Abstract
Background
The extent to which outcome benefits may be achieved through the implementation of aggressive low‐density lipoprotein (LDL) cholesterol targets in real world settings remains unknown, especially among elderly statin users following acute coronary syndromes.
Methods and Results
A population‐based cohort study consisting of 19 544 post‐acute coronary syndrome statin‐users aged ≥66 years between January 1, 2017 and March 31, 2014 was used to project the number of adverse outcome events (acute myocardial infarction or death from any cause) that could be prevented if all post‐acute coronary syndrome elderly statin users were treated to 1 of 2 LDL cholesterol target levels (≤50 and ≤70 mg/dL). The number of preventable adverse outcomes was estimated by using model‐based expected event probabilities as derived from Cox Proportional hazards models. In total, 61.6% and 25.5% of the elderly patients met LDL cholesterol targets of ≤70 and ≤50 mg/dL, respectively, based on current management. No more than 2.3 adverse events per 1000 elderly statin users (95% confidence interval: −0.7 to 5.4, P=0.62) could be prevented over 8.1 years if all patients were to be treated from current LDL cholesterol levels to either of the 2 LDL cholesterol targets of 70 or 50 mg/dL.
Conclusions
The number of acute myocardial infarctions or death that could be prevented through the implementation of LDL cholesterol targets with statins is negligible among an elderly post‐acute coronary syndrome population. Such findings may have implications for the applicability of newer agents, such as proprotein convertase subtilisin/kexin type‐9‐ inhibitors.
Keywords: aging, cardiology, cardiovascular disease, epidemiology, geriatrics, health services research, pharmacology, secondary prevention, statins
Subject Categories: Cardiovascular Disease, Epidemiology, Primary Prevention, Aging
Clinical Perspective
What Is New?
To our knowledge, this is the first study to use real‐world data to estimate the number of adverse events prevented by using aggressive low‐density lipoprotein cholesterol targets among elderly statin‐users following acute coronary syndrome hospitalizations.
What Are the Clinical Implications?
While aggressive low‐density lipoprotein cholesterol lowering using statins or proprotein convertase subtilisin/kexin type‐9 inhibitors have been shown to improve clinical outcomes among younger clinical trial populations, the projected number of acute myocardial infarctions or deaths prevented by using aggressive low‐density lipoprotein cholesterol targets among elderly statin‐users following acute coronary syndromes in the real‐world settings is marginal.
Available evidence from clinical trials has demonstrated that aggressive low‐density lipoprotein (LDL) cholesterol lowering using statins following acute coronary syndromes (ACS) is associated with improved cardiovascular outcomes.1, 2, 3, 4, 5 More recently, evolocumab, a proprotein convertase subtilisin/kexin type‐9 (PCSK‐9) inhibitor with potent LDL cholesterol lowering properties, has also been shown to improve composite cardiovascular outcomes among high‐risk populations, although mortality rates alone were similar between the 2 groups.6 While such studies support the clinical efficacy associated with aggressive LDL cholesterol lowering strategies, the population‐based effect of implementing such strategies on outcomes remains unclear.
Ambiguity in the clinical effectiveness of aggressive LDL cholesterol lowering strategies relates to several factors. First, real‐world populations are older and have greater comorbidities than those enrolled in clinical trials.7, 8, 9, 10 Because of differences in life expectancy and comorbidity burden, elderly patients may have fewer modifiable risks and attenuated outcome benefits, than those seen from clinical trial populations. Second, the potential incremental clinical effectiveness of an aggressive LDL cholesterol lowering strategy will depend upon the levels with which LDL cholesterol can be controlled through usual‐care in the real‐world. For example, if LDL cholesterol levels were already well controlled, the incremental outcome benefits that might be expected from higher doses of statins, and/or other therapies such as PCSK‐9 inhibitors may be marginal. The importance of exploring the clinical effectiveness of aggressive LDL cholesterol lowering strategies in high‐risk elderly populations using contemporary management becomes even more important when considering the costs associated with PCSK‐9 inhibitors.
To the best of our knowledge, no study has estimated the number of adverse events that could be prevented if all elderly statin users were treated to aggressive LDL cholesterol targets following an ACS. Accordingly, the objective of this study was to estimate the projected number of adverse outcome events which could be prevented if all post‐ACS elderly statin users were treated from current LDL cholesterol levels as observed in real‐world settings to 1 of 2 LDL cholesterol target levels (≤50 and ≤70 mg/dL). Canada serves as an ideal setting in which to explore the clinical effectiveness of aggressive LDL cholesterol targets among elderly statin users, given that medications are covered free of charge for patients aged ≥65 years, thereby mitigating the potential confounding effects of affordability.
Methods
Data Sources
The data set from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre‐specified criteria for confidential access, available at https://www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the programs may rely upon coding templates or macros that are unique to ICES.
The study population was derived from the Cardiovascular Health in Ambulatory Care Research Team (CANHEART) cohort (http://www.canheart.ca), which comprised the linkage of multiple individual‐level databases using encoded personal identifiers.11 Databases that were linked include the Canadian Institute for Health Information (CIHI) Discharge Abstract Database and the Ontario Diabetes Database, to identify hospitalizations for ACS and comorbidities; Same Day Surgery, and National Ambulatory Care Reporting System to identify same day surgical and emergency room visits respectively; Registered Persons Database for death information; the Ontario Drug Benefit prescription database, which was used to determine outpatient prescription drug use for patients aged ≥65 years; the Gamma‐Dynacare Medical Laboratory database, which captures 25% to 30% of all outpatient laboratory testing in Ontario was used to determine cholesterol levels; and the Registrar General of Ontario Vital Statistics Database, which was used to determine cause of death of all Ontarians. These data sets were linked using unique encoded identifiers and analyzed at ICES. This study was approved by the institutional review board at Sunnybrook Health Sciences Centre, Toronto, Canada. The requirement for informed consent was waived.
Study Sample
The study population was comprised of Ontario residents aged ≥66 years with (1) a valid health card number; (2) who were hospitalized for ACS (codes for myocardial infarction International Classification of Diseases, Tenth Revision (ICD‐10): I21, I22, ICD‐9: 410; unstable angina ICD‐10: I20, ICD‐9: 411, 413) between January 1, 2007 and March 31, 2014; (3) who had one or more outpatient LDL cholesterol measurements in Ontario between 30 and 365 days of their ACS hospitalization, and (4) who received at least one statin prescription within 6 months of their ACS hospitalization. The first LDL cholesterol measurement was excluded if such measurements were taken within the first 30 days of an ACS. We imposed a 30‐day ACS survival period to ensure accuracy in LDL cholesterol levels, which may be artificially low within the first 30 days following ACS.12, 13 Patients who had not received any statin prescriptions were excluded from the study given that our objective was not to evaluate the efficacy of statins versus non‐statins, but rather, to explore the potential outcome yield of adopting aggressive LDL cholesterol targets among elderly patients already on statins (Figure 1).
Figure 1.

Study participant selection. Study participation selection and exclusion criteria. AMI indicates acute myocardial infarction; LDL, low‐density lipoprotein cholesterol.
LDL Cholesterol and Statin‐Intensity
The LDL cholesterol measurement of interest was the first such measurement performed in the outpatient setting between 30 and 365 days following ACS hospitalization. Nineteen statin preparations and dosages were classified in accordance to the 2013 ACC/AHA guidelines into low‐intensity, medium‐intensity, or high‐intensity which took into account the specific statin drug and pill‐strength of each prescription.14
We explored 2 pre‐specified LDL cholesterol targets: (1) An LDL cholesterol target of ≤70 mg/dL as recommended by the European Society of Cardiology/Canadian Cardiovascular Society guidelines for post‐ACS populations,15, 16 and (2) an LDL cholesterol target of ≤50 mg/dL, which approximates the mean LDL cholesterol levels associated with aggressive LDL lower strategies in recent clinical trials.1, 3, 4
Outcomes
Our primary outcomes were the number of adverse events (the first occurrence of acute myocardial infarction or death from any cause) prevented if all elderly statin users were treated from their current LDL cholesterol levels as observed in real‐world settings to LDL cholesterol targets of ≤70 or ≤50 mg/dL. The primary outcomes incorporated model‐based expected event probabilities of adverse events using Cox Proportional hazards models.
Patients were followed from the date of their first LDL cholesterol measurement following their ACS hospitalization until a primary outcome event, or until the end of follow up (March 31, 2015) (Figure S1).
Statistical Analysis
The baseline characteristics of the cohort were reported by LDL quintiles. Baseline characteristics were compared across the LDL strata using one‐way analysis of variance for continuous variables, and chi‐squared test for categorical variables.
We determined expected probabilities of an adverse outcome according to LDL cholesterol levels for elderly statin users in Ontario using Cox proportional hazard models after adjusting for age, sex, socioeconomic status (neighborhood income quintile), clinical risk factors, invasive cardiac procedure use, comorbidity, and statin intensity as determined at study baseline for each of our 2 LDL target levels across statin intensity groups. To examine whether the hazards associated with LDL cholesterol measurements varied according to age, all statistical models also used an age‐LDL cholesterol interaction within each of the age stratum (66–74 years old versus 75 years and beyond) given uncertainty in the efficacy of statin therapy among patients aged ≥75 years as compared with younger populations.17, 18 Based on the fitted Cox model, the predicted probability of an adverse event within a specified duration of follow‐up could be determined for any patient covariate pattern.
For each subject, we computed 2 probabilities based on the fitted Cox model. First, the patient's model‐based probability of an event over a duration of 8.1 years (which corresponded to the maximum duration of study follow‐up) conditional on his/her existing LDL and his/her measured baseline covariates. Second, the patient's model‐based probability of an event over a time‐duration of 8.1 years under the assumptions that his/her LDL cholesterol was lowered to the desired target threshold, and that other baseline covariates remaining unchanged. For subjects who were currently at or below the LDL threshold, we assumed that these 2 probabilities were equal to one another (ie, that their LDL would not change). We then computed the difference in these 2 probabilities. The average of this difference in probabilities across the sample of patients is the population‐average reduction in the probability of an event. This average probability was multiplied by the size of the initial cohort to estimate the reduction in the number of events if LDL was lowered to the target level. These analyses assumed that a treatment‐to‐target approach was feasible even among patients already receiving high‐intensity statins, given that adherence rates to statins (irrespective of intensity) remain suboptimal.19 Confidence intervals were determined using bootstrapping. When examining the number of acute myocardial infarctions (AMIs) prevented, the Fine‐Gray sub‐distribution hazard model,20 was used to account for the competing risk of non‐AMI mortality.
Several sensitivity analyses were undertaken in which we varied the outcomes to include broader composite outcomes (ie, stroke or all‐cause hospitalization) as well as narrower non‐composite outcomes (all‐cause mortality alone or AMI alone), sex, and comorbidity (Charlson comorbidity index of >2 versus ≤2). Another sensitivity analyses adjusted for adherence to statins using prescription refill data and the proportion of days covered (PDC) of ≥80% versus <80%.
Two‐tailed P values <0.05 were considered significant. Analyses were performed with the use of SAS software, version 9.3 (SAS Institute Inc, Cary, NC) R Statistical Software (rms package) was used for the creation of hazard‐LDL plots.21
Results
Baseline Characteristics
Our cohort consisted of 19 544 patients. The mean age of the cohort was 76.3±7.0 years; 39.7% of patients were female. In total, 61.6% and 25.5% of the elderly population met LDL cholesterol targets of ≤70 and ≤50 mg/dL, respectively at baseline. In general, increasing age, male sex, higher intensity statins, diabetes mellitus, hypertension, and a higher Charlson comorbidity index were associated with lower baseline LDL cholesterol (Table 1). Patients on higher intensity statins at baseline were younger, more likely to be male, had diabetes mellitus, hypertension, and a higher Charlson comorbidity index than those on lower intensity statins.
Table 1.
Baseline Characteristics According to LDL Cholesterol Categories Among Post ACS Patients Aged ≥66 Years on Statins in Ontario
| LDL <50 | LDL 50 to 69 | LDL 70 to 99 | LDL ≥100 | P Value | |
|---|---|---|---|---|---|
| n=4984 | n=7047 | n=5599 | n=1914 | ||
| Age | |||||
| Mean±SD | 76.56±7.07 | 76.37±6.99 | 76.03±6.88 | 75.88±7.03 | <0.001 |
| 66 to 74 y | 2153 (43.2%) | 3120 (44.3%) | 2553 (45.6%) | 911 (47.6%) | 0.004 |
| ≥75 y | 2831 (56.8%) | 3927 (55.7%) | 3046 (54.4%) | 1003 (52.4%) | |
| Sex | |||||
| F | 1663 (33.4%) | 2628 (37.3%) | 2453 (43.8%) | 1009 (52.7%) | <0.001 |
| M | 3321 (66.6%) | 4419 (62.7%) | 3146 (56.2%) | 905 (47.3%) | |
| Income quintile at index date | |||||
| 1 | 975 (19.6%) | 1320 (18.7%) | 1130 (20.2%) | 413 (21.6%) | 0.099 |
| 2 | 1074 (21.5%) | 1574 (22.3%) | 1149 (20.5%) | 419 (21.9%) | |
| 3 | 1043 (20.9%) | 1405 (19.9%) | 1153 (20.6%) | 381 (19.9%) | |
| 4 | 949 (19.0%) | 1400 (19.9%) | 1112 (19.9%) | 373 (19.5%) | |
| 5 | 943 (18.9%) | 1348 (19.1%) | 1055 (18.8%) | 328 (17.1%) | |
| LDL cholesterol | |||||
| Mean±SD | 39.18±8.13 | 59.08±5.59 | 80.99±8.28 | 123.89±25.59 | <0.001 |
| HDL cholesterol | |||||
| Mean±SD | 44.99±14.42 | 47.98±13.92 | 49.87±14.13 | 51.43±14.60 | <0.001 |
| Triglyceride cholesterol | |||||
| Mean±SD | 115.26±61.61 | 113.52±51.79 | 126.72±55.83 | 149.83±64.37 | <0.001 |
| Non‐HDL cholesterol | |||||
| Mean±SD | 62.28±13.91 | 81.84±11.96 | 106.40±14.53 | 153.89±30.16 | <0.001 |
| Total cholesterol | |||||
| Mean±SD | 107.28±17.65 | 129.83±15.92 | 156.26±17.56 | 205.32±32.14 | <0.001 |
| Low‐intensity statin | 47 (0.9%) | 125 (1.8%) | 225 (4.0%) | 161 (8.4%) | <0.001 |
| Medium‐intensity statin | 1533 (30.8%) | 2757 (39.1%) | 2710 (48.4%) | 981 (51.3%) | |
| High‐intensity statin | 3404 (68.3%) | 4165 (59.1%) | 2664 (47.6%) | 772 (40.3%) | |
| Diabetes mellitus | 2794 (56.1%) | 3273 (46.4%) | 2333 (41.7%) | 777 (40.6%) | <0.001 |
| Hypertension | 4527 (90.8%) | 6283 (89.2%) | 4940 (88.2%) | 1693 (88.5%) | <0.001 |
| Stroke or TIA | 317 (6.4%) | 358 (5.1%) | 296 (5.3%) | 125 (6.5%) | 0.004 |
| Congestive heart failure | 1940 (38.9%) | 2464 (35.0%) | 1890 (33.8%) | 667 (34.8%) | <0.001 |
| Atrial fibrillation | 1219 (24.5%) | 1548 (22.0%) | 1193 (21.3%) | 412 (21.5%) | <0.001 |
| Peripheral vascular disease | 228 (4.6%) | 334 (4.7%) | 277 (4.9%) | 96 (5.0%) | 0.786 |
| Chronic obstructive lung disease | 1533 (30.8%) | 1969 (27.9%) | 1648 (29.4%) | 574 (30.0%) | 0.008 |
| Charlson index | |||||
| Mean±SD | 2.74±1.96 | 2.36±1.89 | 2.22±1.89 | 2.25±1.96 | <0.001 |
| Primary cancer | 302 (6.1%) | 388 (5.5%) | 292 (5.2%) | 83 (4.3%) | 0.031 |
| Metastatic cancer | 45 (0.9%) | 63 (0.9%) | 51 (0.9%) | 27 (1.4%) | 0.191 |
| Peptic ulcer disease | 127 (2.5%) | 152 (2.2%) | 111 (2.0%) | 42 (2.2%) | 0.254 |
| Mild liver disease | 32 (0.6%) | 34 (0.5%) | 25 (0.4%) | 11 (0.6%) | 0.51 |
| Moderate or severe liver disease | 10 (0.2%) | 8 (0.1%) | 9 (0.2%) | 6 (0.3%) | 0.264 |
| Connective tissue/rheumatic disease | 57 (1.1%) | 71 (1.0%) | 64 (1.1%) | 27 (1.4%) | 0.509 |
| Dementia | 251 (5.0%) | 256 (3.6%) | 182 (3.3%) | 58 (3.0%) | <0.001 |
| Hemiplegia or paraplegia | 60 (1.2%) | 80 (1.1%) | 53 (0.9%) | 22 (1.1%) | 0.609 |
| Coronary angiography 1 y | 3657 (73.4%) | 5259 (74.6%) | 4020 (71.8%) | 1316 (68.8%) | <0.001 |
| Percutaneous coronary intervention | 2202 (44.2%) | 2944 (41.8%) | 2194 (39.2%) | 660 (34.5%) | <0.001 |
| Coronary artery bypass surgery | 811 (16.3%) | 1243 (17.6%) | 1021 (18.2%) | 342 (17.9%) | 0.056 |
| Number of days from AMI/angina to LDL measurement | |||||
| Median (IQR) | 115 (63–204) | 114 (62–204) | 122 (67–209) | 129 (74–210) | <0.001 |
| Number of days from statin to LDL test | |||||
| Median (IQR) | 18 (5–43) | 22 (6–50) | 25 (7–55) | 40 (11–80) | <0.001 |
ACS indicates acute coronary syndrome; AMI, acute myocardial infarction; HDL, high density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol; TIA, transient ischemic attack.
Fluctuations in LDL Cholesterol and Statin Intensities Over Time
In total, 72% of the cohort had ≥2 LDL cholesterol measurements over a maximum duration of follow‐up of 8.1 years (median duration of follow‐up of 2.8 years). The majority of patients (14 075/19 544, 72%) did not change dose intensities of statins throughout the follow‐up period. Among those with ≥2 measurements, LDL cholesterol on average did not change significantly between an individual's first and last available measurement (mean difference of 0.37 mg/dL, 95% confidence interval: −0.80±0.07, P=0.10). Each patient received a median of 16 statin prescriptions (interquartile range: 7–30) throughout the follow‐up period. In total, ≥80% of patients were on medium‐ or high‐intensity statins, irrespective of age throughout the study period. The fluctuations in LDL cholesterol and statin intensities were similar among patients ≥75 years as amongst those ages 66 to 74 years (Figure S2 and Table S1).
Projected Number of Adverse Outcome Events According to LDL Targets
After adjustment for all baseline factors including statin intensities, the relationship between LDL cholesterol and outcomes varied by age (age‐LDL cholesterol interaction, P<0.001). While in both age groups, the adjusted probabilities of the occurrence of our primary composite outcome within 8.1 years decreased from LDL cholesterol levels of 100 mg/dL down to 70 mg/dL, the incremental adjusted probabilities of AMI or death were not significantly lower at LDL cholesterol levels under 70 mg/dL as compared with those estimated using current LDL cholesterol levels as observed in the real‐world (Figure 2).
Figure 2.
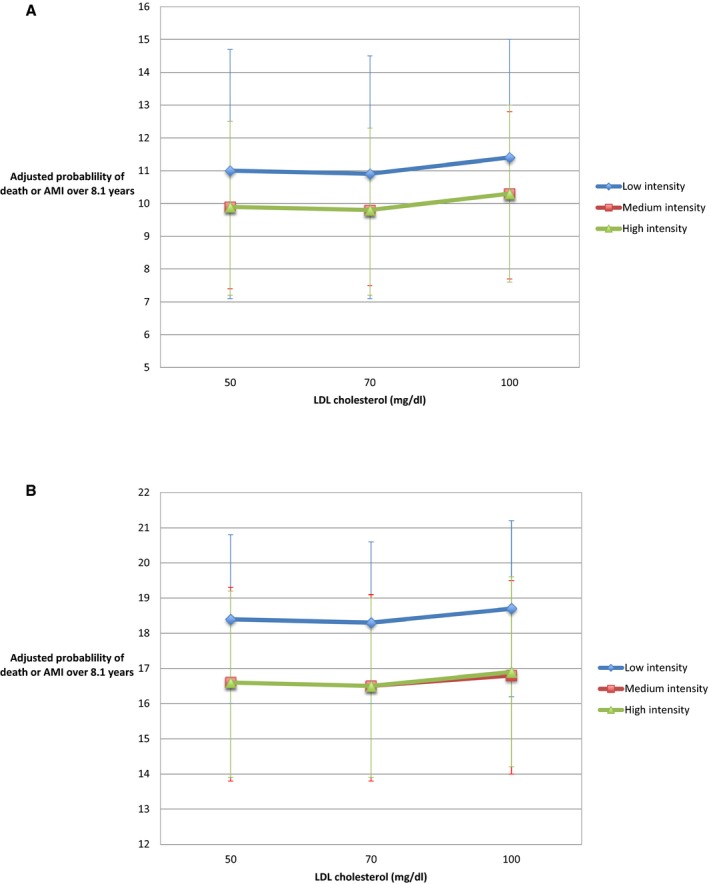
The relationship between LDL cholesterol and the adjusted probability of death or acute myocardial infarction according to statin dose intensity. A, Among patients ages 66 to 74 years old. B, Among patients ages ≥75 years. AMI indicates acute myocardial infarction; LDL, low‐density lipoprotein cholesterol.
After adjusting for all baseline clinical factors, no more than 2.3 adverse events per 1000 post‐ACS patients (95% confidence interval: −0.7 to 5.4, P=0.62) would have been prevented over 8.1 years if all patients' LDL cholesterol levels were to have been reduced from current levels to LDL cholesterol targets of ≤70 or ≤50 mg/dL. There were no significant differences in the numbers of adverse events prevented between patients aged ≥75 years versus those 66 to 74 years of age, or when reducing LDL cholesterol levels down from current levels to a target of ≤70 mg/dL versus a target of ≤50 mg/dL (P>0.6) (Table 2).
Table 2.
The Estimated Number of Deaths or AMI Events Prevented Had All Post‐ACS Elderly Statin Users in Ontario, Canada Been Treated From Current LDL Cholesterol Levels to One of Two LDL Cholesterol Targets (ie, the LDL Cholesterol Levels ≤50 mg/dL and LDL Cholesterol Levels ≤70 mg/dL)
| Age Groups | LDL Target Levels, mg/dL | Number of Patients Currently in Ontario Whose LDL Cholesterol Exceeding the Corresponding LDL Target Level | Number of Adverse Outcomes Prevented (+/− 95% Confidence Interval) Per 1000 Patients Treated to Achieve the Corresponding LDL Target Levela | Number of Adverse Outcomes Prevented in the Sample (+/− 95% CI) | P Value |
|---|---|---|---|---|---|
| All patients | ≤70 | 7513 | 2.3 (−0.7 to 5.4) | 45 (−14 to 106) | 0.62 |
| ≤50 | 14 560 | 0.7 (−7.7 to 8.9) | 13 (−150 to 173) | ||
| 66 to 74 y | ≤70 | 3464 | 3.6 (−1.4 to 8.9) | 31 (−12 to 77) | 0.83 |
| ≤50 | 6584 | 2.5 (−9.8 to 14.9) | 22 (−86 to 130) | ||
| ≥75 y | ≤70 | 4049 | 1.3 (−2.3 to 4.9) | 14 (−25 to 53) | 0.63 |
| ≤50 | 7979 | −0.8 (−11.2 to 9.3) | −9 (−121 to 100) |
ACS indicates acute coronary syndrome; AMI, acute myocardial infarction; CI, confidence interval; LDL, low‐density lipoprotein cholesterol.
Average outcome rates were derived using Cox proportional hazards models adjusted for age, sex, socioeconomic status, clinical risk factors, invasive cardiac procedures, comorbid diseases, statin intensity, and an age‐LDL cholesterol interaction. For each subject, we computed 2 probabilities based on the fitted Cox model. First, the patient's model‐based probability of an event over 8.1 years (which corresponds to the maximum duration of study follow‐up) conditional on his/her existing LDL and their measured baseline covariates. Second, the patient's model‐based probability of an event over 8.1 years, under the assumption that his/her LDL cholesterol was lowered to the desired target threshold (and that other baseline covariates remaining unchanged). For subjects who were currently at or below the LDL threshold, we assumed that these 2 probabilities were equal to one another (ie, that their LDL would not change). We then computed the difference in these 2 probabilities. The average of this difference in probabilities across the sample of patients is the population‐average reduction in the probability of an event. This average probability was multiplied by the size of the initial cohort to estimate the reduction in the number of events if LDL was lowered to the target level. Negative numbers imply more rather than fewer adverse events prevented as a result of the projected treatment strategy.
Sensitivity Analyses
A sensitivity analysis when including stroke in our composite outcomes did not meaningfully alter our results. Additional sensitivity analyses examined non‐composite outcomes of all‐cause mortality alone and AMI alone. While the projected impact of aggressive LDL cholesterol targets varied by outcomes, the absolute numbers of projected adverse events prevented were uniformly low with overlapping confidence intervals irrespective of age, sex, comorbidity, target levels, and outcomes assessed (Tables 3 and 4). Finally, a sensitivity analysis in which we adjusted for prescription refill adherence data (proportion of days covered) did not meaningfully change our results.
Table 3.
The Estimated Number of Adverse Outcomes (ie, AMI or Death) Prevented Had All Post‐ACS Elderly Statin Users in Ontario, Canada Been Treated From Current LDL Cholesterol Levels to One of Two LDL Cholesterol Targets (ie, the LDL Cholesterol Levels ≤50 mg/dL and LDL Cholesterol Levels ≤70 mg/dL) According to Specific Outcomes
| Outcome | Age Groups | LDL Target Levels, mg/dL | Number Of Patients Currently in Ontario Whose LDL Cholesterol Exceeding the Corresponding LDL Target Level | Number of Adverse Events Avoided (+/− 95% Confidence Interval) Per 1000 Patients Treated to Achieve the Corresponding LDL Target Levela | Number of Events Prevented in the Sample (+/− 95% CI) | P Value |
|---|---|---|---|---|---|---|
| AMI | All patients | ≤70 | 7513 | 5.3 (3.1–7.8) | 104 (60–152) | <0.001 |
| ≤50 | 14 560 | 12.8 (7.5–18.2) | 249 (146–356) | |||
| 65 to 74 y | ≤70 | 3464 | 2.4 (−1.2 to 6.3) | 21 (−11 to 55) | 0.28 | |
| ≤50 | 6584 | 5.8 (−1.7 to 13.7) | 51 (−15 to −120) | |||
| 75+ y | ≤70 | 4049 | 6.3 (3.6–9.2) | 68 (39–100) | <0.001 | |
| ≤50 | 7976 | 15.1 (8.7–21.6) | 164 (94–234) | |||
| All‐cause mortality | All patients | ≤70 | 7513 | −1.4 (−4.3 to 1.7) | −27 (−84 to 33) | 0.01 |
| ≤50 | 14 560 | −9.8 (−17.8 to −1.6) | −192 (−348 to −30) | |||
| 65 to 74 y | ≤70 | 3464 | 1.6 (−2.9 to 6.3) | 14 (−25 to 55) | 0.35 | |
| ≤50 | 6584 | −3.1 (−15.1 to 8.8) | −27 (−132 to 77) | |||
| ≥75 y | ≤70 | 4049 | −3.8 (−7.5 to 0.0) | −41 (−81 to 0) | 0.009 | |
| ≤50 | 7976 | −15.2 (−25.3 to −4.6) | −164 (−274 to −50) | |||
| All‐cause readmission or death | All patients | ≤70 | 7513 | 2.5 (−0.3 to 5.5) | 49 (−7 to 108) | 0.74 |
| ≤50 | 14 560 | 1.4 (−6.6 to 9.4) | 28 (−128 to 184) | |||
| 65 to 74 y | ≤70 | 3464 | 3.4 (−1.3 to 8.3) | 30 (−11 to 72) | 0.48 | |
| ≤50 | 6584 | −0.4 (−13.4 to 12.6) | −3 (−117 to 110) | |||
| ≥75 y | ≤70 | 4049 | 1.8 (−1.7 to 5.4) | 19 (−19 to 58) | 0.79 | |
| ≤50 | 7976 | 2.9 (−6.9 to 12.6) | 31 (−74 to 136) |
ACS indicates acute coronary syndrome; AMI, acute myocardial infarction; CI, confidence interval; LDL, low‐density lipoprotein cholesterol.
Average outcome rates were derived using Cox proportional hazards models adjusted for age, sex, socioeconomic status, clinical risk factors, invasive cardiac procedures, comorbid diseases, statin intensity, and an age‐LDL cholesterol interaction. For each subject, we computed 2 probabilities based on the fitted Cox model. First, the patient's model‐based probability of an event over 8.1 years (which corresponds to the maximum duration of study follow‐up) conditional on his/her existing LDL and their measured baseline covariates. Second, the patient's model‐based probability of an event over 8.1 years, under the assumption that his/her LDL cholesterol was lowered to the desired target threshold (and that other baseline covariates remaining unchanged). For subjects who were currently at or below the LDL threshold, we assumed that these 2 probabilities were equal to one another (ie, that their LDL would not change). We then computed the difference in these 2 probabilities. The average of this difference in probabilities across the sample of patients is the population‐average reduction in the probability of an event. This average probability was multiplied by the size of the initial cohort to estimate the reduction in the number of events if LDL was lowered to the target level. Negative numbers imply more rather than fewer adverse events prevented as a result of the projected treatment strategy.
Table 4.
The Estimated Number of Adverse Outcomes (AMI or Deaths) Prevented Had All Post‐ACS Elderly Statin Users in Ontario, Canada Been Treated From Current LDL Cholesterol Levels to One of Two LDL Cholesterol Targets (ie, the LDL Cholesterol Levels ≤50 mg/dL and LDL Cholesterol Levels ≤70 mg/dL) According to Sex and Charlson Index
| Outcome | LDL Target Levels, mg/dL | Number of Patients Currently in Ontario Whose LDL Cholesterol Exceeding the Corresponding LDL Target Level | Number of Adverse Events Avoided (+/− 95% Confidence Interval) Per 1000 Patients Treated to Achieve the Corresponding LDL Target Levela | Number of Events Prevented in the Sample (+/− 95% CI) | P Value |
|---|---|---|---|---|---|
| Female | ≤70 | 3462 | 2.8 (−1 to 6.8) | 22 (−8 to 53) | 0.49 |
| ≤50 | 6090 | 0.9 (−8.3 to 10.4) | 7 (−64 to 80) | ||
| Males | ≤70 | 4051 | 2 (−0.4 to 4.6) | 24 (−5 to 54) | 0.52 |
| ≤50 | 8470 | 0.4 (−6.9 to 8) | 5 (−81 to 95) | ||
| Charlson ≤2 | ≤70 | 4690 | 2.3 (−0.7 to 5.4) | 26 (−8 to 61) | 0.51 |
| ≤50 | 8845 | 0.6 (−7.5 to 8.9) | 6 (−84 to101) | ||
| Charlson >2 | ≤70 | 2823 | 2.4 (−0.5 to 5.5) | 20 (−4 to 45) | 0.49 |
| ≤50 | 5715 | 0.6 (−7.1 to −8.8) | 5 (−59 to 72) |
ACS indicates acute coronary syndromes; AMI, acute myocardial infarction; CI, confidence interval; LDL, low‐density lipoprotein cholesterol.
Average outcome rates were derived using Cox proportional hazards models adjusted for age, socioeconomic status, clinical risk factors, invasive cardiac procedures, comorbid diseases, statin intensity, and an age‐LDL cholesterol interaction. For each subject, we computed 2 probabilities based on the fitted Cox model. First, the patient's model‐based probability of an event over 8.1 years (which corresponds to the maximum duration of study follow‐up) conditional on his/her existing LDL and their measured baseline covariates; second, the patient's model‐based probability of an event over 8.1 years, under the assumption that his/her LDL cholesterol was lowered to the desired target threshold (and that other baseline covariates remaining unchanged). For subjects who were currently at or below the LDL threshold, we assumed that these 2 probabilities were equal to one another (ie, that their LDL would not change). We then computed the difference in these 2 probabilities. The average of this difference in probabilities across the sample of patients is the population‐average reduction in the probability of an event. This average probability was multiplied by the size of the initial cohort to estimate the reduction in the number of events if LDL was lowered to the target level. Negative numbers imply more rather than fewer adverse events prevented as a result of the projected treatment strategy.
Discussion
Our study explored the projected number of incremental adverse outcomes that could have been prevented if all post‐ACS elderly statin users were treated to aggressively low LDL cholesterol target levels. We projected no significant reductions in the numbers of adverse events prevented over a duration of 8.1 years if all elderly statin users post‐ACS had been treated from their current LDL cholesterol levels to LDL cholesterol targets of ≤70 or ≤50 mg/dL.
Our study builds on the growing body of evidence examining the incremental clinical effectiveness of aggressive LDL cholesterol lowering in the management of cardiovascular disease. Our findings were consistent with those of a recent observational study examining statin adherers among a population with pre‐existing ischemic heart disease in Israel, in which LDL cholesterol levels of ≤70 mg/dL were not associated with any differences in the risk of adverse cardiovascular events than LDL cholesterol levels of between 70 and 100 mg/dL.22 To the best of our knowledge, our study is the first to project the population effectiveness of using LDL cholesterol targets among an exclusively elderly population of statin users. Moreover, our study focused on lower LDL cholesterol targets than previous observational studies (≤50 mg/dL), and did so among a post‐ACS population, where the debate over the implementation of aggressively low LDL cholesterol targets remains greatest.1, 3, 4, 23, 24 Finally, our study took place within the Canadian healthcare setting, which covers the costs of medications for patients aged ≥65 years, thereby mitigating the potential effects of medication affordability on outcomes.
Clinical trials, such as IMPROVE‐IT (The Improved Reduction of Outcomes: Vytorin Efficacy International Trial), demonstrated that patients randomized to a combination of simvastatin and ezetemibe achieved a modest 6.4% relative improvement in composite cardiovascular outcomes of death from cardiovascular disease, a major coronary event, or non‐fatal stroke, compared with those randomized to simvastatin alone although mortality rates did not differ between the 2 groups. Such outcome improvements were thought to be attributable to variations in LDL cholesterol levels between the 2 groups (ie, average LDL cholesterol of 53.7 mg/dL versus 69.5 mg/dL)1—LDL cholesterol levels similar to the targets examined in our study.
PCSK‐9 inhibitors have emerged as a potent LDL cholesterol lowering therapy, which may serve as an adjunctive (or alternate) therapy to statins among high‐risk populations. The recently published FOURIER (Further Cardiovascular OUTcomes Research with PCSK9 Inhibitors in Subjects with Elevated risk) trial, a phase 3 double‐blind randomized placebo controlled trial, enrolled 27 500 high‐risk patients on optimal statin therapy whose LDL cholesterol levels were 70 mg/dL or greater (or a non HDL cholesterol of 100 mg/dL or greater).6 The study demonstrated a 15% relative risk reduction of the composite cardiovascular end point among patients randomized to evolocumab as compared with placebo. Improvement of evolocumabs in outcomes was attributed to the 59% reduction in mean LDL cholesterol as compared with placebo (mean LDL cholesterols: 30.2 mg/dL versus 92 mg/dL in evolocumab versus placebo, respectively). Outcome benefits associated with evolocumab were driven predominantly by a reduction in non‐fatal rather than fatal vascular events (ie, myocardial infarction, stroke, coronary revascularization), and were considered modest in magnitude relative to the large decreases in LDL levels achieved.
The debate regarding the relationship between LDL cholesterol and outcomes continues to evolve. A recent consensus document summarizing the results of >200 studies led the authors to unequivocally conclude that the relationship between LDL cholesterol and adverse outcomes was “dose‐dependent” and linear.25 However, most studies that have supported a “lower is better” linear relationship between LDL cholesterol and outcomes have been conducted in younger populations. Indeed, elderly populations have been significantly under‐represented in statin and PCSK‐9 inhibitor outcome trials.5, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 Our study suggests that the linear “dose‐dependent” relationship between LDL cholesterol and outcomes may not apply to elderly populations on statins following ACS hospitalizations, and the effectiveness of using aggressive LDL cholesterol targets among such elderly subpopulations may be unwarranted. In this regard, our findings support the 2013 American College of Cardiology/American Heart Association, United States Preventative Task Force, and the Department of Veterans Affairs and Department of Defense practice guidelines which do not advocate for cholesterol targets among secondary prevention in the ≥75‐year population.14, 36, 37
Our findings have important implications. First, the negligible outcome benefits, combined with the potential side‐effects and higher costs may make the adoption of aggressive LDL cholesterol targets using statins among an elderly post‐ACS population unattractive from a clinical and cost‐effectiveness standpoint.38 Moreover, our results may question the merits of serial LDL cholesterol monitoring among statin‐adherent elderly populations. Not only did our study demonstrate that LDL cholesterol levels fluctuated little among those with ≥2 measurements over the follow‐up period, but the marginal incremental outcome benefits expected may not justify serial LDL cholesterol monitoring among elderly patients already on statins unless serial monitoring is used among patients suspected of being non‐adherent or intolerable to statins. Our results may also have implications for PCSK‐9 inhibitor research, especially given their costs and their uncertain benefits in elderly populations. In summary, our findings underscore the need for greater clinical effectiveness data for aggressive LDL cholesterol lowering strategies among elderly populations—a population who comprise the majority demographic of the cardiovascular disease population, yet for whom clinical efficacy of aggressive LDL cholesterol lowering remains less clear than that of younger populations.
Our study has several important limitations. First, our study relies on observational data and makes the assumption that the number of cardiovascular events prevented among patients on statins whose LDL cholesterols exceeded a pre‐specified target would reflect the outcomes associated with LDL cholesterol levels at or below the pre‐specified LDL target of interest. We do not know whether lowering of LDL cholesterol to aggressive target levels would have been feasible or would have translated into outcomes that mirrored those individuals whose LDL cholesterol levels were already at or below the targets of interest. Moreover, residual confounding may have existed, as we had no information on other lifestyle behaviors or anthropometric measures beyond cholesterol levels themselves. As has been seen in other studies such as SPRINT (Systolic Blood Pressure Intervention Trial) for blood pressure control, the clinical benefits of aggressive risk‐factor modification may not always be apparent originally using observational study designs.39 Furthermore, our median follow‐up time was only 2.8 years. That said, insufficient clinical trial and/or intervention data existed that could inform the efficacy of a treatment‐to‐target approach in real‐world elderly populations beyond the use of observational data.
Second, our study was not designed to determine why some patients' baseline LDL cholesterol levels were lower than others despite the fact that all patients were on statins. In our study, patients with lower LDL cholesterol levels were more likely to be older and of male sex, were more likely to have diabetes mellitus, hypertension, and comorbidity, and were more likely to be receiving higher intensity statins than those who had higher LDL cholesterol levels at baseline. Accordingly, we hypothesize that variations in LDL cholesterols are multifactorial, and likely attributable to differences in treatment aggressiveness (statin intensity and doses), variations in statin adherence, biological responsiveness to therapy, genetic predisposition, and/or frailty. That being said, sensitivity analyses demonstrated that the absolute number of preventable adverse events from aggressive LDL cholesterol targets were similarly low among younger (65–74 years old) versus older (≥75 years old) patients, males versus females, and those with higher versus lower Charlson comorbidity scores. Moreover, adjusting for prescription refill data did not significantly alter our results.
Third, we assumed that every patient whose LDL cholesterol exceeded our pre‐specified targets could have actually achieved lower LDL cholesterol levels regardless of whether or not they were already on maximum intensities of statins. Given that any potential incremental benefits from aggressive LDL cholesterol lowering would have only applied to those who were not receiving maximum intensities of high‐intensity statins, our results, if anything, provide a best‐case scenario regarding the projected incremental benefit among this elderly population.
Fourth, LDL cholesterol levels were well controlled with the majority of patients receiving medium‐ or high‐intensity statins. The optimal penetrance of statin therapy in this population is not unique to Ontario,40 and likely reflects on a multitude of factors including increasing evidence, better education, evolving clinical guidelines, as well as provincial and national quality control best‐practice initiatives that have been undertaken to optimize secondary prevention management throughout the past decade. It is possible that the incremental yield of aggressive LDL cholesterol targets might have been greater had LDL cholesterol levels been less tightly controlled than that of the elderly Ontario statin population.
Finally, our study was restricted to the Ontario population. While the population size of Ontario is the largest of any other province and comprises 40% of the Canadian population, it is possible that results may not be generalizable to other jurisdictions, particularly those whose LDL cholesterol levels were generally less well‐controlled and/or where statins were less well‐penetrated. Nonetheless, these limitations must be counterbalanced against the strengths of this natural history population‐based study, in which detailed serial cholesterol measurements and statin prescriptions were available.
In conclusion, the number of acute myocardial infarctions or death that could be prevented through the implementation of LDL cholesterol targets with statins is negligible among an elderly post‐ACS population. Such findings may have implications for the applicability of newer agents, such as PCSK‐9 inhibitors.
Sources of Funding
The study was supported by ICES, an operating grant from the Institute of Circulatory and Respiratory Health, Canadian Institutes of Health Research (ICRH–CIHR) Chronic Diseases Team (grant no. TCA 118349), a CIHR foundation grant (grant no. FDN‐143313), a CIHR operating grant (grant no. MOP‐111035) and a Canadian Vascular Network (CVN) seed grant. The CVN is supported by grants from the CIHR–ICRH, and the Institute of Aging in partnership with and supported by Hypertension Canada. ICES is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC).
Disclosures
Tu is supported by a Tier 1 Canada Research Chair in Health Services Research and an Eaton Scholar Award from the Department of Medicine, University of Toronto. Austin and, Alter are supported by Heart and Stroke Foundation Career Investigator Awards; Ko is supported by a Heart and Stroke Foundation Mid‐Career Award, and Udell and Bhatia are supported by a Heart and Stroke Foundation National New Investigator and Ontario Clinician Scientist Award, all from the Heart and Stroke Foundation. Alter is also funded by a Chair in Cardiovascular and Metabolic Rehabilitation, University Health Network–Toronto Rehabilitation Institute, University of Toronto.
Supporting information
Table S1. The Relationship Between an Individual's Statin Intensity and the Statin Intensity at the End of Study Follow‐Up, Among All Patients, and Among Age‐Specific Subgroups of <75 Years of Age, and ≥75 Years)
Figure S1. The schematic diagram of the study design.
Figure S2. The distribution of LDL cholesterol during the first and last measurements among patients <75 years, and those aged ≥75 years.
Acknowledgments
Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). The authors thank IMS Brogan Inc for use of its Drug Information Database.
(J Am Heart Assoc. 2018;7:e007535 DOI: 10.1161/JAHA.117.007535.)29754125
References
- 1. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE‐IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 2. Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni‐Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D; RUTHERFORD‐2 Investigators . PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD‐2): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:331–340. [DOI] [PubMed] [Google Scholar]
- 3. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ; ODYSSEY LONG TERM Investigators . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 4. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA; Open‐Label Study of Long‐Term Evaluation against LDL Cholesterol (OSLER) Investigators . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 5. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK; Treating to New Targets (TNT) Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 6. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 7. Gurwitz JH, Col NF, Avorn J. The exclusion of the elderly and women from clinical trials in acute myocardial infarction. JAMA. 1992;268:1417–1422. [PubMed] [Google Scholar]
- 8. Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118:1294–1303. [DOI] [PubMed] [Google Scholar]
- 9. Ko DT, Mamdani M, Alter DA. Lipid‐lowering therapy with statins in high‐risk elderly patients: the treatment‐risk paradox. JAMA. 2004;291:1864–1870. [DOI] [PubMed] [Google Scholar]
- 10. Udell JA, Wang TY, Li S, Kohli P, Roe MT, de Lemos JA, Wiviott SD. Clinical trial participation after myocardial infarction in a national cardiovascular data registry. JAMA. 2014;312:841–843. [DOI] [PubMed] [Google Scholar]
- 11. Tu JV, Chu A, Donovan LR, Ko DT, Booth GL, Tu K, Maclagan LC, Guo H, Austin PC, Hogg W, Kapral MK, Wijeysundera HC, Atzema CL, Gershon AS, Alter DA, Lee DS, Jackevicius CA, Bhatia RS, Udell JA, Rezai MR, Stukel TA. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8:204–212. [DOI] [PubMed] [Google Scholar]
- 12. Arnold SV, Kosiborod M, Tang F, Zhao Z, McCollam PL, Birt J, Spertus JA. Changes in low‐density lipoprotein cholesterol levels after discharge for acute myocardial infarction in a real‐world patient population. Am J Epidemiol. 2014;179:1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rott D, Klempfner R, Goldenberg I, Leibowitz D. Cholesterol levels decrease soon after acute myocardial infarction. Isr Med Assoc J. 2015;17:370–373. [PubMed] [Google Scholar]
- 14. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 15. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D; ESC Committee for Practice Guidelines (CPG) 2008‐2010 and 2010‐2012 Committees . ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 16. Anderson TJ, Gregoire J, Pearson GJ, Barry AR, Couture P, Dawes M, Francis GA, Genest J Jr, Grover S, Gupta M, Hegele RA, Lau DC, Leiter LA, Lonn E, Mancini GB, McPherson R, Ngui D, Poirier P, Sievenpiper JL, Stone JA, Thanassoulis G, Ward R. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263–1282. [DOI] [PubMed] [Google Scholar]
- 17. Pedro‐Botet J, Climent E, Chillaron JJ, Toro R, Benaiges D, Flores‐Le Roux JA. Statins for primary cardiovascular prevention in the elderly. J Geriatr Cardiol. 2015;12:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilmot KA, Khan A, Krishnan S, Eapen DJ, Sperling L. Statins in the elderly: a patient‐focused approach. Clin Cardiol. 2015;38:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin I, Sung J, Sanchez RJ, Mallya UG, Friedman M, Panaccio M, Koren A, Neumann P, Menzin J. Patterns of statin use in a real‐world population of patients at high cardiovascular risk. J Manag Care Spec Pharm. 2016;22:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 21. Harrell FE Jr. rms: regression modeling strategies version 5.1‐0. 2017. Available at: https://cran.r-project.org/web/packages/rms/index.html. Accessed May 7, 2018.
- 22. Leibowitz M, Karpati T, Cohen‐Stavi CJ, Feldman BS, Hoshen M, Bitterman H, Suissa S, Balicer RD. Association between achieved low‐density lipoprotein levels and major adverse cardiac events in patients with stable ischemic heart disease taking statin treatment. JAMA Intern Med. 2016;176:1105–1113. [DOI] [PubMed] [Google Scholar]
- 23. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta‐analysis. JAMA. 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 24. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R; Cholesterol Treatment Trialists' (CTT) Collaborators . Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 25. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen MR, Tokgözoglu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL. Low‐density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, Pyörälä K, Miettinen T, Wilhelmsen L, Olsson AG, Wedel H. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004;5:81–87. [DOI] [PubMed] [Google Scholar]
- 27. de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. [DOI] [PubMed] [Google Scholar]
- 28. Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non‐insulin‐dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485. [DOI] [PubMed] [Google Scholar]
- 29. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–1009. [DOI] [PubMed] [Google Scholar]
- 30. Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet. 2002;360:7–22.12114036 [Google Scholar]
- 31. Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J. High‐dose atorvastatin vs usual‐dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. [DOI] [PubMed] [Google Scholar]
- 32. Long‐Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group . Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. [DOI] [PubMed] [Google Scholar]
- 33. Serruys PW, de Feyter P, Macaya C, Kokott N, Puel J, Vrolix M, Branzi A, Bertolami MC, Jackson G, Strauss B, Meier B. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;287:3215–3222. [DOI] [PubMed] [Google Scholar]
- 34. Post Coronary Artery Bypass Graft Trial Investigators . The effect of aggressive lowering of low‐density lipoprotein cholesterol levels and low‐dose anticoagulation on obstructive changes in saphenous‐vein coronary‐artery bypass grafts. N Engl J Med. 1997;336:153–163. [DOI] [PubMed] [Google Scholar]
- 35. Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Peto R, Collins R. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double‐blind randomised trial. Lancet. 2010;376:1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Affairs. USDoV . VA/DoD clinical practice guideline for the management of dislipidemia for cardiovascular risk reduction. 2014.
- 37. Force. UPST . Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. 2016. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-usein-adults-preventive-medication1?ds=1&s=statin. Accessed May 7, 2018.
- 38. Pasternak RC, Smith SC, Bairey‐Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024–1028. [DOI] [PubMed] [Google Scholar]
- 39. SPRINT Research Group . A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Keeffe AG, Nazareth I, Petersen I. Time trends in the prescription of statins for the primary prevention of cardiovascular disease in the United Kingdom: a cohort study using The Health Improvement Network primary care data. Clin Epidemiol. 2016;8:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The Relationship Between an Individual's Statin Intensity and the Statin Intensity at the End of Study Follow‐Up, Among All Patients, and Among Age‐Specific Subgroups of <75 Years of Age, and ≥75 Years)
Figure S1. The schematic diagram of the study design.
Figure S2. The distribution of LDL cholesterol during the first and last measurements among patients <75 years, and those aged ≥75 years.


