Abstract
Background
Outcomes in peripartum cardiomyopathy (PPCM) vary. We sought to determine whether severity of left or right ventricular dysfunction (RVD) at PPCM diagnosis differentially associates with adverse outcomes.
Methods and Results
We conducted a single‐center retrospective cohort study of 53 patients with PPCM. The primary outcome was a composite of left ventricular assist device implantation, cardiac transplantation, or death. We used Kaplan‐Meier curves to examine event‐free survival and Cox proportional hazards models to examine associations of left ventricular (LV) ejection fraction <30%, LV end‐diastolic diameter ≥60 mm, and moderate‐to‐severe RVD at PPCM diagnosis with the primary outcome. Median (interquartile range) follow‐up time was 3.6 (1.4–7.3) years. Seventeen patients (32%) experienced the primary outcome, of whom 11 had moderate‐to‐severe RVD at time of PPCM diagnosis. Overall event‐free survival differed by initial RVD severity and LV ejection fraction <30%, but not by LV end‐diastolic diameter ≥60 mm. In univariable analyses, LV ejection fraction <30% and moderate‐to‐severe RVD were associated with the outcome (hazard ratios [95% confidence intervals] of 4.85 [1.11–21.3] and 4.26 [1.47–11.6], respectively). In a multivariable model with LV ejection fraction <30%, LV end‐diastolic diameter ≥60 mm, and moderate‐to‐severe RVD, only moderate‐to‐severe RVD was independently associated with the outcome (hazard ratio [95% confidence interval], 3.21 [1.13–9.10]). Although most outcomes occurred within the first year, nearly a third occurred years after PPCM diagnosis.
Conclusions
Initial moderate‐to‐severe RVD is associated with a more advanced cardiomyopathy phenotype and increased risk of adverse outcomes in PPCM, within and beyond the first year of diagnosis. By identifying a worse PPCM phenotype, initial moderate‐to‐severe RVD may prompt earlier consideration of advanced heart replacement therapies.
Keywords: long‐term outcome, peripartum cardiomyopathy, right ventricular dysfunction
Subject Categories: Cardiomyopathy, Women, Mortality/Survival, Heart Failure, Pregnancy
Clinical Perspective
What Is New?
Initial moderate‐to‐severe right ventricular (RV) dysfunction at peripartum cardiomyopathy (PPCM) presentation independently predicts major adverse clinical outcomes, specifically left ventricular assist device implantation, cardiac transplantation, and death.
Moderate‐to‐severe RV dysfunction is more predictive than either left ventricular ejection fraction <30% or left ventricular end‐diastolic diameter ≥60 mm of major adverse clinical outcomes in PPCM, even after controlling for severity of mitral regurgitation.
The increased risk of poor outcomes that is associated with biventricular dysfunction in PPCM is highest within the first year of diagnosis but also persists beyond then.
Normalization of RV function was strongly associated with event‐free survival.
What Are the Clinical Implications?
Our findings support the importance of initial RV functional assessment in PPCM as a tool for short‐ and long‐term risk stratification.
Identification of this more severe PPCM phenotype with biventricular dysfunction may prompt early referral for advanced heart failure therapies and closer follow‐up.
Further studies are needed to assess the potential for RV functional recovery among patients with PPCM who present with moderate‐to‐severe RV dysfunction. Identification of RV functional recovery potential would provide additional long‐term risk stratification of patients with PPCM.
Introduction
Peripartum cardiomyopathy (PPCM) is a major cause of maternal morbidity and mortality in the United States,1, 2 with an increasing incidence during the past 25 years from 1:4350 to 1:1000 live births.1, 3 Characterized by the development of marked left ventricular (LV) systolic dysfunction and heart failure (HF) during the final month of pregnancy or the first 5 postpartum months, PPCM is generally considered an idiopathic form of cardiomyopathy. Outcomes in PPCM are strikingly heterogeneous. As many as 37% to 62% of patients with PPCM with LV ejection fraction (LVEF) ≤30% at diagnosis recover cardiac function within 1 year.4, 5, 6 LV functional recovery in PPCM usually occurs within 6 months, but some cases of recovery occur up to 5 years later.7, 8, 9 Those who do not recover can progress to advanced HF, requiring heart replacement therapy. PPCM accounts for 5% of all women who undergo cardiac transplantation and 8% who undergo mechanical circulatory support device implantation in the United States.10, 11 Despite the potential for late adverse outcomes, prior PPCM studies have largely focused on in‐hospital outcomes2, 12, 13 or those within the first year of diagnosis.6, 12, 13, 14, 15 Severe LV systolic dysfunction and LV dilatation have long been identified as major physiologic risk factors for adverse outcomes in PPCM.4, 6, 9, 13, 14, 16, 17, 18, 19, 20 Right ventricular (RV) systolic function at presentation was recently identified as a strong independent predictor of LV recovery and clinical events at 1 year.15
Given the heterogeneity of PPCM outcomes, particularly with regard to time to event, we hypothesized that the severity of RV dysfunction (RVD) at diagnosis may identify a severe phenotype of PPCM. Therefore, we conducted a single‐center retrospective cohort study to determine whether RVD severity is differentially associated with adverse outcomes and time to adverse outcome. Understanding how RV and LV dysfunction may interact to affect adverse outcomes would help clinicians identify those most in need of aggressive initial medical management with earlier consideration of advanced therapies versus those who might benefit from longer‐term follow‐up.
Methods
The deidentified data, analytic methods, and study materials that support the findings of this study are available from the corresponding author on reasonable request.
Study Design and Subjects
We conducted a retrospective cohort study of women who were cared for at Temple University Hospital (Philadelphia, PA) between January 1, 1992 and February 29, 2016, who were diagnosed with new‐onset PPCM. Cases were identified by a query of the medical record for any of the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD‐9‐CM) codes for PPCM. PPCM is defined as developing LV systolic dysfunction (LVEF ≤45%) between the last trimester and 5 months postpartum without any other probable cause. We included consecutive women who were ≥18 years of age and had both a baseline and follow‐up echocardiogram. We excluded those patients who had been diagnosed with PPCM before their initial encounter at Temple University Hospital (n=3). The study protocol was approved by the Temple University Institutional Review Board, and informed consent requirement was waived.
Measurements
Echocardiographic data were obtained from detailed abstraction from clinical echocardiographic reports. Echocardiograms were performed in accordance with contemporary clinical guidelines,21, 22, 23 on Philips CX50, Philips iE33, GE Vivid E9, and HP Sonos 5500 machines. We assessed echocardiographic parameters at PPCM diagnosis, including LVEF that we categorized as severely reduced if LVEF values were <30%; LV end‐diastolic diameter (LVEDD) that we categorized as dilated if LVEDD values were ≥60 mm; mitral regurgitation (MR) and tricuspid regurgitation that were categorized as none, mild, moderate, or severe; estimated RV systolic pressure; and RVD that was categorized as none, mild, moderate, or severe. The assessment of global RV function was qualitatively made on the basis of visualization of the RV from multiple imaging planes. Internationally endorsed guidelines for quantitative echocardiographic assessment of RV systolic function were established in 201024; however, most baseline echocardiograms (83%) predated this guideline. Hence, we used the qualitative grading of RVD included in echocardiogram reports. To assess the construct validity of the qualitative visual grading of RVD severity, we examined available invasive hemodynamic measurements also performed at diagnosis.
For participants who underwent right‐sided heart catheterization at time of PPCM diagnosis, we ascertained right atrial (RA) pressure, RV pressure, pulmonary artery pressure, mean pulmonary capillary wedge pressure (PCWP), diastolic pulmonary gradient (the difference between pulmonary artery diastolic pressure and PCWP), pulmonary vascular resistance, cardiac output, and cardiac index. For hemodynamic indexes of RV dysfunction, we calculated the ratio of mean RA pressure/mean PCWP,25, 26 the ratio of stroke volume index/mean RA pressure,27 and the pulmonary artery pulsatility index.28, 29 The pulmonary artery pulsatility index was calculated as the pulmonary artery pulse pressure/mean RA pressure.
We ascertained from the medical record at time of PPCM diagnosis each participant's age, race/ethnicity, body mass index (kg/m2), number of pregnancies and live births, and presence of diabetes mellitus, hypertension, or hyperlipidemia.
We reviewed each participant's subsequent echocardiograms during the study period. We defined persistence of severe LV dysfunction as an LVEF <30% on the final echocardiogram during the study period, LV functional recovery as an LVEF ≥50% on follow‐up echocardiogram, and RV functional recovery as improvement from initial moderate‐to‐severe RVD to normal RV function on follow‐up echocardiogram. The primary composite outcome was defined as LV assist device (LVAD) implantation, cardiac transplantation, or death.
Statistical Analysis
Continuous variables were expressed as mean and SD or median and interquartile range (IQR), as appropriate for the data distributions. Means and medians were compared using Student t test or Wilcoxon rank sum test, respectively. Categorical variables were expressed as percentages and compared using the χ2 or Fisher's exact test. Among those who had LV functional recovery, we assessed the time to recovery from the date of echocardiographic PPCM diagnosis to the first follow‐up echocardiogram during the study period with an LVEF ≥50%.
We first tested the construct validity of the visual qualitative echocardiographic assessment of global RV function by comparing right heart catheterization measurements across the echocardiographically determined categories of RVD severity.
We next examined how event‐free survival varied by RVD severity, by LVEF <30%, and by LVEDD ≥60 mm using Kaplan‐Meier curves. We tested the equality of survival distributions using the log‐rank test. Survival time was measured from the date of echocardiographic PPCM diagnosis to the date of LVAD implantation, cardiac transplantation, or death, with right censoring at the date of the last echocardiogram during the study period for those who did not experience the primary composite outcome.
We examined associations between categories of LV and RV dysfunction and adverse outcomes. We used Cox proportional hazards models to examine associations of LV dysfunction and RV dysfunction with time to the primary composite outcome. We categorized RV dysfunction as none to mild and moderate to severe because the Kaplan‐Meier curves showed that each respective category of RV grades had similar observed event‐free survival and because we sought to avoid overparameterizing our multivariable model given our limited number of participants and events. We first tested univariable associations of moderate‐to‐severe RVD, LVEF <30%, LVEDD ≥60 mm, and severity of MR (mild, moderate, or severe) with the primary composite outcome. For the first multivariable model, we included all parameters that were individually associated with the primary composite outcome at P<0.2. However, there was evidence of collinearity between LVEF <30% and LVEDD ≥60 mm given that their effect estimates were largely reduced when both variables were included in the model, and because LVEF <30% was associated with LVEDD ≥60 mm (χ2 P=0.002). Therefore, we constructed additional multivariable Cox models separately combining the moderate‐to‐severe RVD variable with LVEF <30% or LVEDD ≥60 mm. We also constructed multivariable Cox models to test whether the association between RV dysfunction and outcomes is independent of MR grade and time period. Specifically, we created a time period indicator variable based on whether the date of PPCM diagnostic echocardiogram was before or after July 12, 2006. This date marked when the United Network of Organ Sharing implemented, within our study period, the latest change in its donor heart allocation algorithm.30
Statistical significance was defined as a 2‐tailed P<0.05. Statistical analyses were performed using Stata14.0 (Stata‐Corp LP, College Station, TX) and SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient Characteristics
A total of 54 women had new‐onset PPCM, but 1 was excluded for lack of any follow‐up echocardiogram. The 53 women with new‐onset PPCM in the study cohort had a mean±SD age of 31±7.6 years and a median (IQR) body mass index of 30 (24–34) kg/m2; 75% were black. They had median (IQR) 3 (1–5) prior pregnancies and median 2 (1–4) prior births. Approximately one quarter had diabetes mellitus and hyperlipidemia, and 60% had hypertension. Of the PPCM study cohort, 40% had moderate‐to‐severe RVD on initial echocardiogram. Clinical characteristics were similar between subjects with initial moderate‐to‐severe RVD versus those with no‐to‐mild RVD (Table 1). However, subjects with moderate‐to‐severe RVD at PPCM diagnosis had lower LVEF (median [IQR], 12.5% [7.5%–12.5%] versus 32.5% [18%–40%]), larger LVEDD (median [IQR], 68 [60–72] versus 59 [55–66] mm), more severe MR and tricuspid regurgitation, and higher estimated RV systolic pressure (mean±SD, 44±12 versus 36±12 mm Hg) (Table 2).
Table 1.
Clinical Characteristics by RVD Severity
| Characteristic | All | None‐Mild RVD | Moderate‐Severe RVD | P Value |
|---|---|---|---|---|
| (N=53) | (n=32) | (n=21) | ||
| Age, mean±SD, y | 31±7.6 | 29.5±6.4 | 33.2±8.9 | 0.080 |
| BMI, median (IQR), kg/m2 | 30.0 (23.5–34.3) | 31.6 (25.3–35.4) | 25.8 (22.1–31.7) | 0.052 |
| Race/ethnicity, n (%) | 0.21 | |||
| Black | 40 (75) | 24 (75) | 16 (76) | |
| White | 6 (11) | 2 (6.3) | 4 (19) | |
| Hispanic | 4 (7.6) | 4 (13) | 0 (0) | |
| Unknown | 3 (5.7) | 2 (6.3) | 1 (4.8) | |
| Obstetric history, median (IQR) | ||||
| Gravidity | 3 (1–5) | 3 (2–4) | 2.5 (1–5) | 0.77 |
| Parity | 2 (1–4) | 2 (1–4) | 1.5 (1–4.5) | 0.76 |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 14 (26) | 7 (22) | 7 (33) | 0.22 |
| Hypertension | 32 (60) | 21 (66) | 11 (52) | 0.64 |
| Hyperlipidemia | 15 (28) | 9 (28) | 6 (33) | 0.65 |
BMI indicates body mass index; IQR, interquartile range; RVD, right ventricular dysfunction.
Table 2.
Echocardiographic Parameters by RVD Severity
| Measurement | All (N=53) | None‐Mild RVD (n=32) | Moderate‐Severe RVD (n=21) | P Value |
|---|---|---|---|---|
| LVEF, median (IQR), % | 18 (13–35) | 32.5 (18–40) | 12.5 (7.5–12.5) | <0.001 |
| LVEDD, median (IQR), mm | 63 (56–69) | 59 (55–66) | 68 (60–72) | 0.03 |
| MR, n (%) | n=53 | n=32 | n=21 | |
| None | 7 (13) | 7 (22) | 0 (0) | 0.013 |
| Mild | 21 (40) | 14 (44) | 7 (33) | |
| Moderate | 16 (30) | 9 (28) | 7 (33) | |
| Severe | 9 (17) | 2 (6.3) | 7 (33) | |
| TR, n (%) | n=51 | n=30 | n=21 | |
| None | 8 (16) | 7 (23) | 1 (4.8) | 0.003 |
| Mild | 23 (45) | 17 (57) | 6 (29) | |
| Moderate | 16 (31) | 6 (20) | 10 (48) | |
| Severe | 4 (7.8) | 0 (0) | 4 (19) | |
| RVSP, Mean±SD (n), mm Hg | 40±13 (42) | 36±12 (23) | 44±12 (19) | 0.03 |
| LVEF <30%, n (%) | 35 (66) | 15 (47) | 20 (95) | <0.001 |
| LVEDD ≥60 mm, n (%) | 28 (60) | 13 (48) | 15 (75) | 0.059 |
Percentage distributions may not add up to 100% because of rounding to the nearest percentage. IQR indicates interquartile range; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; RVD, right ventricular dysfunction; RVSP, right ventricular systolic pressure; TR, tricuspid regurgitation.
Outcomes and Follow‐Up
The median (IQR) follow‐up time was 3.6 (1.4–7.3) years. A total of 17 patients (32%) experienced the primary composite outcome, of whom 11 (65%) had initial moderate‐to‐severe RVD (Table 3). The median (IQR) time to outcome was 0.27 (0.04–0.53) years for those with initial moderate‐to‐severe RVD and 7.6 (3.1–10.5) years for those with no‐to‐mild RVD (P=0.003).
Table 3.
Adverse Outcomes by RVD Severity
| Outcomes | All (N=53) | None‐Mild RVD (n=32) | Moderate‐Severe RVD (n=21) | P Value |
|---|---|---|---|---|
| Those with outcome, n (%) | 17 (32) | 6 (19) | 11 (52) | 0.01 |
| Time to outcome, median (IQR), y | 0.48 (0.23–3.1) | 7.6 (3.1–10.5) | 0.27 (0.04–0.53) | 0.003 |
| Type of outcome, n (% of outcomes) | ||||
| LVAD implantation | 1 (1.9) | 1 (3.1) | 0 (0) | |
| Cardiac transplantation | 15 (28) | 4 (13) | 11 (52) | |
| Death | 1 (1.9) | 1 (3.1) | 0 (0) | |
IQR indicates interquartile range; LVAD, left ventricular assist device; RVD, right ventricular dysfunction.
Among the remaining 36 participants (68%) who did not experience the primary composite outcome, 19 had LV functional recovery a median (IQR) 2.9 (1.7–3.8) years later, with only 1 participant having LV recovery within the first year. Seven had persistent severe LV dysfunction (LVEF <30%) for a median (IQR) 2.5 (2.5–10.8) years of follow‐up. Thus, the overall LV functional recovery rate was 35.8%, and the overall rate of persistent severe LV dysfunction was 13.2%.
Of the 21 patients who had initial moderate‐to‐severe RVD, 11 underwent cardiac transplantation within the first year of PPCM diagnosis. Of the remaining 10 patients with initial moderate‐to‐severe RVD, 9 recovered normal RV and LV function; all 10 experienced event‐free survival.
Construct Validation of Echocardiographic RVD Severity Grade by Invasive Hemodynamic Indexes
Right‐sided heart catheterizations were performed at the time of PPCM diagnosis in 21 subjects: 6 had none or mild RVD, and 15 had moderate or severe RVD, on diagnostic echocardiogram (Table S1). In this subset of patients with PPCM, those with echocardiographic moderate‐to‐severe RVD had higher mean RA pressure and RV end‐diastolic pressure and lower pulmonary artery pulsatility index and stroke volume index/RA than patients with none‐to‐mild RVD, consistent with worse RV function. Although they had higher mean pulmonary artery pressure, subjects with moderate‐to‐severe RVD had similar pulmonary vascular resistance and diastolic pulmonary gradient as the subjects with none‐to‐mild RVD. Median diastolic pulmonary gradients were <7 mm Hg for both subgroups, suggesting a lack of any significant pulmonary vascular disease.31 Cardiac output and mean PCWP were worse in subjects with moderate‐to‐severe RVD, corroborating the echocardiographic measurements that suggest these patients also had a greater degree of LV dysfunction.
Outcomes Vary by RVD Severity
Event‐free survival differed substantially by initial severity of RVD (Figure [A]). The overall event‐free survival curves revealed that those with moderate or severe RVD experienced the outcome primarily within the first year after diagnosis; and those with no or mild RVD experienced few events over the following 5 years. Event‐free survival appeared worse for those with an LVEF <30% at diagnosis (Figure [B]) but was similar for those with LVEDD ≥60 mm and those with LVEDD <60 mm (Figure [C]). In a multivariable model including RVD severity and United Network of Organ Sharing donor heart allocation era, moderate‐to‐severe RVD remained associated with outcomes independent of United Network of Organ Sharing donor heart allocation era (hazard ratio [HR] [95% confidence interval {CI}], 3.24 [1.16–9.0]).
Figure 1.
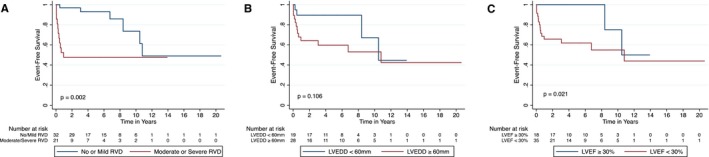
Kaplan‐Meier survival curves depicting event‐free survival to the primary composite outcome of left ventricular assist device implantation, cardiac transplantation, or death by echocardiographic grades of right ventricular dysfunction (RVD; A), left ventricular end‐diastolic diameter (LVEDD) <60 or ≥60 mm (B), and left ventricular ejection fraction (LVEF) ≥30% or <30% (C) at peripartum cardiomyopathy diagnosis. P values are for log‐rank tests.
Univariable Analyses
Univariable analyses showed that the risk of LVAD implantation, transplantation, or death was >4‐fold higher for those with moderate‐to‐severe RVD (HR [95% CI], 4.26 [1.57–11.6]) and ≈5‐fold higher for those with an LVEF <30% (HR [95% CI], 4.85 [1.11–21.3]) (Table 4). LV dilatation at diagnosis (LVEDD ≥60 mm) showed a trend toward association with the primary composite outcome that did not achieve statistical significance (HR [95% CI], 2.45 [0.79–7.5]). MR severity was not associated with the primary composite outcome (Table 4).
Table 4.
Univariable and Multivariable Analyses of LVD and RVD in PPCM Outcomes
| Outcomes | |
|---|---|
| No. of participants | 53 |
| Follow‐up time, median (IQR), y | 3.6 (1.4–7.3) |
| No. of events | 17 |
| Event rate, per 100 person‐years (95% CI) | 65.6 (40.7–100.5) |
| Model | HR | 95% CI | P Value |
|---|---|---|---|
| Univariable models | |||
| Moderate‐severe RVD | 4.26 | 1.57–11.6 | 0.005 |
| LVEF <30% | 4.85 | 1.11–21.3 | 0.036 |
| LVEDD ≥60 mm | 2.45 | 0.79–7.5 | 0.117 |
| MR severity | |||
| None | 1 | NA | NA |
| Mild | 0.97 | 0.19–5.0 | 0.970 |
| Moderate | 1.94 | 0.39–9.7 | 0.420 |
| Severe | 3.80 | 0.66–22 | 0.137 |
| Multivariable model 1 | |||
| Moderate‐severe RVD | 3.21 | 1.13–9.10 | 0.028 |
| LVEF <30% | 2.17 | 0.37–12.8 | 0.391 |
| LVEDD ≥60 mm | 1.41 | 0.38–5.25 | 0.607 |
| Multivariable model 2 | |||
| Moderate‐severe RVD | 3.18 | 1.12–9.08 | 0.030 |
| LVEF <30% | 3.21 | 0.69–14.9 | 0.137 |
| Multivariable model 3 | |||
| Moderate‐severe RVD | 3.59 | 1.30–9.92 | 0.014 |
| LVEDD ≥60 mm | 1.99 | 0.64–6.18 | 0.235 |
| Multivariable model 4 | |||
| Moderate‐severe RVD | 4.79 | 1.45–16 | 0.010 |
| MR severity | |||
| None | 1 | NA | NA |
| Mild | 0.41 | 0.06–2.6 | 0.348 |
| Moderate | 1.01 | 0.17–6.0 | 0.995 |
| Severe | 1.22 | 0.16–9.32 | 0.848 |
CI indicates confidence interval; HR, hazard ratio; IQR, interquartile range; LVD, left ventricular dysfunction; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NA, not applicable; PPCM, peripartum cardiomyopathy; RVD, right ventricular dysfunction.
Multivariable Analyses
After controlling for both severe LV dysfunction and LV dilatation at PPCM diagnosis, moderate‐to‐severe RVD was independently associated with a 3‐fold risk of LVAD implantation, transplantation, or death (Table 4, model 1: HR [95% CI], 3.21 [1.13–9.10]). Because there was evidence of collinearity between both severe LV dysfunction and LV dilatation, we tested associations between outcomes and RV dysfunction with each of these parameters individually. In a model with moderate‐to‐severe RVD and LVEF <30%, moderate‐to‐severe RVD remained independently associated with the outcome (model 2: HR [95% CI], 3.18 [1.12–9.08]), whereas LVEF <30% showed only a trend towards association that did not achieve statistical significance (model 2: HR [95% CI], 3.21 [0.69–14.9]). Similarly, in a model with moderate‐to‐severe RVD and LVEDD ≥60 mm, moderate‐to‐severe RVD remained independently associated with the outcome (model 3: HR [95% CI], 3.59 [1.30–9.92]), whereas LVEDD ≥60 mm showed a trend towards association that did not achieve statistical significance (model 3: HR [95% CI], 1.99 [0.64–6.18]). When MR severity grade was included with moderate‐to‐severe RVD, moderate‐to‐severe RVD remained independently associated with the outcome (model 4: HR [95% CI], 4.79 [1.45–16]).
Discussion
Herein, we report on a cohort of patients with PPCM with relatively severe cardiac dysfunction and high adverse event rates. We found that patients with moderate‐to‐severe RV dysfunction at PPCM diagnosis were 4 times more likely to require LVAD implantation, cardiac transplantation, or die than those with normal RV function or mild RVD, over a median (IQR) follow‐up of 3.6 (1.4–7.3) years. Patients with moderate‐to‐severe RVD were also more likely to have adverse outcomes within the first year of PPCM diagnosis. Thus, risk stratifying patients with PPCM by initial RVD severity could trigger prompt evaluation for cardiac replacement therapy versus aggressive optimization of guideline‐directed medical therapy with close longitudinal follow‐up. Recognizing that the vast majority of adverse events occurred within 12 months eliminates the possibility that subsequent pregnancies confounded our major observations.
Our findings suggest that RVD severity at PPCM diagnosis confers additional prognostic value over measures of LV dysfunction and remodeling. Using multivariable Cox models, we found that only moderate‐to‐severe RVD (not LVEF <30%, LVEDD ≥60 mm, or MR severity) was independently associated with time to the primary composite outcome. With our multivariable models, we yielded fresh insights into PPCM, suggesting a more severe PPCM endophenotype with biventricular dysfunction that is not attributable to either worse pulmonary vascular disease or MR. Patients with PPCM with moderate‐to‐severe RV dysfunction also had worse LV systolic function and larger LV dimensions. This contrasts sharply with the reduction in LV chamber dimension typically seen with isolated RVD as a result of pericardial constraint. Hence, the presence of moderate‐to‐severe RVD, concomitant with a more dilated and dysfunctional LV, speaks to a greater total burden of biventricular dysfunction and more severe global cardiomyopathy among our study cohort. Consistent with this observation, patients with moderate‐to‐severe RVD had higher right and left atrial pressures and more severe mitral and tricuspid regurgitation. Both PPCM cohorts, those with and without moderate‐to‐severe RVD, had similar low diastolic pulmonary gradients and minimally elevated pulmonary vascular resistance, thereby excluding pulmonary vascular disease as a cause of worse RVD. Moreover, MR severity was not associated with the primary composite outcome nor did it modify the association between moderate‐to‐severe RVD and outcome; these observations suggest that RVD in PPCM may be occurring independent of LV dysfunction‐associated MR and any secondary pulmonary hypertension. Thus, it is plausible that our patients with PPCM with moderate‐to‐severe RVD represented an overall more severe cardiomyopathy phenotype. This may partly explain the more accelerated clinical decline and higher adverse event rates experienced by the patients with PPCM with moderate‐to‐severe RVD.
Only 3 prior studies have assessed the RV systolic function in PPCM outcomes. All were limited to 6 or 12 months' follow‐up.15, 32, 33 The IPAC (Investigations of Pregnancy Associated Cardiomyopathy) study, the largest of these multicenter studies, evaluated multiple quantitative echocardiographic indexes of baseline RV and LV size and function in association with LV recovery and clinical outcome at 1 year.15 The 2 combined end points of poor outcome in the IPAC study were driven by the LVEF component because the rate of clinical events was too low (7%) to be statistically analyzed independently. Moreover, the low rate of clinical events and high rate of LV recovery (75%) differ from findings of many prior PPCM outcome studies.1, 8, 12, 13, 18, 19, 34 The 2 other studies of RV systolic function in PPCM, one based in Nigeria32 and the other in Germany,33 had smaller study cohorts than ours. One did not identify any significant predictors of mortality; the other limited its outcome to LV functional recovery.
Our study had the longest overall follow‐up and assessed associations between the severity of initial RVD and timing of patient‐oriented clinical outcomes (LVAD, transplantation, and death), thereby distinguishing our analysis from previous studies and offering new insights. Our patients experienced a higher rate of adverse outcomes at 1 year compared with the IPAC study cohort (22.6% versus 7%). The simple explanation would be that our cohort had a more severe PPCM phenotype, characterized by significant biventricular dysfunction; ≈40% of our cohort had moderate‐to‐severe RVD at baseline, whereas only about a quarter of the IPAC study cohort had significant RVD, as defined by RV fractional area change <30%. Alternatively, the observed differences in adverse outcome rates may be because of the larger proportion of black patients in our cohort (75.5% versus 30% in the IPAC study). Black race has been associated with lower LVEF at 1 year and lack of cardiac recovery.6, 15, 35 Although black race was not associated with our composite outcome, the high prevalence of black race in our cohort limited our power to detect a statistically significant association between race and outcomes. Regardless, our adverse outcome rates are consistent with those observed in other PPCM studies of predominantly black US patient populations.8, 18, 34, 35 Other retrospective cohort studies of PPCM in predominantly black patients in the United States have reported overall mortality rates of 11% to 15.9%, transplantation rates of 10%, and LV functional recovery rates of 23% to 45%.8, 18, 34, 35 Accordingly, our study results are likely generalizable, especially to black patients with PPCM.
Our study is the first to assess associations between the severity of RVD and adverse outcomes beyond the first year of diagnosis. Ours is the only study to examine a composite patient‐centered outcome: death, cardiac transplantation, or LVAD implantation. Prior PPCM studies primarily assessed associations between clinical and demographic variables and improvement in LVEF. We identified echocardiographic risk factors for adverse patient‐centered clinical outcomes, which is arguably more important to clinicians who need to know which patients with PPCM might need the most intensive care and follow‐up. We conducted several prespecified survival analyses and conservatively right‐censored follow‐up at the time of last echocardiogram to minimize misclassification bias. Prior studies conducted survival analyses with right censoring at study period end date (and may not have captured important changes in cardiac function)34 or relied on simple logistic regression and χ2 analysis to test association between predictor variables and outcomes.8, 32, 33
Our findings that initial RVD severity is independently associated with adverse outcomes and that RV recovery appears associated with event‐free survival raise the question of whether improving RV function would improve PPCM outcomes. Others have reported a strong association between RV recovery and improved survival in ambulatory patients with chronic HF with reduced LVEF.36 Future studies of patients with PPCM may be leveraged to investigate mechanisms underlying RV recovery potential and, in turn, identify novel therapeutic targets. Most significantly, we identify a severe phenotype of PPCM that presents with biventricular dysfunction and may be associated with black race. Large, multicenter, longitudinal studies of racially diverse populations are needed to better define potential PPCM endophenotypes.
Limitations
This study is limited in that it is a retrospective, single‐center, cohort study, and future prospective studies of larger racially diverse cohorts of patients with PPCM are needed to validate our results. The use of ICD‐9‐CM coding to identify patients introduces the possibility that patients were missed because of miscoding. We were unable to assess outcomes in any patients with PPCM who potentially sought subsequent clinical care at another medical center. However, we minimized the possibility for misclassification bias by right censoring at the date of the final echocardiogram performed during the study period, rather than right censoring at the study period end date. We recognize that RVD was assessed by qualitative visual assessment rather than quantifiable measurements, such as tricuspid annular planar systolic excursion and RV fractional area change. This reflects the fact that not all echocardiography images were available for review. Also, quantitative assessment of RV function was not consistently reported during the bulk of the studies. The vast majority of our diagnostic echocardiograms predated consensus guidelines for quantitative echocardiographic assessment of RV function24; quantitative RV assessment is not routinely incorporated into standard diagnostic echocardiography in the assessment of new‐onset HF.37 That said, prior studies have shown that moderate‐to‐severe RVD, as assessed by visual estimate, reliably prognosticates HF outcomes,38, 39, 40 as has been demonstrated with quantitative measures.41 Moreover, our invasive hemodynamics corroborate the construct validity of our RVD designations by visual estimate; RA, pulmonary artery pulsatility index, PCWP, stroke volume index/RA, and cardiac output were worse in the moderate‐to‐severe RVD group. Future work would ideally apply quantitative measures of RV function.
Conclusion
Moderate‐to‐severe RV dysfunction at time of PPCM diagnosis is strongly and independently associated with adverse outcomes, especially within the first year of diagnosis. These subjects had an overall more severe cardiomyopathy phenotype marked with more dilated and dysfunctional LVs, worse atrioventricular valvular regurgitation, and higher biventricular filling pressures. Our findings highlight the importance of initial RV functional assessment in PPCM as moderate‐to‐severe RVD signals a more advanced cardiomyopathy phenotype with an increased adverse event rate and accelerated clinical decline. Recognizing these patients promptly may lead to early referral for advanced HF therapies.
Sources of Funding
Dr Tsai is supported by the National Heart, Lung, and Blood Institute Career Development Award (HL109159) and the Foundation for Gender‐Specific Medicine (M. Irené Ferrer Scholar Award).
Disclosures
None.
Supporting information
Table S1. Cardiovascular Hemodynamics of Echocardiographic RVD Severity Subgroups
(J Am Heart Assoc. 2018;7:e008378 DOI: 10.1161/JAHA.117.008378.)29686029
References
- 1. Kolte D, Khera S, Aronow WS, Palaniswamy C, Mujib M, Ahn C, Jain D, Gass A, Ahmed A, Panza JA, Fonarow GC. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: a nationwide population‐based study. J Am Heart Assoc. 2014;3:e001056 DOI: 10.1161/JAHA.114.001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy‐related mortality in the United States, 2006–2010. Obstet Gynecol. 2015;125:5–12. [DOI] [PubMed] [Google Scholar]
- 3. Mielniczuk LM, Williams K, Davis DR, Tang AS, Lemery R, Green MS, Gollob MH, Haddad H, Birnie DH. Frequency of peripartum cardiomyopathy. Am J Cardiol. 2006;97:1765–1768. [DOI] [PubMed] [Google Scholar]
- 4. Goland S, Modi K, Bitar F, Janmohamed M, Mirocha JM, Czer LS, Illum S, Hatamizadeh P, Elkayam U. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail. 2009;15:645–650. [DOI] [PubMed] [Google Scholar]
- 5. Cooper LT, Mather PJ, Alexis JD, Pauly DF, Torre‐Amione G, Wittstein IS, Dec GW, Zucker M, Narula J, Kip K, McNamara DM; for the IMAC2 Investigators . Myocardial recovery in peripartum cardiomyopathy: prospective comparison with recent onset cardiomyopathy in men and nonperipartum women. J Card Fail. 2012;18:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, Modi K, Alexis JD, Ramani GV, Semigran MJ, Haythe J, Markham DW, Marek J, Gorcsan J III, Wu WC, Lin Y, Halder I, Pisarcik J, Cooper LT, Fett JD; IPAC Investigators . Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC study (Investigations of Pregnancy‐Associated Cardiomyopathy). J Am Coll Cardiol. 2015;66:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fett JD, Sannon H, Thelisma E, Sprunger T, Suresh V. Recovery from severe heart failure following peripartum cardiomyopathy. Int J Gynaecol Obstet. 2009;104:125–127. [DOI] [PubMed] [Google Scholar]
- 8. Modi KA, Illum S, Jariatul K, Caldito G, Reddy PC. Poor outcome of indigent patients with peripartum cardiomyopathy in the United States. Am J Obstet Gynecol. 2009;201:171.e171–171.e175. [DOI] [PubMed] [Google Scholar]
- 9. Biteker M, Ilhan E, Biteker G, Duman D, Bozkurt B. Delayed recovery in peripartum cardiomyopathy: an indication for long‐term follow‐up and sustained therapy. Eur J Heart Fail. 2012;14:895–901. [DOI] [PubMed] [Google Scholar]
- 10. Rasmusson K, Brunisholz K, Budge D, Horne BD, Alharethi R, Folsom J, Connolly JJ, Stehlik J, Kfoury A. Peripartum cardiomyopathy: post‐transplant outcomes from the United Network for Organ Sharing Database. J Heart Lung Transplant. 2012;31:180–186. [DOI] [PubMed] [Google Scholar]
- 11. Loyaga‐Rendon RY, Pamboukian SV, Tallaj JA, Acharya D, Cantor R, Starling RC, Naftel D, Kirklin J. Outcomes of patients with peripartum cardiomyopathy who received mechanical circulatory support: data from the interagency registry for mechanically assisted circulatory support. Circ Heart Fail. 2014;7:300–309. [DOI] [PubMed] [Google Scholar]
- 12. Blauwet LA, Libhaber E, Forster O, Tibazarwa K, Mebazaa A, Hilfiker‐Kleiner D, Sliwa K. Predictors of outcome in 176 South African patients with peripartum cardiomyopathy. Heart. 2013;99:308–313. [DOI] [PubMed] [Google Scholar]
- 13. Sliwa K, Forster O, Libhaber E, Fett JD, Sundstrom JB, Hilfiker‐Kleiner D, Ansari AA. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J. 2006;27:441–446. [DOI] [PubMed] [Google Scholar]
- 14. Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P, Tsikas D, Jordan J, Lichtinghagen R, von Kaisenberg CS, Struman I, Bovy N, Sliwa K, Bauersachs J, Hilfiker‐Kleiner D. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blauwet LA, Delgado‐Montero A, Ryo K, Marek JJ, Alharethi R, Mather PJ, Modi K, Sheppard R, Thohan V, Pisarcik J, McNamara DM, Gorcsan J III; IPAC Investigators . Right ventricular function in peripartum cardiomyopathy at presentation is associated with subsequent left ventricular recovery and clinical outcomes. Circ Heart Fail. 2016;9:e002756. [DOI] [PubMed] [Google Scholar]
- 16. Chapa JB, Heiberger HB, Weinert L, Decara J, Lang RM, Hibbard JU. Prognostic value of echocardiography in peripartum cardiomyopathy. Obstet Gynecol. 2005;105:1303–1308. [DOI] [PubMed] [Google Scholar]
- 17. Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, Hameed A, Shotan A. Pregnancy‐associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111:2050–2055. [DOI] [PubMed] [Google Scholar]
- 18. Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J. 2006;152:509–513. [DOI] [PubMed] [Google Scholar]
- 19. Habli M, O'Brien T, Nowack E, Khoury S, Barton JR, Sibai B. Peripartum cardiomyopathy: prognostic factors for long‐term maternal outcome. Am J Obstet Gynecol. 2008;199:415.e411–415.e415. [DOI] [PubMed] [Google Scholar]
- 20. Krishnamoorthy P, Garg J, Palaniswamy C, Pandey A, Ahmad H, Frishman WH, Lanier G. Epidemiology and outcomes of peripartum cardiomyopathy in the United States: findings from the Nationwide Inpatient Sample. J Cardiovasc Med (Hagerstown). 2016;17:756–761. [DOI] [PubMed] [Google Scholar]
- 21. ACC/AHA guidelines for the clinical application of echocardiography: a report of the American College of Cardiology/American Heart Association task force on assessment of diagnostic and therapeutic cardiovascular procedures (subcommittee to develop guidelines for the clinical application of echocardiography). Circulation. 1990;82:2323–2345. [DOI] [PubMed] [Google Scholar]
- 22. Cheitlin MD, Alpert JS, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davidson TW, Davis JL, Douglas PS, Gillam LD, Lewis RP, Pearlman AS, Philbrick JT, Shah PM, Williams RG, Ritchie JL, Eagle KA, Gardner TJ, Garson A, Gibbons RJ, O'Rourke RA, Ryan TJ. ACC/AHA guidelines for the clinical application of echocardiography: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee on clinical application of echocardiography): developed in collaboration with the American Society of Echocardiography. Circulation. 1997;95:1686–1744. [DOI] [PubMed] [Google Scholar]
- 23. Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Faxon DP, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO; ACC, AHA, ASE . ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (ACC/AHA/ASE committee to update the 1997 guidelines for the clinical application of echocardiography). J Am Soc Echocardiogr. 2003;16:1091–1110. [DOI] [PubMed] [Google Scholar]
- 24. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 25. Drazner MH, Velez‐Martinez M, Ayers CR, Reimold SC, Thibodeau JT, Mishkin JD, Mammen PP, Markham DW, Patel CB. Relationship of right‐ to left‐sided ventricular filling pressures in advanced heart failure: insights from the ESCAPE trial. Circ Heart Fail. 2013;6:264–270. [DOI] [PubMed] [Google Scholar]
- 26. Grodin JL, Drazner MH, Dupont M, Mullens W, Taylor DO, Starling RC, Tang WH. A disproportionate elevation in right ventricular filling pressure, in relation to left ventricular filling pressure, is associated with renal impairment and increased mortality in advanced decompensated heart failure. Am Heart J. 2015;169:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Odake M, Takeuchi M, Fukuzaki H. Doppler assessment of right ventricular filling dynamics during volume loading in ischemic heart disease. Clin Cardiol. 1991;14:402–408. [DOI] [PubMed] [Google Scholar]
- 28. Korabathina R, Heffernan KS, Paruchuri V, Patel AR, Mudd JO, Prutkin JM, Orr NM, Weintraub A, Kimmelstiel CD, Kapur NK. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheter Cardiovasc Interv. 2012;80:593–600. [DOI] [PubMed] [Google Scholar]
- 29. Morine KJ, Kiernan MS, Pham DT, Paruchuri V, Denofrio D, Kapur NK. Pulmonary artery pulsatility index is associated with right ventricular failure after left ventricular assist device surgery. J Card Fail. 2016;22:110–116. [DOI] [PubMed] [Google Scholar]
- 30. Nativi JN, Kfoury AG, Myrick C, Peters M, Renlund D, Gilbert EM, Bader F, Singhal AK, Everitt M, Fisher P, Bull DA, Selzman C, Stehlik J. Effects of the 2006 U.S. thoracic organ allocation change: analysis of local impact on organ procurement and heart transplantation. J Heart Lung Transplant. 2010;29:235–239. [DOI] [PubMed] [Google Scholar]
- 31. Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs JS, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–D108. [DOI] [PubMed] [Google Scholar]
- 32. Karaye KM, Lindmark K, Henein M. Right ventricular systolic dysfunction and remodelling in Nigerians with peripartum cardiomyopathy: a longitudinal study. BMC Cardiovasc Disord. 2016;16:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haghikia A, Rontgen P, Vogel‐Claussen J, Schwab J, Westenfeld R, Ehlermann P, Berliner D, Podewski E, Hilfiker‐Kleiner D, Bauersachs J. Prognostic implication of right ventricular involvement in peripartum cardiomyopathy: a cardiovascular magnetic resonance study. ESC Heart Fail. 2015;2:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pillarisetti J, Kondur A, Alani A, Reddy M, Reddy M, Vacek J, Weiner CP, Ellerbeck E, Schreiber T, Lakkireddy D. Peripartum cardiomyopathy: predictors of recovery and current state of implantable cardioverter‐defibrillator use. J Am Coll Cardiol. 2014;63:2831–2839. [DOI] [PubMed] [Google Scholar]
- 35. Goland S, Modi K, Hatamizadeh P, Elkayam U. Differences in clinical profile of African‐American women with peripartum cardiomyopathy in the United States. J Card Fail. 2013;19:214–218. [DOI] [PubMed] [Google Scholar]
- 36. Dini FL, Carluccio E, Simioniuc A, Biagioli P, Reboldi G, Galeotti GG, Raineri C, Gargani L, Scelsi L, Mandoli GE, Cannito A, Rossi A, Temporelli PL, Ghio S; Network Labs Ultrasound in Heart Failure Study Group . Right ventricular recovery during follow‐up is associated with improved survival in patients with chronic heart failure with reduced ejection fraction. Eur J Heart Fail. 2016;18:1462–1471. [DOI] [PubMed] [Google Scholar]
- 37. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 38. Kukulski T, She L, Racine N, Gradinac S, Panza JA, Velazquez EJ, Chan K, Petrie MC, Lee KL, Pellikka PA, Romanov A, Biernat J, Rouleau JL, Batlle C, Rogowski J, Ferrazzi P, Zembala M, Oh JK; Surgical Treatment for Ischemic Heart Failure Investigators . Implication of right ventricular dysfunction on long‐term outcome in patients with ischemic cardiomyopathy undergoing coronary artery bypass grafting with or without surgical ventricular reconstruction. J Thorac Cardiovasc Surg. 2015;149:1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Melenovsky V, Kotrc M, Borlaug BA, Marek T, Kovar J, Malek I, Kautzner J. Relationships between right ventricular function, body composition, and prognosis in advanced heart failure. J Am Coll Cardiol. 2013;62:1660–1670. [DOI] [PubMed] [Google Scholar]
- 40. Ravis E, Theron A, Mancini J, Jaussaud N, Morera P, Chalvignac V, Guidon C, Grisoli D, Gariboldi V, Riberi A, Habib G, Mouly‐Bandini A, Collart F. Severe right ventricular dysfunction is an independent predictor of pre‐ and post‐transplant mortality among candidates for heart transplantation. Arch Cardiovasc Dis. 2017;110:139–148. [DOI] [PubMed] [Google Scholar]
- 41. Damy T, Viallet C, Lairez O, Deswarte G, Paulino A, Maison P, Vermes E, Gueret P, Adnot S, Dubois‐Rande JL, Hittinger L. Comparison of four right ventricular systolic echocardiographic parameters to predict adverse outcomes in chronic heart failure. Eur J Heart Fail. 2009;11:818–824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cardiovascular Hemodynamics of Echocardiographic RVD Severity Subgroups


