Abstract
Objective(s):
Herbal medicines are an alternative choice for treatment or controlling of polycystic ovary syndrome (PCOS). Effect of hydroalcoholic extract of flaxseed was evaluated on ovarian hormones and histological changes of uterus and ovary in a PCOS-induced rat model.
Materials and Methods:
Twenty four rats divided into four groups including negative control, positive control, PCOS and treatment groups. Positive control group received hydroalcoholic extract of flaxseed for 30 days. PCOS was induced by single intramuscular injection of estradiol valerate. Treatment group was treated with flaxseed extract 7 weeks after induction of PCOS for 30 days. Ovaries and uterus were dissected out and their sections were used for histomorphometric study. Levels of estradiol, progesterone, testosterone and dehydroepiandrosterone (DHEA) were measured in the serum.
Results:
In the treatment group, flaxseed extract increased level of progesterone (P<0.05), while decreased testosterone (P<0.05) compared with the PCOS group. Concentrations of estrogen and DHEA did not change significantly in comparison with the PCOS group. Histomorphometric study showed that in the treatment group, the number of preantral follicles, antral follicles and corpus luteum increased compared with the PCOS group (P<0.05), but the number of cystic follicles and diameter of antral follicles decreased (P<0.05), and the number of primary follicle did not alter significantly. In the treatment group, the thickness of granulosa layer increased, but the thickness of theca layer and tunica albuginea decreased compared to the PCOS group (P<0.05).
Conclusion:
Hormonal profile and histomorphometric features of ovary that were disturbed by PCOS induction were ameliorated by hydroalcoholic extract of flaxseed.
Keywords: Flax, Ovary, Polycystic ovary syndrome Rats, Sex hormones, Uterus
Introduction
Polycystic ovary syndrome (PCOS) is a female endocrine disorder. Women suffering from this disease have infertility problems mostly because of hormonal imbalance. Patients often show elevated levels of androgens in plasma. Metabolic disturbances such as insulin resistance can activate hypothalamic-pituitary-adrenal (HPA) axis and result in the increased production of androgens in PCOS patients (1, 2).
A number of endocrine disorders that enhance and intensify each other may be observed in PCOS. These disorders include hypothalamic-pituitary-gonad (HPG) axis dysfunction. In fact, PCOS includes abnormal gonadotropin secretion and increased secretion of ovarian steroids. Luteinizing hormone (LH), particularly in women with PCOS, increases due to an increase in the frequency and secretion of these hormones. When the concentration of LH increases compared to follicle stimulating hormone (FSH), ovaries preferentially increase androgen synthesis (3). The National Institutes of Health postulates that higher concentrations of androgens in blood and/or clinical hyperandrogenism are the key criteria for PCOS diagnosis (4).
Various therapeutic methods such as changing of life habits, surgery and medication like clomiphene citrate, metformin, letrozole and tamoxifen have been proposed for PCOS (3). Today, herbal medicines are widely used as an alternative for treatment or controlling of diseases. In this regard, in a case study, reduced testosterone and increased insulin level was reported in a 31-years old woman with PCOS following treatment by flaxseed (Linum usitatissimum) (30 g/day for 4 months) (5). Administration of 15 g/day of flaxseed for 12 weeks to a 48 years old woman (postmenopausal) was also reported to reduce testosterone with no significant changes in estrone and estradiol level (6). Phytochemical screening of hydroalcoholic extract of flaxseed (7) has been presented in Table 1. Hence this study was designed to evaluate the effect of hydroalcoholic extracts of flaxseed on endocrine and histomorphometry changes of ovary and uterus in estradiol valerate-induced PCOS rats.
Table 1.
Phytochemical screening of hydroalcoholic extract of flaxseed (Linux usitatissimum) (7)
| Name of the compound | Name of the test | Results* |
|---|---|---|
| Tannins | 5% ferric chloride test | + |
| Flavonoids | Alkaline reagent test | - |
| Carbohydrates | Molischdr test | + |
| Phenols | Ferric chloride test | + |
| Alkaloids | Mayeroidslor | + |
| Steroids | Chloroform + acetic acid + H2SO4 | + |
| Glycosides | Legalsi test | - |
| Proteins | Ninhydrin test | + |
| Diterpenes | Copper acetate test | + |
| Saponnins | Distilled water | - |
-, absent; +, present
Materials and Methods
Animals and ethics
This experiment was accomplished under the approval of the state committee on animal ethics, Shiraz University, Shiraz, Iran. Also, the recommendations of European Council Directive (86/609/EC) of November 24, 1986, regarding the standards in the protection of animals for experimental purposes were followed.
Twenty-four adult female Sprague Dawley rats, weighing approximately 200±20 g, were used. Animals were kept in standard polypropylene cages at 22±2 °C temperature, 38% relative humidity, and 12/12 hr light/dark cycle, and fed with a standard pellet diet and water ad libitum.
Preparation of hydroalcoholic extract of flaxseed
Flaxseed was ground into a fine powder using a homogenizer and the powder was used for extraction with 70% ethanol as previously described (8). The extract was concentrated under reduced pressure in rotary evaporator to yield a crude semi-solid mass. The resultant semi-solid extract was then lyophilized to fine powder in a lyophilizer. It was then stored in airtight container at 4 °C.
Induction of PCOS and flaxseed treatment
A diagram demonstrating the study design is shown in Figure 1. Reproductive cycle of all animals was determined by vaginal smear for 14 days (9) (Figure 2). The rats with regular cycles were randomly divided into 4 equal groups (n=6). Negative control and positive control groups received one dose of intramuscular injection of 0.2 ml sesame oil (as solvent of estradiol valerate) at the start of the study. Furthermore, the positive control group was treated with 200 mg/Kg hydroalcoholic extracts of flaxseed orally by gavage for 30 days. PCOS was induced in PCOS group by intramuscular injection of estradiol valerate but was not treated following induction of PCOS. Treatment group, after induction of PCOS, was treated with 200 mg/Kg hydroalcoholic extract of flaxseed, orally by gavage, following induction of PCOS.
Figure 1.
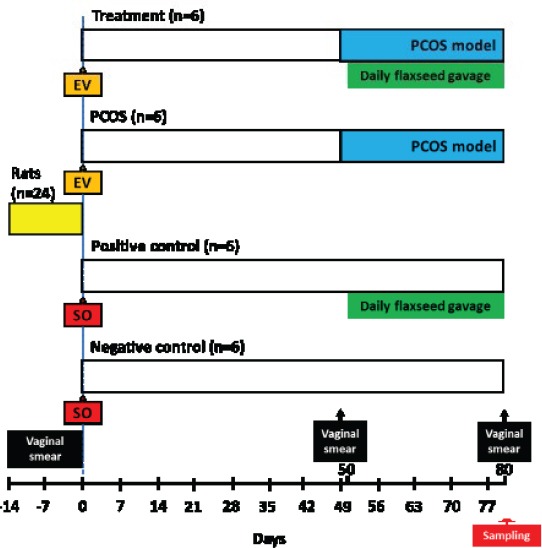
Schematic diagram of the experimental design including induction model, treatment, vaginal smear checking and sampling time for evaluation of the effect of hydroalcoholic extract of flaxseed on polycystic ovary syndrome (PCOS) in a rat model. EV, estradiol valerate; SO, sesame oil
Figure 2.
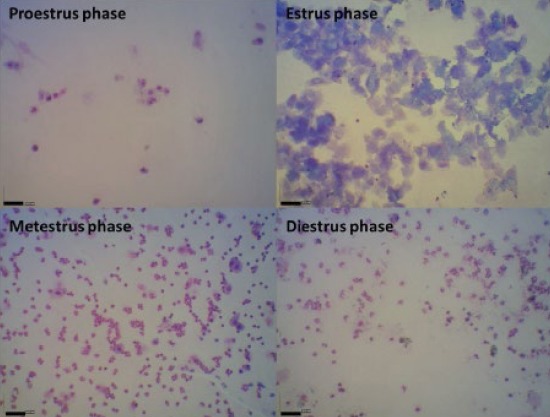
Vaginal smear of rats for detection of reproductive cycle. (Giemsa staining, index bars, 50 μm)
PCOS was induced in rats by single intramuscular injection of 4 mg of estradiol valerate, which is a long-acting estrogen. Administration of estradiol valerate causes dysregulation of gonadotropin-releasing hormone (GnRH), resulting in improper release and storage of LH. This hormone is considered a key pathogenic factor in the development of PCOS. A single dose of estradiol valerate to cyclic rat induces anovulation and polycystic ovaries within 7 weeks (10). The treatment with flaxseed was started 50 days after estradiol valerate injection in PCOS-induced treatment group and this was considered as the first day of treatment for 30 days. Reproductive cycle of animals was monitored after induction of PCOS on day 49 and before sampling on day 80 (Figure 1).
Blood and tissue samplings
On day 80 after model induction (Figure 1), whole blood was collected through heart puncture and their sera were used for hormonal measurement. Ovaries and uteri were then dissected out and subjected to histomorphometric study according to Rahmanifar et al (11).
For ovary and uterus histomorphometric evaluation, after fixation, segments were dehydrated in ethanol and xylene and then were embedded in paraffin wax, and histopathologic sections were made from each block. Serial sections of ovaries were prepared at thicknesses of 10 μm. The 5 µm thickness sections of uterus were also prepared. One section of every 10 serial sections of ovary and three sections of each horn of uteri were deparaffinized in 60 °C. Then, selected sections rehydrated in graded concentrations of xylene and ethanol. Finally, ovarian and uterine slices stained with hematoxylin and eosin.
To assess type of follicles (12), as well as counting primary, preantral, antral and/or cystic follicles and corpora lutea (13), ovarian slices were observed under a light microscope (CX21, Olympus, Japan) and were photographed by an adjusted digital camera (AM423U Eyepiece Camera, Dino-Eye, Taiwan). Tunica albuginea, granulosa layer, and theca layer thicknesses of follicles (11) and endometrial and myometrial thicknesses of the uterus (14) were measured by Dino Capture 2.0 software (AnMo Electronics Corporation, New Taipei City, Taiwan). Testosterone, progesterone, estrogen and dehydroepiandrosterone (DHEA) were measured in serum by ELISA method (Accu-Bind, Monobind Inc., Lake Forest, CA, USA) (15, 16).
Statistical analysis
The results are presented as means ± standard error of mean (Mean ± SEM). Statistical Package for Social Sciences (SPSS-16.0) were used and the results were analyzed using one-way analysis of variance (ANOVA) followed by post hoc multiple comparisons Tukey test for comparison between different treatment groups. Statistical significance was set at P<0.05.
Results
Flaxseed extract improved serum sex steroids in PCOS
Level of estradiol in the PCOS and treatment groups increased compared to the positive and negative controls (P<0.05, Figure 3A). While, there was no significant difference in estradiol level between the PCOS and treatment groups. Progesterone concentration in the PCOS group decreased compared to the control and treatment groups (P<0.05, Figure 3B); however, the level of progesterone in the treatment group did not have significant difference with the control groups (P>0.05). In addition, mean testosterone level in the PCOS group was higher than the control and treatment groups (P<0.05, Figure 3C). On the other hand, there was no significant difference in testosterone level between the control and treatment groups. There was no significant difference between the groups regarding the level of DHEA (P<0.05, Figure 3D).
Figure 3.

Effects of flaxseed extract on polycystic ovary syndrome (PCOS) rats and plasma concentrations of estradiol, progesterone, testosterone, and dehydroepiandrosterone (DHEA). Values presented as mean±SEM, the above lines indicate significant difference between groups
Flaxseed extract corrected ovarian histomorphology in PCOS
Light microscopic analysis of the negative and positive control groups showed no structural abnormalities in different stages of development and regression of follicles and corpus luteum, and no cystic follicles (Figures 4A and B). The area of the largest follicle was greater in the PCOS rats than the controls; a trend toward follicular enlargement was not observed in the treatment group (Figures 4C and D). The number of cystic follicles was increased in the PCOS group. In addition, the PCOS rats had no corpus luteum (Figure 4C). The theca and granulosa cell layers were normal in the control groups (Figures 5A and B). A large fluid-filled cyst was characteristic of cystic follicle with compressed granulosa cell layer and enlarged theca interna cell layer (Figure 5C). The cystic follicles’ walls were thickened, because of thickened theca cell layer; while, this phenomenon was not observed after treatment with flaxseed extract (Figure 5D).
Figure 4.
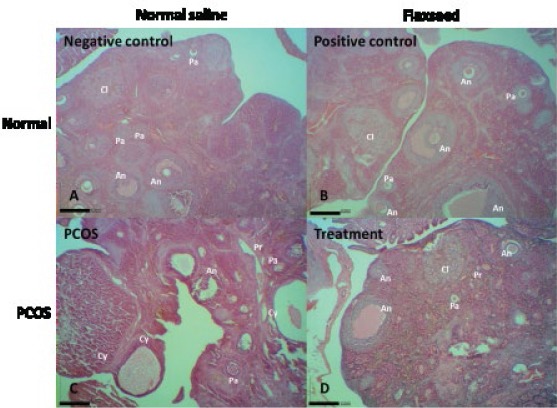
Survey views showing negative control (A), positive control (B), estradiol valerate-exposed rat (C), and flaxseed extract-treated rat (D) ovaries in the same magnification. Pr, primary follicle; Pa, preantral follicle; An, antral follicle; Cy, cystic follicle; Cl, corpus luteum (H&E staining; index bars, 200 μm)
Figure 5.
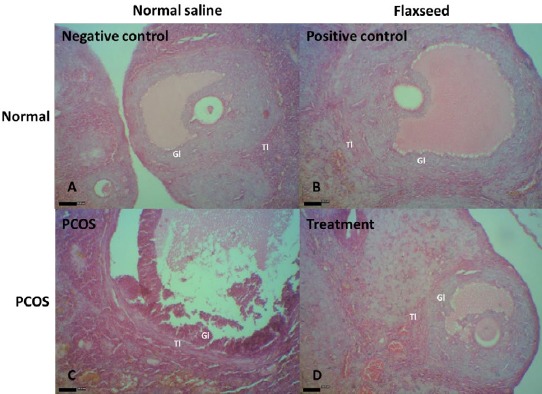
A and B, healthy tertiary follicle in the negative and positive control rats. The theca layers and granulosa layers appear normal. C, A follicle in the early process of atresia with apoptotic granulosa cells, most of which are in the inner parts of the granulosa layer in a polycystic ovary syndrome (PCOS) rat. Thin and elongated epithelioid cells form the inner surface of the wall. The cyst fluid contains macrophages. D, Ovary from a flaxseed-treated PCOS rat with normal tertiary follicles. Gl, granulosa layer; Tl, theca layer (H&E staining; index bars, 50 μm)
Flaxseed extract attenuated ovarian histomorphometric indices in PCOS
Numbers of preantral follicles, antral follicles and corpus luteum decreased in the PCOS group compare to the control groups (P<0.05, Table 2). Treatment by flaxseed extract increased their number and except the number of corpus luteum, other parameters returned to normal range (P<0.05). Histomorphometric evaluation of ovaries revealed that thickness of granulosa and tunica albuginea increased, while thickness of theca layer deceased in the PCOS group compare to the control groups; however, consumption of flaxseed extract altered this value toward normal level (P<0.05, Table 3). No significant difference was observed in thickness of endometrial and myometrial layers of uteri between the groups (P>0.05, Figure 6 and Table 4).
Table 2.
Comparison of the number of ovarian follicles and corpus luteum after treatment of polycystic ovary syndrome (PCOS) in rat with flaxseed extract
| Groups | Follicles | Corpus luteum | |||
|---|---|---|---|---|---|
| Primary | Preantral | Antral | Cystic | ||
| Negative control | 19.37±1.15a | 26.87±2.29a | 10.0 ±0.65a | 0a | 10.93±0.54a |
| Positive control | 21.57±1.42a | 34.55±1.83b | 11.35±0.89a | 0a | 11.25±0.93a |
| PCOS | 10.41±1.54b | 10.2±1.35c | 2.80±0.42b | 5.26±0.04b | 2.60±0.21b |
| Treatment | 13.55±0.58b | 23.38±1.61a | 8.55±0.73a | 2.31±0.07c | 6.45±0.82c |
Values presented as mean±SEM. aa,b,c different superscript letters in each column show significant difference observed between groups
Table 3.
Histomorphometric comparisons of tunica albuginea and antral follicles layers after treatment of polycystic ovary syndrome (PCOS) in rat with flaxseed extract
| Groups | Tunica albuginea thickness (μm) | Granulosa layer thickness (μm) | Theca layer thickness (μm) | Antral follicle diameter (μm) |
|---|---|---|---|---|
| Negative control | 14.17±0.83a | 53.63±2.89a | 22.19 ±1.64a | 556.27 ±14.18a |
| Positive control | 13.69±0.74a | 56.55±2.40a | 19.73±2.19a | 532.43 ±19.25a |
| PCOS | 22.55±1.31b | 31.67±1.62b | 34.26±1.24b | 703.36±8.51b |
| Treatment | 17.37±1.38c | 51.23±3.54a | 24.36±1.84a | 576.46±18.31c |
Values presented as mean±SEM a,b,c different superscript letters in each column show significant difference observed between groups
Figure 6.
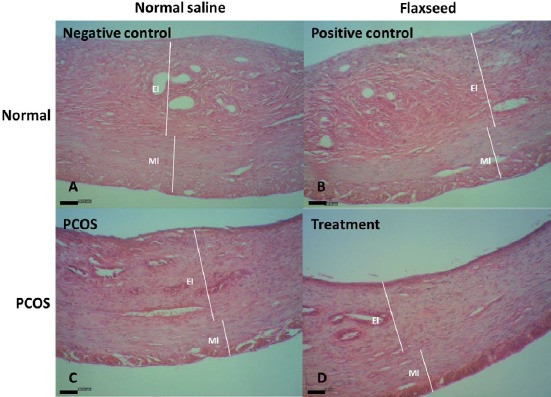
Uterine tissue sections of negative control (A), positive control (B), estradiol valerate-exposed rats (C), and flaxseed extract-treated (D) rats. El, endometrial layer; Ml, myometrial layer (H&E staining; index bars, 50 μm)
Table 4.
Histomorphometric comparisons of uterine tissue layers after treatment of polycystic ovary syndrome (PCOS) in rat with flaxseed extract
| Groups | Endometrium thickness (µm) | Myometrium thickness (µm) |
|---|---|---|
| Negative control | 283.54±26.43 | 137.67±31.11 |
| Positive control | 305.14±27.68 | 121.45±32.67 |
| PCOS | 262.71±25.97 | 121.91±28.02 |
| Treatment | 293.32±24.49 | 115.45±26.56 |
Values presented as mean±SEM. No significant difference observed between groups
Therefore, flaxseed extract attenuated PCOS signs that were induced by estradiol valerate in rats. In addition, sex hormonal profile and histomorphometry of ovary that were disturbed by PCOS induction were ameliorated by flaxseed.
Discussion
Treatment of PCOS with flaxseed extract significantly deceased level of testosterone in the treatment group compare to the PCOS group with no significant difference with the control groups. Similar to our findings, in a case study on a 31-year-old woman with PCOS, it was reported that flaxseed supplementation of 30 g/day for 4 months reduced testosterone levels (5). This effect may be due to the negative feedback of flaxseed extract on LH. Treatment of the PCOS rats with flaxseed extracts had no significant changes on level of estradiol. Consistent with our findings, in a clinical trial on 48 postmenopausal women, flaxseed extract did not change estrone and estradiol levels (6). Flaxseed as an herbal medicine can be considered for the management of women with PCOS and hyperandrogenism. Although no exact mechanism of the effect of flaxseed in PCOS has been reported (17), but the effects of flaxseed on menstrual regulation (18, 19) and hormonal concentration in postmenopausal women (19-21) has been reported.
In the present study, we used estradiol valerate for induction of PCOS. Following induction of PCOS, estradiol and testosterone were significantly increased, while level of progesterone significantly decreased in this group; however, no significant change in levels of DHEA was observed. Similar results were also reported by other researchers following induction of PCOS by estradiol valerate (22, 23). Estradiol valerate (2 mg) in adult rat or estradiol benzoate (0.5 mg) in neonatal rat (0 to 2 days) induced PCOS reproductive features with a single subcutaneous injection (24, 25). Two months later, polycystic ovaries of induced rats exhibited elevation of testosterone and estradiol levels (24). Increase in the secondary follicle population, ovarian theca-interstitial area, and expression of LH receptor and Cyp17a1 genes are induced by estrogen (25). Moreover, pre-pubertal rats subcutaneously implanted with 10 mg diethylstilbestrol showed follicle growth, without luteinizing the follicles and therefore can be served as another model of PCOS (26). Also in mice, pre-pubertal estrogen stimulation led to follicular development synchronization, but no typical symptoms of PCOS remained at adulthood (27). Physiology and anatomy of the ovary PCOS in estradiol model resemble those of human. In addition, inconsistent characteristics of sex hormone profile and ovarian morphology of estrogen models in different studies have been reported (10, 22, 28-34).
Regarding to ovarian histomorphology, induction of PCOS resulted in significant reduction in the number of primary, preantral and antral follicles compared to the negative control group, while treatment with flaxseed improved the condition. In other words, flaxseed is somewhat effective in the growth and development of follicles, and corpus luteum and reduces the cyst follicles after induction of PCOS. Consistent with our results, induction of PCOS in female rats with estradiol valerate led to decrease of the average number of primary, secondary and Graffian follicles compared to the normal control group (35). Increase in the number and growth of Graffian follicles in immature female rats was reported following treatment with flaxseed extract (36). Morphological study of ovaries showed a significant decrease in the number of granulosa cell layers around the cystic follicles compared to the control group. The HPG axis dysfunction in PCOS may lead to the increase of GnRH, and improper secretion of LH and FSH causes disruption of ovarian function and growing of more preantral follicles, which may not convert to antral follicles (37).
No significant difference in the average of endometrium and myometrium thickness was observed between study groups that are in contrary with previous report, which indicated increase in the height of the epithelium and decrease in the thickness of the endometrium in rats following induction of PCOS with estradiol valerate (38).
Conclusion
Findings of the current study showed that hydroalcoholic extract of flaxseed attenuated PCOS signs in rats. Although, estradiol valerate model of PCOS did not show all phenomena of human PCOS, but sex-steroid hormonal profile and histomorphometric characteristics of ovary in treated rats were ameliorated by flaxseed. Although, in a recent clinical trial, flaxseed oil omega-3 supplementation in PCOS women for 12 weeks had beneficial effects on some metabolic status (39), but further studies in PCOS women needs to confirm therapeutic effects of hydroalcoholic extract of flaxseed.
Acknowledgment
The current project was financially supported by Shiraz University, Vice Chancellor of Research, Shiraz, Iran.
References
- 1.Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HcF, Franks S, Gambineri A, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171:P1–P29. doi: 10.1530/EJE-14-0253. [DOI] [PubMed] [Google Scholar]
- 2.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2014;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmina E. Diagnosis of polycystic ovary syndrome: from NIH criteria to ESHRE-ASRM guidelines. Minerva Ginecol. 2004;56:1–6. [PubMed] [Google Scholar]
- 5.Nowak DA, Snyder DC, Brown AJ, Demark-Wahnefried W. The effect of flaxseed supplementation on hormonal levels associated with polycystic ovarian syndrome: A case study. Curr Top Nutraceutical Res. 2007;5:177–181. [PMC free article] [PubMed] [Google Scholar]
- 6.Sturgeon SR, Heersink JL, Volpe SL, Bertone-Johnson ER, Puleo E, Stanczyk FZ, et al. Effect of dietary flaxseed on serum levels of estrogens and androgens in postmenopausal women. Nutr Cancer. 2008;60:612–618. doi: 10.1080/01635580801971864. [DOI] [PubMed] [Google Scholar]
- 7.Altwair K, Edrah S. Phytochemical screening and antimicrobial activity for plants Dracaena cinnabari Verbena officinal Polygala tenuifolia and Linux usitatissimum. J Curr Chem Pharm Sci. 2015;5:47–55. [Google Scholar]
- 8.Paydar S, Jelodar GA, Mohammadi J, Mohammadi N. The effect of hydroalcoholic extract of Nectaroscordum tripedale on liver and kidney functional parameters in streptozotocin-induced diabetic male rats. Iran J Endocrinol Metab. 2016;18:112–119. [Google Scholar]
- 9.Salehi MS, Jafarzadeh Shirazi MR, Zamiri MJ, Pazhoohi F, Namavar MR, Niazi A, et al. Hypothalamic expression of KiSS1 and RFamide-related peptide-3 mRNAs during the estrous cycle of rats. Int J Fertil Steril. 2013;6:304–309. [PMC free article] [PubMed] [Google Scholar]
- 10.Ouladsahebmadarek E, Khaki A, Khanahmadi S, Ahmadi Ashtiani H, Paknejad P, Ayubi MR. Hormonal and metabolic effects of polyunsaturated fatty acid (omega-3) on polycystic ovary syndrome induced rats under diet. Iran J Basic Med Sci. 2014;17:123–127. [PMC free article] [PubMed] [Google Scholar]
- 11.Rahmanifar F, Nooranizadeh MH, Tamadon A, Rajabi-Aslani J, Koohi-Hosseinabadi O, Shirazi MRJ, et al. Histomorphometric comparison of induction of polycystic ovary syndrome by exposure to constant light in primiparous and nulliparous rats. Iran J Sci Technol A: Sci. 2017 doi:10.1007/s40995-40017-40226-40999. [Google Scholar]
- 12.Azarnia M, Koochesfahani HM, Rajabi M, Tahamtani Y, Tamadon A. Histological examination of endosulfan effects on follicular development of BALB/C mice. Bulg J Vet Med. 2008;12:33–41. [Google Scholar]
- 13.Shaaban Z, Shirazi MRJ, Nooranizadeh MH, Tamadon A, Rahmanifar F, Ahmadloo S, et al. Decreased expression of arginine-phenylalanine-amide-related peptide-3 gene in dorsomedial hypothalamic nucleus of constant light exposure model of polycystic ovarian syndrome. Int J Fertil Steril. 2018;12:43–50. doi: 10.22074/ijfs.2018.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazoobandi S, Tanideh N, Rahmanifar F, Tamadon A, Keshtkar M, Mehrabani D, et al. Induction of Asherman's syndrome in rabbit. J Reprod Isnfertil. 2016;17:10–16. [PMC free article] [PubMed] [Google Scholar]
- 15.Kafi M, Tamadon A, Saeb M. The relationship between serum adiponectin and postpartum luteal activity in high-producing dairy cows. Theriogenology. 2015;83:1264–1271. doi: 10.1016/j.theriogenology.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Kheirabad MK, Khodabandeh Z, Rahmanifar F, Tamadon A, Jahromi BN, Owjfard M, et al. Testicular germ cells apoptosis following exposure to chronic stress in rats. Asian Pac J Reprod. 2016;5:371–375. [Google Scholar]
- 17.Arentz S, Abbott JA, Smith CA, Bensoussan A. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism;a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement Altern Med. 2014;14:511. doi: 10.1186/1472-6882-14-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phipps WR, Martini MC, Lampe JW, Slavin JL, Kurzer MS. Effect of flax seed ingestion on the menstrual cycle. J Clin Endocrinol Metab. 1993;77:1215–1219. doi: 10.1210/jcem.77.5.8077314. [DOI] [PubMed] [Google Scholar]
- 19.Lampe JW, Martini MC, Kurzer MS, Adlercreutz H, Slavin JL. Urinary lignan and isoflavonoid excretion in premenopausal women consuming flaxseed powder. Am J Clin Nutr. 1994;60:122–128. doi: 10.1093/ajcn/60.1.122. [DOI] [PubMed] [Google Scholar]
- 20.Hutchins AM, Martini MC, Olson BA, Thomas W, Slavin JL. Flaxseed consumption influences endogenous hormone concentrations in postmenopausal women. Nutr Cancer. 2001;39:58–65. doi: 10.1207/S15327914nc391_8. [DOI] [PubMed] [Google Scholar]
- 21.Frische EJ, Hutchins AM, Martini MC, Thomas W, Slavin JL. Effect of flaxseed and wheat bran on serum hormones and lignan excretion in premenopausal women. J Am Coll Nutr. 2003;22:550–554. doi: 10.1080/07315724.2003.10719335. [DOI] [PubMed] [Google Scholar]
- 22.Linares R, Hernández D, Morán C, Chavira R, Cárdenas M, Domínguez R, et al. Unilateral or bilateral vagotomy induces ovulation in both ovaries of rats with polycystic ovarian syndrome. Reprod Biol Endocrinol. 2013;11:68–76. doi: 10.1186/1477-7827-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azarnia M, Kamyab SZ, Mirabolghasemi S, Saeidnia S. Effect of hydroalcoholic extract of Melia azedarach L. seeds on serum concentration of sex hormones in polycystic ovary syndrome induced in female wistar rats. Feyz. 2015;19:112–118. [Google Scholar]
- 24.Pereira VM, Honorato-Sampaio K, Martins AS, Reis FM, Reis AM. Downregulation of natriuretic peptide system and increased steroidogenesis in rat polycystic ovary. Peptides. 2014;60:80–85. doi: 10.1016/j.peptides.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 25.Marcondes RR, Carvalho KtC, Duarte DC, Garcia Nl, Amaral VC, Simões MJ, et al. Differences in neonatal exposure to estradiol or testosterone on ovarian function and hormonal levels. Gen Comp Endocrinol. 2015;212:28–33. doi: 10.1016/j.ygcen.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Hoang YD, McTavish KJ, Chang RJ, Shimasaki S. Paracrine regulation of theca androgen production by granulosa cells in the ovary. Fertil Steril. 2013;100:561–567. doi: 10.1016/j.fertnstert.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu B, Shi Y, Gong X, Yu L, Chen Q, Wang J, et al. Evaluation of follicular synchronization caused by estrogen administration and its reproductive outcome. PLoS ONE. 2015;10:e0127595. doi: 10.1371/journal.pone.0127595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peyghambari F, Fayazi M, Amanpour S, Haddadi M, Muhammadnejad S, Muhammadnejad A, et al. Assessment of α4, αv, β1 and β3 integrins expression throughout the implantation window phase in endometrium of a mouse model of polycystic ovarian syndromes. Iran J Reprod Med. 2014;12:687–694. [PMC free article] [PubMed] [Google Scholar]
- 29.Dăneasă A, Cucolaş C, Lenghel LM, Olteanu D, Orăsan R, Filip GA. Letrozole vs estradiol valerate induced PCOS in rats: glycemic, oxidative and inflammatory status assessment. Reproduction. 2016;151:401–409. doi: 10.1530/REP-15-0352. [DOI] [PubMed] [Google Scholar]
- 30.Dăneasă A, Cucolaş C, Furcea M, Bolfa P, Dudea S, Olteanu D, et al. Spironolactone and dimethylsulfoxide effect on glucose metabolism and oxidative stress markers in polycystic ovarian syndrome rat model. Exp Clin Endocrinol Diabetes. 2014;122:154–162. doi: 10.1055/s-0033-1363685. [DOI] [PubMed] [Google Scholar]
- 31.Kabiri N, Tabandeh MR, Tabatabaie SRF. Beneficial effects of pioglitazone and metformin in murine model of polycystic ovaries via improvement of chemerin gene up-regulation. Daru. 2014;22:39. doi: 10.1186/2008-2231-22-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesbah F, Moslem M, Vojdani Z, Mirkhani H. Does metformin improve in vitro maturation and ultrastructure of oocytes retrieved from estradiol valerate polycystic ovary syndrome-induced rats. J Ovarian Res. 2015;8:74. doi: 10.1186/s13048-015-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miri M, Karimi Jashni H, Alipour F. Effect of exercise intensity on weight changes and sexual hormones (androstenedione and free testosterone) in female rats with estradiol valerate-induced PCOS. J Ovarian Res. 2014;7:37–37. doi: 10.1186/1757-2215-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghafurniyan H, Azarnia M, Nabiuni M, Karimzadeh L. The effect of green tea extract on reproductive improvement in estradiol valerate-induced polycystic ovarian syndrome in rat. Iran J Pharm Res. 2015;14:1215–1233. [PMC free article] [PubMed] [Google Scholar]
- 35.Atis A, Aydin Y, Ciftci F, Sakız D, Arslan A, Toklu AnS, et al. Hyberbaric oxygen increases atresia in normal &steroid induced PCO rat ovaries. Reprod Biol Endocrinol. 2012;10:11. doi: 10.1186/1477-7827-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad N, Akhtar N, Ali S. Effects of aqueous methanolic extract of flax seeds (Linum usitatissimum) on serum estradiol, progesterone, kidney and liver functions and some serum biochemical metabolites in immature female rats. Pak Vet J. 2012;32:211–215. [Google Scholar]
- 37.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 38.Fozalaee SS, Farokhi F. The effect of metformin and aqueous extract Foeniculum vulgare(fennel) on endometrial histomorphometry and the level of steroid hormones in rats with polycystic ovary syndrome. Qom Univ Med Sci J. 2015;8:12–19. [Google Scholar]
- 39.Mirmasoumi G, Fazilati M, Foroozanfard F, Vahedpoor Z, Mahmoodi S, Taghizadeh M, et al. The Effects of flaxseed oil omega-3 fatty acids supplementation on metabolic status of patients with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes. 2018;126:222–228. doi: 10.1055/s-0043-119751. [DOI] [PubMed] [Google Scholar]


