Abstract
Background
It remains unclear whether beta‐blockade is similarly effective in black patients with heart failure and reduced ejection fraction as in white patients, but self‐reported race is a complex social construct with both biological and environmental components. The objective of this study was to compare the reduction in mortality associated with beta‐blocker exposure in heart failure and reduced ejection fraction patients by both self‐reported race and by proportion African genetic ancestry.
Methods and Results
Insured patients with heart failure and reduced ejection fraction (n=1122) were included in a prospective registry at Henry Ford Health System. This included 575 self‐reported blacks (129 deaths, 22%) and 547 self‐reported whites (126 deaths, 23%) followed for a median 3.0 years. Beta‐blocker exposure (BBexp) was calculated from pharmacy claims, and the proportion of African genetic ancestry was determined from genome‐wide array data. Time‐dependent Cox proportional hazards regression was used to separately test the association of BBexp with all‐cause mortality by self‐reported race or by proportion of African genetic ancestry. Both sets of models were evaluated unadjusted and then adjusted for baseline risk factors and beta‐blocker propensity score. BBexp effect estimates were protective and of similar magnitude both by self‐reported race and by African genetic ancestry (adjusted hazard ratio=0.56 in blacks and adjusted hazard ratio=0.48 in whites). The tests for interactions with BBexp for both self‐reported race and for African genetic ancestry were not statistically significant in any model (P>0.1 for all).
Conclusions
Among black and white patients with heart failure and reduced ejection fraction, reduction in all‐cause mortality associated with BBexp was similar, regardless of self‐reported race or proportion African genetic ancestry.
Keywords: ancestry, beta‐blocker, disparity, genetics, genomics, heart failure, pharmacogenetics, pharmacogenomics, race
Subject Categories: Heart Failure; Clinical Studies; Genetic, Association Studies; Mortality/Survival; Race and Ethnicity
Clinical Perspective
What Is New?
Previous research suggested racial differences in beta‐blocker effectiveness for treating heart failure with reduced ejection fraction by self‐reported race.
We re‐examined beta‐blocker effectiveness by self‐reported race and by genetic ancestry to help distinguish biological differences by race (ie, the genetic component) from nonbiological components/correlates of race (eg, diet, socioeconomic status, and others).
We found that beta‐blocker treatment was associated with a similar reduction in the risk for mortality in self‐identified blacks compared with whites, regardless of genetic ancestry (overall proportion African genetic ancestry).
What Are the Clinical Implications?
This study provides strong reassurance that there is similar benefit of beta blockade in blacks with heart failure and reduced ejection fraction compared with white counterparts.
Introduction
The landmark clinical trials that established the efficacy of beta‐blockers in patients with heart failure (HF) with reduced ejection fraction (HFrEF) predominately consisted of white patients (80–99%),1, 2, 3, 4, 5, 6 despite evidence that black patients have higher risk for developing and dying from HF.7 Subgroup analyses of these pivotal trials show effect estimates that are consistent with treatment benefit across races (though very limited in terms of power),8, 9 and consensus guidelines reasonably recommend beta‐blocker use in all patients with HFrEF unless contraindicated.10 The extrapolation of clinical trial data from 1 patient race to another is an important issue because at least 29 medications (including beta‐blockers) are reported to have racial disparities in safety or efficacy,11 and this is clearly salient in the setting of HF.12 BEST (Beta Blocker Evaluation of Survival trial), which tested bucindolol in chronic HFrEF patients, showed differing effects depending on race with a trend toward harm among black patients.13, 14 Moreover, some observational data sets have suggested reduced efficacy of approved beta‐blockers in black HFrEF patients.15, 16
A complicating issue surrounding race‐based analyses is that race is a subjective social construct associated with a myriad of demographic, socioeconomic, comorbidity, and treatment differences that can confound estimates of treatment benefit.17 Moreover, it is imperfectly linked to genetic ancestry, which could indicate biological bases for difference in therapeutic responses, both risks and benefits. There are significant data suggesting that genetic variation, specifically many variants that are correlated to ancestral population, may also impact beta‐blocker effectiveness or HF disease progression.18, 19, 20, 21 Unraveling the limitations of these past reports of racial disparities in beta‐blocker efficacy among HFrEF patients requires genetic ancestry data that can objectively and quantitatively assess ancestral background. It is particularly important to include genetic ancestry in racial analyses because self‐reported race can substantially disagree with genetic ancestry, especially in genetically admixed populations such as in the United States. For example, self‐reported blacks tend to average ≈20% European genetic ancestry, but this ranges widely from near zero to majority European genetic ancestry.11, 22 To help evaluate the effectiveness of beta‐blocker use in blacks, as compared with whites, we developed a genetic HF registry and compared the association between beta‐blocker exposure (BBexp) and risk for all‐cause mortality between self‐identified whites and blacks, and then also tested the beta‐blockers’ associations with all‐cause mortality by proportion of African genetic ancestry.
Methods
Patient Data
The study was approved by the Institutional Review Board at the Henry Ford Health System, and all patients gave written informed consent before participation. Because of the sensitive nature of this research, the data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Patients for this study came from a prospective genetic registry of HF patients with the overall goal of discovering novel ways to better predict prognosis and response to HF treatments. The registry started in October 2007 and completed in March 2015 at the Henry Ford Health System, which is a vertically integrated health system serving the primary and specialty healthcare needs of individuals in southeastern Michigan. The health system includes several hospitals, a multispecialty physician group of ≈1200 physicians, as well as an affiliated insurance product (Health Alliance Plan; all subjects are members). Patients were included in the HF registry if they were aged ≥18 years, insured, and met the definition for HF as defined by the Framingham Heart Study.23 Specifically, patients must have had at least 2 major, or 1 major and 2 minor, HF criteria present at the time of exam or documented in the medical record. Major criteria were paroxysmal nocturnal dyspnea or orthopnea, neck vein distension, rales, cardiomegaly, acute pulmonary edema, S3 gallop, increased venous pressure (16 cm of water), circulation time (≥25 seconds), and hepatojugular reflux. Minor criteria were ankle edema, night cough, dyspnea on exertion, hepatomegaly, pleural effusion, vital capacity decreased 33% from maximum, and heart rate ≥120 beats per minute. Weight loss ≥4.5 kg in 5 days in response to treatment was a major or minor criterion. Patients were excluded from the registry if they were on dialysis or dependent on supplemental oxygen or dialysis. Detailed phenotypic information (eg, demographics, physical examination, past medical history, laboratory values, functional status, and medications) and blood samples were collected upon enrollment into the HF registry. Patient deaths were collected from the Social Security Administration Death Master File, Michigan State Division of Vital Records, and the Henry Ford Health System administrative data, through July 28, 2016. Only patients with HFrEF were included in this analysis. Patients with left ventricular ejection fraction (LVEF) <50% verified by echocardiography, nuclear stress tests, or radionuclide blood pool imaging were included in the primary analysis (n=1122). This ejection fraction cutoff was chosen to reflect patients with systolic HF because the study was designed and started before the more‐recent reclassifications, suggesting that HFrEF should be defined as an LVEF ≤40%.24 To address whether this could alter our findings, we performed a secondary analysis restricted to patients with LVEF ≤40% (n=794).
Calculation of BBexp
BBexp was calculated using dose standardization and pharmacy claims data as previously described.15 Briefly, doses of specific beta‐blockers were standardized into dose equivalents by the target dose used in clinical trials of HFrEF or, for beta‐blockers not tested in HF clinical trials (eg, atenolol), by the maximum daily dose. Specifically, these target/maximal daily doses were 50 mg for carvedilol, 200 mg for metoprolol (for both long‐acting and short‐acting formulations), 10 mg for bisoprolol, 100 mg for atenolol, and 600 mg for labetalol. For example, 25 mg of carvedilol per day (ie, 12.5 mg twice‐daily) was considered a 0.5 beta‐blocker standardized dose equivalent. Carvedilol (39%) and metoprolol succinate (38%) were the agents most often used, but there were smaller groups of patients using metoprolol tartrate (18%) or another beta‐blocker (4%).
BBexp was then calculated by multiplying the standardized dose equivalent by the quantity of medication dispensed in a 6‐month time block, divided by the total number of days in the 6‐month time block. BBexp was calculated for each patient for each day of observation, and thus this method accounts for both dose and adherence over a rolling period of time (6 months). For example, if a patient was prescribed 12.5 mg of carvedilol twice‐daily and had picked up their prescription from the pharmacy so that there was continuous availability over the previous 6 months, then their calculated BBexp would be 0.5. We have previously demonstrated that this approach for calculating BBexp is superior to a single time point and dichotomous classification of BBexp (eg, discharge medication status), in terms of correlation to heart rate and death or hospitalization.25
Genotyping and Genetic Ancestry Analysis
Blood samples were collected at enrollment into the HF registry and were immediately processed and stored at −70°C. Each sample was genotyped using the Axiom Biobank array (Affymetrix, Santa Clara, CA), which includes the following ≈600K genetic variants: (1) ≈300K genome‐wide variants with minor allele frequencies >1%; (2) ≈250K low frequency (<1%) coding variants from global exome sequencing projects; and (3) an additional ≈50K variants to improve African ancestry coverage (Yoruba in Ibadan, Nigeria [YRI] booster). The proportion of West African genetic ancestry (heretofore referred to as African ancestry) in each patient was estimated using ANCESTRYMAP2.0.26 Briefly, the software program uses a Hidden Markov Model to combine data across unlinked single‐nucleotide polymorphisms and incorporates information from many neighboring markers to infer ancestry.
Statistical Analysis
Continuous baseline variables were summarized by the mean±SD and compared by self‐reported race with 2‐sample Student t tests. Continuous baseline variables that were not normally distributed were compared by self‐reported race using the 2‐sample Mann–Whitney test. Categorical baseline variables were summarized by counts and percentages and compared by self‐reported race using χ2 tests or Fisher's exact tests, when appropriate. Time‐dependent Cox proportional hazards regression models were used to assess the relationship of BBexp with the primary end point of all‐cause mortality. BBexp was modeled as a continuous variable with a daily value ranging from 0 (pharmacy claims indicated that no beta‐blocker was available to the patient on any day throughout the 6 months preceding that day) to 1 (pharmacy claims indicated that target doses of beta‐blocker were available to the patient on every day throughout the 6 months preceding that day). Because BBexp was modeled as a continuous variable, the hazard ratios (HRs) for the association between BBexp and all‐cause mortality were scaled as zero exposure (0) versus target exposure (1). BBexp was only dichotomized when plotting survival curves (high exposure defined as ≥50th percentile and low exposure defined as <50th percentile). Two separate sets of models were made, 1 for self‐identified race (dichotomous variable) and another for genetic ancestry (continuous variable), that is, both factors were not in models together. The 2 sets of models were otherwise similar (ie, same covariates and end points). Interaction between either self‐reported race or proportion of genetic African ancestry and BBexp was tested by incorporating a multiplicative interaction term within the models for time to all‐cause mortality (eg, self‐identified race×BBexp). Models stratified by self‐identified race were also developed. The models were adjusted for the Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score27 (excluding beta‐blocker as an input variable), N‐terminal pro‐b‐type natriuretic peptide level, and beta‐blocker propensity score. Beta‐blocker propensity score was calculated using logistic regression of all variables in Table 1 with the output separated into quartiles and used as an ordinal (1–4) adjuster in the Cox regression models.28 For main effects, P<0.05 was considered statistically significant, and for the interaction, P<0.1 was considered statistically significant. In the analyses stratified by self‐reported race, we estimated 80% power to detect an HR ≤0.65 for beta‐blocker response, which is similar to the reduction in the risk for mortality reported in the landmark beta‐blocker clinical trials. The primary analysis of African genetic ancestry included genetic ancestry as a continuous variable, which was available in all subjects, and thus there was similar power for the analysis of African genetic ancestry and beta‐blocker survival benefit. However, when race was stratified by African genetic ancestry <5% and >80%, subjects with African genetic ancestry between 5% and 80% were excluded (n=27 self‐reported whites and n=165 self‐reported blacks excluded). However, the estimated power only decreased slightly when race was stratified by African genetic ancestry (but not when African genetic ancestry was used as a continuous variable) to have 80% power to detect an HR ≤0.63 for beta‐blocker survival benefit. All statistical analyses were performed in SAS software (version 9.4; SAS Institute Inc, Cary, NC).
Table 1.
Baseline Characteristics Overall and Stratified by Both Self‐Reported Race and Proportion of African Genetic Ancestry
| Characteristic | Overall (n=1122) | Self‐Reported Race | P Valuea | Proportion of African Genetic Ancestry | P Valueb | ||
|---|---|---|---|---|---|---|---|
| Black (n=575) 51% | White (n=547) 49% | >80% (n=410) 37% | <5% (n=520) 46% | ||||
| Female | 394 (35.1%) | 231 (40.2%) | 163 (29.8%) | <0.001c | 171 (41.7%) | 153 (29.4%) | <0.001c |
| Age, y | 67.5±11.9 | 64.4±12.1 | 70.8±10.8 | <0.001c | 63.8±11.8 | 70.7±10.8 | <0.001c |
| LVEF, % | 34.7±11.1 | 33.4±11.5 | 36.1±10.5 | <0.001c | 32.7±11.3 | 36.1±10.4 | <0.001c |
| Ischemic etiology | 494 (44.0%) | 192 (33.4%) | 302 (55.2%) | <0.001c | 124 (30.2%) | 295 (56.7%) | <0.001c |
| Hypertension | 977 (88.91%) | 530 (92.2%) | 467 (85.4%) | <0.001c | 383 (93.4%) | 442 (85.0%) | <0.001c |
| Chronic obstructive pulmonary disease | 246 (21.9%) | 118 (20.5%) | 128 (23.4%) | 0.244 | 87 (21.2%) | 121 (23.3%) | 0.456 |
| Chronic kidney disease | 251 (22.4%) | 158 (27.5%) | 93 (17.0%) | <0.001c | 123 (30.0%) | 84 (16.2%) | <0.001c |
| Atrial fibrillation | 311 (27.7%) | 113 (19.7%) | 198 (36.2%) | <0.001c | 76 (18.5%) | 188 (36.2% | <0.001c |
| Stroke/transient ischemic attack | 140 (12.5%) | 74 (12.9%) | 66 (12.1%) | 0.684 | 53 (12.9%) | 60 (11.5%) | 0.520 |
| Diabetes mellitus | 462 (41.21%) | 260 (45.2%) | 202 (36.9%) | 0.005c | 189 (46.1%) | 193 (37.1%) | 0.006c |
| Body mass index, kg/m2 | 31.1±7.3 | 31.4±7.6 | 30.8±7.1 | 0.198 | 31.3±7.7 | 30.8±7.1 | 0.301 |
| Systolic blood pressure, mm Hg | 129±23 | 131.2±24.0 | 126.6±21.7 | 0.001c | 131.7±24.2 | 125.9±21.5 | <0.001c |
| Heart rate, beats per min | 71.2±13.1 | 72.3±13.3 | 69.9±12.8 | 0.002c | 73.1±13.6 | 70.0±12.4 | <0.001c |
| NTpro‐BNP, pmol/L | 358±380 | 353±394 | 364±366 | 0.635 | 354±392 | 360±363 | 0.812 |
| Serum creatinine, mg/dL | 1.28±0.92 | 1.38±1.12 | 1.18±0.60 | 0.001c | 1.45±1.27 | 1.17±0.61 | <0.001c |
| MAGGIC risk score (w/o beta‐blocker) | 17.8±7.3 | 17.3±7.5 | 18.4±7.1 | 0.010c | 17.4±7.7 | 18.4±7.2 | 0.045c |
| BBexp | 26.6±29.0 | 26.4±28.5 | 26.7±29.6 | 0.852 | 26.0±29.0 | 27.0±36.0 | 0.626 |
| Any BBexp | 781 (76.9%) | 401 (76.2%) | 380 (77.6%) | 0.619 | 280 (74.7%) | 363 (77.7%) | 0.298 |
| ACE/ARB exposure | 616 (54.9%) | 331 (57.6%) | 285 (52.1%) | 0.066 | 238 (58.1%) | 272 (52.3%) | 0.081 |
| Proportion African genetic ancestry | 43.4±44.0 | 83.5±20.4 | 1.0±6.6 | <0.001c | NA | NA | NA |
| Length of follow‐up, d | 1089±699 | 1082±695 | 1097±703 | 0.737 | 1090±708 | 1109±708 | 0.699 |
| Deaths | 255 (22.7%) | 129 (22.4%) | 126 (23.0%) | 0.811 | 92 (22.4%) | 115 (22.1) | 0.906 |
ACE indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BBexp, beta‐blocker exposure; LVEF, left ventricular ejection fraction; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure risk score27; NA, not applicable; NTpro‐BNP, N‐terminal pro‐b‐type natriuretic peptide.
P value for self‐reported black vs white.
P value for >80% African genetic ancestry vs <5% African genetic ancestry.
P<0.05.
Results
The analytical cohort comprised 1122 HFrEF patients meeting inclusion criteria: 575 self‐reported black patients (51%) and 547 self‐reported white patients (49%). The overall group had a median follow‐up of 1089 days (range, 3–3516). There were a total of 255 deaths in the analysis, 129 among black patients (22%) and 126 in white patients (23%). Baseline characteristics overall and stratified by self‐reported race are presented in Table 1. Statistically significant differences at baseline were present between the groups for many characteristics, including age, sex, ischemic etiology, LVEF, and atrial fibrillation. Notably, there were no significant differences between race groups in terms of medication exposure, specifically including quantified BBexp, categorized BBexp (none versus any), or categorized angiotensin‐converting enzyme inhibitor or angiotensin receptor blockers. Genetic admixture was observed in the cohort within both race groups, but was more prominent in self‐identified blacks (Figure 1). Self‐reported whites had an average of 1% African genetic ancestry, whereas self‐reported blacks had an average of 16% European genetic ancestry.
Figure 1.
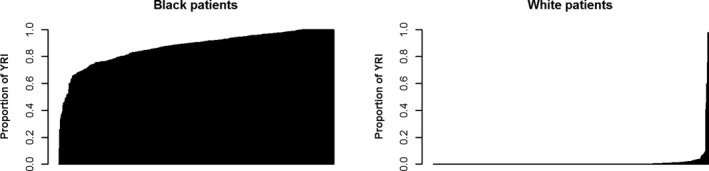
Proportion of African genetic ancestry in the self‐reported whites (left panel) and blacks (right panel). YRI indicates Yoruba in Ibadan, Nigeria.
Overall, the quantified beta‐blocker exposure metric (BBexp) varied from 0% to 100% across the cohort and over time. Figure 2 depicts the mean BBexp for each individual from least to most across the cohort. Roughly 25% of patients had no BBexp, whereas 55% had intermediate levels of BBexp and 20% had relatively high‐intensity BBexp. In the overall cohort, BBexp was associated with improved survival. Unadjusted analysis revealed BBexp HR of 0.47 (P=0.001). When adjusted for baseline Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) score (without the beta‐blocker variable input), N‐terminal pro‐b‐type natriuretic peptide, and self‐reported race (race is not a component of MAGGIC) the BBexp continued to be strongly protective with an adjusted HR (aHR) of 0.49 (P=0.005).
Figure 2.
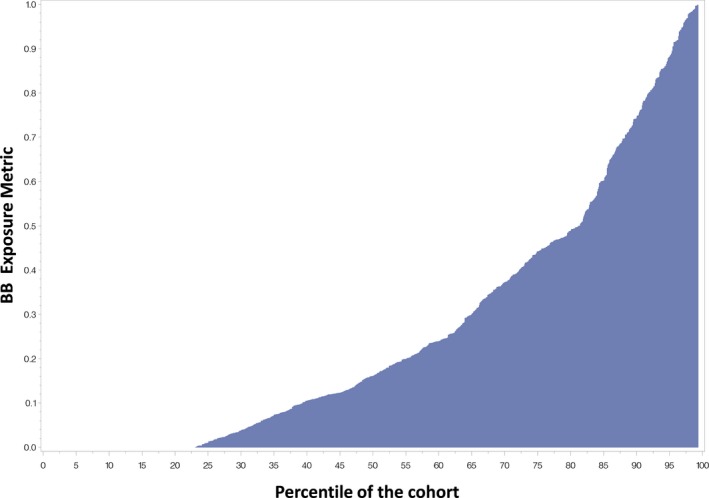
Mean beta‐blocker exposure metric (BBexp, Y axis) for each participant across the cohort (X axis).
Beta‐Blocker Association With Time to Death by Self‐Identified Race and Genetic Ancestry
We tested Cox models of BBexp on the time to death in univariable analysis and then analyses adjusted for baseline MAGGIC score (minus beta‐blocker input). In unadjusted analyses stratified by race, BBexp was strongly protective in both groups, though showing some numeric separation. Among white patients, BBexp HR was 0.41 (95% confidence interval, 0.22, 0.76; P=0.005), whereas in black patients the HR was 0.55 (95% confidence interval, 0.31, 0.98; P=0.041). Once adjusted for baseline risk using MAGGIC score alone, the HRs in each group were more closely aligned. For whites, the BBexp aHR was 0.45 (0.24, 0.86; P=0.016), whereas for black patients the aHR was 0.54 (0.30, 0.97; P=0.038), again statistically significant in both groups. Formal testing for the interaction of race with BBexp (ie, adding race×BBexp term in the model) was also not significant (β=0.17; P=0.70). The relationship of BBexp to survival, stratified by race, is presented in Table 2 and illustrated in Figure 3.
Table 2.
Association of BBexp With Time to All‐Cause Mortality in All Patients and Stratified by Both Self‐Reported Race and Proportion of African Genetic Ancestry
| Variable | All Patients HR (95% CI) n=1122 | P Value | Self‐Reported Race | Proportion of African Genetic Ancestry | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| White HR (95% CI) n=547 | P Value | Black HR (95% CI) n=575 | P Value | <5% HR (95% CI) n=520 | P Value | >80% HR (95% CI) n=410 | P Value | |||
| BBexp | 0.46 (0.24, 0.89) | 0.020a | 0.48 (0.25, 0.93) | 0.029a | 0.56 (0.31, 1.03) | 0.062 | 0.45 (0.23, 0.89) | 0.022a | 0.47 (0.23, 0.98) | 0.045a |
| Black | 0.95 (0.70, 1.31) | 0.765 | NA | NA | NA | NA | ||||
| NTpro‐BNP | 1.47 (1.32, 1.65) | 0.001a | 1.55 (1.30, 1.84) | 0.001a | 1.38 (1.18, 1.60) | 0.001a | 1.58 (1.31, 1.80) | 0.001a | 1.39 (1.13–1.70) | 0.002a |
| MAGGIC | 1.11 (1.09, 1.13) | 0.001a | 1.11 (1.08, 1.15) | 0.001a | 1.11 (1.07, 1.14) | 0.001a | 1.12 (1.08, 1.15) | 0.001a | 1.10 (1.07, 1.14) | 0.001a |
| BB propensity | 0.88 (0.78, 0.99) | 0.038a | 0.76 (0.64, 0.91) | 0.003a | 1.01 (0.85, 1.19) | 0.933 | 0.76 (0.63, 0.91) | 0.003a | 0.97 (0.80, 1.18) | 0.781 |
| BBexp×race | BBexp×self‐reported race | β=0.21 | 0.642 | BBexp×African Genetic Ancestry | β=0.11 | 0.823 | ||||
BB indicates beta‐blocker; BBexp, beta‐blocker exposure; CI, confidence interval; HR, hazard ratio; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure risk score27; NA, not applicable; NTpro‐BNP, N‐terminal pro‐b‐type natriuretic peptide (HR scaled by 400 units).
P<0.05.
Figure 3.
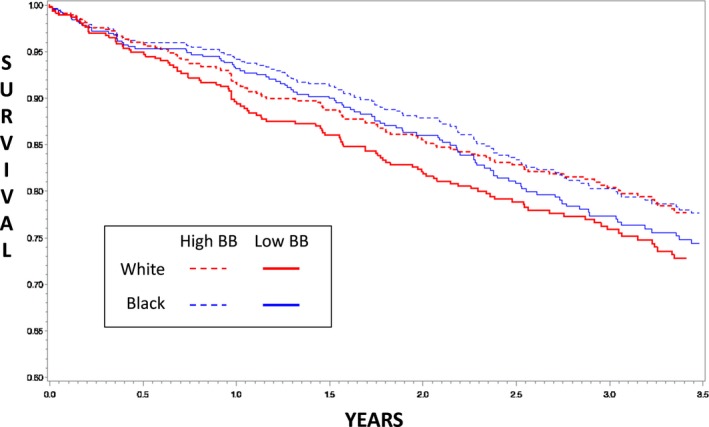
Survival curves stratified by self‐reported race and high vs low beta‐blocker (BB) exposure. High beta‐blocker exposure was defined as ≥50th percentile (dashed lines) and low beta‐blocker exposure as <50th percentile (solid lines). Red lines are self‐reported blacks and blue lines are self‐reported whites.
We then sought to examine whether genetic ancestry, a better reflection of genetic differences across race (rather than self‐identified race), modulated the association of BBexp with survival times. Specifically, we estimated proportion of African ancestry for each individual and then tested similar time‐dependent Cox models as above, but with proportion of African genetic ancestry included as a numerical covariate and with interaction terms. The BBexp effect did not appear to differ across the spectrum of African genetic ancestry. In models including BBexp, MAGGIC, and proportion African genetic ancestry, genetic ancestry was not statistically significant (P=0.77), and the BBexp effect estimate was similar to the above (aHR=0.50; P=0.002). Formal testing with interaction term (BBexp×ancestry) was also not statistically significant (P=0.71). To better illustrate this lack of impact of African genetic ancestry on beta‐blocker effectiveness, if the BBexp HR is tabulated using a proportion African genetic ancestry of <5% versus >80%, the unadjusted HR generated are 0.70 and 0.72, respectively. A comparison of HRs for high versus low BBexp in each race category is presented in Table 3.
Table 3.
HRs and 95% CIs for High Beta‐Blocker Exposure (≥50th Percentile) versus Low Beta‐Blocker Exposure (<50th Percentile) in the Self‐Reported Races and Patients With <5% and >80% Proportion African Genetic Ancestry
| BBexp | Race Category | |||
|---|---|---|---|---|
| Self‐Report | Genetic | |||
| White | Black | <5% African | >80% African | |
| High vs low |
n=516 0.74 (0.52–1.06) P=0.102 |
n=543 0.69 (0.49–0.99) P=0.045a |
n=490 0.71 (0.49–1.03) P=0.071 |
n=388 0.64 (0.42–0.98) P=0.038a |
| Interaction P value | P=0.979 | P=0.992 | ||
All models were adjusted for MAGGIC risk score (minus beta‐blocker) and NTpro‐BNP level, and the sample sizes were for patients with complete data available for analysis. BBexp indicates beta‐blocker exposure; CI, confidence interval; HR, hazard ratio; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure risk score27; NTpro‐BNP, N‐terminal pro‐b‐type natriuretic peptide.
indicates P < 0.05.
Sensitivity Analyses
We performed several secondary analyses to assess whether our results were impacted by certain potential confounders or classification schemes. First, because this was an observational study, confounding by indication or disease severity is always a concern. The primary analysis plan included adjustment for baseline risk by using a validated clinical risk score (MAGGIC). To supplement this, we additionally tested models adjusted for baseline N‐terminal pro‐b‐type natriuretic peptide levels and propensity for beta‐blocker use. In terms of overall beta‐blocker effect, when including propensity as the only covariate, the BBexp effect remains very consistent with the unadjusted overall analysis (aHR of 0.50; P=0.002). The results were also similar in models adjusted for MAGGIC, race, N‐terminal pro‐b‐type natriuretic peptide, and propensity (Table 2). Importantly, in the full model, the race×BBexp interaction remains insignificant (β=0.21; P=0.64), race was not a significant predictor of outcome, and in the race‐stratified models the BBexp aHR were relatively similar (BBexp aHR 0.48 for whites and 0.56 blacks), with the estimates for each race group indicating a strong protective association.
To address potential concerns regarding the fact that ejection fraction <50% was part of the inclusion criteria (as opposed to ≤40%), we performed additional analyses restricted to those patients with LVEF ≤40%. Among the 1122 total subjects, 794 had ejection fraction ≤40% (n=370 white patients and n=424 black patients). In these subgroups, the BBexp HR was 0.58 (P=0.14) and 0.59 (P=0.09) for whites and blacks, respectively. In the full model of all patients with ejection fraction ≤40% (n=717) adjusted for MAGGIC, race, and race×BBexp interaction, the BBexp HR was 0.61 (0.38, 0.98; P=0.040) and the interaction was not significant (P=0.78).
Discussion
Definitive evidence for the efficacy of beta‐blockers in blacks with HFrEF will likely never be obtained given that the pivotal trials did not include a sufficient number of such participants and further randomized trials would likely be deemed unethical. Whereas the preponderance of evidence suggests a strong benefit, this prospective, observational study extends past studies by both using self‐reported race as a potential marker of a cluster of factors that differ and genetic ancestry as a quantifiable and objective biological construct. For example, if we had found a significant difference in beta‐blocker response by self‐reported race and not genetic ancestry, then that could have suggested that the difference is likely attributed to sociocultural differences rather than biological. The fact that we found similar reductions in mortality stratified by self‐reported race (representative of biological+sociocultural effects) and genetic ancestry (representative of only biological effects) is reassuring that beta‐blockers are equally effective in white and black patients. We found a marked reduction in mortality associated with beta‐blocker treatment that was similar in blacks and whites, regardless of race or genetic ancestry. Compared with the landmark trials for HF‐approved beta‐blockers, our study includes roughly the same number of black patients (n=575) as the number of black patients in the MERIT‐HF (Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure), US Carvedilol Trial, COPERNICUS (Carvedilol Prospective Randomized Cumulative Survival Trial), and CIBIS‐II (second Cardiac Insufficiency Bisoprolol Study) combined (n=546). The BEST trial of the non–US Food and Drug Administration–approved bucindolol included 627 black patients, but treatment with bucindolol trended toward increased all‐cause mortality in the black patients and a significant test for interaction of treatment benefit with patient race, further supporting the importance of this race‐stratified analysis. This study is the first to examine this issue by quantified genetic ancestry, which can potentially offer a more‐granular view of the biological portion of race. Previous studies on the genetic differences in beta‐blocker response have relied on candidate gene approaches, particularly candidate polymorphisms affecting adrenergic activity.29 Adverse genetic polymorphisms in the adrenergic system that are associated with decreased beta‐blocker response are most frequent in blacks. The strength of our approach using whole‐genome ancestry informative markers is that it captures ancestral variation across the entire genome, and it accounts for population substructure, a well‐known phenomenon that confounds genetic association studies.30
Our data provide additional insight in the context of past literature. Our findings are similar to previous observational studies investigating racial differences in beta‐blocker response in HF patients,16 which found protective, though nonsignificant, HRs for mortality, similar to the clinical trial data for carvedilol and metroprolol succinate in blacks. Our data show a significant benefit in blacks, and add a major advantage compared with previous observational studies, in that we utilized time‐dependent quantified BBexp calculated from pharmacy claims. This method is a far more‐granular and sensitive way of quantifying drug exposure (accounts for adherence, dose variability, and changes over time) compared with dichotomized baseline classification schemes, which were usually used in the previous studies. Our data do contrast with the BEST trial findings, as well as our previous retrospective study,15 which yielded a statistically significant race interaction for beta‐blocker–associated benefit (ie, increased beta‐blocker benefit in whites compared with blacks for the composite end point of all‐cause mortality plus hospitalization). Given the totality of data, the BEST findings seem most easily explained as an agent‐specific effect, given that several studies with the other beta‐blocking agents concur with our current observations.31, 32 The contrast with our previous data is most likely attributed to differences in the end point used. Our previous work utilized a composite end point including hospitalization, and indeed when examining our previous data, the differences between racial groups were driven primarily by hospitalization.15 All‐cause mortality alone (used in this prospective study) may be a better end point than the composite end point of all‐cause mortality+hospitalization (used in our previous, retrospective study) for evaluating beta‐blocker efficacy. Hospitalization of HF patients is influenced by factors that are unrelated to beta‐blocker efficacy more than all‐cause mortality, such as patient refusal, lack of available beds, the number of cardiologists, whether the patient came to the hospital from home or a skilled nursing facility, and even the day of the week.33, 34, 35, 36 Ultimately, hospitalization is the discretion of the treating physician in the emergency room or clinic. Beta‐blockers decreased the risk of hospitalization in the landmark clinical trials, but as noted previously, the enrollment of blacks in the landmark clinical trials was extremely low. Recent data show that hospitalization rates significantly differ by race,34 and thus inclusion of hospitalization in the end point may confound the results in race‐stratified analyses such as this study.
An additional strength of this study was the inclusion of genetic ancestry to explore more biologically based mechanisms of potential racial differences in beta‐blocker effectiveness. This is an important consideration because of the known and significant genetic admixture in the United States,17 as well as the complexity of self‐identified race as a social construct. The availability of genetic ancestry can help differentiate true inherited differences versus the wide range of environmental factors that are associated with self‐identified race, such as socioeconomic status, diet, and healthcare quality and accessibility, all of which can make attempts to understand the underlying cause of race disparities in health outcomes very difficult.37 It is important to interrogate the role of genetic ancestry because socioeconomic factors do not fully explain the critical race disparities in HF outcomes,38 and to try to quantify potential genetic and biological effects. For example, African genetic ancestry is associated with poorer diastolic function parameters in HF patients.39 Consistent with the literature, substantial genetic admixture was observed in our patient population, and despite the potential for differences in outcomes in self‐reported race versus genetically defined ancestry, the reduction in the risk for mortality from beta‐blockers in our study was mostly similar across the entire spectrum. The fact that even quantified African genetic ancestry proportion, a granular marker of genetic race, was also not associated with differences in beta‐blocker benefit provides some additional reassurance of equal effectiveness in blacks.
Limitations
Our study should be interpreted in the context of the following potential limitations. Whereas an observational study can never definitively assess absolute efficacy such as in a clinical trial, we have taken great care to adjust for potential confounders. We sought to mitigate confounders inherent in observational study design by adjusting with several methods, including a comprehensive and previously validated clinical score, a biomarker, and a beta‐blocker propensity score. Supporting our external validity is that the beta‐blocker benefit estimates were similar to those expected from clinical trials, and overall were statistically significant. Another limitation of our study was that beta‐blockers approved specifically for HF (ie, carvedilol, metoprolol succinate, and bisoprolol) were not distinguished from other beta‐blockers. However, of the patients who were taking beta‐blockers at baseline, the vast majority (78%) were taking HF‐approved agents; the most frequent nonapproved agent being metoprolol tartrate (18%), and only 4% of patients taking some other beta‐blocker. Despite inclusion of other beta‐blockers and low beta‐blocker treatment rate, an advantage of this observational study design is that it more closely represents current, real‐world clinical practice than the older randomized controlled trials. Moreover, a range of beta‐blocker treatment (from zero, to low, to target exposure) allows analysis of beta‐blocker benefit, which would not be possible if all patients were treated with target doses of beta‐blockers. Even with inclusion of other beta‐blockers, our results are reassuring given that overall the beta‐blocker survival benefit was statistically significant in both groups and of a magnitude which approximates the findings from randomized trials of approved beta‐blocking agents. Finally, our data are from insured patients in a single health system, so although our service population is diverse and reflects the greater regional population,40 the fact that all patients had insurance and access to care may somewhat limit the generalizability.
Conclusions
Our prospective, observational study demonstrates that beta‐blocker–associated reduction in the risk for mortality in HFrEF patients is similar between self‐reported black race and genetically assigned African race, as compared with whites. We further demonstrate that the overall proportion of African genetic ancestry, defined by genome‐wide ancestry informative markers, does not modify the beta‐blocker benefit. These data lend further credence to current guidelines that recommend beta‐blocker use in all HFrEF patients, reassuring patients and providers that black HFrEF patients are likely deriving similar benefit from beta‐blocker treatment. These findings are not suggestive of genetic mechanisms meaningfully impacting beta‐blocker effectiveness relative to race.
Sources of Funding
This research was supported by the National Heart, Lung, and Blood Institute (Lanfear R01HL103871, R01HL132154; Williams R01HL118267; Sabbah P01HL074237, R01HL132154). Dr Williams is also supported by the National Institute of Allergy and Infectious Diseases (R01AI079139) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK064695, R01DK113003). Dr Luzum was supported by the NIH student loan repayment program (L30 HL110279).
Disclosures
None.
(J Am Heart Assoc. 2018;7:e007956 DOI: 10.1161/JAHA.117.007956.)29739794
References
- 1. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 2. MERIT‐HF Investigators . Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT‐HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 3. Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann‐Zalan I, DeMets DL; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group . Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194–2199. [DOI] [PubMed] [Google Scholar]
- 4. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 5. Goldstein S. Beta blocker therapy in African American patients with heart failure. Heart Fail Rev. 2004;9:161–167. [DOI] [PubMed] [Google Scholar]
- 6. Randomised, placebo‐controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Australia/New Zealand Heart Failure Research Collaborative Group. Lancet. 1997;349:375–380. [PubMed] [Google Scholar]
- 7. Writing Group Members , Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee, Stroke Statistics Subcommittee . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e60. [DOI] [PubMed] [Google Scholar]
- 8. Yancy CW, Fowler MB, Colucci WS, Gilbert EM, Bristow MR, Cohn JN, Lukas MA, Young ST, Packer M; U.S. Carvedilol Heart Failure Study Group . Race and the response to adrenergic blockade with carvedilol in patients with chronic heart failure. N Engl J Med. 2001;344:1358–1365. [DOI] [PubMed] [Google Scholar]
- 9. Goldstein S, Deedwania P, Gottlieb S, Wikstrand J; MERIT‐HF Study Group . Metoprolol CR/XL in black patients with heart failure (from the metoprolol CR/XL randomized intervention trial in chronic heart failure). Am J Cardiol. 2003;92:478–480. [DOI] [PubMed] [Google Scholar]
- 10. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 11. Tate SK, Goldstein DB. Will tomorrow's medicines work for everyone? Nat Genet. 2004;36:S34–S42. [DOI] [PubMed] [Google Scholar]
- 12. Taylor JS, Ellis GR. Racial differences in responses to drug treatment: implications for pharmacotherapy of heart failure. Am J Cardiovasc Drugs. 2002;2:389–399. [DOI] [PubMed] [Google Scholar]
- 13. Beta‐Blocker Evaluation of Survival Trial Investigators . A trial of the beta‐blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. [DOI] [PubMed] [Google Scholar]
- 14. Domanski MJ, Krause‐Steinrauf H, Massie BM, Deedwania P, Follmann D, Kovar D, Murray D, Oren R, Rosenberg Y, Young J, Zile M, Eichhorn E; BEST Investigators . A trial of the beta‐blocker bucindolol in patients with advanced chronic heart failure. J Card Fail. 2003;9:354–363. [DOI] [PubMed] [Google Scholar]
- 15. Lanfear DE, Hrobowski TN, Peterson EL, Wells KE, Swadia TV, Spertus JA, Williams LK. Association of beta‐blocker exposure with outcomes in heart failure differs between African American and white patients. Circ Heart Fail. 2012;5:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cresci S, Kelly RJ, Cappola TP, Diwan A, Dries D, Kardia SL, Dorn GW II. Clinical and genetic modifiers of long‐term survival in heart failure. J Am Coll Cardiol. 2009;54:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mersha TB, Abebe T. Self‐reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum Genomics. 2015;9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cresci S, Dorn GW II, Jones PG, Beitelshees AL, Li AY, Lenzini PA, Province MA, Spertus JA, Lanfear DE. Adrenergic‐pathway gene variants influence beta‐blocker‐related outcomes after acute coronary syndrome in a race‐specific manner. J Am Coll Cardiol. 2012;60:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, Diwan A, Martini JS, Sparks L, Parekh RR, Spertus JA, Koch WJ, Kardia SL, Dorn GW II. A GRK5 polymorphism that inhibits beta‐adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lobmeyer MT, Gong Y, Terra SG, Beitelshees AL, Langaee TY, Pauly DF, Schofield RS, Hamilton KK, Herbert Patterson J, Adams KF Jr, Hill JA, Aranda JM Jr, Johnson JA. Synergistic polymorphisms of beta1 and alpha2C‐adrenergic receptors and the influence on left ventricular ejection fraction response to beta‐blocker therapy in heart failure. Pharmacogenet Genomics. 2007;17:277–282. [DOI] [PubMed] [Google Scholar]
- 21. Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1‐ and alpha2C‐adrenergic receptors and the risk of congestive heart failure. N Engl J Med. 2002;347:1135–1142. [DOI] [PubMed] [Google Scholar]
- 22. Smith MW, Patterson N, Lautenberger JA, Truelove AL, McDonald GJ, Waliszewska A, Kessing BD, Malasky MJ, Scafe C, Le E, De Jager PL, Mignault AA, Yi Z, De The G, Essex M, Sankale JL, Moore JH, Poku K, Phair JP, Goedert JJ, Vlahov D, Williams SM, Tishkoff SA, Winkler CA, De La Vega FM, Woodage T, Sninsky JJ, Hafler DA, Altshuler D, Gilbert DA, O'Brien SJ, Reich D. A high‐density admixture map for disease gene discovery in African Americans. Am J Hum Genet. 2004;74:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 24. Writing Committee Members , Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 25. Lanfear DE, Peterson E, Wells K, Williams LK. Discharge medication status compares poorly with claims‐based outpatient medication exposure estimates. Circ Cardiovasc Qual Outcomes. 2011;4:AP234. [Google Scholar]
- 26. Patterson N, Hattangadi N, Lane B, Lohmueller KE, Hafler DA, Oksenberg JR, Hauser SL, Smith MW, O'Brien SJ, Altshuler D, Daly MJ, Reich D. Methods for high‐density admixture mapping of disease genes. Am J Hum Genet. 2004;74:979–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN; Meta‐Analysis Global Group in Chronic Heart Failure . Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–1413. [DOI] [PubMed] [Google Scholar]
- 28. D'Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med. 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 29. Talameh JA, Lanfear DE. Pharmacogenetics in chronic heart failure: new developments and current challenges. Curr Heart Fail Rep. 2012;9:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome‐wide association studies. Hum Mol Genet. 2008;17:R143–R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abraham WT, Massie BM, Lukas MA, Lottes SR, Nelson JJ, Fowler MB, Greenberg B, Gilbert EM, Franciosa JA; COHERE Participant Physicians . Tolerability, safety, and efficacy of beta‐blockade in black patients with heart failure in the community setting: insights from a large prospective beta‐blocker registry. Congest Heart Fail. 2007;13:16–21. [DOI] [PubMed] [Google Scholar]
- 32. Shekelle PG, Rich MW, Morton SC, Atkinson CS, Tu W, Maglione M, Rhodes S, Barrett M, Fonarow GC, Greenberg B, Heidenreich PA, Knabel T, Konstam MA, Steimle A, Warner Stevenson L. Efficacy of angiotensin‐converting enzyme inhibitors and beta‐blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta‐analysis of major clinical trials. J Am Coll Cardiol. 2003;41:1529–1538. [DOI] [PubMed] [Google Scholar]
- 33. Shah M, Patnaik S, Patel B, Arora S, Patel N, Lahewala S, Figueredo VM, Martinez MW, Jacobs L. The day of the week and acute heart failure admissions: relationship with acute myocardial infarction, 30‐day readmission rate and in‐hospital mortality. Int J Cardiol. 2017;249:292–300. [DOI] [PubMed] [Google Scholar]
- 34. Mirkin KA, Enomoto LM, Caputo GM, Hollenbeak CS. Risk factors for 30‐day readmission in patients with congestive heart failure. Heart Lung. 2017;46:357–362. [DOI] [PubMed] [Google Scholar]
- 35. O'Connor M, Murtaugh CM, Shah S, Barron‐Vaya Y, Bowles KH, Peng TR, Zhu CW, Feldman PH. Patient characteristics predicting readmission among individuals hospitalized for heart failure. Med Care Res Rev. 2016;73:3–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365:2287–2295. [DOI] [PubMed] [Google Scholar]
- 37. Spertus JA, Jones PG, Masoudi FA, Rumsfeld JS, Krumholz HM. Factors associated with racial differences in myocardial infarction outcomes. Ann Intern Med. 2009;150:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rathore SS, Foody JM, Wang Y, Smith GL, Herrin J, Masoudi FA, Wolfe P, Havranek EP, Ordin DL, Krumholz HM. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. JAMA. 2003;289:2517–2524. [DOI] [PubMed] [Google Scholar]
- 39. Bernardez‐Pereira S, Gioli‐Pereira L, Marcondes‐Braga FG, Santos PC, Spina JM, Horimoto AR, Santos HC, Bacal F, Fernandes F, Mansur AJ, Pietrobon R, Krieger JE, Mesquita ET, Pereira AC. Genomic ancestry as a predictor of haemodynamic profile in heart failure. Open Heart. 2016;3:e000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schulz A, Israel B, Williams D, Parker E, Becker A, James S. Social inequalities, stressors and self reported health status among African American and white women in the Detroit metropolitan area. Soc Sci Med. 2000;51:1639–1653. [DOI] [PubMed] [Google Scholar]


