Abstract
Objective(s):
There are controversial results regarding the effect of the interaction of CETP polymorphisms with dietary fats on the lipid profiles. The aim of this study was to examine the effect of CETP polymorphisms (rs5882 and rs3764261) and macronutrient intakes interaction in relation to metabolic syndrome (MetS) or its components.
Materials and Methods:
In this nested case-control study, subjects were selected from among participants of the Tehran Lipid and Glucose Study. Cases (n=441) were individually matched with two controls (844 non-MetS subjects). DNA samples were genotyped with HumanOmniExpress-24-v1-0 bead chips, including 649,932 SNP loci.
Results:
The mean ages at baseline were 38.1±10 and 37.0±10 years in women and 36.2±11 and 36.3±11 years in men, respectively in cases and controls. We did not find significant gene-diet interactions between rs5882 and dietary macronutrient intakes in relation to MetS risk. The risk of low HDL-C was lower in the first quartile of MUFA and total fat intake in G allele carriers, compared to AA genotype group. The risk of high BP appeared to increase significantly in higher quartiles of trans-fatty acid intakes (>1.81% of total energy intake) in G allele carriers compared with the AA genotype group. No significant interactions were found between rs3764261 and macronutrient intakes in association with MetS or its components.
Conclusion:
Our findings demonstrate that dietary fats modify the association of rs5882 and risk of low HDL-C and high blood pressure.
Keywords: CETP polymorphism, Dietary fats, Dietary macronutrients Interaction, Metabolic syndrome
Introduction
Metabolic syndrome (MetS) is a cluster of multiple risk factors, which increase the risk of serious disease. In Iran, the prevalence of MetS is rising with age (1); High triglyceride (TG) and low high-density lipoprotein cholesterol (HDL-C) levels are the most prevalent risk factors of MetS (2, 3). Recent studies have shown that MetS arise from interactions of genetic and environmental factors, particularly dietary factors. The contribution of genetics to the incidence of MetS is estimated to be 10–30%, and it is possible to override the effects of adverse changes in genetics by changing dietary intakes. The cholesteryl ester transfer protein (CETP) gene plays a critical role in determining the plasma lipid profile, especially TG and HDL-C levels. The CETP gene is located in the q21 region of chromosome 16 and spans 25 kilobases genomic DNA encoding 16 exons. This gene yields a protein of 476 amino acids, forming a 74-kDa glycoprotein (4, 5). CETP is a blood glycoprotein that removes cholesteryl esters from HDL towards apolipoprotein B-containing lipoproteins in exchange for TGs. Some studies reported the interaction between CETP genetic variants and dietary variables in relation to lipid profiles. Two meta-analyses found the relationship of CETP polymorphisms with CETP activity inhibition and improving plasma lipid profiles (6, 7). Several interventional studies have documented that CETP gene variations influence the plasma lipid profile in response to dietary factors, particularly dietary fat intake (4, 8-11), although these results were not found in other intervention studies (12-15). In observational studies, there were controversial results regarding the interaction between CETP polymorphisms and dietary fats in relation to HDL-C concentration (16).
Few studies have investigated the possible interaction between CETP variants and macronutrient intakes in Middle-Eastern populations; therefore, the aim of this study is to investigate whether CETP variants rs3764261 and rs5882 modify the relationship of macronutrient intakes and MetS or its components.
Materials and Methods
Study population
Subjects of this nested case-control study were selected from among Tehran Lipid and Glucose Study (TLGS) participants, a population-based prospective study performed to find out risk factors of chronic diseases in a group of residents of District 13 of Tehran, the capital of Iran (2). The first survey of the TLGS was done from 1999 to 2001 on 15005 individuals, aged ≥3 years, and follow-up surveys were performed every 3 years (2002–2005; 2006–2008; 2008–2011, and 2011–2014) to determine recently developed diseases.
The numbers of participants who were aged ≥18 years and showed newly developed MetS during the third, fourth, and fifth phases of TLGS were 1019, 940, and 768, respectively, of which 550 cases were randomly selected. After excluding subjects with a history of more than 5 kg weight change in the previous 6 months, pregnancy, lactation, or individuals who took drugs that affect weight, 500 cases were included in the study. Each case was pair-matched with two controls randomly, based on age (±5 years) and sex of those who did not have MetS at the time that the corresponding case developed MetS. Subjects lacking DNA purification in the range of 1.7<A260/A280<2 and those whose reported energy intake divided by the predicted energy intake did not qualify for the ±3 SD range were excluded from the study participants; finally data of 441 cases and 844 controls were analyzed (Figure 1). Of total participants, 74% were Fars, 12% were Turk, and the others were Arab, Balouch, Kurd, Lur, and Mazani.
Figure 1.
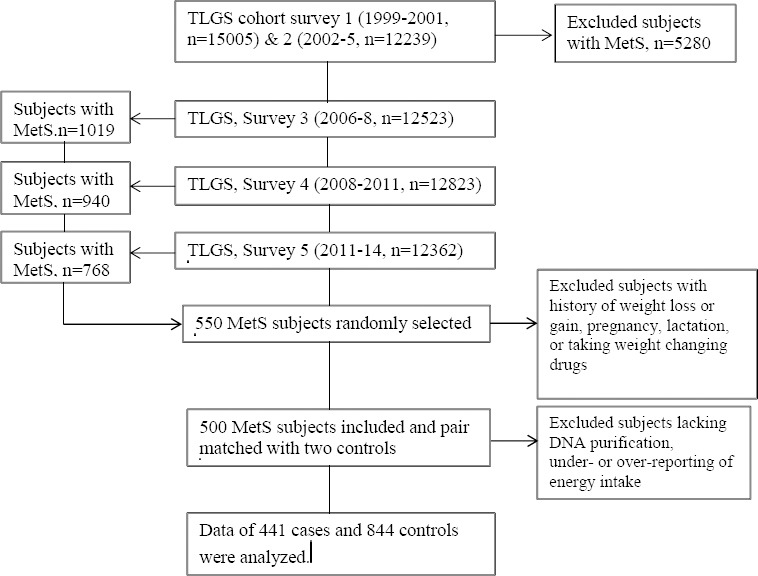
Flowchart of study participants. TLGS: Tehran lipid and glucose Study; MetS: Metabolic Syndrome
Written informed consents were obtained from all participants before they participated in this study. The study was performed in accordance with the Declaration of Helsinki and the study protocol was approved by the ethics committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. All methods were performed in accordance with their relevant guidelines and regulations.
Dietary intakes
Dietary intake was assessed using a valid and reliable semi-quantitative food frequency questionnaire (FFQ), for which expert dietitians collected information on the intake of food items by face-to-face personal interviews. The consumption frequency of each food item was converted to daily intakes using standard serving sizes; portion sizes were then converted to mass (in grams) (17).
Physical activity
Physical activity level was assessed using a highly reliable and relatively valid activity questionnaire, the Persian translated modifiable activity questionnaire (MAQ). It was reported based on metabolic equivalent-h/week (Met/hr/week) (18-20).
Blood pressure and anthropometric measurements
For measuring BP, the participants rested for 15 min and a physician measured BP two times with a 30 sec interval between these two measurements.
Weight was measured to the nearest 100 g, using digital scales (Seca 707, Hamburg, Germany) while the subjects were minimally clothed and not wearing shoes. Height was measured to the nearest 0.5 cm with a tape measure (model 208 Portable Body Meter Measuring Device; Seca) in a standing position and with shoulders in a normal alignment and without shoes. Waist circumference (WC) was taken at the end of a normal expiration, over light clothing, with the un-stretched tape meter at the level of the umbilicus without any pressure to the body surface; measurements were recorded to the nearest 0.1 cm.
Laboratory assays
Blood samples were drawn into vacutainer tubes from subjects who were in a sitting position between 7:00 a.m and 9:00 a.m. after a 12–14 hr overnight fast. Blood samples were centrifuged within 30 to 45 min of collection. All biochemical analyses were performed using a Selectra 2 auto-analyzer at the TLGS research laboratory on the day of blood collection. Fasting blood glucose (FBG) concentration was measured by the enzymatic colorimetric method using the glucose oxidase technique. HDL-C concentration was assessed after precipitation of the apolipoprotein B-containing lipoproteins with phosphotungstic acid. TG level was determined by enzymatic colorimetric tests using glycerol phosphate oxidase and TG kits. Assay performance was monitored once every 20 tests using lipid control serum, Percinorm (normal range) and Percipath (pathological range), where applicable (Boehringer Mannheim; catalog no. 1446070 for Percinorm and 171778 for Percipath). A lipid standard (Cfas, Boehringer Mannheim; catalog no. 759350) was used to calibrate the Selectra 2 auto-analyzer on each day of the laboratory analysis, and all samples were analyzed only when the internal quality control met the standard criteria. Inter- and intra-assay coefficients of variations were both 2.2% for serum glucose and 1.6% and 0.6% for TG, respectively (2).
Genetic analysis
Genomic DNA was extracted from the buffy-coat of samples using a proteinase K/salting out standard method. A Thermo Scientific NanoDrop 1000 Spectrophotometer was used for qualitative estimation of the extracted DNA. Samples lacking DNA purification in the range of 1.7<A260/A280<2 were excluded due to low quality and concentration.
Portions of DNA samples were genotyped with HumanOmniExpress-24-v1-0 bead chips (containing 649,932 SNP loci with an average mean distance of 4 kb) at the deCODE genetics company (Reykjavik, Iceland) according to the manufacturer’s specifications (Illumina Inc., San Diego, CA, USA). PLINK program (V 1.07) and R statistic (V 3.2) were used for quality control procedures with the total genotyping rate of 0.9774. After quality control procedures, the genotyping data of two CETP polymorphisms (rs5882 and rs3764261) was used for data analysis (21).
Definitions
Subjects with three or more of the following conditions were considered to have MetS based on the Iranian modified National Cholesterol Education Program/Adult Treatment Panel III (22, 23): (1) HDL-C <1.30 mmol/l (<50 mg/dl) in women, and <1.04 mmol/l (<40 mg/dl) in men or drug treatment; (2) TG ≥1.70 mmol/l (≥150 mg/dl) or drug treatment; (3) FBG≥6.11 mmol/l (≥110 mg/dl) or drug treatment for hyperglycemia; (4) BP ≥130/85 mmHg or drug treatment; (5) WC≥95 cm for men and women.
Statistical analyses
Statistical analyses were performed using the STATA (Statistics/Data analysis 12.0) and the Statistical Package for Social Sciences (version 21.0; SPSS); a P-value <0.05 was considered statistically significant. The Student’s t-test and χ2 test were used for quantitative and qualitative variables, respectively to compare the characteristics of cases and controls. The Pearson’s χ2 statistic and Power Marker software were used to determine the Hardy–Weinberg equilibrium for SNPs and the differences in percentages, respectively. In the case of non-normal nutritional and biochemical variables (TG concentration), log-transformed values were used for statistical analysis. Participants were divided into eight groups based on the macronutrient intakes (carbohydrate, protein, and fats) and harboring of AA/AG+GG (rs5882) or CC/CA+AA (rs3764261) genotypes; intake of the first quartile of macronutrients (as a percentage of energy) and harboring of the AA or CC genotypes were considered as the reference. The multiplicative interaction between polymorphisms (dominant model) and quartiles of macronutrient intakes with the risk of MetS were examined using the likelihood ratio test. Conditional logistic regression analyses were used to examine the combined role of quartiles of macronutrient intakes and genotypes in predicting MetS risk, which were adjusted for education levels (>14 and ≤14 years), smoking (never smoked, ex-smoker, and current smoker), and body mass index (BMI). Logistic regression was performed to estimate the interactions of SNPs with quartiles of dietary macronutrients in relation to components of MetS risk. The P-value for trend across the quartiles of dietary macronutrients was determined using logistic regression, with the median of each quartile of dietary macronutrients as a continuous variable.
Results
The mean ages were 38.1±10 and 37.0±10 years in female patients and controls and 36.2±11 and 36.3±11 years in male patents and controls, respectively at baseline. The controls had a lower mean BMI than the cases (Table 1). Genotype frequency did not deviate from the Hardy–Weinberg equilibrium (Table 2).
Table 1.
Characteristics of the subjects with the metabolic syndrome (MetS) and the controls: the Tehran lipid and glucose study
| Control without MetS | Case with MetS | P | |
|---|---|---|---|
| Baseline age (years) | 36.8±11 | 37.3±11 | 0.46 |
| Men (n=653) | 36.3±11 | 36.2±11 | 0.8 |
| Women (n=643) | 37.0±11 | 38.1±11 | 0.4 |
| Current smokers (%) | 46 | 38 | 0.02 |
| Low physical activity (%) | 37 | 40 | 0.25 |
| Education level ≥14 years (%) | 22.4 | 17.4 | 0.04 |
| Baseline BMI (kg/m2) | 24.1±4 | 26.0±3 | <0.001 |
| Obesity (%)† | 59.6±7 | 72.3±16 | <0.001 |
| Baseline WC (cm) | 79.0±12 | 85.3±11 | <0.001 |
| Abdominal obesity (%)† | 54(7) | 66(15) | <0.001 |
| Baseline systolic BP (mmHg) | 110±12 | 115±13 | <0.001 |
| Baseline diastolic BP (mmHg) | 73.0±8 | 76.2±8 | <0.001 |
| Elevated BP (%)† | 98(11) | 77(17) | 0.005 |
| Baseline HDL-C (mmol/l) | 45.3±11 | 40.2±9 | <0.001 |
| Low HDL-C (%)† | 474(56) | 313(71) | <0.001 |
| Baseline TG (mmol/l) | 126±71 | 153±94 | <0.001 |
| High TG (%)† | 228(27) | 161(36) | <0.001 |
| Baseline FBG (mmol/l) | 86.4±10 | 90.2±10 | <0.001 |
| High FBG (%)† | 41(5) | 37(8) | 0.01 |
| Energy intake (kcal/d) | 2837±1117 | 2848±1204 | 0.87 |
| Carbohydrate (% of energy) | 59.6±13 | 59.5±13 | 0.85 |
| Protein (% of energy) | 15.3±14 | 14.2±2 | 0.39 |
| Total fat (% of energy) | 31.8±32 | 29.2±5 | 0.27 |
| PUFA | 23.7±10 | 18.2±9 | 0.25 |
| MUFA | 11.3±4 | 9.8±2 | 0.33 |
| Trans-fatty acids | 1.4±1 | 1.4±2 | 0.31 |
| Cholesterol | 234±125 | 226±114 | 0.27 |
| Omega3 fatty acids | 4.8±98 | 0.51±0.83 | 0.33 |
| Dietary energy density (kcal/g) | 0.96±0.20 | 0.95±0.18 | 0.25 |
WC, waist circumference; BP, blood pressure; HDL-C, HDL-cholesterol; TG, Triglycerides; FBG, fasting blood glucose; PUFA, Polyunsaturated fatty acids; MUFA, Monounsaturated fatty acids; * Mean value was significantly different from that of the control group (P<0.05)
Table 2.
Genotype and allele frequency of rs3764261 and rs5882CETP polymorphisms in subjects with the metabolic syndrome (MetS) and controls: the Tehran lipid and glucose study
| Case with MetS | Control without MetS | |
|---|---|---|
| Allele frequency rs3764261 n(%) | ||
| C | 543(62.7) | 1053(62.9) |
| A | 323(37.3) | 621(37.1) |
| Allele frequency rs5882 n(%) | ||
| A | 530(60.4) | 1037(62.1) |
| G | 348(39.6) | 633(37.9) |
| Genotype frequency rs3764261 n(%) | ||
| CC | 167(39) | 339(40) |
| CA | 209(48) | 375(45) |
| AA | 57(13) | 123(15) |
| Genotype frequency rs5882 n(%) | ||
| AA | 161(37) | 316(38) |
| AG | 208(47) | 405(48) |
| GG | 70(16) | 114(14) |
The interaction between the risk of MetS and CETP SNPs across the quartiles of dietary macronutrients was not significant (data not shown);
The risk of low HDL-C was not uniform among the CETP rs5882 genotypes across quartiles of MUFA intake (Figure 2); the risk of low HDL-C was lower in the first quartile of dietary MUFA in the G allele carriers (AA genotype, OR: 1, 0.61, 0.62, 0.68, Ptrend=0.19; and AG+GG genotype, OR:0.49, 0.66, 0.88, 0.66, Ptrend=0.12, Pinteraction=0.02), compared to AA genotype group.
Figure 2.
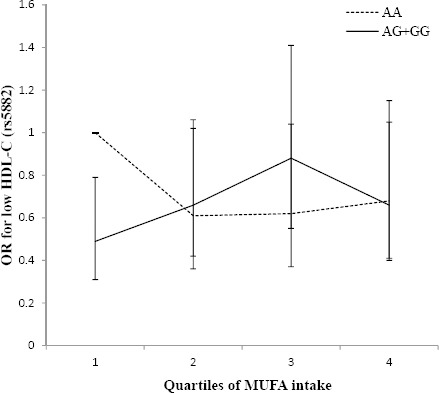
Adjusted OR (95% CI) for low HDL-C across quartiles of monounsaturated fatty acids (MUFA) by the rs5882 genotypes (Pinteraction=0.02; Q1<8.4, Q2:8.4–9.5, Q3:9.6–10.9, Q4>11% of energy). The risk of low HDL-C was lower in the first quartile of MUFA intake in the G allele carriers (Ptrend for the AA and AG+GG genotypes are 0.19 and 0.12, respectively)
Furthermore, an interaction between the rs5882 polymorphism and total fat intake in relation to risk of low HDL-C was observed (Figure 3); the G allele carriers had lower odds of low HDL-C in the first quartile of total fat intake (AA genotype, OR: 1, 0.53, 0.65, 0.70, Ptrend=0.3; and AG+GG genotype, OR: 0.50, 0.69, 0.91, 0.68, Ptrend=0.04, Pinteraction=0.05), compared to the AA genotype group.
Figure 3.
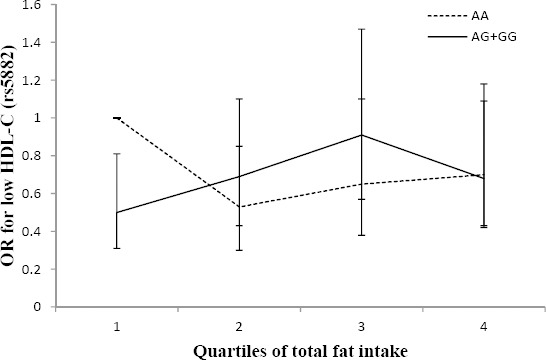
Adjusted OR for low HDL-C across quartiles of total fat intake by the rs5882 genotypes (Pinteraction=0.05; Q1<26, Q2:26.1–29.4, Q3:29.5–33, Q4>33.1% of energy). The G allele carriers had a lower odds ratio of low HDL-C in the first quartile of total fat intake (Ptrend for AA and AG+GG genotypes are 0.3 and 0.04, respectively)
G allele carriers (mutated allele) had a higher risk of low HDL-C with higher intakes of MUFA (9.6–11% of total energy intake) and total fat (29.5–33% of total energy intake) in comparison with the AA genotype group. Also in G allele carriers, the risk of low HDL-C decreased when dietary MUFA was less than 8.5% of total energy intake and dietary total fat was less than 26% of total energy, compared to the AA genotype group.
The interaction between the high BP risk and CETP genotypes across quartiles of trans-fatty acid intake was significant (Pinteraction=0.04) (Figure 4); the risk of high BP increased significantly in higher quartiles of trans-fatty acid intake (>1.81% of total energy intake) in G allele carriers (OR: 0.89, 0.80, 0.70, 1.49, Ptrend=0·005) compared with the AA genotype group who had lower odds of high BP in higher quartiles of trans-fatty acid intake (OR: 1, 0.77, 1.01, 0.54, Ptrend=0.04).
Figure 4.
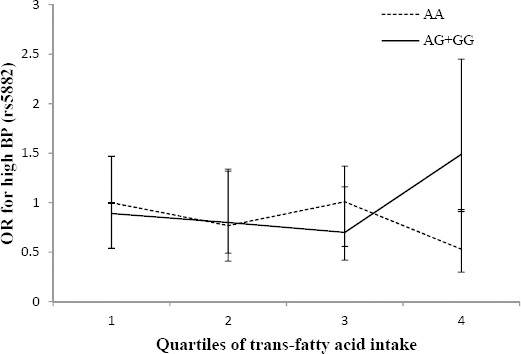
Adjusted OR for high blood pressure (BP) across quartiles of trans-fatty acid intake by the rs5882 genotypes (Pinteraction=0.04; Q1<0.73, Q2:0.73–1.14, Q3:1.15–1.80, Q4>1.81% of energy). The risk of high BP appeared to increase significantly in higher quartiles of trans-fatty acid intake in G allele carriers (Ptrend=0.005) compared with AA genotype carriers who had lower odds of high BP in higher quartiles of trans-fatty acid intake (Ptrend=0.04)
The examination of other components of MetS did not reveal an interaction between genetic variants of CETP and macronutrient intakes.
Discussion
The effect of interactions of macronutrient intakes and genetic variants of CETP rs5882 and rs3764261on MetS risk and its components was assessed in a group of Tehrani adults. The macronutrients did not show an association with CETP genotypes in relation to MetS risk, whereas MUFA and total fat intake showed an association with the rs5882 genotypes in relation to the risk of low HDL-C. A significant association was found between trans-fatty acid intake and the rs5882 genotype in relation to high BP risk. G allele carriers may be more susceptible than wild-type homozygous carriers to low HDL-C and high BP when dietary fat is high.
In this study, no association was found between CETP rs3764261 genotypes and dietary intakes with MetS risk or its components; however, in another interventional study, there were differences in the increment of HDL-C levels and decrease in TG levels after consumption of a high-fat diet versus low-fat diet among carriers of the CC genotype, while carriers of the A allele did not show any significant differences (4).
The association between CETP rs5882 genotypes and dietary intakes, especially fat intake, in determining lipid profiles have been examined in previous studies. An interventional study reported no rs5882 effects on plasma lipid and lipoprotein levels in response to dietary fat (14). Rudkowska et al. (24) demonstrated that total cholesterol levels were higher in GG homozygotes when consuming a high-fat diet. Moreover, Darabi et al. (10) reported that G allele carriers had a significant reduction in HDL-C levels compared to AA genotype carriers, after a low poly-unsaturated:saturated fatty acid ratio diet. Based on our findings and previous studies, it seems that the CETP rs5882 gene variation modifies the influence of fat intake on plasma lipids.
However, the definitive mechanisms underlying the observed gene–nutrient interactions are still undefined, suggesting that CETP gene expression and activity may be regulated by the quality and quantity of dietary fat. An animal study showed that transgenic mice expressing human CETP in a high-fat diet group had higher hepatic CETP mRNA concentrations, CETP activity, and CETP mass than these parameters in the low-fat diet group (4, 25).
No previous study has examined the effect of CETP variants and dietary variables on high BP risk, thus comparing the magnitude of the effect in the present study with previous studies is not possible.
The strengths of this study include matched controls for all segments of the population and its longitudinal design. The findings of this study also emphasize the importance of regarding the effect of gene-diet interaction in association studies. However, food intakes at only one time point were considered. Replication studies on particular populations are needed in order to demonstrate these relationships.
Conclusion
The findings of this study indicated that individuals with the AG+GG genotypes of rs5882 exhibit a significant increment in HDL-C and reduction in BP with lower intakes of total dietary fat, MUFA, and trans-fatty acids compared to individuals with the AA genotype.
Acknowledgment
The results presented in this paper were part of a student thesis. This study was supported by Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran (grant no. 834).
References
- 1.Delavari A, Forouzanfar MH, Alikhani S, Sharifian A, Kelishadi R. First nationwide study of the prevalence of the metabolic syndrom and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes care. 2009;32:1092–1097. doi: 10.2337/dc08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azizi F, Ghanbarian A, Momenan AA, Hadaegh F, Mirmiran P, Hedayati M, et al. Prevention of non-communicable disease in a population in nutrition transistion: Tehran Lipid and Glucose Study phase II. Trials. 2009;10:5. doi: 10.1186/1745-6215-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosseini-Esfahani F, Jessri M, Mirmiran P, Bastan S, Azizi F. Adherence to dietary recommendations and risk of metabolic syndrome: Tehran Lipid and Glucose Study. Metab Clin Exp. 2010;59:1833–1842. doi: 10.1016/j.metabol.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Qi Q, Durst R, Schwarzfuchs D, Leitersdorf E, Shpitzen S, Li Y, et al. CETP genotype and changes in lipid levels in response to weight-loss diet intervention in the POUNDS LOST and DIRECT randomized trials. J Lipid Res. 2015;56:713–721. doi: 10.1194/jlr.P055715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips CM. Nutrigenetics and metabolic disease: current status and implications for personalised nutrition. Nutrients. 2013;5:32–57. doi: 10.3390/nu5010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson A, Di Angelantonio E, Sarwar N, Erqou S, Saleheen D, Dullaart RP, et al. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 2008;299:2777–2788. doi: 10.1001/jama.299.23.2777. [DOI] [PubMed] [Google Scholar]
- 7.Boekholdt SM, Sacks FM, Jukema JW, Shepherd J, Freeman DJ, McMahon AD, et al. Cholesteryl ester transfer protein TaqIB variant, high-density lipoprotein cholesterol levels, cardiovascular risk, and efficacy of pravastatin treatment: individual patient meta-analysis of 13,677 subjects. Circulation. 2005;111:278–287. doi: 10.1161/01.CIR.0000153341.46271.40. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Rios A, Alcala-Diaz JF, Gomez-Delgado F, Delgado-Lista J, Marin C, Leon-Acuna A, et al. Beneficial effect of CETP gene polymorphism in combination with a Mediterranean diet influencing lipid metabolism in metabolic syndrome patients: CORDIOPREV study. Clin Nutr. 2018;37:229–234. doi: 10.1016/j.clnu.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Mackay DS, Eck PK, Rideout TC, Baer DJ, Jones PJ. Cholesterol ester transfer protein polymorphism rs5882 is associated with triglyceride-lowering in response to plant sterol consumption. Appl Physiol Nutr Metab. 2015;40:846–849. doi: 10.1139/apnm-2015-0039. [DOI] [PubMed] [Google Scholar]
- 10.Darabi M, Abolfathi A, Noori M, Kazemi A, Ostadrahimi A, Rahimipour A, et al. Cholesteryl ester transfer protein I405V polymorphism influences apolipoprotein AI response to a change in dietary fatty acid composition. Appl Physiol Nutr Metab. 2009;41:554–558. doi: 10.1055/s-0029-1192034. [DOI] [PubMed] [Google Scholar]
- 11.Terán-García M, Després J-P, Tremblay A, Bouchard C. Effects of cholesterol ester transfer protein (CETP) gene on adiposity in response to long-term overfeeding. Atherosclerosis. 2008;196:455–460. doi: 10.1016/j.atherosclerosis.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frances E, Carrasco P, Sorli JV, Ortega C, Portoles O, Rubio MM, et al. Impact of APOE, APOA5 and CETP polymorphism on plasma lipid concentrations and response to a mediterranean diet in the predimed study. Atherosclerosis Suppl. 2006;7:47. [Google Scholar]
- 13.Aitken WAE, Chisholm AWAH, Duncan AW, Harper MJ, Humphries SE, Mann JI, et al. Variation in the cholesteryl ester transfer protein (CETP) gene does not influence individual plasma cholesterol response to changes in the nature of dietary fat. Nutr Metab Cardiovasc Dis. 2006;16:353–363. doi: 10.1016/j.numecd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Friedlander Y, Leitersdorf E, Vecsler R, Funke H, Kark J. The contribution of candidate genes to the response of plasma lipids and lipoproteins to dietary challenge. Atherosclerosis. 2000;152:239–248. doi: 10.1016/s0021-9150(99)00474-8. [DOI] [PubMed] [Google Scholar]
- 15.Wallace AJ, Humphries SE, Fisher RM, Mann JI, Chisholm A, Sutherland WH. Genetic factors associated with response of LDL subfractions to change in the nature of dietary fat. Atherosclerosis. 2000;149:387–394. doi: 10.1016/s0021-9150(99)00328-7. [DOI] [PubMed] [Google Scholar]
- 16.Li TY, Zhang C, Asselbergs FW, Qi L, Rimm E, Hunter DJ, et al. Interaction between dietary fat intake and the cholesterol ester transfer protein TaqIB polymorphism in relation to HDL-cholesterol concentrations among US diabetic men. Am J Clin Nutr. 2007;86:1524–1529. doi: 10.1093/ajcn/86.5.1524. [DOI] [PubMed] [Google Scholar]
- 17.Mirmiran P, Hosseini Esfahani F, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran Lipid and Glucose Study. Public Health Nutr. 2010;13:654–662. doi: 10.1017/S1368980009991698. [DOI] [PubMed] [Google Scholar]
- 18.Delshad M, Ghanbarian A, Ghaleh NR, Amirshekari G, Askari S, Azizi F. Reliability and validity of the modifiable activity questionnaire for an Iranian urban adolescent population. Int J Prev Med. 2015;6:3. doi: 10.4103/2008-7802.151433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes care. 1990;13:401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 21.Daneshpour MS, Fallah MS, Sedaghati-Khayat B, Guity K, Khalili D, Hedayati M, et al. Rationale and Design of a Genetic Study on Cardiometabolic Risk Factors. Protocol for the Tehran Cardiometabolic Genetic Study (TCGS) 2017;6:e28. doi: 10.2196/resprot.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundy SM, Hansen B, Smith SC, Cleeman JI, Kahn RA. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler Thromb Vasc Biol. 2004;24:e19–24. doi: 10.1161/01.ATV.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 23.Azizi F, Hadaegh F, Khalili D, Esteghamati A, Hosseinpanah F, Delavari A, et al. Appropriate definition of metabolic syndrome among Iranian adults: report of the Iranian National Committee of Obesity. Arch Iran Med. 2010;13:426–428. [PubMed] [Google Scholar]
- 24.Rudkowska I, Dewailly E, Hegele RA, Boiteau V, Dube-Linteau A, Abdous B, et al. Gene-diet interactions on plasma lipid levels in the Inuit population. Br J Nutr. 2013;109:953–961. doi: 10.1017/S0007114512002231. [DOI] [PubMed] [Google Scholar]
- 25.Cheema SK, Agarwal-Mawal A, Murray CM, Tucker S. Lack of stimulation of cholesteryl ester transfer protein by cholesterol in the presence of a high-fat diet. J Lipid Res. 2005;46:2356–2366. doi: 10.1194/jlr.M500051-JLR200. [DOI] [PubMed] [Google Scholar]


