Abstract
Background
It is unknown whether causes and temporal patterns of 30‐day readmission vary between heart failure (HF) with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF). We sought to address this question by examining a 5% national sample of Medicare beneficiaries.
Methods and Results
We included individuals who experienced a hospitalization for HFpEF or HFrEF between 2007 and 2013. We identified causes of 30‐day readmission based on primary discharge diagnosis and further classified causes of readmission as HF‐related, non–HF cardiovascular‐related, and non–cardiovascular‐related. We calculated the cumulative incidence of these classifications for HFpEF and HFrEF in a competing risks model and calculated subdistribution hazard ratios of these classifications by comparing those with HFpEF and those with HFrEF. Among 60 640 Medicare beneficiaries, we identified 13 785 unique older adults hospitalized with HFpEF and 15 205 who were hospitalized with HFrEF. Noncardiovascular diagnoses represented the most common causes of 30‐day readmission (HFpEF: 59%; HFrEF: 47%), a pattern that was observed for each week of the 30‐day study period for both HFpEF and HFrEF participants. In comparing readmission diagnoses in an adjusted model, non–cardiovascular‐related diagnoses were more common and HF‐related diagnoses were less common in HFpEF participants.
Conclusions
Non–cardiovascular‐related diagnoses represented the most common causes of 30‐day readmission following HF hospitalization for each week of the 30‐day postdischarge period. HF diagnoses were less common among those with HFpEF compared with HFrEF. Future interventions aimed at reducing 30‐day readmissions following an HF hospitalization would benefit from an increased focus on noncardiovascular comorbidity and interventions that target HFpEF and HFrEF separately.
Keywords: comorbidities heart failure, epidemiology, heart failure, patient readmission
Subject Categories: Heart Failure, Epidemiology
Clinical Perspective
What Is New?
The most common 30‐day readmission diagnoses for heart failure (HF) with either preserved or reduced ejection fraction were noncardiovascular conditions.
Noncardiovascular diagnoses composed the majority of readmissions for each week of the 30‐day postdischarge period for HF with either preserved or reduced ejection fraction.
Readmission specifically for heart failure was less common among those with HF with preserved ejection fraction compared with those with HF with reduced ejection fraction.
What Are the Clinical Implications?
A paradigm shift with an increased emphasis on noncardiovascular comorbidities in patients admitted for HF may be warranted, independent of ejection fraction.
Our observation that the most common readmission diagnoses following discharge were noncardiovascular suggests that follow‐up with a primary care physician may be as important as follow‐up with a cardiologist.
Given differences in patterns of readmission, interventions aimed at reducing 30‐day readmissions may need to be tailored for HF with preserved or reduced ejection fraction separately.
Introduction
One in 4 older adults hospitalized with heart failure (HF) in the United States are readmitted within 30 days of discharge.1 Costing >$1.7 billion annually,2 readmissions put a substantial burden on the healthcare system. To control costs associated with potentially preventable readmissions, the Centers for Medicare and Medicaid Services (CMS) now impose penalties on hospitals with excessive readmission rates,3 prompting hospitals across the country to search for ways to reduce their 30‐day readmission rates.
Interventions aimed at reducing readmission rates in HF have produced mixed results4 for reasons that are not yet fully elucidated. A possible contributor may be the heterogeneity of the HF disease state. Clinically, HF is typically dichotomized by the presence or absence of a reduced ejection fraction, described as HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF). Given important differences in patient demographics,5 comorbidity profiles,6 natural history,7, 8 and availability of evidence‐based therapies,9 causes of and temporal patterns in 30‐day readmission may vary for HFpEF and HFrEF. This could have important implications for developing interventions aimed at curbing readmission rates.
Accordingly, we compared and contrasted the causes and temporal patterns of 30‐day readmissions among patients with HFpEF and HFrEF using a 5% national random sample of Medicare beneficiaries.
Methods
The data, analytic methods, and study materials cannot be made available to other researchers for purposes of reproducing the results or replicating the procedure, as our Medicare data reuse agreement prohibits sharing these data.
We drew the study population from a national 5% sample of Medicare beneficiaries and included individuals who experienced a hospitalization for HFpEF or HFrEF between 2007 and 2013, lived in the United States, had full fee‐for‐service and prescription drug coverage in the year before hospitalization, and were aged 65 to 109 years on the day of admission. HFpEF hospitalizations were identified as inpatient claims with an International Classification of Diseases, Ninth Revision (ICD‐9) primary discharge diagnosis of 428.3x (diastolic HF), and HFrEF hospitalizations were identified as inpatient claims with an ICD‐9 primary discharge diagnosis of 428.2x (systolic HF) or 428.4x (combined systolic and diastolic HF). Only the first HF hospitalization was used for each individual (Figure S1).
We assessed characteristics of the Medicare beneficiaries by using claims and enrollment data for the year before the hospitalization. These characteristics included age, race, sex, Medicaid eligibility, eligibility for Part D prescription drug coverage subsidies, US Census region, history of coronary heart disease, chronic kidney disease, diabetes mellitus, atrial fibrillation, hypertension, chronic obstructive pulmonary disease, Charlson comorbidity index, length of stay, nursing home residence,10 and skilled nursing facility stay in the prior year. We also identified intensive care unit stays during the HF hospitalization as a proxy for severity of illness.
Medicare beneficiaries were followed for readmission for up to 30 days after discharge. To avoid counting hospital transfers as readmissions, we considered hospital admissions that occurred on the day of a hospital discharge or the day after hospital discharge with a hospital transfer code as a single episode of care. We assessed causes of readmission by using the primary discharge diagnosis, grouped using the Healthcare Cost and Utilization Project Clinical Classifications software.11 We further classified causes of readmission into HF‐related (primary discharge diagnoses 402.01, 402.11, 402.91, 404.01, 404.11, 404.91, or 428), non‐HF cardiovascular‐related (the first 3 digits of primary discharge diagnoses 390–459 except those listed previously for HF), and non–cardiovascular‐related (any inpatient claims except primary discharge diagnoses 390–459).
We calculated means and standard deviations for continuous characteristics and numbers and percentages for categorical beneficiary characteristics stratified by HF type (HFpEF versus HFrEF). We also calculated numbers and percentages for causes of readmission following HFpEF and HFrEF hospitalizations separately. We then calculated the cumulative incidence of HF‐related, non‐HF cardiovascular‐related, and non–cardiovascular‐related readmissions for HFpEF and HFrEF participants, treating other causes of hospitalization and mortality as competing risks.12 We also calculated subdistribution hazard ratios of HF‐related, non‐HF cardiovascular‐related, and non–cardiovascular‐related readmissions comparing beneficiaries hospitalized for HFpEF and those hospitalized for HFrEF.12 These models considered other causes of hospitalization and mortality as competing risks. For the HF‐related readmissions, for example, non‐HF cardiovascular‐related and non–cardiovascular‐related readmissions, as well as mortality, were treated as competing risks. Models were initially adjusted for age, race, sex, eligibility for Medicaid and Part D subsidies, and region (model 1). We then additionally adjusted for history of coronary heart disease, chronic kidney disease, diabetes mellitus, atrial fibrillation, hypertension, chronic obstructive pulmonary disease, Charlson comorbidity index, nursing home residence, skilled nursing facility stay in the prior year, length of stay (quartiles), and intensive care unit stay during the HF hospitalization as a proxy for severity of illness (model 2). To examine whether the association of HF type and cause‐specific readmission varied by time since discharge, we included interactions between time since discharge and cause‐specific readmission in our models. In addition, we calculated hazard ratios for the association between HF type and cause‐specific readmission for each week of the month following discharge.
This research was approved by the CMS privacy board and the institutional review board of the University of Alabama at Birmingham with a waiver of consent.
Results
Among 60 640 Medicare beneficiaries hospitalized for HF from 2007 to 2013, we identified 13 785 unique older adults hospitalized with HFpEF and 15 205 unique older adults hospitalized with HFrEF. Older adults with HFpEF were slightly older, were more commonly women, and had a higher mean Charlson comorbidity index compared with those with HFrEF (Table 1). Older adults with HFpEF were more likely to have hypertension and chronic obstructive pulmonary disease; older adults with HFrEF were more likely to have coronary heart disease. Length of stay was similar for HFpEF and HFrEF participants. Notably, older adults with HFpEF were less likely to require intensive care unit–level care and were more likely to be nursing home residents.
Table 1.
Summary Statistics of Sample by Type of HF
| Variable | HFpEF (n=13 785) | HFrEF (n=15 205) |
|---|---|---|
| Age, y, mean (SD) | 78.6 (11.5) | 76.5 (12.1) |
| Race | ||
| Black | 1827 (13.3) | 2184 (14.4) |
| Other | 800 (5.8) | 852 (5.6) |
| White | 11 158 (80.9) | 12 169 (80.0) |
| Women | 9853 (71.5) | 7869 (51.8) |
| Medicare Part D subsidy | 6869 (49.8) | 7291 (48.0) |
| Dual eligibility for Medicare and Medicaid | 5960 (43.2) | 6146 (40.4) |
| US Census region | ||
| Northeast | 3144 (22.8) | 3074 (20.2) |
| Midwest | 3545 (25.7) | 3909 (25.7) |
| South | 5463 (39.6) | 6257 (41.2) |
| West | 1633 (11.8) | 1965 (12.9) |
| Coronary heart disease | 7726 (56.0) | 10 932 (71.9) |
| Chronic kidney disease | 8333 (60.4) | 9244 (60.8) |
| COPD | 7180 (52.1) | 7338 (48.3) |
| Diabetes mellitus | 6822 (49.5) | 7439 (48.9) |
| Atrial fibrillation | 6676 (48.4) | 7193 (47.3) |
| Hypertension | 12 081 (87.6) | 12 337 (81.1) |
| Dementia | 2487 (18.0) | 2375 (15.6) |
| Liver disease | 713 (5.2) | 710 (4.7) |
| Cerebrovascular disease | 2531 (18.4) | 2654 (17.5) |
| Cancer | 3013 (21.9) | 3059 (20.1) |
| Charlson comorbidity index | ||
| 0 | 5328 (38.7) | 5811 (38.2) |
| 1–3 | 1588 (11.5) | 1865 (12.3) |
| 4–5 | 2139 (15.5) | 2314 (15.2) |
| 6–7 | 1763 (12.8) | 2032 (13.4) |
| 8–9 | 1528 (11.1) | 1717 (11.3) |
| ≥10 | 1439 (10.4) | 1466 (9.6) |
| Length of stay, mean (SD) | 6.3 (4.3) | 6.3 (4.9) |
| ICU stay | 2863 (20.8) | 3530 (23.2) |
| Nursing home residence | 2438 (17.7) | 2112 (13.9) |
| Skilled nursing facility stay | 2916 (21.2) | 2481 (16.3) |
Data are shown as n (%) except as noted. COPD indicates chronic obstructive pulmonary disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICU, intensive care unit.
Older adults with HFpEF experienced a 30‐day readmission rate of 22.3%, and older adults with HFrEF experienced a 30‐day readmission rate of 22.1%. Noncardiovascular causes of readmission occurred more frequently than HF‐related and non‐HF cardiovascular‐related causes for both HFpEF and HFrEF participants (Table 2).
Table 2.
Causes of 30‐Day Readmission Among Medicare Beneficiaries With HFpEF vs HFrEF
| Cause | HFpEF Readmissions (n=3075) | HFrEF Readmissions (n=3367) |
|---|---|---|
| n (%) | n (%) | |
| HF | 743 (24.2) | 1105 (32.8) |
| Non‐HF cardiovascular‐related | 517 (16.8) | 673 (20.0) |
| Dysrhythmia | 139 (4.5) | 142 (4.2) |
| Acute myocardial infarction | 54 (1.8) | 108 (3.2) |
| Coronary atherosclerosis | 60 (2.0) | 97 (2.9) |
| Hypertension with complications | 77 (2.5) | 89 (2.6) |
| Non–cardiovascular‐related | 1815 (59.0) | 1589 (47.2) |
| Acute renal failure | 168 (5.5) | 167 (5.0) |
| Septicemia | 160 (5.2) | 155 (4.6) |
| Pneumonia | 150 (4.9) | 106 (3.1) |
| Adult respiratory failure | 141 (4.6) | 104 (3.1) |
| COPD | 99 (3.2) | 84 (2.5) |
| Fluid/electrolyte diagnosis | 82 (2.7) | 81 (2.4) |
| Urinary tract infection | 73 (2.4) | 60 (1.8) |
Values are numbers of readmission and percentages of those readmitted within 30 days. COPD indicates chronic obstructive pulmonary disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
We examined temporal patterns of readmission diagnoses separately among participants with HFpEF and HFrEF (Figure). Among those with HFpEF, non–cardiovascular‐related diagnoses composed the majority of readmissions over the course of the 30‐day study period. This observation was noted during the first week following discharge and continued to compose the majority of readmissions during each week of the 30‐day period. Similar to HFpEF participants, the majority of readmission diagnoses for those with HFrEF were non–cardiovascular‐related for each week of the 30‐day study period, although this was less marked compared with HFpEF participants. HF‐related diagnoses were the next most common causes of readmission for each week of the study, followed by non‐HF cardiovascular‐related diagnoses.
Figure 1.
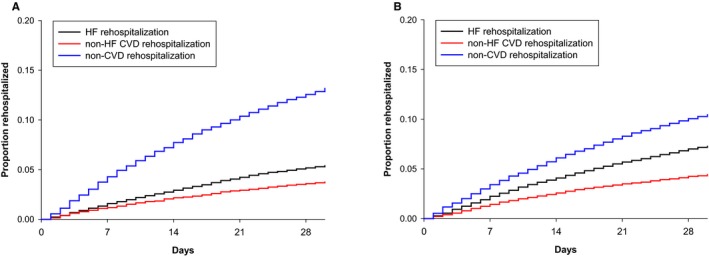
Temporal patterns for causes of readmission among Medicare beneficiaries with HFpEF versus HFrEF. A, Medicare beneficiaries hospitalized for HFpEF. B, Medicare beneficiaries hospitalized for HFrEF. CVD indicates cardiovascular‐related; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
In comparing readmission diagnoses for HFpEF and HFrEF participants, HF‐related diagnoses were less common with HFpEF, even after adjusting for sociodemographics and comorbidity burden (Table 3). This pattern remained for each of the 4 weeks of the 30‐day readmission period studied (P=0.63 for interaction with time). Non‐HF cardiovascular‐related diagnoses were also less common in HFpEF compared to HFrEF, in both unadjusted and adjusted models. Differences did not meet statistical significance for any single week of the 30‐day readmission period (P=0.50 for interaction with time). Meanwhile, non–cardiovascular‐related diagnoses were more common in HFpEF even after adjusting for sociodemographics and comorbidity burden. This pattern was stable for each of the 4 weeks of the 30‐day readmission period (P=0.39 for interaction with time).
Table 3.
HRs for Causes of Readmissions Comparing Medicare Beneficiaries With HFpEF vs HFrEF, Stratified by Week Following Hospital Discharge
| Overall | Week 1 | Week 2 | Week 3 | Week 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| HF‐related readmission | ||||||||||
| Model 1 | 0.73 (0.67–0.81) | <0.001 | 0.71 (0.59–0.84) | <0.001 | 0.72 (0.60–0.87) | <0.001 | 0.80 (0.66–0.97) | 0.021 | 0.72 (0.59–0.88) | 0.002 |
| Model 2 | 0.74 (0.67–0.82) | <0.001 | 0.71 (0.60–0.85) | <0.001 | 0.73 (0.61–0.88) | 0.001 | 0.81 (0.66–0.98) | 0.028 | 0.73 (0.60–0.89) | 0.002 |
| Non‐HF cardiovascular‐related readmission | ||||||||||
| Model 1 | 0.85 (0.75–0.95) | 0.005 | 0.84 (0.68–1.03) | 0.087 | 0.86 (0.69–1.08) | 0.195 | 0.82 (0.63–1.05) | 0.116 | 0.88 (0.69–1.13) | 0.31 |
| Model 2 | 0.88 (0.78–0.99) | 0.033 | 0.87 (0.71–1.07) | 0.176 | 0.89 (0.71–1.12) | 0.329 | 0.85 (0.65–1.09) | 0.197 | 0.91 (0.71–1.17) | 0.464 |
| Non–cardiovascular‐related readmission | ||||||||||
| Model 1 | 1.28 (1.19–1.37) | <0.001 | 1.26 (1.12–1.42) | <0.001 | 1.30 (1.14–1.48) | <0.001 | 1.24 (1.06–1.43) | 0.006 | 1.33 (1.14–1.54) | <0.001 |
| Model 2 | 1.23 (1.15–1.32) | <0.001 | 1.21 (1.08–1.37) | 0.002 | 1.26 (1.10–1.44) | 0.001 | 1.19 (1.03–1.39) | 0.021 | 1.28 (1.11–1.49) | 0.001 |
Model 1 adjusted for age, race, sex, eligibility for Medicaid and Part D subsidies, and region. Model 2 adjusted for variables in model 1 plus history of coronary heart disease, chronic kidney disease, diabetes mellitus, atrial fibrillation, hypertension, chronic obstructive pulmonary disease, Charlson comorbidity index, nursing home residence, skilled nursing facility stay in the prior year, length of stay, and intensive care unit stay during the HF hospitalization. CI indicates confidence interval; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio.
Discussion
Our study revealed several important findings regarding causes and temporal patterns of 30‐day readmission among older adults hospitalized with HFpEF and HFrEF. First, the most common diagnoses for 30‐day readmission for both HFpEF and HFrEF were non–cardiovascular‐related. Second, non–cardiovascular‐related diagnoses composed the majority of readmissions for each week of the 30‐day postdischarge period for both HFpEF and HFrEF participants. Finally, readmission for HF was less common among those with HFpEF compared with those with HFrEF.
The sociodemographic and clinical characteristics observed in this cohort were largely consistent with other cohorts that have reported key differences between HFpEF and HFrEF.13, 14, 15 Those with HFpEF were older, more commonly women, and more commonly white. They were also more likely to have hypertension and chronic obstructive pulmonary disease, comorbidities that have been implicated in the pathophysiology of HFpEF.16
Prior examination of Medicare data has revealed that HF accounts for a minority of 30‐day readmissions among Medicare beneficiaries.17 Our data extend these findings by specifically comparing HFpEF and HFrEF participants, showing that the most common readmission diagnoses among both groups were noncardiovascular, with HF‐related diagnoses accounting for a smaller proportion of readmissions. These data likely reflect the significant burden of comorbidity that afflicts older adults with HF13, 18 and highlight the importance of addressing concurrent comorbid conditions both during and after hospitalization. Approximately 90% of older adults with HF have at least 3 other comorbid conditions and >50% have at least 5 other comorbid conditions19; consequently, the most common comorbidity among older adults with HF is multimorbidity, the condition of having multiple conditions.20 Although efforts to improve posthospitalization outcomes have historically focused on HF‐specific strategies like increasing guideline‐directed medical therapy and promoting HF self‐care through nurse education,21 our findings suggest the need for a paradigm shift with increased emphasis on noncardiovascular comorbidity. To date, major guidelines have addressed the importance of treating comorbidity in HFpEF but have been less explicit for HFrEF.22, 23 Based on our study, increased focus on comorbidity is applicable to all types of HF, regardless of ejection fraction.
A recommended strategy to reduce readmissions of HF patients has been ensuring adequate postdischarge outpatient follow‐up.22 Although data suggest that early follow‐up is associated with a reduction in 30‐day readmissions,24, 25 almost no data inform the subspecialty with which to schedule appointments following hospital discharge. Although hospitalization for a principal diagnosis of HF would intuitively suggest that follow‐up with a cardiologist is most warranted, our observation that the most common readmission diagnoses following discharge are non–cardiovascular‐related suggests that follow‐up with a primary care physician may be as important, if not more so. Future studies that examine whether physician specialty moderates the relationship between early follow‐up and rates of readmission would be helpful to delineate the optimal postdischarge follow‐up strategy for HF patients.
Our study also showed that readmissions for HF were less common among HFpEF compared with HFrEF participants. This finding also has important implications for designing interventions to prevent readmissions. To date, few interventions have consistently demonstrated efficacy in reducing 30‐day readmissions.4 A potential contributor to the current state of the literature is that most studies have overlooked HFpEF or have combined HFpEF and HFrEF in a single cohort. Failure to examine HFpEF in isolation is problematic, given the rising prevalence of HFpEF hospitalizations, which compose about half of all HF hospitalizations.13, 26 Combining HFpEF and HFrEF is similarly problematic because the heterogeneity that stems from combining disease entities with key differences in sociodemographics, pathophysiology, and causes of readmission, as we have shown, may contribute to negative findings, similar to the manner in which heterogeneity has been cited as the cause of failed clinical trials in HF.27, 28 Taken together, these findings suggest that future interventions aimed at reducing 30‐day readmissions should probably target HFpEF and HFrEF separately.
Some important limitations should be noted. First, this retrospective cohort study was derived from a 5% sample of Medicare, which does not provide direct measures of health status, severity of concurrent comorbidities, or in‐hospital treatment. Data on, for example, blood pressure and heart rate, need for home oxygen, medication use, and laboratory values like baseline renal function and B‐type natriuretic peptide were unavailable. Nonetheless, Medicare data have been used extensively to examine patterns of readmission among older adults with HF,17, 29 representing a nationally representative and geographically diverse sample. Second, we identified HF cases based on the presence of HF‐based ICD‐9 codes as the principal diagnosis. Although the sensitivity of utilizing claims data to identify HF has been validated,30 it is possible that we included cases for which HF was a secondary issue but classified as a principal diagnosis (ie, upcoded) for billing purposes. Finally, echocardiographic data were not available to confirm ejection fraction. Prior studies have demonstrated that patients with HFpEF and HFrEF based on claims data have characteristics and outcomes similar to patients enrolled in registries and community‐based studies,13, 29 supporting their validity for examining subtypes of HF.
In conclusion, our study revealed that non–cardiovascular‐related diagnoses represented the most common causes of 30‐day readmission following HF hospitalization for each week of the 30‐day postdischarge period for both HFpEF and HFrEF participants. We also showed that HF readmissions were less common among those with HFpEF compared with HFrEF. Future interventions aimed at reducing 30‐day readmissions following an HF hospitalization would benefit from an increased focus on noncardiovascular comorbidity and interventions that target HFpEF and HFrEF separately.
Sources of Funding
Funding for this project was provided by an academic collaboration between University of Alabama at Birmingham and Amgen Inc. The funders provided comments on the design and interpretation of this work. The academic authors conducted all analyses and maintained the rights to publish this article.
Disclosures
Dr Goyal was the recipient of the 2016–2017 Glorney‐Raisbeck Fellowship Award in Cardiovascular Disease from the New York Academy of Medicine during a portion of the research. Dr Goyal is currently supported by National Institute on Aging grant R03AG056446. Dr Loop reports salary support from Amgen during a portion of the research. Dr Brown reports research support from Amgen. Dr Safford reports research support from Amgen. Dr Levitan reports research support from Amgen, serving on Amgen advisory boards, and consulting income from Novartis. The other authors report no conflicts.
Supporting information
Figure S1. Flowchart for included cases.
Acknowledgments
Drs Levitan, Chen, and Loop had access to the data, and all authors had a role in writing the article.
(J Am Heart Assoc. 2018;7:e007785 DOI: 10.1161/JAHA.117.007785.)29686028
References
- 1. Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Wang Y, Lin Z, Straube BM, Rapp MT, Normand SL, Drye EE. Patterns of hospital performance in acute myocardial infarction and heart failure 30‐day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407–413. [DOI] [PubMed] [Google Scholar]
- 2. Agency for Healthcare Research and Quality . Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; April 2014. Statistical brief 172. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb172-Conditions-Readmissions-Payer.pdf. Accessed September 16, 2016. [Google Scholar]
- 3. Center for Medicare and Medicaid . Readmissions Reduction Program. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed March 23, 2017.
- 4. Feltner C, Jones CD, Cene CW, Zheng ZJ, Sueta CA, Coker‐Schwimmer EJ, Arvanitis M, Lohr KN, Middleton JC, Jonas DE. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160:774–784. [DOI] [PubMed] [Google Scholar]
- 5. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community‐based cohort: 11‐year follow‐up of PREVEND. Eur Heart J. 2013;34:1424–1431. [DOI] [PubMed] [Google Scholar]
- 6. Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, Kjeldsen K, Jankowska EA, Atar D, Butler J, Fiuzat M, Zannad F, Pitt B, O'Connor CM. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Pocock S, Pfeffer MA. Effect of candesartan on cause‐specific mortality in heart failure patients: the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2004;110:2180–2183. [DOI] [PubMed] [Google Scholar]
- 8. Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez‐Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE; Investigators IP . Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I‐Preserve) trial. Circulation. 2010;121:1393–1405. [DOI] [PubMed] [Google Scholar]
- 9. Huang D, Cheng JW. Pharmacologic management of heart failure with preserved ejection fraction. Ann Pharmacother. 2010;44:1933–1945. [DOI] [PubMed] [Google Scholar]
- 10. Yun HKM, Curtis JR, Delzell E, Gary LC, Saag KG, Morrisey MA, Becker D, Matthews R, Smith W, Locher JL. Identifying types of nursing facility stays using Medicare claims data: an algorithm and validation. Health Serv Outcomes Res Methodol. 2010;1:100–102. [Google Scholar]
- 11. Elixhauser A, Steiner C, Palmer L. Clinical Classifications Software (CCS), 2014. US Agency for Healthcare Research and Quality Available at: https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed March 23, 2017.
- 12. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13. Goyal P, Almarzooq ZI, Horn EM, Karas MG, Sobol I, Swaminathan RV, Feldman DN, Minutello RM, Singh HS, Bergman GW, Wong SC, Kim LK. Characteristics of hospitalizations for heart failure with preserved ejection fraction. Am J Med. 2016;129:635.e15–635.e626. [DOI] [PubMed] [Google Scholar]
- 14. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC; Committee ASA Investigators . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 15. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB; Investigators O‐H Hospitals . Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF Registry. J Am Coll Cardiol. 2007;50:768–777. [DOI] [PubMed] [Google Scholar]
- 16. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 17. Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto‐Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. [DOI] [PubMed] [Google Scholar]
- 19. Centers for Medicare & Medicaid Services . Chronic Conditions among Medicare Beneficiaries. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Chartbook_Charts.html. Accessed March 23, 2017.
- 20. Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition–multimorbidity. JAMA. 2012;307:2493–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kociol RD, Peterson ED, Hammill BG, Flynn KE, Heidenreich PA, Pina IL, Lytle BL, Albert NM, Curtis LH, Fonarow GC, Hernandez AF. National survey of hospital strategies to reduce heart failure readmissions: findings from the Get With the Guidelines‐Heart Failure registry. Circ Heart Fail. 2012;5:680–687. [DOI] [PubMed] [Google Scholar]
- 22. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 23. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force M, Document R . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 24. Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. [DOI] [PubMed] [Google Scholar]
- 25. Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post‐discharge Follow‐up characteristics associated with 30‐day readmission after heart failure hospitalization. Med Care. 2016;54:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC; Get With the Guidelines Scientific Advisory C, Investigators . Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- 27. Francis GS, Cogswell R, Thenappan T. The heterogeneity of heart failure: will enhanced phenotyping be necessary for future clinical trial success? J Am Coll Cardiol. 2014;64:1775–1776. [DOI] [PubMed] [Google Scholar]
- 28. Marantz PR, Alderman MH, Tobin JN. Diagnostic heterogeneity in clinical trials for congestive heart failure. Ann Intern Med. 1988;109:55–61. [DOI] [PubMed] [Google Scholar]
- 29. Loop MS, Van Dyke MK, Chen L, Brown TM, Durant RW, Safford MM, Levitan EB. Comparison of length of stay, 30‐day mortality, and 30‐day readmission rates in Medicare patients with heart failure and with reduced versus preserved ejection fraction. Am J Cardiol. 2016;118:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Q, Glynn RJ, Dreyer NA, Liu J, Mogun H, Setoguchi S. Validity of claims‐based definitions of left ventricular systolic dysfunction in Medicare patients. Pharmacoepidemiol Drug Saf. 2011;20:700–708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flowchart for included cases.


