Abstract
Objective(s):
The study was intended to investigate the combined influence of two neuroprotective agents pramipexole and n-acetylcysteine on global cerebral ischemic reperfusion injury (GCIRI) model in rats.
Materials and Methods:
GCIRI was induced by bilateral common carotid artery ligation (BCCA) in rats. Animals were divided into six groups. Groups I, II, and III received saline intraperitoneally (IP) (5 ml/kg/day, 0.9 % saline). The remaining groups IV, V, and VI were treated with n-acetylcysteine (NAC-150 mg/kg/day, IP), pramipexole (PPX-0.23 mg/kg/day, IP) alone and in combination, respectively. BCCA was done in all groups except in groups I (control) and II (sham control) of animals. The treatment was given for one week before the surgery and continued for two days after surgery. Subsequently, behavioral performances, biochemical estimations, proinflammatory cytokines, and histopathological evaluations were done.
Results:
NAC, PPX, and combination treatment groups showed significant ameliorative effects on behavioral, biochemical, proinflammatory cytokines, and histopathological studies as compared with the BCCA group. Whereas, the combination group showed a significant difference in ameliorating the pathological changes of biochemical parameters and histopathological changes in comparison with the PPX alone treated group but not with the NAC alone group.
Conclusion:
The study concluded that in the combination treatment group the histopathological parameter improved and the oxidative stress parameters were mitigated significantly compared with the PPX alone treatment group but not with the NAC alone treatment group.
Keywords: Histopathology, N-acetylcysteine, Neuroprotective, Oxidative stress, Pramipexole Proinflammatory cytokines
Introduction
Stroke is the third most devastating problem in the world of which, ischemic cerebral stroke is the most common cause of death and disability (1). One of the leading causes for cerebral ischemia is thrombosis or embolus formation in the cerebral artery, which leads to inadequate supply of blood (2). At the present, the only well-established treatment strategy for stroke is to dissolve the thrombus or embolus by using recombinant tissue plasminogen activator (t-PA) (3), but it has a low therapeutic index with complications like hemorrhage. However, the rapid reperfusion itself is exacerbating the neuronal injury by promoting the generation of oxygen free radical and also stimulating the inflammatory response. So, both ischemia and reperfusion were associated with a wide range of pathological changes like glutamate-mediated excitotoxicity, inflammation, calcium overload, oxidative stress, apoptosis, and blood-brain barrier dysfunction, etc., contributing to neuronal death. So far there is no single drug treatment reported for the reversal of ischemic reperfusion injury in the brain. Despite having multiple mechanisms involved in cerebral ischemic reperfusion injury, using monotherapy is not worthwhile (4).
Recent scientific reports suggest that dopamine receptor agonists regulate innate immunity and control the expression of neurotrophic factors that result in improvement of motor coordination after cerebral stroke (5). Pramipexole (PX) is a dopamine receptor agonist that has proved its neuroprotective activity against cerebral ischemia. It shows its neuroprotective activity by having antioxidant (6), hypothermic, and D3 receptor agonistic properties in ischemic brain injury (7). Hypothermia is the most promising neuroprotective strategy so far due to its metabolic demand lowering, reduction in calcium influx, suppression of inflammatory response, and reduction in reactive oxygen species (ROS) generation (8). Ischemic stroke produces an excessive amount of ROS (9). A drug like n-acetylcysteine (NAC) is well known for its quenching effect on ROS thereby it prevents cellular damage. Previous literature proved that NAC is neuroprotective for the reason that it has antioxidant property and neurovascular protective effects (10).
As there is no reported literature available for the comparative and combination study of PPX and NAC the present work was planned to investigate the therapeutic benefits of PPX and NAC alone and in combination against global cerebral ischemic reperfusion injury.
Materials and Methods
Animals
Sixty Sprague-Dawley rats weighing 220–280 g were used for the study. They were procured from Mahaveer Enterprises, Hyderabad, India and maintained in the animal house facility provided by Acharya Nagarjuna University in standard humidity (44%–60%) and temperature (22±5 °C) with 12 hr light/dark cycle. Animals were allowed to have access to food and water ad libitum.
Experimental design
Animals were divided into six groups of ten animals each. Group-I (control), was not allowed any surgical intervention. Group-II (sham control), was allowed surgical intervention without any ligation of bilateral common carotid arteries. Group-III (ligation control), was allowed surgical intervention with bilateral common carotid artery ligation (BCCA). Groups IV, V, and VI (ligation with treatment) were allowed surgical intervention and ligation followed by treatment. Groups I, II, and III were treated with saline (5 ml/kg/day, 0.9 % saline, IP). The remaining groups IV, V, and VI were treated with N-acetylcysteine (NAC-150 mg/kg/day, IP) and pramipexole (PPX-0.23 mg/kg/day, IP) alone and in the combination, respectively. Treatment was given for one week before the surgery and continued for two days after surgery.
Procedure for the induction of global cerebral ischemia
Animals were anesthetized with ketamine (50 mg/kg, IP) and xylazine (10 mg/kg, IM) and were laid on their ventral side to expose the neck region. A midline incision was made after shaving the neck area. Dissection was done at sternocleidomastoid and sternothyroid regions to detect common carotid arteries. Both common carotid arteries were identified and separated from the vagus nerve to produce ischemia by ligation with a silk thread for 20 min. After 20 min, the ligature was removed to allow the perfusion. The incision was closed with sutures, and iodine ointment was applied to prevent infection. After 24 hr of reperfusion, animals were allowed to assess the behavioral performances, and after 48 hr of reperfusion, six animals were sacrificed for biochemical examinations and four animals were used for histopathological examinations (11).
Behavioral assessment
Locomotor activity
Locomotor activity measurement is commonly used to study sensitivity to the locomotor activating or depressing effects of a drug. After post-surgical treatment, all animals were allowed to get acclimatized to Photoactometer for 5 min. After acclimatization, animals were observed for locomotor activity for 10 min, and locomotor activity count/10 min were calculated (12).
Hanging wire test
Rats were assessed for grip strength by using the hanging wire test. In this test, animals were allowed to hang from a wire (45 cm long x 0.3 cm in diameter) with forelimbs. The time of fall was measured and 90 sec was kept as cut-off time (13).
Rotarod test
Rotarod test was used to analyze motor coordination in rodents. The rats were allowed to train on the rotarod apparatus with a 5 cm diameter for 5 min by adjusting the rotations per min of the rod. They were observed for motor coordination by noting the time it takes them to fall off by keeping the animals on a rod (14).
Y- maze test
Short-term spatial working memory was assessed using the Y-maze test by observing spontaneous alterations. The Y-maze consists of 3 arms labeled A, B, and C. Animals were placed in one arm of the maze and allowed to explore the maze for 5 min, and alternations were counted manually (consecutive entries in three different arms). Percentage of spontaneous alternations (%SA) was calculated using the formula (15)
Maximum number of alterations=Total number of alterations - 2
Biochemical estimations
Preparation of homogenate
After scarification, the brain samples of animals were isolated and washed with an ice-cold saline solution. The brain tissue was minced into small pieces and homogenized in ice-cold phosphate buffer (0.1 M, pH 7.4). The obtained homogenate was centrifuged (8000 rpm, 20 min, -4 °C) and the supernatant was collected for further estimations of biochemical parameters (16).
Estimation of malondialdehyde
Lipid peroxidation was estimated by thiobarbituric acid reactive substance. To 0.1 ml of homogenate was added 10% trichloroacetic acid and thiobarbituric acid, 1 ml each in test tubes. Test tubes were placed in a boiling water bath for 20 min by covering with aluminum foil to prevent evaporation. After 20 min, they were cooled by keeping them in an ice bath for 10 min. Samples were centrifuged for 10 min, and the supernatant was estimated spectrophotometrically at a wavelength of 540 nm. The malondialdehyde (MDA) levels were calculated using extinction coefficients (17).
Estimation of total thiols
To 0.2 ml of homogenate was added 0.36 ml of phosphate buffer, 0.04 ml of DTMB (10 mM), and 1.5 ml of methanol with constant mixing after the addition of each element. The mixture was centrifuged (8000 rpm, 15 min, -4 °C). The yellow colored supernatant was collected and used to measure the spectroscopic absorption at 412 nm (18).
Estimation of reduced glutathione
To 0.5 ml of homogenate, 0.5 ml of 10 % trichloroacetic acid was added and centrifuged at 3000 rpm for 20 min to separate the protein from homogenate. 0.1 ml of supernatant was taken in a test tube and mixed with 1 ml of phosphate buffer (0.2 M, pH 8) and 0.5 ml of DTNB reagent (0.6 mM in 0.2 M phosphate buffer). After mixing, the absorbance of the mixture was observed spectrophotometrically at 412 nm (19).
Estimation of superoxide dismutase
To estimate superoxide dismutase (SOD) levels in the tissue sample, we used autoxidation of pyrogallol monitored at 420 nm. In this assay procedure, we used 1.5 ml of tris buffer (0.05 M), 0.5 ml of EDTA (1 M), 0.05 ml of homogenate, and 1 ml of pyrogallol (0.2 mM) mixture and the change in absorbance was measured at 420 nm for 1 min (20).
Estimation of acetylcholinesterase
To 0.4 ml of homogenate, 2.6 ml of Phosphate buffer (0.2 M, pH 8) and 100 µl of DTNB were added. The solution was mixed well, and the absorbance was measured at 412 nm. After reaching the stable absorption, 20 µl of acetylthiocholine was added, and change in absorption was recorded for 5 min with an interval of 1 min to determine the change in absorbance for 1 min. The determination of acetylcholinesterase was estimated by using the formula: R=5.74×10-4 × change in absorbance per min / original concentration of the tissue (mg/ml) (21).
Estimation of serum proinflammatory cytokines
After 2 hr of global cerebral ischemia, blood samples were collected for the estimation of cytokines like TNF-α and IL-1β. Blood samples were allowed to clot and centrifuged for 20 min at -4 °C to collect the serum. Then the serum was stored at -80 °C until the time of estimation. Krishgen Biosystems TNF-α and IL-1β ELISA kits were used to estimate the cytokine levels in the animals by following manufacturer’s catalogs (22).
Histopathological study of hippocampus
After 48 hr of ischemic reperfusion, the animals were anesthetized. The brain samples of animals were isolated, collected, and stored in 10% formalin solution. They were embedded in paraffin wax and sliced coronally into 5 µm thick slices. The slices were stained using eosin and hematoxylin to observe the hippocampus neuronal density and the percentage of necrotic neurons at the CA1 region (22).
Statistical analysis
The values were expressed as Mean±SEM. The data were analyzed by using one-way ANOVA followed by Tukey’s post hoc test using the GraphPad Prism software. Statistical significance was considered at P≤0.05 for analysis.
Results
Effect on behavioral studies
In examination of the data obtained from the locomotor activity test, the sham group showed no significant difference in behavioral responses as compared to the control group. The BCCA control showed significant (P<0.01) decrease in locomotor activity count as compared to the sham group. The NAC, PPX alone, and combination of both NAC & PPX treatment groups showed significant (P<0.01, P<0.01, and P<0.001) improvement in locomotor performance in comparison with the BCCA group, whereas the combination treatment group showed no significant difference with the individual treatment groups.
Based on the data obtained from the hanging wire test, the BCCA control group showed significant (P<0.01) decrease in hanging latency time when compared to the sham control. The NAC, PPX alone, and combination treatment groups showed significant (P<0.05, P<0.05, and P<0.001) increase in hanging latency as compared to the BCCA control group of animals, whereas there was no statistical difference between the combination treatment group and the individual treatment groups.
In analysis of the data obtained from the rotarod test, the BCCA control showed significant (P<0.05) decrease in time of fall when compared to the sham control group and the individual treated group showed improved time of fall but not significantly when compared to the BCCA control group. The combination of NAC and PPX treated group showed significant (P<0.001) improvement in time of fall in comparison with the BCCA control group but no significant difference was observed when compared with individual treatment groups (Table 1).
Table 1.
Behavioral assessment
| Treatments | Locomotor activity count/10 min | Hanging latency time/90 sec | Time of fall / 90 sec | %Spontaneous alterations |
|---|---|---|---|---|
| Control | 252±29.1 | 22.3±3.16 | 58.2±8.36 | 69.2±2.93 |
| Sham Control | 185±18.5 | 19.2±1.01 | 39.8±5.51 | 63.0±3.65 |
| BCCA Control | 77.0±6.03** | 8.17±0.872** | 15.0±1.53* | 40.3±4.98*** |
| BCCA+NAC | 175±17.5** | 16.2±0.946* | 33.5±3.59 | 62.2±4.59** |
| BCCA+PPX | 180±15.7* | 27.7±3.28 | 57.8±3.28* | |
| BCCA+NAC+PPX | 243±12.6*** | 20.2±1.40*** | 45.8±2.54*** | 68.3±2.32*** |
Results were expressed in mean ± SEM, (n=6) in each group, ‘*’ ‘**’ and ‘***’ were significantly different at P<0.05, P<0.01 and P<0.001 respectively, in comparison with bilateral common carotid artery ligation groupBilateral common carotid artery ligation group was compared with sham control; combination treatment group was compared with Individual treatment groups
In the Y-maze test, the BCCA control group showed significant (P<0.001) decrease in %SA when compared to the sham control group. The NAC, PPX alone, and combination treatment groups showed significant (P<0.01, P<0.05, and P<0.001) increase in % SA of animals with respect to the BCCA control group, whereas there was no significant difference between the combination treatment group and the individual treatment groups.
Effect on biochemical estimations
The MDA level in the sham control group of animals did not show significant difference with the normal control animals. The BCCA control group of animals showed significant (P<0.001) increase in the MDA levels in comparison with the sham control animals. The NAC, PPX alone, and combination treatment groups showed significant (P<0.001) decrease in MDA levels as compared with the BCCA control group of animals. The combination treatment group showed significant (P<0.05) difference with the PPX alone treatment group in decreasing the MDA levels, whereas there was no significant difference between the combination group and NAC alone treatment group. The data was represented in Figure 1.
Figure 1.
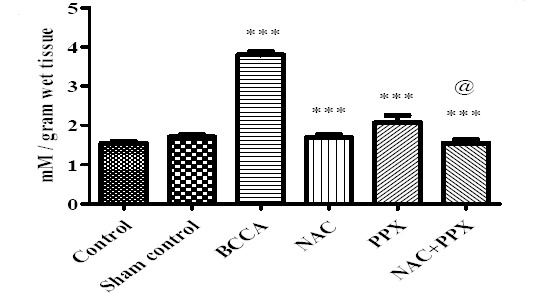
Effect on lipid peroxidation of the tissue. Results were expressed in mean±SEM, ‘*’, ‘**’ and ‘***’ significantly different at P<0.05, P<0.01 and P<0.001 respectively in comparison with bilateral common carotid artery ligation group. Bilateral common carotid artery ligation group was compared with sham control; combination treatment group was compared with Individual treatment groups. @significantly different at P<0.05 (PPX vs. NAC+PPX)
The thiol content of sham control group of animals did not show significant difference with the control group of animals, at the same time the thiol content of the BCCA group showed significant (P<0.001) decrease in comparison with the sham control group of animals. The NAC, PPX alone, and combination treatment groups showed significant (P<0.001, P<0.01, and P<0.001) improvement in thiol levels, respectively. The combination treatment group showed significant (P<0.01) difference with the PPX alone treatment group in increased thiol levels, whereas the combination group showed no significant difference with the NAC alone treatment group. The data was represented in Figure 2.
Figure 2.
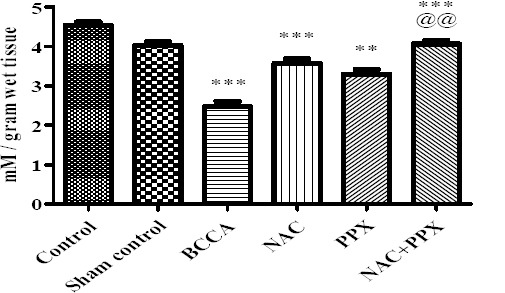
Effect on thiol content of the tissue. Results were expressed as Mean±SEM, *, **, and *** were significantly different at P<0.05, P<0.01, and P<0.001, respectively in comparison with bilateral common carotid artery ligation group. Bilateral common carotid artery ligation group was compared with the sham control; the combination treatment group was compared with individual treatment groups; @@ significantly different at P<0.01 (PPX vs NAC+PPX)
The GSH content of sham control group of animals did not show significant difference with the control group of animals, at the same time GSH content of the BCCA group showed significant (P<0.001) decrease in comparison with the sham control group of animals. The NAC alone and combination treatment groups showed significant increase (P<0.01 and P<0.001, respectively) in GSH levels, whereas in the PPX alone treatment group the levels of GSH were not increased significantly in comparison with the BCCA group. The combination treatment group showed significant (P<0.05) difference with the PPX alone treatment group in increased GSH levels; there was no significant difference between the combination group and NAC alone treatment group. The data was represented in Figure 3.
Figure 3.
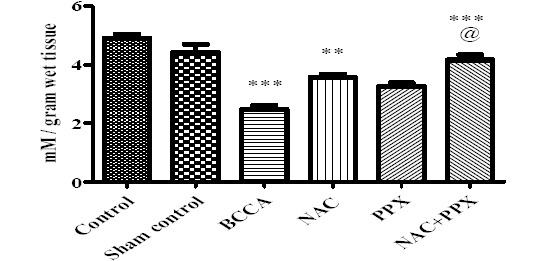
Effect on thiol content of the tissue. Results were expressed as Mean±SEM, *, **, and *** were significantly different at P<0.05, P<0.01, and P<0.001, respectively in comparison with bilateral common carotid artery ligation group. Bilateral common carotid artery ligation group was compared with the sham control; the combination treatment group was compared with individual treatment groups; @@ significantly different at P<0.01 (PPX vs NAC+PPX)
The SOD levels of the sham control group of animals were significantly (P<0.01) decreased in comparison with the normal control group of animals. BCCA group of animals showed significant (P<0.001) decrease in SOD levels as compared to the sham control group of animals. The treatment groups NAC, PPX alone, and combination groups showed significant (P<0.001) increase in SOD levels in comparison with the BCCA group of animals. The SOD levels of combination treatment group significantly (P<0.01 and P<0.001) differed with NAC and PPX alone treatment groups. All data is represented in Figure 4.
Figure 4.
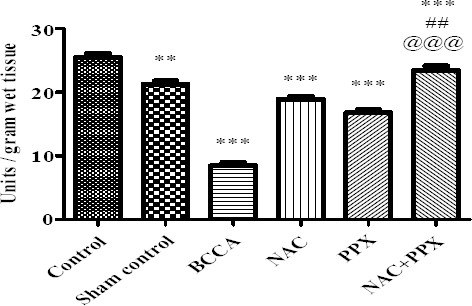
Effect on superoxide dismutase levels of the tissue. Results were expressed as Mean±SEM, *, **, and *** were significantly different at P<0.05, P<0.01, and P<0.001, respectively in comparison with bilateral common carotid artery ligation group. Bilateral common carotid artery ligation group was compared with individual treatment groups; ## significantly different at P<0.01 (NAC vs NAC+PPX); @@@ significantly different at P<0.001 (PPX vs NAC+PPX)
The AChE levels of the sham control group of animals were significantly (P<0.01) increased compared with the normal control group of animals. BCCA group of animals showed significant (P<0.001) increase in AChE levels as compared to the sham control group of animals. The treatment groups NAC, PPX alone, and combination groups showed significant (P<0.001) decrease in AChE levels in comparison with BCCA group of animals. The combination treatment group showed significant (P<0.01) difference with PPX alone treatment group in decreasing AChE levels, whereas the combination group did not show significant difference with the NAC alone treatment group. All data is represented in Figure 5.
Figure 5.
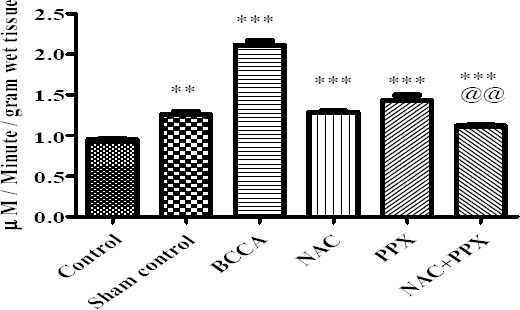
Effect on acetylcholinesterase levels of the tissue. Results were expressed as mean±SEM, *, **, and *** were significantly different at P<0.05, P<0.01 and P<0.001, respectively in comparison bilateral common carotid artery ligation group. Bilateral common carotid artery ligation group was compared with the sham control; the combination treatment group was compared with individual treatment groups; @@ significantly different at P<0.01 (PPX vs NAC+PPX)
Effect on proinflammatory cytokines in serum
The serum proinflammatory cytokines TNF-α and IL-1β were not increased significantly in the sham control group of animals in comparison with the control group of animals. There was a significant (P<0.001) increase in cytokine levels in the BCCA control group of animals as compared to the sham control group. The NAC individual treatment group had significantly lowered TNF-α and IL-1β (P<0.05 and P<0.01), and significantly (P<0.01) decreased IL-1β was also observed with the PPX alone treatment group. But in the PPX treatment group, TNF-α was not reduced significantly in comparison with the BCCA control group of animals. The combination treatment group had significantly lowered TNF- α and IL-1β (P<0.01 and P<0.001), whereas there was no significant difference between the individual treatment groups and the combination treatment group. The results are shown in Figure 6.
Figure 6.
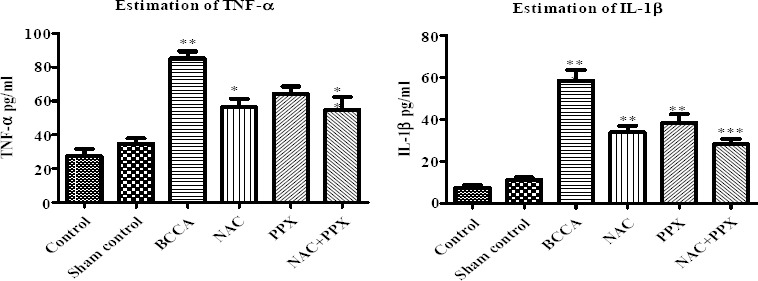
Effect on proinflammatory cytokines in serum. Results were expressed as mean±SEM, *, **, and *** were significantly different at P<0.05, P<0.01, and P<0.001, respectively in comparison with bilateral common carotid artery ligation group. Bilateral common carotid artery ligation group was compared with the sham control; the combination treatment group was compared with individual treatment groups
Histopathological study of hippocampus
Histopathological study of the hippocampus revealed that the normal control and the sham group of animals showed the normal structural morphology of CA1 pyramidal cells, whereas the BCCA group of animals showed significantly (P<0.001) increased percentage of pyknotic cells characterized by shrunken cytoplasm and damaged nuclei with thick staining. The individual treatment groups NAC, PPX, and the combination group showed significant (P<0.001, P<0.01, and P<0.001) decrease in the percentage of pyknotic cells, respectively. The combination treatment group significantly (P<0.05) differed with the PPX alone treatment group but not with the NAC alone treatment group in decreased percentage of pyknotic cells. The results are shown in Figures 7 and 8.
Figure 7.
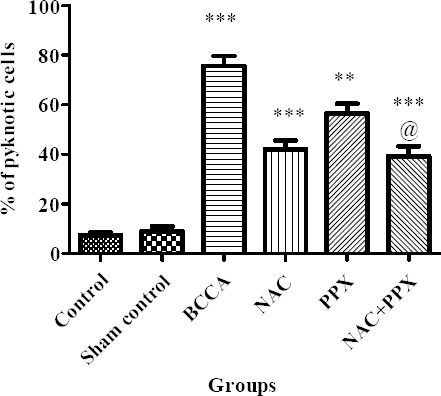
Effect on hippocampus pyramidal cells. Results were expressed a mean±SEM, ** and *** were significantly different at P<0.01 and P<0.001, respectively in comparison with bilateral common carotid artery ligation group. Bilateral common carotid artery ligation group was compared with the sham control; the combination treatment group was compared with the individual treatment groups; @ significantly different at P<0.05 (PPX vs NAC+PPX)
Figure 8.
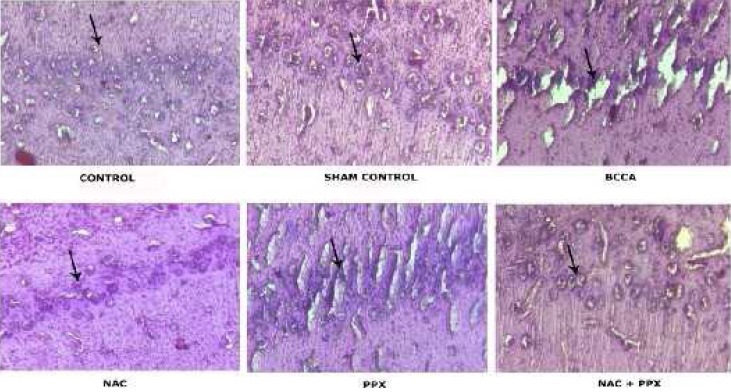
Photographic representations of coronal sections of CA1 region in hippocampus
Discussion
The cerebral ischemic reperfusion injury involves multiple pathways for its pathology, so the Stroke Therapy Academic Industry Round Table body combination drug therapy was always advisable (23). Stroke-induced disabilities are the principal reason for the socioeconomic burden of the world (24). So, the study was intended to evaluate the combined effect of an antioxidant substance (NAC) and a D2 receptor family agonist (PPX) on cerebral ischemic reperfusion injury and its associated postischemic motor, cognitive, and behavioral changes.
Reperfusion is the primary treatment strategy for ameliorating the brain injury after cerebral ischemia while reperfusion itself exuberates neuronal injury by the generation of oxygen free radicals and also by promoting neuronal inflammation (25). Decreased or complete cessation of blood supply to the brain initiates multiple pathological pathways like excitotoxicity, calcium overload, and stimulation of induced nitric oxide synthase activity. As a result, it increases the levels of NO and other free radicals in the ischemic cells (26). The free radicals are normally produced by the cell metabolic process. In our body, there are defensive mechanisms like enzymatic and nonenzymatic anti-oxidants to protect from the progression of free radical chain reactions (27). Many authors have reported that ischemic reperfusion promotes the excessive generation of free radicals and phosphorylation of various molecular pathways like ERK-1/2, SAPK/JNK ½, and P38/MAPK leading to transcription of apoptotic factors and gene mediating inflammation in the ischemic regions (28). Under ischemic conditions, our natural anti-oxidant defense system fails to protect from the excessive generation of free radicals and cellular injury. Previous literature has supported providing anti-oxidants to ameliorate the ischemic reperfusion injury.
In cerebral ischemic reperfusion injury, excitotoxicity also plays a significant role in neuronal death. The massive release of glutamate stimulates NMDA receptors, which allow an excess amount of calcium into the neuronal cells likely to cause neuronal death (29). Along with excitotoxicity, proinflammatory cytokines like TNF-α and IL-1β are also responsible for exacerbation of ischemic reperfusion injury (30, 31). In the present study, we tried to use a combination of drugs to ameliorate neuronal injury. One of the drugs, NAC, is well known as an antidote for acetaminophen toxicity. It has an extensive range of clinical applications because of its ability to support the natural antioxidant defense system. NAC is a precursor for cysteine, and it provides sulfhydryl groups, which mitigate the effects of oxidative free radicals (32). Previous literature has demonstrated that NAC ameliorates cellular injury by its antioxidant property and also by upregulation of hypoxia-induced factor 1α (33). Another drug, D2 receptor agonist PPX, was selected mainly as a therapeutic agent for Parkinson’s disease; along with that PPX has been proven for its neuroprotective activity. There are some scientific reports stating that neuroprotective mechanisms of PPX may be due its antioxidant activity (34), upregulation of brain-derived neurotrophic factor (BDNF), and mitochondrial membrane stabilization (35). There are some scientific reports that support the therapeutic benefit of hypothermia in cerebral stroke by reduction of various salutary factors like metabolic demand, excitotoxicity, and generation of free radicals (36, 37). It has also been proven that mild hypothermic effects PPX lead to inhibition of neuronal death via the PI3K/AKT/GSK3β pathway (38).
Thus, the study concentrated on the combined influence of NAC and PPX in the global cerebral ischemic injury model to get more therapeutic benefit than individual treatments. The individual treatment groups of NAC and PPX showed significant improvement in locomotor activity, hanging latency time, and % spontaneous alterations, whereas the time of fall showed no significant improvement in comparison with the BCCA group. However, the combination treatment group did not differ with the individual treatments of NAC and PPX except in case of time of fall in the rotarod experiment, which showed significant difference with NAC only. The study demonstrated that the combination of NAC and PPX was not able to improve significantly the behavioral performances compared to individual treatment groups. Because of the multifactorial role in the pathophysiology of cerebral ischemic injury (4), this is not the possible combination to improve the behavioral performances.
In the present study, as reported in the previous literature the individual treatment groups, NAC and PPX, showed a significant decrease in MDA and a significant increase in total thiols and SOD and GSH levels in comparison with the BCCA group (34, 39). The increase in GSH levels in case of the PPX alone treatment group, did not differ significantly with the BCCA group of animals. The combination treatment group showed significant difference with the PPX alone treatment group but not with the NAC alone treatment group except in SOD levels, in which NAC alone treatment group differed significantly with the combination treatment group. The results of our study indicated that the combination treatment group had shown better results than the individual PPX alone treatment group in improving antioxidant potential, whereas the combination treatment did not differ significantly with the NAC alone treatment group. The antioxidant study revealed that the combination group showed significant improvement in antioxidant potential compared with individual treatment of PPX but not with NAC. In existing literature, it has been demonstrated that the administration of NAC reduces the levels of acetylcholinesterase thereby improving the memory performance (40) as represented in the Y-maze test by increasing the % spontaneous alterations. In the present study, we tried to evaluate the combined influence of NAC along with PPX. The PPX treatment group also showed a significant decrease in acetylcholinesterase and also increased % spontaneous alterations in the Y-maze test. The combination treatment group had significantly decreased levels of acetylcholinesterase in comparison with the BCCA group of animals, and it showed significant differences with the PPX alone treatment group but not with the NAC alone treatment group. Here the combination treatment group showed significant differences neither with NAC nor PPX alone treatment groups in improving % spontaneous alterations.
In existing literature, it has already been shown that levels of proinflammatory cytokines like TNF-α and IL-1β increase in cerebral-stroke induced rats and these proinflammatory cytokines promote neuronal death. NAC has been proven for its neuroprotective activity by its associative decrease in expression of TNF-α and IL-1β (41). The pathologically increased levels of TNF-α and IL-1β show detrimental effects on rat hippocampus by influencing the % spontaneous alterations (42). PPX also has been reported for its anti-inflammatory potential and its neuronal protection in the hippocampus (43). In line with existing literature, in our present study, the NAC and PPX individual treatment groups showed a significant reduction in IL-1β, whereas the significant decrease in TNF-α was only in comparison with NAC but not with the PPX alone treatment group when comparing with the BCCA group of animals. The combination treatment group showed a significant decrease in TNF-α and IL-1β as compared with the BCCA group of animals but showed no significant difference in reducing the proinflammatory cytokines compared with the individual treatment groups. Ischemic reperfusion decreases the pyramidal cell count in the CA1 region of the hippocampus (44). In the present study, the individual treatment groups NAC and PPX showed significantly decreased pyknotic count of pyramidal cells in comparison with the BCCA group of animals and the results are in line with existing literature (45). The combination treatment group had significantly decreased percentage of pyknotic cell count in comparison with the BCCA group of animals. In the present study, it was found that the combination treatment group had decreased percentage of pyknotic cell count in comparison with the PPX alone treatment group but not with the NAC alone treatment group.
Conclusion
The present work concludes that in the combination treatment group the behavioral performance and inhibition of proinflammatory cytokines was not improved compared with the individual treatment groups. But, in biochemical and histopathological studies, it was seen that the combination treatment group was able to mitigate the pathological changes when compared to the individual treatment group of PPX but, not with the NAC treatment group.
Acknowledgment
This work was supported by Acharya Nagarjuna University College of Pharmaceutical Sciences (ANUCPS), Guntur, Andhra Pradesh, India. The Authors would like to thank Prof A Prameela Rani, Principal ANUCPS, and the staff of ANUCPS, Acharya Nagarjuna University, Guntur, for their kind cooperation.
Conflicts of interest
The authors have no conflicts of interest to report.
References
- 1.Helen MB, Dalton DW. Pathophysiology of cerebral ischemia and brain trauma: Similarities and differences. J Cerebr Blood Flow Metab. 2003;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- 2.Dirnagl U, Ladecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 3.Neetu S, Gaurav S, Nilendra S, and Kashif H. Acomparative study of neuroprotective effect of single and combined blockade of AT1 receptor and PARP-1 in focal cerebral ischemia in rat. Int J Stroke. 2012;9:560–568. doi: 10.1111/j.1747-4949.2012.00916.x. [DOI] [PubMed] [Google Scholar]
- 4.Suresh LM, Namratta M, Ram R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Huck JH, Freyer D, Bottcher C, Mladinov M, Muselmann-Genschow C, Thielke M, et al. De novo expression of dopamine D2 receptors on microglia after stroke. J Cerebr Blood Flow Metab. 2015;35:1804–1811. doi: 10.1038/jcbfm.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling ZD, Robie HC, Tong CW, Carvey PM. Both the antioxidant and D3 agonist actions of pramipexole mediate its neuroprotective actions in mesencephalic cultures. J Pharmacol Exp Ther. 1999;289:202–210. [PubMed] [Google Scholar]
- 7.Ma J, Wang Z, Liu C, Shen H, Chen Z, Yin J, et al. Pramipexole-induced Hypothermia Reduces Early Brain Injury Via PI3K/AKT/GSK3βpathway in Subarachnoid Hemorrhage rats. Sci Rep. 2016;6:1–11. doi: 10.1038/srep23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goossens J, Hachimi-Idrissi S. Combination of therapeutic hypothermia and other neuroprotective strategies after an ischemic cerebral insult. Curr Neuropharmacol. 2014;12:399–412. doi: 10.2174/1570159X12666140424233036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erkut B, Ozyazicioglu A, Karapolat BS, Kocogullari CU, Keles S, Ates A, et al. Effect of ascorbic acid, alpha-tocopherol and allopurinol on ischemic-reperfusion injury in rabbit skeletal muscle: an experimental study. Drug Target Insights. 2007;2:249–258. [PMC free article] [PubMed] [Google Scholar]
- 10.Eakin K, Baratz-Goldstein R, Pick CG, Zindel O, Balaban CD, Hoffer ME, et al. Effect of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9:e90617. doi: 10.1371/journal.pone.0090617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozdemir HH, Ilhan S, Demir CF, Akgun B, Kapan O, Atas E, et al. Effects of memantine and clopidogrel alone and in combination in cerebral ischemia-reperfusion model. Neurol Psychiat BR. 2015;21:73–77. [Google Scholar]
- 12.Kulkarni SK. Handbook of Experimental Pharmacology. New delhi: VallabhPrakashan; 1999. [Google Scholar]
- 13.Hunter AJ, Hatcher J, Virley D, Nelson P, Irving E, Hadingham SJ, et al. Functional assessments in mice and rats after focal stroke. Neuropharmacol. 2000;39:806–816. doi: 10.1016/s0028-3908(99)00262-2. [DOI] [PubMed] [Google Scholar]
- 14.Hong SH, Belayev L, Khoutorova L, Obenaus A, Bazan NG. Docosahexaenoic acid confers enduring neuroprotection in experimental stroke. J Neurol Sci. 2014;338:135–141. doi: 10.1016/j.jns.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf A, Bauer B, Abner EL, Ashkenazy-Frolinger T, Hartz AM. A comprehensive behavioral test battery to assess learning and memory in 129s6/tg2576 mice. PLoS One. 2016;11:e0147733. doi: 10.1371/journal.pone.0147733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bora KS, Sharma A. Neuroprotective effect of Artemisia absinthium L. on focal ischemia and reperfusion-induced cerebral injury. J Ethnopharmacol. 2010;129:403–409. doi: 10.1016/j.jep.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Akhtar M, Pillai KK, Vohora D. Effect of thioperamide on oxidative stress markers in middle cerebral artery occlusion model of focal cerebral ischemia in rats. Hum Exp toxicol. 2008;27:761–767. doi: 10.1177/0960327108094608. [DOI] [PubMed] [Google Scholar]
- 18.Sedlak J, Lindsay RH. Estimation of total, protein bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 19.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 21.Srikumar BN, Ramkumar K, Raju TR, Shankaranarayana Rao BS. Assay of Acetyl cholinesterase activity in the brain. Neuro Sci. 2004:142–144. [Google Scholar]
- 22.Seo JH, Park HP, Jeon YT, Lim YJ, Nam K, Hwang JW. Combined treatment with celecoxib and sevoflurane after global cerebral ischaemia has no additive neuroprotective effects in rats. Brit J Anaesth. 2013:aet009. doi: 10.1093/bja/aet009. [DOI] [PubMed] [Google Scholar]
- 23.Fisher M, Hanley DF, Howard G, Jauch EC, Warach S. Recommendations from the STAIRV meeting on acute stroke trials, technology and outcomes. Stroke. 2007;38:2391–2398. doi: 10.1161/01.STR.0000255951.37434.aa. [DOI] [PubMed] [Google Scholar]
- 24.Wu C, Zhang J, Chen Y. Study on the behavioral changes of a poststroke depression rat model. Exp Ther Med. 2015;10:159–163. doi: 10.3892/etm.2015.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang M, Li J, Peng Q, Liu Y, Liu W, Luo C, et al. Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J Neuroinflammation. 2014;11:167. doi: 10.1186/s12974-014-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson VL, Dawson TM. Free radicals and neuronal cell death. Cell Death Differ. 1996;3:71–78. [PubMed] [Google Scholar]
- 27.Erkut B, Onk OA. Effect of N-acetylcysteine and allopurinol combination to protect spinal cord ischemia/reperfusion injury induced by aortic cross-clamping in rat model. J Cardiothorac Surg. 2015;10:95. doi: 10.1186/s13019-015-0284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilic U, Yilmaz B, Reiter RJ, Yüksel A, Kilic E. Effects of memantine and melatonin on signal transduction pathways vascular leakage and brain injury after focal cerebral ischemia in mice. Neuroscience. 2013;237:268–276. doi: 10.1016/j.neuroscience.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 29.Meldrum B. Protection against ischaemic neuronal damage by drugs acting on excitatory neurotransmission. Cerebrovas Brain Metab Rev. 1989;2:27–57. [PubMed] [Google Scholar]
- 30.Maddahi A, Kruse LS, Chen QW, Edvinsson L. The role of tumor necrosis factor-αand TNF-αreceptors in cerebral arteries following cerebral ischemia in rat. J Neuroinflammation. 2011;8:107. doi: 10.1186/1742-2094-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 32.Bavarsad Shahripour R, Harrigan MR, Alexandrov AV. N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain Behav. 2014;4:108–122. doi: 10.1002/brb3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Yan J, Taheri S, Liu KJ, Shi H. Hypoxia-inducible factor 1 contributes to N-acetylcysteine's protection in stroke. Free Radic Biol Med. 2014;68:8–21. doi: 10.1016/j.freeradbiomed.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le WD, Jankovic J, Xie W, Appel SH. Antioxidant property of pramipexole independent of dopamine receptor activation in neuroprotection. J Neural Transm. 2000;107:1165–1173. doi: 10.1007/s007020070030. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Guo Y, Xie W, Li X, Janokovic J, Le W. Neuroprotection of pramipexole in UPS impairment induced animal model of Parkinson's disease. Neurochem Res. 2010;35:1546–1556. doi: 10.1007/s11064-010-0214-3. [DOI] [PubMed] [Google Scholar]
- 36.Kim JY, Yenari MA. Hypothermia for treatment of stroke. Brain Circ. 2015;1:14. [Google Scholar]
- 37.Allahtavakoli M, Kahnouei MH, Rezazadeh H, Roohbakhsh A, Mahmoodi MH, Moghadam-Ahmadi A, Zarisfi M. Delayed combination therapy of local brain hypothermia and decompressive craniectomy on acute stroke outcome in rat. Iran J Basic Med Sci. 2014;17:476. [PMC free article] [PubMed] [Google Scholar]
- 38.Ma J, Wang Z, Liu C, Shen H, Chen Z, Yin J, et al. Pramipexole-induced hypothermia reduces early brain injury via PI3K/AKT/GSK3βpathway in subarachnoid hemorrhage rats. Sci Rep. 2016:6. doi: 10.1038/srep23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuzzocrea S, Mazzon E, Costantino G, Serraino I, Dugo L, Calabrò G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J pharmacol. 2000;130:1219–1226. doi: 10.1038/sj.bjp.0703421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakash A, Kumar A. Effect of N-acetyl cysteine against aluminium-induced cognitive dysfunction and oxidative damage in rats. Basic Clin Pharmacol Toxicol. 2009;105:98–104. doi: 10.1111/j.1742-7843.2009.00404.x. [DOI] [PubMed] [Google Scholar]
- 41.Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, et al. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res. 2004;76:519–527. doi: 10.1002/jnr.20087. [DOI] [PubMed] [Google Scholar]
- 42.Khairova RA, Machado-Vieira R, Du J, Manji HK. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2009;12:561–578. doi: 10.1017/S1461145709009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieberknecht V, Cunha MP, Junqueira SC, dos Santos Coelho I, de Souza LF, dos Santos AR, et al. Antidepressant-like effect of pramipexole in an inflammatory model of depression. Behav Brain Res. 2017;320:365–373. doi: 10.1016/j.bbr.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Dabaghian FH, Hashemi M, Entezari M, Movassaghi S, Goushegir SA, Kalantari S, et al. Effect of Cyperusrotundus on ischemia-induced brain damage and memory dysfunction in rats. Iran J Basic Med Sci. 2015;18:199. [PMC free article] [PubMed] [Google Scholar]
- 45.Cuzzocrea S, Mazzon E, Costantino G, Serraino I, Dugo L, Calabrò G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130:1219–1226. doi: 10.1038/sj.bjp.0703421. [DOI] [PMC free article] [PubMed] [Google Scholar]


