psychiatric Rating Scale.
Discussion
The ATLAS trial shows that a single daily dose of 100 mg amisulpride is an effective treatment for the symptoms of very late-onset schizophrenia-like psychosis. The mean improvement in psychosis symptoms of 12 BPRS points with amisulpride we report is similar to the mean BPRS change of 14 points seen in a previous open-label study.9 However, a significant 4-point improvement in BPRS scores from baseline was also seen in our placebo group, showing that the net benefit of amisulpride is around 8 BPRS points compared with placebo. Although this 8-point difference in change in BPRS score would probably have been larger with better compliance, it is close to one standard deviation (8·8 points), indicating a large effect size.22
If a worthwhile benefit is defined as a 0·5 SD improvement24—which at 4·7 BPRS points is close to our predefined minimal worthwhile improvement of 5 BPRS points—then at the week 12 assessment, 45 (74%) of 61 participants in the amisulpride group compared with 14 (45%) of 31 participants in the placebo group had an improvement in BPRS score of 0·5 SD (p=0·007; post-hoc analysis). Alternative definitions of worthwhile benefit, such as a 25% reduction in BPRS score,25 produce similar estimates of about three patients as the number needed to treat to have one worthwhile benefit.
This benefit of amisulpride in very late-onset schizophrenia-like psychosis is substantially greater than the moderate effect sizes of around 0·5 SD reported from a meta-analysis26 of trials of antipsychotics in the reduction of psychosis symptoms in schizophrenia, and the small effect sizes of 0·15–0·2 SD reported from meta-analyses27, 28 of antipsychotic trials in psychosis associated with Alzheimer's disease. Our results should, therefore, encourage clinicians to be optimistic about the benefits of antipsychotic treatment in a group of patients who are generally undertreated.13
Although we chose to use amisulpride, a limbic-selective D2 and D3 antagonist,29 in ATLAS because it is relatively non-sedating (which helps to maintain compliance and blinding of treatment allocation), we would expect similar results with other antipsychotics. The recommended daily dose of amisulpride for patients with early onset schizophrenia is 400–800 mg, but our data show that a much lower dose (100 mg/day) can improve symptoms in patients with very late-onset schizophrenia-like psychosis.
We tested 100 mg/day of amisulpride because we believed that it would be unlikely to induce important extrapyramidal side-effects, since an open-label trial9 had already shown that this daily dose of amisulpride is probably effective and well tolerated in our study population. Although our data suggest a moderate increase in extrapyramidal side-effects even at this low dose, these side-effects are not of sufficient frequency or severity to affect compliance or the benefits of amisulpride, even with up to 36 weeks of treatment. This finding is consistent with results from a PET study30 showing that people with Alzheimer's disease receiving 50 mg/day amisulpride to treat psychosis symptoms had a higher occupancy of striatal dopamine receptors (40–80%) than was anticipated, which increases risk of extrapyramidal side-effects. The mean age of ATLAS participants was around 80 years, therefore exposure to high-dose antipsychotics would be expected to produce a substantially increased risk of such side-effects. These effects can be distressing and disfiguring, lead to reduced mobility and increased falls, and represent an important disincentive to both prescription and treatment adherence in this patient group. Differences between schizophrenia, psychosis in Alzheimer's disease, and very late-onset schizophrenia-like psychosis in antipsychotic efficacy, and the dose at which extrapyramidal side-effects appear are further evidence that these are distinct illnesses with specific treatment requirements.
Although amisulpride improved clinician-rated psychosis symptoms in the ATLAS trial, successful treatment did not result in participant-rated, subjective changes in health-related quality of life, as measured by the EuroQol-5D or WHO Quality of Life Scale,19, 20 which might reflect a poor awareness that psychotic symptoms could be occurring as part of an illness.31 If participants do not recognise that they are unwell or that antipsychotic treatment might improve their symptoms or situation, they are not likely to rate themselves or their situation as better. We used generic quality of life instruments because of a absence of validated measures for this population. The absence of notable changes in participant quality of life could be due to insufficient statistical power to detect small differences, but could also reflect an insensitivity of such generic quality-of-life assessments, which focus on physical and mood difficulties in a participant group for whom changes in these domains are not likely to take place with treatment. We had similar problems with the sensitivity of the Simpson Angus Scale, which could not detect clinically significant differences in potentially extrapyramidal symptoms reported by participants.
The main limitation of our study was that recruitment was lower than expected. Despite our sample size, there was sufficient statistical power to show the substantial benefit of amisulpride over the first 12 weeks of treatment, even with only 100 patients randomised and suboptimal compliance. The benefits of continuing amisulpride compared with stopping after 12 weeks appeared to be similar to those in the first 12 weeks. However, with only 41 participants completing the first 12 weeks of treatment and entering the second randomisation between continuing and stopping amisulpride, this result, although plausible, requires confirmation in a larger study population. A further limitation was the variable duration of this second stage of treatment, which was initially 24 weeks but was reduced to 12 weeks to improve compliance, making it difficult to recommend an optimal duration of treatment. Missing outcome assessments can also introduce bias, particularly if participants with missing assessments are atypical, for example having worse than average symptoms. However, few patients in this study had no post-randomisation assessments and we tried to limit potential bias from missing data by carrying forward assessments from week 4, for the few participants who were not assessed at week 12. Results were similar with or without this imputation, thus we do not believe that the few missing assessments had any substantial effect on our conclusions.
The greatest difficulty we encountered with recruitment to the ATLAS trial was the inability of many potential participants to appreciate that antipsychotic treatment could benefit them. Insight into the presence of illness and the possible benefits of treatment is uncommon in this group,31 who are often only persuaded to take antipsychotic medication by the argument that it might improve their sleep or anxiety, rather than because it might help with their psychosis symptoms. Notably, some patients said that they had been motivated to participate in this study because their clinical teams were advocating the use of antipsychotic treatment and they recognised that involvement in the trial carried a 1 in 3 chance of allocation to placebo, which they found attractive.
Although substantial numbers of patients declined to take part in the ATLAS trial, the clear benefits seen in our study population should be reproducible in usual clinical practice. Those who took part in ATLAS are likely to be representative of the patients with very late-onset schizophrenia-like psychosis that clinicians can successfully engage with drug treatment in usual practice. Similarly, some participants in the ATLAS trial might have had dementia pathologies, but this potential diagnostic heterogeneity will also be true of any group of patients considered by their psychiatrists to have very late-onset schizophrenia-like psychosis and so the results of the ATLAS trial will apply to the treatment of typical future patients despite occasional diagnostic uncertainty.
The difficulties we experienced in recruiting patients to the ATLAS trial reflect the more general challenge of engaging this clinical population in treatment. Even when patients with very late-onset schizophrenia-like psychosis cooperate with a specialist mental health team for assessment, less than half will agree to take an antipsychotic and less than a third will be on treatment at 12-month follow-up or at the point of discharge to primary care.13 Such low prevalence of antipsychotic treatment would be considered unacceptable in younger people with schizophrenia and the clear evidence of benefits from antipsychotic treatment shown in the ATLAS trial should encourage clinicians to be more confident that active treatment will benefit patients, and drive a more assertive approach to antipsychotic treatment in this vulnerable group. With less uncertainty about the benefits of amisulpride treatment, and without the additional complication of explaining randomised trials and placebo control, a greater proportion of future patients might agree to treatment than agreed to join the ATLAS trial. If patients do agree to treatment, even those with the least insight would be expected to benefit, because we found, if anything, greater benefits from amisulpride in those with more severe symptoms.
This online publication has been corrected. The corrected version first appeared at thelancet.com/psychiatry on June 12, 2018
Acknowledgments
Acknowledgments
The trial was funded by the UK National Institute for Health Research's Health Technology Assessment Programme (reference 09/55/06). RH is supported by the University College Hospital NIHR Biomedical Research Centre. RG is supported by the Medical Research Council. The views expressed are those of the authors and are not necessarily those of the UK National Health Service, the UK National Institute for Health Research, or the Department of Health and Social Care.
Contributors
RH, PB, CR, and RG designed the trial. LK, EH, EC, RG, and RH ran the trial; RH, EC, PB, CR, WF, GL, AS, SO, EN, RN, RBa, CF, AVM, PR, VP, JS, and VC recruited patients; RBr and RG analysed the data. RH, RBr, and RG interpreted the data and wrote the initial paper draft; RH, EC, LK, PB, CR, SR, WF, GL, SO, EN, RN, RBa, CF, AVM, PR, VP, JS, VC, CK, TD, and MK contributed to the paper. All authors assume responsibility for the accuracy and completeness of the data and for the overall content and integrity of the paper. RH is the guarantor of this manuscript and affirms that the manuscript is an honest, accurate, and transparent account of the ATLAS trial, that no important aspects of the study have been omitted, and that any discrepancies from the design of the study as planned have been explained.
ATLAS Trialists Group
Investigators at each centre are listed in the appendix. The writing committee were Robert Howard (University College London, London, UK), Rosie Bradley (University of Oxford, Oxford, UK), Elizabeth Cort (South London and Maudsley NHS Foundation Trust, London, UK), Emma Harper (University of Oxford, Oxford, UK), Linda Kelly (University of Oxford, Oxford, UK), Craig Ritchie (University of Edinburgh, Edinburgh, UK), Robert Barber (Northumberland, Tyne, and Wear NHS Trust, Newcastle, UK), Waleed Fawzi (East London NHS Foundation Trust, London, UK), Chris Fox (Norfolk & Suffolk NHS Foundation Trust, Norwich, UK), Rob Jones (Nottinghamshire Healthcare NHS Trust, Nottingham, UK), Gill Livingston (Camden and Islington NHS Foundation Trust, London, UK), Ajay Macharouthu (NHS Ayrshire & Arran, Kilmarnock, UK), Ejaz Nazir (South Staffordshire & Shropshire Healthcare NHS Foundation Trust, Shrewsbury, UK), Ramin Nilforooshan (Surrey and Borders Partnership NHS Foundation Trust, Leatherhead, UK), Sabu Oomman (Cheshire and Wirral Partnership NHS Foundation Trust, Chester, UK), Vivek Pattan (NHS Forth Valley, Larbert, UK), Pranathi Ramachandra (Cambridgeshire & Peterborough NHS Foundation Trust, Cambridge, UK), John Sykes (Derbyshire Healthcare NHS Foundation Trust, Derby, UK), Peter Bentham (Birmingham & Solihull Mental Health NHS Foundation Trust, Birmingham, UK), Richard Gray (University of Oxford, Oxford, UK). The data monitoring committee were Brian Lawlor (Trinity College, Dublin, Ireland), Robert Hills (Cardiff University School of Medicine, Cardiff, UK), and Dr Alan Thomas (Newcastle University, Newcastle, UK). The steering committee were Cornelius Katona (University College London, London, UK), Lee Middleton (Birmingham Clinical Trials Unit, Birmingham, UK), and Tom Dening (Institute of Mental Health, Nottingham, UK). Trial management were Emma Harper, Linda Kelly, Natalie Lam, Lynn Pank, Teresa Bahu, Zoe Gray, Rosie Bradley, and Richard Gray (University of Oxford, Oxford, UK). Nursing coordinators were Liz Cort (King's College London, London, UK), Analisa Smythe, and Jan Wright (Birmingham and Solihull Mental Health NHS Foundation Trust, Birmingham, UK). Health economics advisers were Renee Romeo (King's College London, London, UK) and Martin Knapp (London School of Economics and Political Science, London, UK). Nigel Barnes (South London and Maudsley NHS Foundation Trust, London, UK) was the study pharmacist. The trial was monitored by Ingrid Brumarescu, Hannah Mason, Kelly Gormley, Mohammed Hussain, Eve Wallace, and Bina Patel (King's Health Partners Clinical Trials Office, London, UK).
Declaration of interests
RH and PB declare grants from the Health Technology Assessment Programme during the conduct of the study. RH is also a member of the Health Technology Assessment Commissioning Board. MK declares grants and personal fees from Lundbeck, grants from Merck Sharp and Dohme, and personal fees from Takeda. RN received an honorarium from Janssen for a sponsored talk on depot antipsychotic medication. All other authors declare no competing interests.
Supplementary Material
References
- 1.Roth M, Morrisey JD. Problems in the diagnosis and classification of mental disorders in old age. J Ment Sci. 1961;98:66–80. doi: 10.1192/bjp.98.410.66. [DOI] [PubMed] [Google Scholar]
- 2.Howard R, Rabins PV, Seeman MV, Jeste DV, the International Late-Onset Schizophrenia Group Late-onset schizophrenia and very late-onset schizophrenia-like psychosis: an international consensus. Am J Psychiatry. 2000;157:172–178. doi: 10.1176/appi.ajp.157.2.172. [DOI] [PubMed] [Google Scholar]
- 3.Reeves SJ, Sauer J, Stewart R, Granger A, Howard R. Increased first contact rates for very late-onset schizophrenia-like psychosis in African- and Caribbean-born elders. Br J Psych. 2001;179:172–174. doi: 10.1192/bjp.179.2.172. [DOI] [PubMed] [Google Scholar]
- 4.van Os J, Howard R, Takei N, Murray R. Increasing age is a risk factor for psychosis in the elderly. Soc Psychiatry Psychiatr Epidemiol. 1995;30:161–164. doi: 10.1007/BF00790654. [DOI] [PubMed] [Google Scholar]
- 5.Howard R, Graham C, Sham P. A controlled family study of late-onset non-affective psychosis (late paraphrenia) Br J Psychiatry. 1997;170:511–514. doi: 10.1192/bjp.170.6.511. [DOI] [PubMed] [Google Scholar]
- 6.Talaslahti T, Alanen HM, Hakko H, Isohanni M, Hakkinen U, Leinonen E. Patients with very late-onset schizophrenia-like psychosis have higher mortality rates than elderly patients with earlier onset schizophrenia. Int J Geriatr Psychiatry. 2015;30:453–459. doi: 10.1002/gps.4159. [DOI] [PubMed] [Google Scholar]
- 7.Talaslahti T, Alanen HM, Hakko H. Psychiatric hospital admission and long-term care in patients with very late-onset schizophrenia-like psychosis. Int J Geriatr Psychiatry. 2016;31:355–360. doi: 10.1002/gps.4333. [DOI] [PubMed] [Google Scholar]
- 8.Scott J, Grenwald BS, Kramer E, Shuwall M. Atypical (second generation) antipsychotic treatment response in very late-onset schizophrenia-like psychosis. Int Psychogeriatr. 2011;23:742–748. doi: 10.1017/S1041610210002188. [DOI] [PubMed] [Google Scholar]
- 9.Psarros C, Theleritis CG, Paparrigopoulos TJ, Politis AM, Papadimitriou GN. Amisulpride for the treatment of very late-onset schizophrenia-like psychosis. Int J Geriatr Psychiatry. 2009;25:518–522. doi: 10.1002/gps.2146. [DOI] [PubMed] [Google Scholar]
- 10.Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012;15 doi: 10.1002/14651858.CD004162.pub2. CD004162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider LS, Tariot PN, Dagerman KS, CATIE-AD Study Group Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 12.Ballard C, Howard R. Neuroleptic drugs in dementia: benefits and harms. Nat Rev Neurosci. 2006;7:492–500. doi: 10.1038/nrn1926. [DOI] [PubMed] [Google Scholar]
- 13.Sin Fai Lam CC, Reeves SJ, Stewart R, Howard R. Service and treatment engagement of people with very late-onset schizophrenia-like psychosis. Br J Psych Bullet. 2016;40:185–186. doi: 10.1192/pb.bp.115.051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ventura MA, Green MF, Shaner A, Lieberman RP. Training and quality assurance with the Brief Psychiatric Rating Scale: “The drift buster”. Int J Methods Psychiatr Res. 1993;3:221–224. [Google Scholar]
- 15.Molloy DW, Alemayehu E, Roberts R. Reliability of a standardised Mini-Mental State Examination compared with the traditional Mini-Mental State Examination. Am J Psychiatry. 1991;148:102–105. doi: 10.1176/ajp.148.1.102. [DOI] [PubMed] [Google Scholar]
- 16.Howard R, Almeida O, Levy R. Phenomenology, demography and diagnosis in late paraphrenia. Psychol Med. 1994;24:515–524. doi: 10.1017/s0033291700027379. [DOI] [PubMed] [Google Scholar]
- 17.Crippa JA, Snaches RF, Hallak JE, Loureiro SR, Zuardi AW. A structured interview guide increases Brief Psychiatric Rating Scale reliability in raters with low clinical experience. Acta Psychiatrica Scand. 2001;103:465–470. doi: 10.1034/j.1600-0447.2001.00185.x. [DOI] [PubMed] [Google Scholar]
- 18.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scand. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 19.WHO . WHO; Geneva: 1996. WHOQOL-BREF Introduction, administration, scoring and generic version of the assessment. [Google Scholar]
- 20.EuroQol Group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:198–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 21.Beecham J, Knapp M. Costing psychiatric interventions. In: Thornicroft G, editor. Measuring mental health needs. Gaskell; London: 2001. [Google Scholar]
- 22.Cohen J. 2nd edn. Academic Press; New York, NY: 1988. Statistical power analysis for the behavioural sciences. [Google Scholar]
- 23.Hoffman L, Rovine MJ. Multilevel models for the experimental psychologist: foundations and illustrative examples. Behav Res Methods. 2007;39:101–117. doi: 10.3758/bf03192848. [DOI] [PubMed] [Google Scholar]
- 24.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 25.Leucht S, Heres S, Hamann J, Kane JM. Methodological issues in current antipsychotic drug trials. Schizophr Bull. 2008;34:275–285. doi: 10.1093/schbul/sbm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leucht S, Arbter D, Engel R, Kissling W, Davis J. How effective are second-generation antipsychotic drugs? a meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14:429–447. doi: 10.1038/sj.mp.4002136. [DOI] [PubMed] [Google Scholar]
- 27.Katz I, de Deyn P, Mintzer J, Greenspan A, Zhu Y, Brodaty H. The efficacy and safety of risperidone in the treatment of psychosis of Alzheimer's disease and mixed dementia: a meta-analysis of 4 placebo-controlled trials. Int J Geriatr Psychiatry. 2007;22:475–484. doi: 10.1002/gps.1792. [DOI] [PubMed] [Google Scholar]
- 28.Maher A, Maglione M, Bagley S. Efficacy and comparative effectiveness of atypical antipsychotic medication for off-label uses in adults: a systematic review and meta-analysis. JAMA. 2011;306:1359–1369. doi: 10.1001/jama.2011.1360. [DOI] [PubMed] [Google Scholar]
- 29.Perrault G, Depoortere R, Morel E, Sanger DJ, Scatton B. Psychopharmacological profile of amisulpride: An antipsychotic drug with presynaptic D2/D3 receptor antagonism and limbic selectivity. J Pharmacol Exp Ther. 1997;280:73–82. [PubMed] [Google Scholar]
- 30.Reeves S, McLachlan E, Bertrand J. Therapeutic window of dopamine D2/3 receptor occupancy to treat psychosis in AD. Brain. 2017;140:1117–1127. doi: 10.1093/brain/aww359. [DOI] [PubMed] [Google Scholar]
- 31.Almeida O, Levy R, Howard R, David A. Insight and paranoid disorders in late life (late paraphrenia) Int J Geriatr Psychiatry. 1996;11:653–658. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.