Abstract
Objective(s):
The current study was conducted to examine the possible protective and retentive effects of one-week intra-peritoneal (IP) administration of selenium nanoparticles (Se-NPs), compared to its bulk counterpart, selenite sodium (Ss), after one complete cycle of spermatogenesis in mature male mice.
Materials and Methods:
Thirty adult male mice were divided into 3 groups. Control group was administrated phosphate-buffered saline (IP) and the other groups received Ss (0.50 mg kg-1) and Se-NPs (0.50 mg kg-1) for seven successive days. Then, the animals were monitored for 28 days and finally sacrificed and tissue and blood samples were taken. Histopathological features, sperm quality, in vitro fertilization (IVF) capability and selenium (Se) content in testicular tissue were analyzed. Antioxidant enzyme activities including catalase, glutathione peroxidase, and superoxide dismutase as well as total antioxidant capacity and malondialdehyde levels were assessed in blood and the tissue samples.
Results:
Remarkable differences were found in sperm characteristics, histopathological features and oxidative stress biomarkers between control and treatment groups. Moreover, IVF evaluation and tissue Se concentration examination weren’t similar for Se-NPs and Ss.
Conclusion:
Conclusively, Se-treated groups had more antioxidant capacity than the control group, but sperm quality and histopathological features revealed that Se-NPs might possess more antioxidative and retentive potential compared to Ss in one spermatogenesis cycle.
Keywords: Mice, Nanoparticle, Selenium, Spermatogenesis, Testis
Introduction
Jöns Jacob Berzelius discovered selenium (Se) as a crucial element in 1817, but it was found as one of the main trace elements in the human body in 1950 (1). Selenium as a nutritional trace element has pleiotropic effects on human health (2). Se contributes to many biologic compounds, playing crucial roles in the reduction of oxidative stress (3). It acts as a redox center in the form of selenocysteine (4). Some seleno-proteins, such as glutathione peroxidase (GSH-Px) (5), phospholipid hydroperoxide glutathione peroxidase (PHG-Px) (6), and thioredoxin reductase (TrxR) (7) act as enzymes for the redox hemostasis. These enzymes play key roles in anti-oxidant and anti-inflammatory cycles as well as production of activated thyroid hormones (8).
Se deficiency leads to many impairments, such as cardiometabolic dysfunction (9), ischemic stroke (10, 11), induction of different kinds of cancer (10, 12), and type 2 diabetes (13).
Several investigations have determined that selenium is pivotal for proper male fertility. It has been reported that Se as a spermatozoa component is needed for spermatogenesis progression. The high levels of polyunsaturated fatty acids in sperm membranes make spermatogenesis highly susceptible to reactive oxygen species (ROS) attacks and Se as an antioxidant can be protective against ROS (14).
Mediocre Se insufficiency can deform sperm structure and slow down its movements (15) and a constant dietary deficiency eventuates pathological changes of sperm concentration and fertilizing ability (16). Severe Se deficiency results in the production of defective spermatozoa (17), abnormal mitochondrial capsule (18) and imperfect sperm (19). Long-term Se deficiency in pigs decreases sperm concentration and motility, also induces sperm droplet production (20). In animals having sufficient levels of Se, testes possess the highest amount of Se (per dry mass) compared to other tissues. Initially, during Se depletion, the male animals’ reproductive tissues preferentially retain Se while other tissues are depleted (21). Evaluation of seleno-protein in mice revealed that it had an essential role in sperm development (22-24).
Various kinds of dietary supplements of Se including sodium selenite (Na2SeO3) or sodium selenate (Na2SeO4) and Se enriched yeast (SY) are currently available and they are prescribed in order to prevent or treat human and animal diseases (25).
Although Se has chemoprotective and anti-oxidant effects, its low margin of safety is a concerning issue. Protective effects of Se usually occur at 1–3 μg Se/g diet, however, the toxicity threshold is 3–4 μg Se/g diet (26, 27).
Recently, selenium nanoparticles (Se-NPs) have been introduced as alternatives for selenocompounds and many researchers have focused on excellent characteristics of Se-NPs such as lower toxicity, higher anti-oxidant effects, better biocompatibility and bio-efficacy (28, 29) as well as more protective effects on the male reproductive system (30, 31) compared to inorganic and organic selenocompounds.
Although it has been determined that Se-NPs possess protective and positive effects on male reproduction, assessment of Se-NPs supplementation on spermatogenesis cycle, in vitro fertilization (IVF) outcomes, and Se accumulation after one complete cycle of spermatogenesis have not yet been completely studied.
In line with that, the goal of this experimental study was to elucidate the retentive effects of selenite sodium (Ss) and Se-NPs supplementation on testicular histo-structure, epididymal sperm characteristics, oxidative stress biomarkers, Se concentration, and IVF outcomes in mature male mice.
Materials and Methods
Nanoparticle synthesis and characterization
One ml of 25 mM Ss (Sigma-Aldrich, Egypt) was mixed with 4 ml of 25 mM glutathione containing either 2 mg or 20 mg bovine serum albumin. The mixture pH was adjusted to 7.20 using 1.0 M sodium hydroxide to generate red elemental selenium and oxidized glutathione. The red solution was dialyzed for 96 hr at 4 °C against double distilled water, which was replaced every 24 hr to separate oxidized glutathione from the Se-NPs (28). The nanoparticle was synthesized in the Central Laboratory, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran and after that, dried powder samples of the synthesized nanoparticle were sent to Sharif Central Laboratory for Cervices, Sharif University of Technology, Tehran, Iran for further characterizations. X-ray diffraction (XRD) patterns of Se-NPs were determined using an X’Pert PRO MPD PANalytical X-ray diffractometer using Cu Ka (1.54059 A°) radiation with the X-ray generator operating at 45 kV and 40 mA.
Animals
Thirty mature male Mus Musculus mice weighing 20–25 g were used in this study. All of the animals were obtained from Animal Resources Center, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran. The mice were housed in filter polycarbonate cages in well-ventilated conditions at normal temperature (22 °C±5 °C) under a 12:12-hr light-dark cycle and fed standard pellet adjusted based on AIN-93M for adult maintenance containing 0.15 mg selenium per 1 kg of diet (32). We employed previously described dosage in which Se-NPs were administered intraperitoneally with the dose of 0.5 mg kg-1 for 5 consecutive days (33).
Ethics
All animals underwent experimental protocols licensed by Urmia Animal Care and Use Committee. All procedures of the present study were carried out at the Laboratory Animal Facilities, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran.
Experimental design
After one week adaptation period, the animals were randomly assigned into three equal groups: control, Ss, and Se-NPs groups containing 10 mice in each group. Both of the supplements (Ss and Se-NPs) were injected at the dose of 0.50 mg kg-1 (based on pure Se content) for 7 consecutive days IP (Figure 1), (30). First group (control) received sterile phosphate-buffered saline (PBS), (0.50 mL; IP) daily, for seven continuous days. Second group (Ss-treated) received 0.50 mg kg-1 bodyweight Ss dispersed in 0.50 ml PBS daily for seven continuous days IP. Third group (Se-NPs-treated) mice received Se-NPs (0.50 mg kg-1 body weight dissolved in 0.50 ml PBS; IP) for the same duration. From then on, the animals were kept under standard conditions and monitored for 28 days. Since the duration of spermatogenesis in Mus Musculus mice is about 35 days (34), we selected the experimental period based on this fact.
Figure 1.
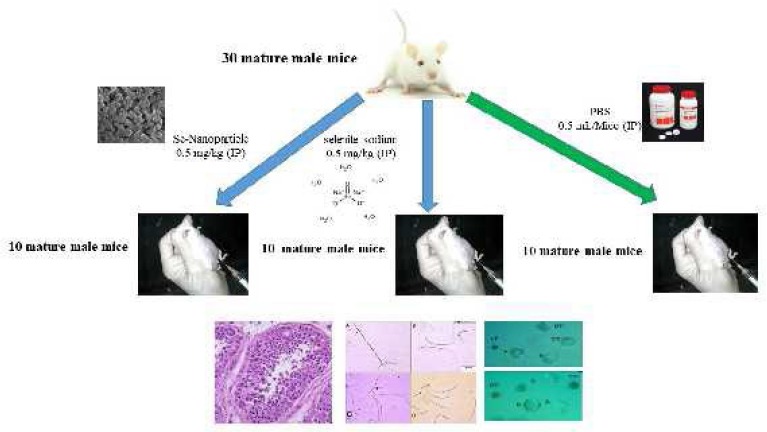
Schematic picture of animals grouped in the current study
Blood and testicular tissue sampling
At the end, day 35, all of the animals were anesthetized with a mixture of ketamine and xylazine (cocktail) containing 0.10 ml xylazine and 1 ml ketamine and 8.90 ml distilled water with the dose of 0.1 ml/10 g BW (35). Afterward, blood samples were directly collected from the heart. Blood was allowed to coagulate and centrifuged (3000 rpm for 10 min) and the separated sera were transferred to Eppendorf tubes and stored at −80 °C until the analyses onset. Following blood sampling, the animals were euthanized, the abdominal cavity was opened and both testes were harvested. The cauda epididymis was removed and put in human tubal fluid (HTF)+4 mg bovine serum albumin (BSA) medium, after slicing. After that, in each group, all testes were collected and suspended in PBS and kept at – 20 °C until further examinations.
Histological Analyses
Following tissue fixation in 10% buffered formalin, 6 μm tissue sections were prepared and stained with hematoxylin and eosin. In order to classify spermatogenesis, Johnsen’s criteria were used. This categorization is based on graded scoring between 1–10 for each tubule cross-section, according to existence or lack of main cell types organized in the order of maturity: 10, complete spermatogenesis and normally organized tubules; 9, many spermatozoa exist but germinal epithelium disorganized; 8, only a few spermatozoa exist in the section; 7, no spermatozoa but many spermatids exist; 6, only a few spermatids exist; 5, no spermatozoa or spermatids but many spermatocytes exist; 4, only a few spermatocytes exist; 3, only spermatogonia exist; 2, no germ cells but only Sertoli cells exist and 1, no germ cells and no Sertoli cells exist. Tubule cross-sections with scores of 9 and 10 were considered as mature tubules (36).
Assessment of sperm characteristics
All of the sperm analytical procedures were performed according to the previously described guidelines (35). The cauda epididymis was sliced and suspended in 1 ml HTF + 4 mg BSA and incubated for 30 min at 37 °C in 5% CO2 atmosphere until all of the sperms came out from the tubules.
For sperm motility percentage assessment, one droplet of sperm suspension was mounted onto a microscope slide and a coverslip was placed over the droplet. At least 10 microscopic fields were examined visually using light microscopy at 400× magnification and motile sperm percentages were calculated according to WHO (2010) recommendations (37). The standard hemocytometric procedure was used for epididymal sperm count. Briefly, epididymal sperm solution was diluted to 1:20 in HTF medium. Then, 10 µl of the diluted specimen was transferred to each of the counting chambers of the hemocytometer and the cell sediments were counted using light microscopy at 400× magnification. The number of sperms was recorded as number of sperms per milliliter.
Morphologic evaluation of sperm was done using Eosin-Y and nigrosine staining method. Briefly, 20 μl of the sperm specimen was mixed with an equal volume of 0.05% Eosin-Y, incubated for 30 sec and then 40 μl of nigrosine was added to the mixture. A thin layer was spread on a clean slide and examined at 400× magnification with a bright-field microscope. Dead sperm plasma membrane appeared pink in color, however, live sperms were not stained. Two hundred spermatozoa were counted for each sample and sperm viability percentages (ratio of sperm with intact/altered plasma membrane) were determined. For morphological abnormalities analysis, the slides were examined at 400× magnification.
In vitro fertilization and embryo culture
In vitro fertilization was performed based on a previously described method (35). Briefly, 12–24 hr before the experiment, separate dishes containing 1–5 ml drops of HTF fertilization and HTF cleavage, covered with mineral oil were prepared for each group. The HTF and washing media were incubated for 12–24 hr in a gas mixture of 5% CO2 at 37 °C before the experiment.
Virgin females (6–8 weeks old) were super-ovulated by 5 IU pregnant mare serum gonadotrophin (PMSG) and 42–46 hr later by 5 IU human chorionic gonadotropin (hCG). Approximately 14 hr after hCG injection, female mice were anesthetized with a cocktail (0.1 ml/10 g BW) and euthanized by cervical dislocation. Cumulous-oocyte-complexes were collected from ampullary portions of the oviducts and washed. Both gametes were co-incubated for 4–6 hr in HTF fertilization medium which was capacitated with cauda epididymal sperm at a final concentration of 1-5 ×105 cells ml-1. Consequently, cumulus-free oocytes were rinsed and transferred to HTF cleavage medium. Twenty-four hr after insemination, 2-cell stage embryos were observed and 4–5 days after fertilization, blastocyst and hatch percentages were evaluated.
Assessment of oxidative stress biomarkers
Various oxidative stress biomarkers including antioxidant enzyme activities, catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) as well as malondialdehyde (MDA) content and total antioxidant capacity (TAC) were measured in the blood and testicular tissue samples as described previously (38, 39).
The GSH-Px activity was determined with GSH-Px detection kit (Ransel, RanDox Co, UK) based on manufacturer’s instructions. The decrease in absorbance was recorded spectrophotometrically against blank at 340 nm. One unit of GSH-Px was defined as µmol of oxidized NADPH per min mg-1 of protein.
The SOD activity was determined with SOD detection kit (RanSod, RanDox Co, UK) based on manufacturer’s instructions. Superoxide dismutase activity was determined at 505 nm wavelength and through a standard curve.
Catalase activity was determined based on a previously described method (40). Briefly, blood and semen plasma samples were homogenized in Triton X-100 1% (Merck, Darmstadt, Germany) and the homogenates were diluted with phosphate buffer (pH: 7.0). Hydrogen peroxide was added to the mixture to commence the reaction and the level of enzyme activity was assessed based on the competency of the catalase in decompensation of hydrogen peroxide. This was acquired by scanning the reduction in absorbance at 240 nm against a blank containing phosphate buffer instead of the substrate. The value of log A1/A2 for a measured interval was used for unit definition owing to the first-order reaction of the enzyme. The aforementioned enzyme activities were expressed as U g-1 Hb and U g-1 protein in blood and semen plasma, respectively. Since the units are according to the protein content of the testicular tissue, the protein level of the supernatant was measured using the colorimetric method of Lowry with BSA as standard.
The MDA level as an indicator of lipid peroxidation was measured in serum and semen plasma based on the procedure described by Buege & Aust (41) with trifling alterations. Briefly, 100 µl of serum or semen plasma specimens were homogenized in 0.15 mol l-1 KCl at a ratio of 1–9 ml using a glass homogenizer. One volume of homogenate was blended thoroughly with two volumes of a stock solution of 15% w/v trichloroacetic acid, 0.375% w/v thiobarbituric acid, and 0.25 mol l-1 hydrochloric acid. After heating and cooling cycles, the solution was clarified by centrifugation at 1000 rpm for 10 min. The absorbance of the clear solution was read at 535 nm and MDA content figured out using 1.56 ×105 mol-1 cm-1 as molar absorbance coefficient. Malondialdehyde levels were presented as nmol per mg protein. The total antioxidant capacity was assessed based on the kit manual (TAS test kit, Randox Laboratories Ltd, GB).
Measurement of Se content in testis
Testicular samples (approximately 250 mg) were digested based on the standard method of microwave tissue digestion with a mixture of HNO3 and H2O2 in a ratio of 5:1 (42). The cooling stage to room temperature was hastened by dipping the digests into the icy water bath. Then, volumes of the digests and reagent blanks were adjusted to 10.0 ml with ultra-pure water. The working solution was prepared in 3.0 M HCl, and Se was directly measured using hydride generation-atomic fluorescence spectrometry (Shimadzu, AA6800, Tokyo, Japan). The primary standard Se solution of 1000 mg/L was prepared in 3.0 M HCl from SeO2 (99.8 %; Sigma-Aldrich Chemical Co.) and stored in a refrigerator at 4 °C (42).
Statistical analysis
The data were analyzed using SPSS program version 23.0 (SPSS Inc, Chicago, IL, USA). All data were subjected to Levene’s test for homogeneity of variances. The activities of GSH-Px, SOD and CAT, and MDA level, as well as fertilization rate between control and treated groups, were compared using one-way ANOVA (Tukey). Data were expressed as the mean ± SEM. The P-values of <0.05 were considered as statistically significant.
Results
Nanoparticle
The crystal structure and the phase composition of Se-NPs were determined using XRD techniques shown in Figure 2. Moreover, Table 1 demonstrates peaks of the XRD pattern. As can be seen, broad diffraction peaks are present in lower angles hence confirming the amorphous/nano-crystalline nature of the sample. The highest peak was recorded in the 29.73 (°2Th) position. As presented in the Joint Committee on Powder Diffraction Standards (JCPDS) card (No., 01-073-0465, a= 4.3662Å, c= 4.9536Å), the diffraction peaks could be indexed to the hexagonal phase of Se-NPs, as the crystallite size was calculated as 362 Å using Scherrer equation.
Figure 2.
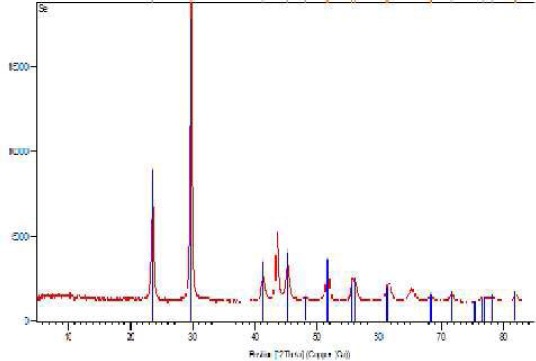
X-ray diffraction patterns of Se-nanoparticles. The highest peak is recorded in 29.73 and 23.523 (°2Th) positions
Table 1.
The details of the observed peaks in the X-ray diffraction pattern of the synthesized selenium nanoparticles
| Pos[°2 Th.] | Height [cts] | d-spacing [Å] | RelInt[∞] |
|---|---|---|---|
| 23.523 (1) | 4010 (33) | 3.77892 | 32.36 |
| 29.7360 (5) | 12390 (59) | 3.00203 | 100.00 |
| 41.331 (4) | 861 (19) | 2.18271 | 6.95 |
| 43.686 (1) | 2717 (24) | 2.07035 | 21.93 |
| 45.374 (3) | 1381 (17) | 1.99716 | 11.14 |
| 48.14 (2) | 137 (11) | 1.88854 | 1.10 |
| 51.708 (2) | 1316 (15) | 1.76642 | 10.62 |
| 55.665 (8) | 778 (34) | 1.64986 | 6.28 |
| 56.14 (1) | 694 (18) | 1.63714 | 5.60 |
| 61.32 (2) | 681 (22) | 1.51049 | 5.49 |
| 61.82 (3) | 399 (31) | 1.49961 | 3.22 |
| 65.210 (7) | 481 (24) | 1.42954 | 3.88 |
| 68.23 (2) | 164 (120) | 1.37338 | 1.32 |
| 68.89 (5) | 39 (27) | 1.36194 | 0.31 |
| 71.595 (8) | 310 (17) | 1.31692 | 2.51 |
| 76.82 (3) | 178 (9) | 1.23980 | 1.43 |
| 78.12 (4) | 138 (9) | 1.22243 | 1.12 |
| 81.67 (2) | 238 (26) | 1.17809 | 1.92 |
| 81.97 (2) | 215 (25) | 1.17452 | 1.73 |
Effect of Ss and Se- NPs on testicular tissue parameters and sperm features
Effects of Ss and Se-NPs treatments on testicular parameters, described as Johnsen’s score and sperm characteristics are depicted in Table 2. As can be seen, Se treatment (as Ss) resulted in decrement of Johnsen’s score and mature seminiferous tubules suggesting testis toxicity. However, Se-NPs had no significant effects on the testicular tissue (Figure 3). Furthermore, none of the treatments had detrimental effects on different features of sperm. However, Se-NPs were found to have some beneficial effects on sperm count, evidenced by the increased sperm count and a tendency toward elevated motility. Figure 4 shows the differences between live and dead sperms. As can be seen, live sperm is not stained.
Table 2.
Effect of Se and Se-nanoparticles treatments on testicular tissue, described as Johnsen’s criteria and sperm characteristics
| Parameters & characteristics | Control | Se | Se-NPs |
|---|---|---|---|
| Johnsen’s score | 8.70 ± 0.06 a | 8.00 ± 0.16 b | 8.53 ± 0.09 a |
| Mature seminiferous tubules (%) | 70.00 ± 5.77 a | 20.00 ± 11.56 b | 56.66 ± 6.66 a |
| Sperm count (106/ml) | 28.12 ± 1.19a | 25.23 ± 1.02a | 41.17 ± 1.16b |
| Dead sperm (%) | 22.63 ± 0.19 | 22.98 ± 0.42 | 22.93 ± 0.47 |
| Sperm motility (%) | 75.87 ± 0.81 | 73.75 ± 1.01 | 76.25 ± 1.21 |
*All data was expressed as the mean±standard error of the mean (SEM)Means within a column with different superscript letters (a–b) denote significant differences (P<0.05)
Table 3.
Evaluated mean values of blood and semen plasma antioxidant enzyme activities, as well as total antioxidant capacity (TAC) and malondialdehyde (MDA) content of Se-nanoparticles and selenium selenite treatment mice
| Samples | Parameter | Blood GSH-Px (U/g Hb) | Blood SOD (U/g Hb) | Blood catalase (U/g Hb) | Serum TAC (mmol/ml) | Serum MDA (mmol/ml) |
|---|---|---|---|---|---|---|
| Groups | ||||||
| Blood & serum | Control | 125.4 ± 10.4b | 1000.5 ± 11.7a | 316.4 ± 15.9 | 1.8 ± 0.6c | 5.1 ±0.5c |
| Selenite | 131.2 ± 7.8b | 845.5 ± 9.2b | 338.4 ± 12.4 | 2.3 ± 0.2b | 3.4 ± 0.2b | |
| Se-NPs | 175.3 ± 8.6a | 981.4 ± 12.5a | 299.1 ± 14.8 | 3.6 ± 0.7a | 2.1 ±0.1a | |
| P-Value | 0.012 | 0.042 | 0.271 | 0.007 | 0.018 | |
| Semen plasma | Parameter | GSH-Px (U/mg protein) | SOD (U/mg protein) | Catalase (U/mg protein) | TAC (mmol/ mg protein) | MDA (mmol/ mg protein) |
| Groups | ||||||
| Control | 112.4 ± 19.3b | 19.53 ± 1.3 | 2.96 ± 0.2 b | 1.6 ± 0.3b | 4.8 ± 1.2b | |
| Selenite | 184.9 ± 17.1a | 21.94 ± 1.2 | 2.72 ±0.4b | 3.3 ± 0.9a | 3.4 ± 1.5ab | |
| Se-NPs | 237.5 ± 24.8a | 22.48 ± 1.9 | 3.36 ± 0.1a | 4.2 ± 0.4a | 2.3 ± 1.4a | |
| P-Value | 0.004 | 0.561 | 0.027 | 0.018 | 0.034 | |
*All data was expressed as the mean ± standard error of the mean (SEM)Means within a column with different superscript letters (a–c) denote significant differences (P<0.05)
Figure 3.
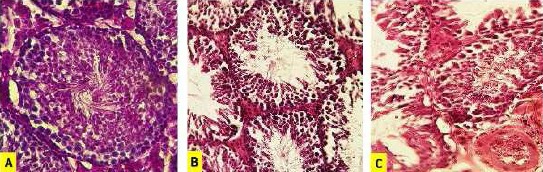
Effect of sodium selenite and selenium nanoparticle on testicular histoarchitecture. A photomicrograph of a section in mouse testis of: (A) control group exhibiting active spermatogenesis, characterized by well-organized distribution of cells in the germinal epithelium of seminiferous tubules, (B) selenium nanoparticle-treated group showing irregular arrangement and detachment of spermatogenic cells, and (C) sodium selenite group showing disrupted spermatogenesis as well as germinal epithelium vacuolization and vascular congestion. Hematoxylin and eosin (× 400)
Figure 4.
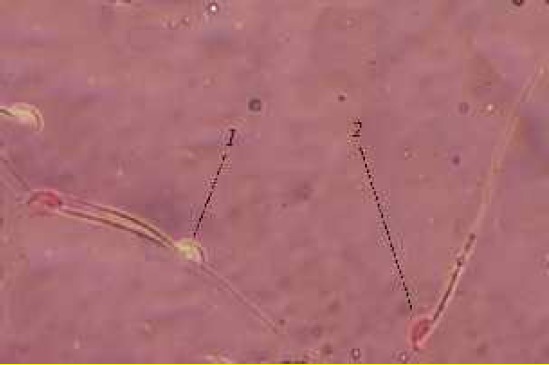
Evaluation of epididymal sperms: sperm colored in pink is dead (2) and live sperm (1) is not stained (Eosin-Y and nigrosin staining method ×1000)
Effect of Ss and Se-NPs administrations on Se content of testicular tissue
The Se content was analyzed in the testicular tissue (Figure 5). As can be seen, both of the treatments resulted in elevation of tissue Se content. In spite of no significant difference between Se-NPs and Ss groups, the Se-NPs had a trend toward more increment of Se level than the bulk counterpart.
Figure 5.
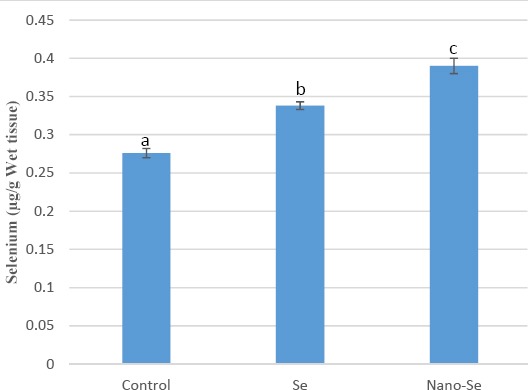
Selenium concentration in the testicular tissues.
*All data was expressed as the mean ± standard error of mean (SEM). Means within a column with different superscript letters (a–c) denote significant differences compared to the control group (P<0.05)
Effect of Ss and Se- NPs on IVF outcomes
Table 4 exhibits epididymal sperm in vitro fertilizing capacity for all of the experimental groups. Furthermore, representative photomicrographs are available in Figure 6. As demonstrated, Se (as Ss) administration resulted in dramatic reduction of fertilization rate after insemination, evidenced by decreased two-cell stage, zygote development, and hatch rate compared to the control group. However, the Se-NPs administration had no significant effects on in vitro fertilizing potential.
Table 4.
Comparison of fertilization rate, two cell embryos, blastocysts, and expanded blastocyst percentage between Se nanoparticles and selenium selenite treated groups
| Fertilization rate% | Two. Cell% | Blastocyst% | Hatch% | |
|---|---|---|---|---|
| Control | 84.2 ±3.7 | 81.99 ±1.13a | 78.99 ±1.67a | 74.92 ±0.77a |
| Se | 72.48 ±0.63 | 63.87 ±0.62b | 69.64 ±0.52b | 62.63 ±0.96b |
| Nano-Se | 81.66 ±1.20 | 80.19 ±0.86a | 79.95 ±0.58a | 74.44 ±0.32a |
* Means within a column with different superscript letters (a–b) denote significant differences compared to the control group (P<0.05)
Figure 6.
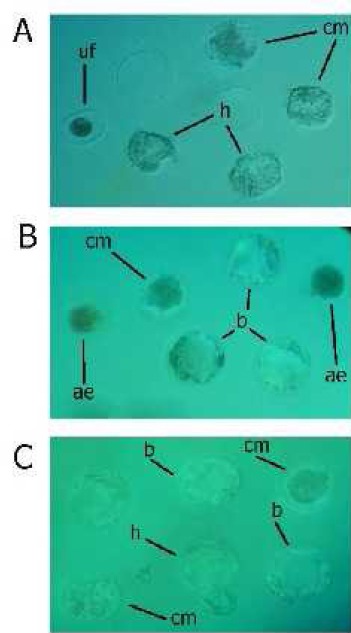
Representative photomicrographs of in vitro embryo development at 72 and 120 hours of culture from A. control, B. Se, and C. Se-nanoparticles administrated mice. Significant difference can be observed between control, Se and Se-nanoparticles groups in droplets. Compact morula (cm), blastocyst (b), hatched blastocyst (h) unfertilized oocytes (uf), arrested embryos (ae)
Effect of Ss and Se- NPs on oxidative stress parameters
The Se and Se-NPs administration effects on various parameters of oxidative stress biomarkers in blood, serum, and semen plasma samples are shown in Table 3. As can be seen, both of the treatments exert anti-oxidative and protective effects evidenced by increased TAC and decreased MDA contents, however, Se-NPs were more potent than Ss. Furthermore, some alterations were observed in antioxidant enzyme activities. Both of the treatments resulted in restoration of GSH-Px activity. However, significant differences were not observed between control and treatment groups regarding blood catalase and semen plasma SOD.
Effect of Ss and Se-NPs administrations on Se content of the testicular tissue
The Se content was analyzed in the testicular tissue (Figure 3). As can be seen, both of the treatments resulted in elevation of tissue Se content. In spite of no significant difference between Se-NPs and Ss groups, the Se-NPs had a trend toward more increment of Se level than the bulk counterpart.
Discussion
In the past few years, nanoparticles have undergone intense investigation as promising agents in order to prevent and treat many kinds of diseases and disorders. Particularly, comparative studies are intriguing, because they usually open new windows to the available science and offer fruitful information regarding pharmacological activities of nanoparticles in comparison with their bulk counterparts (43). Previously, we compared zinc oxide nanoparticles to zinc sulfate and observed that the nanoparticle possesses greater anti-diabetic effects (38). It has been reported that Se supplementation (organic and inorganic) to healthy animals could increase semen quality (44), and even higher levels of Se than normal range can improve male and female reproductive systems and fertility (8). In the current study, we aimed at Se-NPs and evaluated their possible protective and retentive effects versus Ss in one spermatogenesis cycle of mice.
According to the recent investigations, it is recommended that sperm characteristics such as sperm count in males should be evaluated at the appropriate time after treatment. Seminiferous epithelia development cycles in mice are completed in 6–8 days and spermatogenesis cycle lasts nearly 35 days (33.5–35.5), so that, one complete cycle is suggested for experimental spermatological studies in mice (45-47).
Previous studies have indicated that Se supplementation in different methods has a protective effect on spermatogenesis based on testicular ultrastructure and antioxidant status evaluations (30, 31). Abd-Allah and Hashem (2015) have investigated the effects of Ss and Se-NPs on spermatogenesis in a short course study and Shi et. al. (2010) have evaluated pre-pubertal sperm characteristics. In this study, we evaluated Se supplementation effects (as nano and bulk forms) in one complete cycle of spermatogenesis after puberty in which anti-oxidant properties, retentive effects and tissue accumulation status of two Se compounds were evaluated and compared (30, 31).
Based on our knowledge, this investigation is the first study examining Ss retentive effects versus Se-NPs in mature male mice. Selenium as a functional component of selenoproteins plays a central role in many dominant physiological processes in animals and humans (48). Despite its prominent functions in the body, this trace element has a narrow margin between advantageous and adverse effects accelerating public concern about toxicity. So, introducing new Se components with low toxicity and higher marginal safety is a challenging position. There is a growing body of evidence indicating that Se-NPs can be administered confidently with negligible side effects due to lower toxicity and higher activity (28, 49, 50).
Our findings showed that Se-NPs administration in mice has no toxic effects on testicular histology and sperm features after 28 days. As shown in Table 2, the values regarding Se-NPs administration are the same as the control group. Furthermore, Se-NPs exerted more beneficial effects on the reproductive system, evidenced by increased sperm count. These findings completely match the previous reports which evaluated all characteristics immediately after Se administration (30, 31). Slight differences in histopathological features were observed compared to Abd-Allah and Hashem study, possibly due to different administration routes or study course. Furthermore, accumulation of Se in the testicular tissue during this study and various Se retention and elimination rates between different Se sources could influence our results (51-53). In a study by Zhang and colleagues (2010) evaluating in vivo toxicological effects of gold nanoparticles through different administration routes, it was noticed that oral administration of gold nanoparticles results in significant reductions in body weight, red blood cells, and spleen index (54). Also, of the three administration routes (PO, IP, and IV), PO and IP routes exhibited the highest toxicity and tail vein injection showed the lowest toxicity.
Here, we tested the dose of 0.50 mg kg-1 of Se-NPs. It has been indicated that Se-NPs administration at a dose of 4 mg kg-1 in rats, has no toxic effects on various tissues, particularly testicular tissue. However, doses greater than 2.0 mg Se kg-1 BW can lead to chronic toxicities (49).
Our experiment shows that Se treatment in both forms of nano and bulk can improve in vitro fertilization outcomes. It has been well documented that Se deficiency results in significant reduction of fertilized eggs and altered chromatin condensation in male mice (55). Further, it has been claimed that decreased reproductive potential of male mice due to insufficient levels of Se in the diet is associated with spermatozoa oxidative damage (55). Even Se supplementation in healthy animals had ameliorative effects on reproductive system (8, 44). Kaur and Bansal (2005) have shown that Se supplementation in mice fed low-Se diet causes a significant increase in Se concentration in testis and restores pachytene spermatocytes as well as early and late spermatids (56). Moreover, a prospective randomized trial has shown that multi-nutrient supplementation containing Se in women results in better embryo quality (57).
The male reproductive system is vulnerable to oxidative stress due to the high polyunsaturated acid ratio in testis and sperm (17). The antioxidant enzymes, CAT, GSH-Px, and SOD work together to scavenge ROS and inhibit oxidant deleterious effects in tissues and cells. Minor alterations in normal contents of these enzymes may lead to body defense shield malfunction and biomolecules susceptibility to oxidative damages (38). Additionally, measurement of TAC is a suitable way to achieve insight into the delicate in vivo oxidants/antioxidants balance (38, 58).
In this study, both forms of Se supplementation after one complete spermatogenesis cycle increased TAC levels in the whole body and semen. This can be related to direct antioxidant effects of Se or its effects on the antioxidant enzyme activities. We also noticed that Se administration can significantly reduce lipid peroxidation and thus protects the sperm membrane against oxidative damage evidenced by decreased levels of MDA content. Parallel to these findings, it has been reported that in Se deficient mice, elevated levels of lipid peroxidation either in testes or sperm can negatively affect fertilization rate suggesting that Se deficiency aggravates oxidative stress status (55). Glutathione peroxidase plays crucial roles in the defense against oxidative damages through catalyzing the reduction in a variety of hydroperoxides using glutathione as the reducing substrate (59).
We observed that Se treatment in mice after 28 days can increase GSH-Px activity. This is consistent with previous reports in which it has been demonstrated that Se-NPs have more anti-oxidant influences than Se (30, 31). The increased activity of GSH-Px can be attributed to the role of Se as a cofactor for the enzyme. Moreover, it has been demonstrated that Se can increase GSH content even a short time after the administration (30), possibly through either GSH biosynthesis increase or oxidative stress reduction causing GSH less degradation. In this aspect, elevation of GSH-Px activity is justifiable. Our data of decreased MDA content also support this finding. Based on our results and previous studies Se-NPs might have more antioxidant and retentive capability compared to Ss, which was examined in the same dosage after the last administration.
According to previous studies, Se supplementation with different methods had protective effects on spermatogenesis based on antioxidant status evaluation and testis histostructure examination (8).
We found that Se administration in animals causes testicular Se content elevation. However, Se-NPs had a tendency toward more increment than the bulk counterpart. These findings can be ascribed to the size of Se-NPs. Extremely tiny dimensions of nanoparticles allow them to readily pierce into biological membranes and accumulate in the blood and various tissues (38). Similarly, Kaur and Bansal (2005) have reported that Se supplementation results in momentous intensification of testicular Se concentrations in male mice suggesting that testis Se amount is tightly correlated with the testicular antioxidant defense system and exerts protective activities in spermatogenesis (56).
Conclusion
Our data clearly shows that Se-NPs can increase the antioxidant capacity of sperm and testicular tissue and have no toxic impacts on spermatogenesis, indicating their safety. Although the bulk counterpart increased the antioxidant capacity, it exerted some negative impacts on IVF. So, we can conclude that the Se-NPs can be considered as promising agents for Se delivery with less toxic effects. It is suggested that dose adjustment studies should be carried out on Se-NPs to achieve the precise dosage with maximal beneficial and minimal detrimental effects. A major limitation of the current study was its design based on single time point evaluation. However, multiple time-point studies with various administration routes can provide a better understanding of the Se-NPs retention and tissue accumulation process. Such determinations should be covered in further studies.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Schwarz K, Foltz CM. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J Am Chem Soc. 1957;79:3292–3293. [PubMed] [Google Scholar]
- 2.Drake E. Cancer chemoprevention: selenium as a prooxidant, not an antioxidant. Med Hypotheses. 2006;67:318–322. doi: 10.1016/j.mehy.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 3.Kieliszek M, Błażejak S. Selenium: significance, and outlook for supplementation. Nutrition. 2013;29:713–718. doi: 10.1016/j.nut.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Driscoll DM, Copeland PR. Mechanism and regulation of selenoprotein synthesis Annu Rev Nutr. 2003;23:17–40. doi: 10.1146/annurev.nutr.23.011702.073318. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto Y, Koh YH, Park YS, Fujiwara N, Sakiyama H, Misonou Y, et al. Oxidative stress caused by inactivation of glutathione peroxidase and adaptive responses. J Biol Chem. 2003;384:567–574. doi: 10.1515/BC.2003.064. [DOI] [PubMed] [Google Scholar]
- 6.Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 7.Becker K, Gromer S, Schirmer RH, Müller S. Thioredoxin reductase as a pathophysiological factor and drug target. FEBS J. 2000;267:6118–6125. doi: 10.1046/j.1432-1327.2000.01703.x. [DOI] [PubMed] [Google Scholar]
- 8.Rayman MP. Selenium and human health. The Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 9.Lei C, Niu X, Wei J, Zhu J, Zhu Y. Interaction of glutathione peroxidase-1 and selenium in endemic dilated cardiomyopathy. Clin Chim Acta. 2009;399:102–108. doi: 10.1016/j.cca.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Reeves M, Hoffmann P. The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci. 2009;66:2457–2478. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiavon R, Guidi G, Biasioli S, De Fanti E, Targa L. Plasma glutathione peroxidase activity as an index of renal function. Clin Chem Lab Med. 1994;32:759–766. doi: 10.1515/cclm.1994.32.10.759. [DOI] [PubMed] [Google Scholar]
- 12.Burk RF, Hill KE. Selenoprotein P—expression, functions, and roles in mammals. Biochim Biophys Acta. 2009;1790:1441–1447. doi: 10.1016/j.bbagen.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schomburg L, Köhrle J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol Nutr Food Res. 2008;52:1235–1246. doi: 10.1002/mnfr.200700465. [DOI] [PubMed] [Google Scholar]
- 14.Aitken RJ, Baker MA. Oxidative stress and male reproductive biology. Reprod Fertil Dev. 2004;16:581–588. doi: 10.10371/RD03089. [DOI] [PubMed] [Google Scholar]
- 15.Burk RF, Hill KE, Motley AK. Selenoprotein metabolism and function: evidence for more than one function for selenoprotein P. J. Nutr. 2003;133:1517S–1520S. doi: 10.1093/jn/133.5.1517S. [DOI] [PubMed] [Google Scholar]
- 16.Moslemi MK, Tavanbakhsh S. Selenium–vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. Int J Gen Med. 2011;4:99. doi: 10.2147/IJGM.S16275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace E, Calvin HI, Cooper GW. Progressive defects observed in mouse sperm during the course of three generations of selenium deficiency. Gamete Res. 1983;7:377–387. [Google Scholar]
- 18.Wu A, Oldfield J, Shull L, Cheeke P. Specific effect of selenium deficiency on rat sperm. Biol Reprod. 1979;20:793–798. doi: 10.1095/biolreprod20.4.793. [DOI] [PubMed] [Google Scholar]
- 19.Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, Wissing J, et al. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285:1393–1396. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Chen Y, Zhang J, Huang M, Su Q, Lu Z, et al. Preliminary studies on influence of selenium deficiency to the developments of genital organs and spermatogenesis of infancy boars. J Anim Sci. 1982;2:000. [Google Scholar]
- 21.Brennan KM, Pierce JL, Cantor AH, Pescatore AJ, Xiao R, Power RF. Source of selenium supplementation influences testis selenium content and gene expression profiles in Single Comb White Leghorn roosters. Biol Trace Elem Res. 2012;145:330–337. doi: 10.1007/s12011-011-9205-8. [DOI] [PubMed] [Google Scholar]
- 22.Foresta C, Flohé L, Garolla A, Roveri A, Ursini F, Maiorino M. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol Reprod. 2002;67:967–971. doi: 10.1095/biolreprod.102.003822. [DOI] [PubMed] [Google Scholar]
- 23.Olson GE, Winfrey VP, NagDas SK, Hill KE, Burk RF. Selenoprotein P is required for mouse sperm development. Biol Reprod. 2005;73:201–211. doi: 10.1095/biolreprod.105.040360. [DOI] [PubMed] [Google Scholar]
- 24.Shalini S, Bansal M. Dietary selenium deficiency as well as excess supplementation induces multiple defects in mouse epididymal spermatozoa: understanding the role of selenium in male fertility. Int J Androl. 2008;31:438–449. doi: 10.1111/j.1365-2605.2007.00789.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Jia X. Safety evaluation of Se-methylselenocysteine as nutritional selenium supplement: Acute toxicity, genotoxicity and subchronic toxicity. Regul Toxicol Pharmacol. 2014;70:720–727. doi: 10.1016/j.yrtph.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Ip C, Ganther HE. Activity of methylated forms of selenium in cancer prevention. Br J Cancer. 1990;50:1206–1211. [PubMed] [Google Scholar]
- 27.Maier KJ, Knight AW. Ecotoxicology of selenium in freshwater systems. Rev Environ Contam Toxicol. 1994:31–48. doi: 10.1007/978-1-4684-7068-0_2. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J-S, Gao X-Y, Zhang L-D, Bao Y-P. Biological effects of a nano red elemental selenium. Biofactors. 2001;15:27–38. doi: 10.1002/biof.5520150103. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Wang X, Xu T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol Sci. 2008;101:22–31. doi: 10.1093/toxsci/kfm221. [DOI] [PubMed] [Google Scholar]
- 30.Abd-Allah S, Hashem KS. Selenium nanoparticles increase the testicular antioxidant activity and spermatogenesis in male rats as compared to ordinary selenium. Int J Adv Res. 2015;3:792–802. [Google Scholar]
- 31.Shi L-g, Yang R-j, Yue W-b, Xun W-j, Zhang C-x, Ren Y-s, et al. Effect of elemental nano-selenium on semen quality, glutathione peroxidase activity, and testis ultrastructure in male Boer goats. Anim Reprod Sci. 2010;118:248–254. doi: 10.1016/j.anireprosci.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 33.Hassanin KM, El-Kawi SHA, Hashem KS. The prospective protective effect of selenium nanoparticles against chromium-induced oxidative and cellular damage in rat thyroid. Int J Nanomedicine. 2013;8:1713–1720. doi: 10.2147/IJN.S42736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Dev Dyn. 1956;99:507–516. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- 35.Babaei M, Najafi G, Jalali AS, Behfar M. Effects of unilateral Iatrogenic vas deferens trauma on fertility: An experimental in vitro fertilization mice model study. Bull Emerg Trauma. 2015;3:122. [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson M. Changes in the blood-testis barrier of the guinea-pig in relation to histological damage following iso-immunization with testis. Reprod Abstr Ser. 1970;22:119–127. doi: 10.1530/jrf.0.0220119. [DOI] [PubMed] [Google Scholar]
- 37.Cooper TG, Noonan E, Von Eckardstein S, Auger J, Baker HG, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 38.Nazarizadeh A, Asri-Rezaie S. Comparative study of antidiabetic activity and oxidative stress induced by zinc oxide nanoparticles and zinc sulfate in diabetic rats. AAPS Pharm Sci Tech. 2015;17:1–10. doi: 10.1208/s12249-015-0405-y. [DOI] [PubMed] [Google Scholar]
- 39.Asri-Rezaei S, Dalir-Naghadeh B. Evaluation of antioxidant status and oxidative stress in cattle naturally infected with Theileria annulata . Vet Parasitol. 2006;142:179–186. doi: 10.1016/j.vetpar.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 40.Aebi H. [13] Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 41.Buege JA, Aust SD. [30] Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q, Chen L, Guo K, Zheng L, Liu B, Yu W, et al. Effects of different selenium levels on gene expression of a subset of selenoproteins and antioxidative capacity in mice. Biol Trace Elem Res. 2013;154:255–261. doi: 10.1007/s12011-013-9710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asri-Rezaei S, Dalir-Naghadeh B, Nazarizadeh A, Noori-Sabzikar Z. Comparative study of cardio-protective effects of zinc oxide nanoparticles and zinc sulfate in streptozotocin-induced diabetic rats. J Trace Elem Med Biol. 2017;42:129–141. doi: 10.1016/j.jtemb.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 44.El-Sharawy M, Eid E, Darwish S, Abdel-Razek I, Islam M, Kubota K, et al. Effect of organic and inorganic selenium supplementation on semen quality and blood enzymes in buffalo bulls. Animal Sci J. 2017;88:999–1005. doi: 10.1111/asj.12736. [DOI] [PubMed] [Google Scholar]
- 45.Working PK. Male reproductive toxicology: comparison of the human to animal models. Environ Health Perspect. 1988;77:37–44. doi: 10.1289/ehp.887737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Högstrand K, Böhme J. Gene conversion of major histocompatibility complex genes in the mouse spermatogenesis is a premeiotic event. Mol Biol Cell. 1997;8:2511–2517. doi: 10.1091/mbc.8.12.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mangoli E, Talebi AR, Anvari M, Pourentezari M. Effects of experimentally-induced diabetes on sperm parameters and chromatin quality in mice. Int J Reprod Biomed. 2013;11:53–60. [PMC free article] [PubMed] [Google Scholar]
- 48.Ramamurthy Ch, Sampath KS, Arunkumar P, Kumar MS, Sujatha V, Premkumar K, Thirunavukkarasu C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst Eng. 2013;36:1131–1139. doi: 10.1007/s00449-012-0867-1. [DOI] [PubMed] [Google Scholar]
- 49.He Y, Chen S, Liu Z, Cheng C, Li H, Wang M. Toxicity of selenium nanoparticles in male Sprague–Dawley rats at supranutritional and nonlethal levels. Life Sci. 2014;115:44–51. doi: 10.1016/j.lfs.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 50.Jang D-Y, Kim S-J, Jeong J-H, Nam SY, Kim J-S, Yun YW, et al. Protective effects of sodium selenite and selenium nanoparticles against experimental colon carcinogenesis in mice. Prev Vet Med. 2016;40:101–108. [Google Scholar]
- 51.Ehlig C, Hogue D, Allaway W, Hamm D. Fate of selenium from selenite or seleno-methionine, with or without vitamin E in lambs. J Nutr. 1967;92:121–126. doi: 10.1093/jn/92.1.121. [DOI] [PubMed] [Google Scholar]
- 52.Lopez PL, Preston R, Pfander W. Whole-body retention, tissue distribution and excretion of selenium-75 after oral and intravenous administration in lambs fed varying selenium intakes. J Nutr. 1969;97:123–132. doi: 10.1093/jn/97.1.123. [DOI] [PubMed] [Google Scholar]
- 53.Hu C, Li Y, Xiong L, Zhang H, Song J, Xia M. Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim Feed Sci Technol. 2012;177:204–210. [Google Scholar]
- 54.Sánchez-Gutiérrez M, García-Montalvo E, Izquierdo-Vega J, Del Razo L. Effect of dietary selenium deficiency on the in vitro fertilizing ability of mice spermatozoa. Cell Biol Toxicol. 2008;24:321–329. doi: 10.1007/s10565-007-9044-8. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Wu H, Wu D, Wang Y, Chang J, Zhai Z, Meng A, Liu P, Zhang L, Fan F. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int J Nanomedicine. 2010;5:771–781. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaur P, Bansal MP. Effect of selenium-induced oxidative stress on the cell kinetics in testis and reproductive ability of male mice. Nutrition. 2005;21:351–357. doi: 10.1016/j.nut.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 57.Nouri K, Walch K, Weghofer A, Imhof M, Egarter C, Ott J. The impact of a standardized oral multinutrient supplementation on embryo quality in in vitro fertilization/intracytoplasmic sperm injection: A prospective randomized trial. Gynecol Obstet Invest. 2016;82:8–14. doi: 10.1159/000452662. [DOI] [PubMed] [Google Scholar]
- 58.Goel A, Dani V, Dhawan D. Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem Biol Interact. 2005;156:131–140. doi: 10.1016/j.cbi.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Brigelius-Flohé R, Wingler K, Müller C. Estimation of individual types of glutathione peroxidases. Methods Enzymol. 2002;347:101–12. doi: 10.1016/s0076-6879(02)47011-5. [DOI] [PubMed] [Google Scholar]


