Abstract
Background
While adherence to healthful dietary patterns has been associated with a lower risk of coronary artery disease (CAD) in the general population, limited data are available among US veterans. We tested the hypothesis that adherence to Dietary Approach to Stop Hypertension (DASH) food pattern is associated with a lower risk of developing CAD among veterans.
Methods and Results
We analyzed data on 153 802 participants of the Million Veteran Program enrolled between 2011 and 2016. Information on dietary habits was obtained using a food frequency questionnaire at enrollment. We used electronic health records to assess the development of CAD during follow‐up. Of the 153 802 veterans who provided information on diet and were free of CAD at baseline, the mean age was 64.0 (SD=11.8) years and 90.4% were men. During a mean follow‐up of 2.8 years, 5451 CAD cases occurred. The crude incidence rate of CAD was 14.0, 13.1, 12.6, 12.3, and 11.1 cases per 1000 person‐years across consecutive quintiles of Dietary Approach to Stop Hypertension score. Hazard ratios (95% confidence interval) for CAD were 1.0 (ref), 0.91 (0.84–0.99), 0.87 (0.80–0.95), 0.86 (0.79–0.94), and 0.80 (0.73–0.87) from the lowest to highest quintile of Dietary Approach to Stop Hypertension score controlling for age, sex, body mass index, race, smoking, exercise, alcohol intake, and statin use (P linear trend, <0.0001).
Conclusions
Our data are consistent with an inverse association between Dietary Approach to Stop Hypertension diet score and incidence of CAD among US veterans.
Keywords: coronary artery disease, epidemiology, nutrition
Subject Categories: Cardiovascular Disease, Epidemiology, Lifestyle
Clinical Perspective
What Is New?
This is the first study to show benefits of adherence to Dietary Approach to Stop Hypertension dietary approach to stop hypertension dietary pattern on the risk of coronary artery disease among US veterans.
What Are the Clinical Implications?
Based on these findings and existing literature in the general population, clinicians can encourage veterans to adhere to Dietary Approach to Stop Hypertension diet, which is rich in fruits, vegetables, whole grains, poultry, fish, nuts, low‐fat dairy, and low sodium to reduce the risk of coronary artery disease.
Introduction
Every 40 seconds, 1 American will suffer a myocardial infarction.1 Coronary artery disease (CAD) remains the leading cause of death in the United States, thereby underscoring the need for primary prevention. Despite the proven efficacy of statins to reduce serum cholesterol and other effective cardiovascular drugs available for primary2 and secondary prevention,3, 4 it remains important to incorporate modifiable lifestyle factors, such as diet, in a comprehensive approach to reduce the burden of CAD. Recent data underscore the efficacy of overall dietary pattern (over focus on individual foods) in reducing the burden of chronic disease.5 Previous trials have demonstrated health benefits of Dietary Approach to Stop Hypertension (DASH) on blood pressure6 or the efficacy of the Mediterranean diet on cardiometabolic disorders7, 8, 9 in the general population. However, little is known about the benefits of adherence to healthy dietary patterns such as DASH on CAD risk in a large cohort of US veterans. More important, data from the National Health and Nutrition Examination Survey10 and other cohorts11, 12, 13, 14 have reported an extremely low proportion of adults that are ideal on dietary recommendation (<6%). This suggests that there is room for improvement in modifiable risk factors such as diet, which can translate into reduction of CAD burden and cost‐saving.
Therefore, the aim of this project is to curate collected nutritional data in the Million Veteran Program (MVP) and test the primary hypothesis that a healthy dietary pattern, as constructed using the modified DASH, is associated with a lower risk of CAD among veterans. In a secondary hypothesis, we sought to determine whether body mass index (BMI) and race/ethnicity (white, black, and other ethnic groups) modifies the relation between DASH score and the incidence of CAD among veterans.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure, pursuant to the US Department of Veterans Administration policies on compliance with confidentiality of US veterans’ data.
Population
The MVP is an ongoing prospective cohort study and mega‐biobank in the Department of Veterans Affairs Healthcare System designed to study genetic influences on health and disease among veterans. Eligible subjects must be active users of the Veteran Health Administration (with available electronic health record) and willing to provide informed consent. We began enrollment in 2011, initially at VA facilities in Boston, Massachusetts, and West Haven, Connecticut. Subsequently, other recruitment centers were added nationwide. As of February 2017, a total of 504 988 veterans have been enrolled. Detailed description of MVP design and methods has been published.15 Current analyses are restricted to 153 802 participants who provided data on diet during enrollment and were free of CAD at baseline. All participants provided informed consent, and the study protocol was approved by the Veterans Affairs Central Institutional Review Board.
Exposure
We used a Willett food frequency questionnaire to collect baseline information on dietary habits among veterans. Subjects were asked the following question: “For each food listed, please mark the column indicating how often, on average, you have used the amount specified during the past year.” Possible answers were: “Never or less than once per month”; “1 to 3 per month”; “once (1) a week”; “2 to 4 per week”; “5 to 6 per week”; “once (1) a day”; “2 to 3 per day”; “4 to 5 per day”; and “6+ per day.” Food groups listed included dairy foods, fruit and vegetables, meats, sweets, baked goods, cereals, and beverages. We constructed a modified DASH score based on the following 7 components: fruits, vegetables, nuts and legumes, low‐fat dairy products, whole grain, sweetened beverages, and red and processed meats.16 Currently, the MVP does not have nutrient data to allow for dietary sodium inclusion in the DASH score. We created quintiles for each of the 7 components and assigned quintile ranking for foods that are encouraged in DASH (all but sweetened beverages and meats).16 For meats and sweetened beverages, where low intake is desired in DASH, we assigned a lowest value for quintile 5 and highest value for quintile 1 (example: a veteran in the fifth quintiles of red/processed meats was assigned a value of 1). Finally, we summed up the component scores to obtain an overall DASH score ranging from 7 to 35.
Primary Outcome
Primary CAD was defined as nonfatal myocardial infarction (International Classification of Diseases, Ninth Revision [ICD‐9] Codes 410–414 and International Classification of Diseases, Tenth Revision [ICD‐10] codes I20–I25).
Secondary Outcome
The secondary outcome included primary CAD as defined above plus coronary death, coronary angioplasty, and coronary revascularization using following procedure codes (Current Procedural Terminology Codes 33510–33536, 9292x, 9293x, 9294x, 92973, 92974, and 92975; ICD9 Procedure codes 36.x and 00.66).
Covariates
We used baseline survey data to obtain information on demographics, anthropometric measures, alcohol use, statin use, and comorbidities. We used self‐reported weight and height at baseline to compute BMI (weight [kg] divided by height squared [m2]). For physical activity variable, subjects were asked the following question: “How often do you exercise vigorously enough to work up a sweat?” Possible answers were: daily; 5 to 6 times a week; 2 to 4 times a week; once a week; 1 to 3 times a month; and rarely/never.
Statistical Analysis
We computed person‐time of follow‐up from food frequency questionnaire assessment until the first occurrence of CAD, death, or last clinic visit recorded within the Corporate Data Warehouse database. Because we did not assume a linear relation a priori, we modeled DASH score as continuous variable using restricted cubic splines (using LGTPHCURV9 macro) in our primary analysis.17 We placed knots at DASH score of 18, 21, and 25. Secondarily, we also modeled DASH score as quintiles. For each quintile of DASH score, we computed the incidence rate of CAD by using a propensity‐adjusted Poisson regression model with log‐transformed person‐time as the offset variable. We used Cox proportional hazard models to estimate hazard ratios with 95% confidence interval (CI) of CAD. Proportional hazard assumptions were tested by using the product term of log‐transformed person‐time and DASH score in a hierarchical model (P value, 0.57). We created sequential models to assess and minimize confounding based on a priori knowledge: after the crude model, we adjusted for age (continuous) in model 2. The final model controlled for age, sex, BMI, race, smoking (never, former, and current smokers), exercise, alcohol intake, and statin use. These variables were selected based on their reported association with dietary intake and risk of developing CAD in the literature. In secondary analyses, we stratified by sex, BMI (<25 versus 25+ kg/m2), ethnic group (white, black, and others), and age (<65 and 65+). Last, we repeated main analyses using competing risk model to test the robustness of the findings.
Results
Of the 153 802 veterans included in current analyses, 90.4% were men, 81.6% white, 10.8% blacks, and mean age was 64.0 (SD=11.8) years. Median DASH score was 21 (25th and 75th percentile: 18 and 25, respectively). Higher DASH score was associated with older age, lower BMI, female sex, lower prevalence of current smoking, statin use, diabetes mellitus, and hypertension (Table 1). During a mean follow‐up of 2.8 years (range, 0.1–6.2), 5451 new diagnoses of myocardial infarction occurred. Analysis using restricted cubic spline showed an inverse and linear association between DASH score and incidence of CAD (P for linearity, <0.0001; Figure) and no evidence for nonlinearity (P for nonlinearity, 0.37).
Table 1.
Baseline Characteristics of 153 802 Million Veteran Program Participants by Quintiles of DASH Scorea
| Characteristics | Quintiles of DASH Score | ||||
|---|---|---|---|---|---|
| Q1 (n=27 969) | Q2 (n=28 552) | Q3 (n=33 235) | Q4 (n=30 704) | Q5 (n=33 342) | |
| Median DASH score (IQR) | 15 (7–16) | 18 (17–19) | 21 (20–22) | 24 (23–25) | 28 (26–35) |
| Age, y | 61.5±11.3 | 63.1±11.5 | 64.2±11.7 | 65.0±11.9 | 65.9±12.3 |
| BMI, kg/m2 | 29.4±5.8 | 29.6±5.7 | 29.5±5.5 | 29.2±5.4 | 28.5±5.3 |
| Sex (% male) | 92.3 | 91.0 | 91.0 | 90.3 | 87.8 |
| Race/ethnicity, % | |||||
| Black | 14.9 | 12.1 | 10.3 | 9.5 | 7.9 |
| White | 77.5 | 80.1 | 82.4 | 83.0 | 84.3 |
| Hispanic, % | 5.3 | 5.0 | 4.9 | 4.6 | 5.0 |
| Smoking, % | |||||
| Never | 26.1 | 28.0 | 28.8 | 31.0 | 34.2 |
| Former | 44.9 | 50.9 | 53.8 | 55.0 | 55.6 |
| Current | 29.1 | 21.1 | 17.4 | 14.1 | 10.2 |
| Exercise (5+times/w) | 10.1 | 11.6 | 13.4 | 16.0 | 21.1 |
| Alcohol, % | |||||
| Never | 7.3 | 6.9 | 6.8 | 6.9 | 7.6 |
| Former | 42.2 | 38.5 | 36.3 | 34.7 | 33.3 |
| Current drinker | 50.6 | 54.5 | 57.0 | 58.3 | 59.1 |
| Statin use, % | 52.9 | 53.4 | 52.4 | 50.6 | 47.6 |
| Diabetes mellitus, % | 24.8 | 26.1 | 25.9 | 24.7 | 21.2 |
| Hypertension, % | 61.3 | 61.7 | 60.5 | 58.6 | 55.0 |
BMI indicates body mass index; DASH, dietary approach to stop hypertension.
Mean±SD or percentage.
Figure 1.
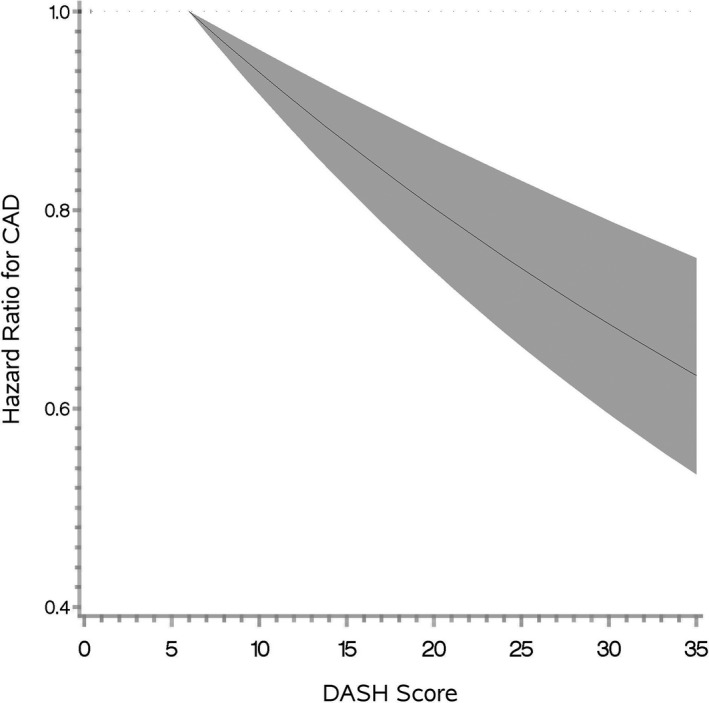
Relation between DASH score and incidence of myocardial infarction in the Million Veteran Program (hazard ratio and 95% confidence interval band); P for linearity <0.0001. CAD indicates coronary artery disease; DASH, dietary approach to stop hypertension.
When DASH was modeled as quintiles, crude incidence rates of myocardial infarction were 14.0, 13.1, 12.6, 12.3, and 11.1 cases per 1000 person‐years from the lowest to highest quintile of DASH score, respectively. The inverse relation between DASH score and incidence of myocardial infarction persisted in minimally and fully adjusted Cox regression model with hazard ratios (95% CI) of 1.0 [ref], 0.91 [0.84–0.99], 0.87 [0.80–0.95], 0.86 [0.79–0.94], and 0.80 [0.73–0.87] across consecutive quintile of DASH score (P for linear trend, <0.0001; Table 2), in a model controlling for age, sex, race, BMI, smoking, exercise, alcohol use, and statin use.
Table 2.
Incidence Rate and Hazard Ratio (95% CI) for Myocardial Infarction by Quintiles of DASH Score in the Million Veteran Program
| Quintiles of DASH Score | ||||||
|---|---|---|---|---|---|---|
| Q1 (Low) (n=27 969) | Q2 (n=28 552) | Q3 (n=33 235) | Q4 (n=30 704) | Q5 (High) (n=33 342) | P Linear Trend | |
| Cases of myocardial infarction | 1084 | 1040 | 1178 | 1078 | 1071 | |
| Crude incidence rate (/1000 PY) | 14.0 | 13.1 | 12.6 | 12.3 | 11.1 | |
| Crude hazard ratio (95% CI) | 1.0 (ref) | 0.93 (0.86–1.02) | 0.9 (0.83–0.98) | 0.88 (0.81–0.96) | 0.80 (0.73–0.87) | <0.0001 |
| Model 1a | 1.0 (ref) | 0.88 (0.81–0.96) | 0.82 (0.76–0.89) | 0.78 (0.72–0.85) | 0.68 (0.63–0.74) | <0.0001 |
| Model 2b | 1.0 (ref) | 0.91 (0.84–0.99) | 0.87 (0.8–0.95) | 0.86 (0.79–0.94) | 0.80 (0.73–0.87) | <0.0001 |
CI indicates confidence interval; DASH, dietary approach to stop hypertension; PY, person‐years.
Age‐adjusted.
Adjusted for age, sex, race, smoking, body mass index, exercise, alcohol, and statin use.
We observed similar inverse relation between DASH score and incidence of total CAD including myocardial infarction, CAD death, coronary angioplasty, and revascularization (Table 3). In secondary analyses, we observed no statistically significant interaction between DASH score and sex (P=0.72), DASH score with age (P=0.86), DASH score with ethnicity (P=0.59), and DASH score with BMI (P=0.22). In a sensitivity analysis, exclusion of CAD events that occurred during the first 1 year (a marker of extreme frailty) did not alter the results (data not shown). Finally, using a competing risk model, we found a similar inverse relation of DASH score with CAD risk (multivariable adjusted hazard ratios [95% CI] were 1.0 [ref], 0.91 [0.84–0.99], 0.87 [0.80–0.95], 0.86 [0.79–0.94], and 0.80 [0.74–0.88] across consecutive quintiles of DASH score).
Table 3.
Incidence Rate and Hazard Ratio (95% CI) for Total CAD (Myocardial Infarction, CAD Death, CABG, and PTCA) by Quintiles of DASH Score in the MVP
| Quintiles of DASH Score | P Linear Trend | |||||
|---|---|---|---|---|---|---|
| Q1 (n=27 969) | Q2 (n=28 552) | Q3 (n=33 235) | Q4 (n=30 704) | Q5 (n=33 342) | ||
| Cases of total CAD | 1122 | 1093 | 1233 | 1124 | 1127 | |
| Crude incidence rate (/1000 PY) | 14.5 | 13.7 | 13.2 | 12.9 | 11.7 | |
| Crude hazard ratio (95% CI) | 1.0 (ref) | 0.95 (0.87–1.03) | 0.91 (0.84–0.99) | 0.89 (0.82–0.97) | 0.81 (0.75–0.88) | <0.0001 |
| Model 1a | 1.0 (ref) | 0.89 (0.82–0.97) | 0.83 (0.76–0.90) | 0.78 (0.72–0.85) | 0.69 (0.63–0.75) | <0.0001 |
| Model 2b | 1.0 (ref) | 0.93 (0.85–1.01) | 0.88 (0.81–0.96) | 0.87 (0.80–0.95) | 0.82 (0.75–0.89) | <0.0001 |
CABG indicates coronary artery bypass graft; CAD, coronary artery disease; CI, confidence interval; DASH, dietary approach to stop hypertension; MVP, Million Veteran Program; PTCA, percutaneous transluminal coronary angioplasty.
Age‐adjusted.
Adjusted for age, sex, race, smoking, body mass index, exercise, alcohol, and statin use.
Discussion
In this large, prospective cohort of US veterans, we reported, for the first time, an inverse association between DASH score and incidence of CAD after controlling for major confounders. Our findings were robust in subanalyses including CAD death, revascularization, and angioplasty; stratification by sex, BMI, and race; and exclusion of events that occurred during the first year of follow‐up.
These findings are in line with the report from the NHS (Nurses’ Health Study), which showed an inverse relation between DASH score and incidence of CAD: Multivariable adjusted relative risks (95% CI) were 1.0 (ref), 0.98 (0.88–1.10), 0.84 (0.75–0.94), 0.85 (0.76–0.96), and 0.73 (0.64–0.84) across consecutive quintiles of DASH score (P trend, <0.001).18 It is noteworthy that the magnitude of benefit was larger in the NHS18 (27% lower risk) than in the MVP (22% lower risk) when comparing extreme quintiles of DASH score; this may be partially explained by a lower risk of CAD in women compared with men (91.3% men in MVP) and longer follow‐up in the NHS18 (24 years). Alternatively, measurement error in diet and/or difference in composition and distribution of DASH components between the 2 studies might account for the apparent difference in reported effect sizes. Earlier analysis of the NHS found an inverse and graded association between prudent diet score (which shares several components with DASH) and risk of CAD.19 Data from the HPFS (Health Professionals Follow‐Up Study) reported an inverse relation between prudent dietary pattern similar to DASH and incidence of CAD with a 25% lower risk comparing highest to lowest quintile.20 In a Mexican cohort of predominantly female subjects, adherence to prudent dietary pattern, which was characterized by high consumption of fruit, vegetables, and whole grains, was associated with a lower risk of CAD; specifically, being in the fifth quintile of such a dietary pattern was associated with a 60% lower risk of cardiovascular disease compared with the first quintile.21 Data from the Whitehall II study also showed a 29% lower risk of CAD (95% CI, 2–49) when comparing a healthy dietary pattern (rich in fruit, vegetables, whole‐meal bread, low‐fat dairy, and little alcohol) with unhealthy diet.22 Findings from the third National Health and Nutrition Examination Survey also showed suggestive (albeit nonstatistically significant) lower risk of ischemic heart disease death when comparing DASH‐like diet with typical diet (hazard ratio=0.77 [95% CI, 0.47–1.14]).23 A major limitation of the National Health and Nutrition Examination Survey study was a lack of statistical power with only 36 events in the DASH‐like group; but the magnitude of the effect size is comparable to what we reported in the MVP. A meta‐analysis of observational studies reported a 21% lower risk of CAD when comparing the highest to the lowest DASH score (relative risk=0.79 [95% CI, 0.71–0.88]).24 Furthermore, a more‐recent meta‐analysis of 11 prospective studies reported similar effect size for the incidence of cardiovascular disease or cardiovascular mortality (pooled relative risk=0.80 [95% CI, 0.78–0.85]) with limited heterogeneity (I2=30%).25
Not all studies have reported inverse relation between DASH diet and incidence of CAD. In a prospective study of 20 993 women aged 55 to 69 years at baseline, concordance to DASH diet was not associated with a statistically significant lower risk of CAD deaths (relative risk [95% CI] 1.0 [ref], 0.85 [0.64–1.27], 1.02 [0.81–1.27], 1.14 [0.91–1.44], and 0.86 [0.67–1.12] from the lowest to the highest category of DASH score, respectively, after adjustment for age, energy intake, education, BMI, smoking, alcohol intake, physical activity, and use of estrogen and multivitamins; P trend, 0.69).26 It is important to note that this study had limited statistical power to detect small effect size, with only 100 events in the highest DASH score category. In addition, women in the IWHS (Iowa Women's Health Study)26 had lower BMI (26 kg/m2) than MVP subjects (29 kg/m2) and had a lower proportion of current smokers.
What potential biological mechanism can causally relate adherence to DASH diet and CAD incidence? Randomized trials have found that intervention with DASH diet lowers blood pressure6, 27 and improves lipid profiles.28, 29 Following a DASH diet has also been reported to be associated with a lower risk of type 2 diabetes mellitus; a meta‐analysis of 4 prospective studies showed a 22% lower risk of diabetes mellitus when comparing the highest to the lowest DASH score categories (95% CI, 15% to 28%).25 An intervention with DASH diet has also been shown to reduce C‐reactive protein30 and reduce serum insulin.31, 32 Meta‐analysis of 7 randomized trials showed that a DASH diet significantly reduced fasting insulin concentration (mean difference, −0.15 [95% CI, −0.22 to −0.08]).32
Our study has some limitations. First, assessment of diet was self‐reported and we cannot exclude bias caused by exposure misclassification, although it is reasonable to assume that people tend to be consistent with their dietary habits over time.33 However, such misclassification of dietary variable is more likely to be nondifferential given that subjects were free of CAD at the time of diet assessment. Nonetheless, misclassification and/residual confounding could explain observed findings. Our assumption is that it is unlikely that people would drastically change their dietary habits, and it is reasonable to assume that baseline diet reflects subjects’ diet during preceding years.33 Second, the MVP cohort has a short follow‐up to allow adequate lag time for the exposure to influence CAD risk. Third, most subjects in our cohort were men; nonetheless, we observed similar results when stratified by sex. Fourth, CAD was ascertained using ICD codes from electronic health records (a validated method to identify CAD in electronic health records34); though very unlikely, it is possible that we might have missed a few CAD events treated at non–Veterans Affairs facilities, but we do not believe that such missingness would be related to DASH score. Fifth, we did not have information on nutrients including dietary sodium intake to construct a full DASH score. It is likely that low‐sodium intake is correlated with other healthful behaviors, such as high intake of whole grain, fruit, and vegetables. Furthermore, a lack of data on fatty acids (trans, polyunsaturated, and omega‐3 fatty acids) precluded the use of the Alternate Healthy Eating Index, which is based on achieving dietary recommendations rather than cohort‐specific quintiles used in DASH score computation. We acknowledge our inability to truly contrast results obtained from DASH score between our cohort and other studies because such a score is cohort dependent and does not allow for a fair comparison (unlike the Alternate Healthy Eating Index).
Despite these limitations, our study has numerous strengths, including a large sample size with several thousands of events allowing for adequate adjustment and subgroup analyses; availability of data on several relevant confounders both from baseline survey and electronic health records; and the robustness of our findings to subgroup analyses.
In conclusion, our data support the hypothesis that adherence to DASH diet is associated with a lower risk of CAD among US veterans. This finding emphasizes the importance of modifiable lifestyle factors including healthy diet in the prevention of CAD.
Appendix
We are grateful to the Million Veteran Program participants and staff. Participating centers are: VA Boston Healthcare System (Ildiko Halasz); VA Connecticut Health Care System (Daniel Federman); Durham VA Medical Center (Jean Beckham); VA New York Harbor Healthcare System (Scott E. Sherman); N. FL/S. GA Veterans Health System (Peruvemba Sriram); VA Palo Alto Health Care System (Philip S. Tsao); VA Puget Sound Health Care System (Edward J. Boyko); VA Western New York Healthcare System (Junzhe Xu); Minneapolis VA Health Care System (Frank Lederle); Birmingham VA Medical Center (Louis J. Dellitalia); Bay Pines VA Healthcare System (Rachel McArdle); VA Health Care System Upstate New York (Laurence Kaminsky); Michael E. DeBakey VA Medical Center (Alan C. Swann); Ralph H. Johnson VA Medical Center (Mark B. Hamner); Miami VA Health Care System (Hermes J. Florez); Kansas City VA Medical Center (Prashant Pandya); New Mexico VA Health Care System (Gerardo Villarreal); Atlanta VA Medical Center (Peter Wilson); VA Long Beach Healthcare System (Timothy R. Morgan); Tuscaloosa VA Medical Center (Lori Davis); W.G. (Bill) Hefner VA Medical Center (Robin A. Hurley); VA Salt Lake City Health Care System (Laurence Meyer); South Texas Veterans Health Care System (Sunil K. Ahuja); Louis Stokes Cleveland VA Medical Center (Eric P. Konicki); Portland VA Medical Center (David Cohen); Washington DC VA Medical Center (Jack Lichy); Clement J. Zablocki VA Medical Center (Jeffrey Whittle); Wm. Jennings Bryan Dorn VA Medical Center (Kathlyn Sue Haddock); Central Arkansas Veterans Health Care System (Karl D. Straub); Richard Roudebush VA Medical Center (John T. Callaghan); Phoenix VA Health Care System (Samuel M. Aguayo); VA San Diego Healthcare System (Samir Gupta); Overton Brooks VA Medical Center (Ronald G. Washburn); VA Eastern Kansas Health Care System (Mary E. Oehlert); VA Tennessee Valley Healthcare System (Adriana M. Hung); VA Greater Los Angeles Health Care System (Agnes Wallbom); VA Eastern Colorado Health Care System (Robert Keith); Central Texas Veterans Health Care System; VA Pittsburgh Health Care System (Elif Sonel); Southern Arizona VA Health Care System (Ronald B. Schifman); Memphis VA Medical Center (Richard D. Childress); Hunter Holmes McGuire VA Medical Center (Michael F. Godschalk); VA Maryland Health Care System (Alan R. Shuldiner); VA North Texas Health Care System (Padmashri Rastogi); Edward Hines, Jr. VA Medical Center (Salvador Gutierrez); VA Loma Linda Healthcare System (Ronald Fernando); Hampton VA Medical Center (Pran R. Iruvanti); Philadelphia VA Medical Center (Darshana Jhala); VA Caribbean Healthcare System (Carlos Rosado‐Rodriguez); James A. Haley Veterans’ Hospital (Stephen M. Mastorides); Cincinnati VA Medical Center (John B. Harley); Central Western Massachusetts Healthcare System (Kristin Mattocks); White River Junction VA Medical Center Brooks Robey (none); William S. Middleton Memorial Veterans Hospital (Robert T. Striker); St. Louis VA Health Care System (Michael Rauchman); Edith Nourse Rogers Memorial Veterans Hospital (John Wells); Iowa City VA Health Care System (Zuhair K. Ballas); VA Maine Healthcare System (Susan S. Woods); Northport VA Medical Center (Shing Yeh); Manchester VA Medical Center (Nora R. Ratcliffe); Louisville VA Medical Center (Jon B. Klein); Orlando VA Medical Center (Adam G. Golden); Jack C. Montgomery VA Medical Center (Harold M. Ginzburg); Providence VA Medical Center (Satish Sharma); Salem VA Medical Center (Kris Ann K. Oursler); San Francisco VA Health Care System (Mary A. Whooley); Fayetteville VA Medical Center (Gretchen Gibson); Brecksville; VA Pittsburgh VA Healthcare System (Heinz); Highland Drive.
Sources of Funding
This research is based on data from the Million Veteran Program, Office of Research and Development, Veterans Health Administration, and was supported by award CSP# G002. This research was also supported by the VA Merit Award I01‐CX001025. This publication does not represent the views of the Department of Veterans Affairs or the U.S. government.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e008089 DOI: 10.1161/JAHA.117.008089.)29680824
Contributor Information
Luc Djoussé, Email: Ldjousse@rics.bwh.harvard.edu.
the VA Million Veteran Program:
Ildiko Halasz, Daniel Federman, Jean Beckham, Scott E. Sherman, Peruvemba Sriram, Philip S. Tsao, Edward J. Boyko, Junzhe Xu, Frank Lederle, Louis J. Dellitalia, Rachel McArdle, Laurence Kaminsky, Alan C. Swann, Mark B. Hamner, Hermes J. Florez, Prashant Pandya, Gerardo Villarreal, Peter Wilson, Timothy R. Morgan, Lori Davis, Robin A. Hurley, Laurence Meyer, Sunil K. Ahuja, Eric P. Konicki, David Cohen, Jack Lichy, Jeffrey Whittle, Kathlyn Sue Haddock, Karl D. Straub, John T. Callaghan, Samuel M. Aguayo, Samir Gupta, Ronald G. Washburn, Mary E. Oehlert, Adriana M. Hung, Agnes Wallbom, Robert Keith, Elif Sonel, Ronald B. Schifman, Richard D. Childress, Michael F. Godschalk, Alan R. Shuldiner, Padmashri Rastogi, Salvador Gutierrez, Ronald Fernando, Pran R. Iruvanti, Darshana Jhala, Carlos Rosado‐Rodriguez, Stephen M. Mastorides, John B. Harley, Kristin Mattocks, Robert T. Striker, Michael Rauchman, John Wells, Zuhair K. Ballas, Susan S. Woods, Shing Yeh, Nora R. Ratcliffe, Jon B. Klein, Adam G. Golden, Harold M. Ginzburg, Satish Sharma, Kris Ann K. Oursler, Mary A. Whooley, and Gretchen Gibson
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics—2017 Update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steering Committee of the Physicians’ Health Study Research Group . Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–135. [DOI] [PubMed] [Google Scholar]
- 3. Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, Gupta R, Kelishadi R, Iqbal R, Avezum A, Kruger A, Kutty R, Lanas F, Lisheng L, Wei L, Lopez‐Jaramillo P, Oguz A, Rahman O, Swidan H, Yusoff K, Zatonski W, Rosengren A, Teo KK; Prospective Urban Rural Epidemiology (PURE) Study Investigators . Use of secondary prevention drugs for cardiovascular disease in the community in high‐income, middle‐income, and low‐income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378:1231–1243. [DOI] [PubMed] [Google Scholar]
- 4. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 5. Freeland‐Graves JH, Nitzke S; Academy of N and Dietetics . Position of the academy of nutrition and dietetics: total diet approach to healthy eating. J Acad Nutr Diet. 2013;113:307–317. [DOI] [PubMed] [Google Scholar]
- 6. Saneei P, Salehi‐Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta‐analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253–1261. [DOI] [PubMed] [Google Scholar]
- 7. Ros E, Martinez‐Gonzalez MA, Estruch R, Salas‐Salvado J, Fito M, Martinez JA, Corella D. Mediterranean diet and cardiovascular health: teachings of the PREDIMED study. Adv Nutr. 2014;5:330S–336S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lopez‐Garcia E, Rodriguez‐Artalejo F, Li TY, Fung TT, Li S, Willett WC, Rimm EB, Hu FB. The Mediterranean‐style dietary pattern and mortality among men and women with cardiovascular disease. Am J Clin Nutr. 2014;99:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salas‐Salvadó J, Guasch‐Ferré M, Lee CH, Estruch R, Clish CB, Ros E. Protective effects of the Mediterranean diet on type 2 diabetes and metabolic syndrome. J Nutr. 2016;146:920S–927S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd‐Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012;125:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Djousse L, Petrone AB, Blackshear C, Griswold M, Harman JL, Clark CR, Talegawkar S, Hickson DA, Gaziano JM, Dubbert PM, Correa A, Tucker KL, Taylor HA. Prevalence and changes over time of ideal cardiovascular health metrics among African‐Americans: the Jackson Heart Study. Prev Med. 2015;74:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graciani A, Leon‐Munoz LM, Guallar‐Castillon P, Rodriguez‐Artalejo F, Banegas JR. Cardiovascular health in a southern Mediterranean European country: a nationwide population‐based study. Circ Cardiovasc Qual Outcomes. 2013;6:90–98. [DOI] [PubMed] [Google Scholar]
- 13. Rasmussen‐Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation. 2013;127:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forget G, Doyon M, Lacerte G, Labonte M, Brown C, Carpentier AC, Langlois MF, Hivert MF. Adoption of American Heart Association 2020 ideal healthy diet recommendations prevents weight gain in young adults. J Acad Nutr Diet. 2013;113:1517–1522. [DOI] [PubMed] [Google Scholar]
- 15. Gaziano JM, Concato J, Brophy M, Fiore L, Pyarajan S, Breeling J, Whitbourne S, Deen J, Shannon C, Humphries D, Guarino P, Aslan M, Anderson D, LaFleur R, Hammond T, Schaa K, Moser J, Huang G, Muralidhar S, Przygodzki R, O'Leary TJ. Million Veteran Program: a mega‐biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214–223. [DOI] [PubMed] [Google Scholar]
- 16. Bertoia ML, Triche EW, Michaud DS, Baylin A, Hogan JW, Neuhouser ML, Tinker LF, Van Horn L, Waring ME, Li W, Shikany JM, Eaton CB. Mediterranean and Dietary Approaches to Stop Hypertension dietary patterns and risk of sudden cardiac death in postmenopausal women. Am J Clin Nutr. 2014;99:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. [DOI] [PubMed] [Google Scholar]
- 18. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH‐style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–720. [DOI] [PubMed] [Google Scholar]
- 19. Fung TT, Willett WC, Stampfer MJ, Manson JE, Hu FB. Dietary patterns and the risk of coronary heart disease in women. Arch Intern Med. 2001;161:1857–1862. [DOI] [PubMed] [Google Scholar]
- 20. Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–921. [DOI] [PubMed] [Google Scholar]
- 21. Denova‐Gutierrez E, Tucker KL, Flores M, Barquera S, Salmeron J. Dietary patterns are associated with predicted cardiovascular disease risk in an urban Mexican adult population. J Nutr. 2016;146:90–97. [DOI] [PubMed] [Google Scholar]
- 22. Brunner EJ, Mosdol A, Witte DR, Martikainen P, Stafford M, Shipley MJ, Marmot MG. Dietary patterns and 15‐y risks of major coronary events, diabetes, and mortality. Am J Clin Nutr. 2008;87:1414–1421. [DOI] [PubMed] [Google Scholar]
- 23. Parikh A, Lipsitz SR, Natarajan S. Association between a DASH‐like diet and mortality in adults with hypertension: findings from a population‐based follow‐up study. Am J Hypertens. 2009;22:409–416. [DOI] [PubMed] [Google Scholar]
- 24. Salehi‐Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH)‐style diet on fatal or nonfatal cardiovascular diseases—incidence: a systematic review and meta‐analysis on observational prospective studies. Nutrition. 2013;29:611–618. [DOI] [PubMed] [Google Scholar]
- 25. Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta‐analysis of cohort studies. J Acad Nutr Diet. 2015;115:780–800.e5. [DOI] [PubMed] [Google Scholar]
- 26. Folsom AR, Parker ED, Harnack LJ. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am J Hypertens. 2007;20:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 28. Obarzanek E, Sacks FM, Vollmer WM, Bray GA, Miller ER III, Lin PH, Karanja NM, Most‐Windhauser MM, Moore TJ, Swain JF, Bales CW, Proschan MA. Effects on blood lipids of a blood pressure‐lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr. 2001;74:80–89. [DOI] [PubMed] [Google Scholar]
- 29. Asemi Z, Tabassi Z, Samimi M, Fahiminejad T, Esmaillzadeh A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: a randomised clinical trial. Br J Nutr. 2013;109:2024–2030. [DOI] [PubMed] [Google Scholar]
- 30. Saneei P, Hashemipour M, Kelishadi R, Esmaillzadeh A. The Dietary Approaches to Stop Hypertension (DASH) diet affects inflammation in childhood metabolic syndrome: a randomized cross‐over clinical trial. Ann Nutr Metab. 2014;64:20–27. [DOI] [PubMed] [Google Scholar]
- 31. Saneei P, Hashemipour M, Kelishadi R, Rajaei S, Esmaillzadeh A. Effects of recommendations to follow the Dietary Approaches to Stop Hypertension (DASH) diet v. usual dietary advice on childhood metabolic syndrome: a randomised cross‐over clinical trial. Br J Nutr. 2013;110:2250–2259. [DOI] [PubMed] [Google Scholar]
- 32. Shirani F, Salehi‐Abargouei A, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta‐analysis on controlled clinical trials. Nutrition. 2013;29:939–947. [DOI] [PubMed] [Google Scholar]
- 33. Djousse L, Petrone AB, Weir NL, Hanson NQ, Glynn RJ, Tsai MY, Gaziano JM. Repeated versus single measurement of plasma omega‐3 fatty acids and risk of heart failure. Eur J Nutr. 2014;56:1403–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Floyd JS, Blondon M, Moore KP, Boyko EJ, Smith NL. Validation of methods for assessing cardiovascular disease using electronic health data in a cohort of Veterans with diabetes. Pharmacoepidemiol Drug Saf. 2016;25:467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]


